Abstract
The migration of vascular endothelial cells in vivo occurs in a fluid dynamic environment due to blood flow, but the role of hemodynamic forces in cell migration is not yet completely understood. Here we investigated the effect of shear stress, the frictional drag of blood flowing over the cell surface, on the migration speed of individual endothelial cells on fibronectin-coated surfaces, as well as the biochemical and biophysical bases underlying this shear effect. Under static conditions, cell migration speed had a bell-shaped relationship with fibronectin concentration. Shear stress significantly increased the migration speed at all fibronectin concentrations tested and shifted the bell-shaped curve upwards. Shear stress also induced the activation of Rho GTPase and increased the traction force exerted by endothelial cells on the underlying substrate, both at the leading edge and the rear, suggesting that shear stress enhances both the frontal forward-pulling force and tail retraction. The inhibition of a Rho-associated kinase, p160ROCK, decreased the traction force and migration speed under both static and shear conditions and eliminated the shear-enhancement of migration speed. Our results indicate that shear stress enhances the migration speed of endothelial cells by modulating the biophysical force of tractions through the biochemical pathway of Rho-p160ROCK.
INTRODUCTION
The migration of vascular endothelial cells (ECs), which form the inner lining of blood vessels, is involved in many normal and diseased conditions. For example, the migration of ECs into the underlying basement membrane is a critical step to initiate the formation of new blood vessels (i.e., angiogenesis). In wound healing circumstances, such as the denudation of ECs by balloon angioplasty or bypass surgery, the migration of nearby ECs into the injured area is crucial for reconstituting an intact monolayer and thus preventing the development of restenosis. A better understanding of the process and mechanism of EC migration may provide a rational base for developing strategies to control EC migration under pathological conditions.
The physical process involved in the migration of ECs is similar to that in the migration of anchorage-dependent cell types in general, which consists of cycles of 1), the extension of the leading edge and its adhesion to the extracellular matrix (ECM), 2), the generation of contractile force by the actin-myosin cytoskeleton for the forward translocation of the cell body, which is transmitted to the underlying substrate as a traction force, and, the last step, 3), the release of adhesions at the rear; these steps all involve the continuous rearrangement of cytoskeletal elements and dynamic cell-ECM interactions (Lauffenburger and Horwitz, 1996; Sheetz et al., 1997). There have been many studies on the mechanisms regulating the migration of ECs in response to several extracellular cues such as growth factors and ECM modifications (see Shuster and Herman, 1998, for review). However, the modulation of EC migration by the fluid dynamic environment, which is unique to ECs because of their direct contact with blood flow, and the molecular basis underlying this flow effect have not yet been well understood.
ECs in vivo are constantly subjected to shear stress, which is the tangential element of hemodynamic forces and has been shown to modulate the gene expression and function of ECs (Chien et al., 1998; Papadaki and Eskin, 1997). In this study, we investigated the effect of shear stress on the migration speed of individual ECs on surfaces coated with fibronectin (FN), a physiologically relevant ECM protein. Because it has been shown that growth factors may decrease or increase cell motility depending on the matrix density (Maheshwari et al., 1999; Ware et al., 1998), the shear effect on EC migration speed was examined over a range of FN density. Furthermore, we tested the hypothesis that shear stress may modify the activity of the migration machineries of the EC and its interaction with ECM, thereby modulating cell migration speed. Special emphases were placed on the activation of Rho GTPase and the traction force exerted by ECs on the underlying substrate, which is the driving force for cell movement.
Rho belongs to a Ras-related Rho protein family that also includes Rac and Cdc42. Like other members of the Ras superfamily, these Rho proteins act as molecular switches to control cellular processes by cycling between an active GTP-bound state and an inactive GDP-bound state (Hall and Nobes, 2000; Van and D'Souza, 1997). The activation of Rho is known to increase the actin-myosin contractility and subsequently the formation of stress fibers and focal adhesions through a Rho-associate kinase p160ROCK (Amano et al., 1997; Chrzanowska and Burridge, 1996). Recent investigations have implicated Rho as a mediator in the shear stress-induced cytoskeletal alignments and directional migration of the ECs (Tzima et al., 2001; Wojciak-Stothard and Ridley, 2003). Our study was focused on the role of Rho as a mechanosensitive motor in the shear modulation of EC migration speed and on the interplay between biochemical pathways and biophysical forces underlying this shear modulation.
Here we show that shear stress increases the migration speed and Rho activity of the ECs over a range of FN density. The inhibition of the Rho-associated kinase p160ROCK eliminates the shear-enhancement of migration speed. Using the traction force microscopy technique (Munevar et al., 2001a; Reinhart-King et al., 2003), we found that migrating ECs under flow exert stronger traction force and that this induction by shear may reflect an increase in cellular contractility mediated by the Rho-p160ROCK pathway. Taken together, these findings provide and correlate the biochemical and biophysical bases (the Rho-p160ROCK pathway and traction force, respectively) underlying the shear stress enhancement of EC migration speed.
MATERIALS AND METHODS
Cell culture
Cell culture reagents were obtained from GibcoBRL (Grand Island, NY) unless otherwise mentioned. Bovine aortic ECs (BAECs) were isolated from the bovine aorta with collagenase as described (Li et al., 1999) and were cultured in flasks in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin, 100 μg/ml streptomycin, and 0.292 mg/ml l-glutamine. Cell culture was maintained in a humidified 5% CO2/95% air incubator at 37°C. All experiments were conducted with cultures before passage 15.
Preparation of FN-coated slides and inhibitor treatment for ECs
Bovine FN (Sigma, St. Louis, MO) was diluted in phosphate-buffered saline (PBS) and applied as a thin film onto 75 × 38 mm sterile glass slides (Fisher, Pittsburgh, PA) at a final surface concentration of 0.5, 1, 5, 20, or 40 μg/cm2. The FN solution was incubated on slides for 2 h at room temperature to allow protein adsorption. The coated slides were washed with PBS three times, and the nonspecific binding sites were blocked with 1% heat-deactivated bovine serum albumin for 1 h at room temperature. BAECs were then plated on FN-coated slides at ∼10% confluence (∼104 cells/cm2) and covered with DMEM containing 0.5% FBS for 2 h before shear stress experiments started. The low serum condition and short spreading time served to avoid the deposition of ECM proteins from serum on the FN-coated surface and the de novo synthesis of ECM proteins by ECs.
Y27632, a specific inhibitor of the Rho-associated kinase p160ROCK (Ishizaki et al., 2000; Uehata et al., 1997), was provided by Welfide (Osaka, Japan). When Y27632 was used, subconfluent BAECs were plated on FN-coated slides for 2 h as described above and then incubated with Y27632 at 10 μM for 30 min. The treated cells were washed three times with PBS to remove the inhibitors, and next were subjected to shear stress or kept as static controls.
Shear stress experiments
A recirculating flow system was used to impose shear stress on cultured ECs as described (Frangos et al., 1985). In brief, a 75 × 38 mm glass slide with ECs formed the floor of a rectangular flow channel (0.025 cm in height, 2.5 cm in width, and 5 cm in length) created by sandwiching a silicone gasket (Specialty Manufacturing, Saginaw, MI) between the glass slide and a custom-made acrylic plate. The channel had inlet and outlet for perfusing the cultured cells. A laminar shear stress of 12 dyn/cm2 was generated by the flow resulting from the height difference between two reservoirs. During the flow experiments, the system was kept at 37°C in a constant-temperature hood, and the circulation medium (DMEM supplemented with 0.5% FBS) was ventilated with a humidified gas mixture of 95% air and 5% CO2. The shear stress of 12 dyn/cm2 is within the physiological range found in human major arteries and has been shown to activate EC signal transduction and induce the expression of several genes in vitro (Chien et al., 1998). All shear stress experiments included static controls, i.e., ECs cultured on slides not exposed to shear stress.
Cell migration studies
The migration of ECs under static and shear conditions was monitored by time-lapse microscopy using a Nikon inverted microscope with a 10× objective and a 10× eyepiece. Phase contrast images of the cells were acquired at 10-min intervals with an analytical charge-coupled device (CCD) camera (Model 72S, DAGE/MTI, Michigan City, IN) and transferred from a frame grabber to computer storage. The Dynamic Image Analysis System software (Solltech, Oakdale, IA) was used to determine the x, y coordinates of cell centroids from the phase images and to track the path of each cell during migration. The migration speed of ECs (VEC) was calculated by quantifying the centroid displacement every 10 min over a 2-h period; the direction of the displacement over the 10-min interval was not considered. The 10-min interval was empirically chosen as the time window because it was long enough to distinguish the actual locomotion from cell shape alterations or instrument noises but was short enough to prevent overly smoothed cell paths. The 2-h period was long enough to obtain reproducible information on cell speed while ECs remained motile in the low serum environment. Only isolated and well-spread cells were used for migration analysis. VEC is shown as mean ± SE. Each condition had at least 60 cells pooled from three independent experiments. Statistical tests were performed using the commercial software StatView (SAS, Cary, NC). Difference between sets of experiments was considered as statistically significant if p-value < 0.05 by the paired t-test or analysis of variance (ANOVA) post hoc tests (with Fisher's PLSD).
Rho activity assays
The activity of Rho GTPase was determined by an affinity-precipitation assay (Ren et al., 1999). BAECs were plated on glass slides at ∼10% confluence and covered with DMEM supplemented with 0.5% FBS for 2 h. These cells were then subjected to shear stress at 12 dyn/cm2 or kept as static controls. Thereafter, the BAECs were lysed with a radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl, pH 7.2, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 500 mM NaCl, 10 mM MgCl2, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 1 mM phenylmethylsulfonyl fluoride (PMSF); cell lysates from three slides in the same experiment were pooled together and centrifuged at 13,000 × g at 4°C for 10 min. The supernatant was incubated with 20 μg GST-RBD beads (Ren et al., 1999) for 45 min at 4°C. Bead-bound Rho (i.e., GTP-bound, active Rho) was detected by Western blotting using a monoclonal antibody against RhoA (Santa Cruz Biotechnology, Santa Cruz, CA). The amount of bead-bound Rho was normalized to the total amount of Rho in cell lysates to determine the percentage of GTP-bound Rho in different samples. The Rho activity thus obtained in each experiment was further normalized to that of the concurrent static controls plated on FN at 0.5 μg/cm2 (set to be 1). Relative Rho activities are shown as mean ± SE. (from three independent experiments) and were analyzed by using Statview as described above.
Traction force studies
Traction force microscopy was used to quantify the tractions generated by migrating ECs on the underlying substrate. The detailed protocol of making elastic polyacrylamide substrates was described previously (Beningo et al., 2002; Dembo and Wang, 1999; Pelham and Wang, 1999); materials were purchased from Bio-Rad (Hercules, CA) unless otherwise mentioned. Briefly, a 70-μl droplet containing 5% acrylamide, 0.1% N,N-methylene-bis-acrylamide, 0.06% ammonium sulfate, 0.4% N,N,N′,N′-tetramethylenediamine (TEMED), and 0.4% fluorescent beads (0.2-μm FluoSpheres: carboxylate-modified orange beads; Molecular Probe, Eugene, OR) was spread onto an activated coverslip (No. 1, 22 × 60 mm; Fisher) as a sheet and allowed to polymerize. The surface of the polyacrylamide gel was then coated with FN at 5 μg/cm2 for EC culture. This protocol gives a gel ∼75 μm in thickness and a Young's Modulus of 2.8 × 105 dyn/cm2 (Dembo and Wang, 1999; Pelham and Wang, 1999).
BAECs in DMEM containing 0.5% FBS were plated on the polyacrylamide substrate for 2 h before the static and shear experiments. Phase images of a single, isolated cell and fluorescence images of the beads were recorded at 2-min intervals using a Nikon (Melville, NY) inverted fluorescent microscope equipped with a 60× Plan-Apo oil objective. All images were acquired with a SIT camera (Model C2400, Hamamatsu, Bridgewater, NJ) and the IPLab software (Scanalytics, Fairfax, VA). At the end of the experiment, the cell was removed via perfusing trypsin through the flow chamber, and a final fluorescent image of the beads in the relaxed, unstrained state was acquired. Tractions generated by an EC were determined as described previously (Marganski et al., 2003; Munevar et al., 2001a) based on the bead displacement, the mechanical properties of the elastic substrate, and the cell boundaries. Estimated EC tractions (TEC) are presented both as a vector map (with both direction and magnitude) and a contour map generated by converting the magnitude of tractions (mag(TEC)) into a color coding scheme. Finally, the mean mag(TEC) is computed by averaging the values of mag(TEC) over the entire cell area (Munevar et al., 2001a).
RESULTS
Quantitative analysis of the EC migration speed under static and flow conditions
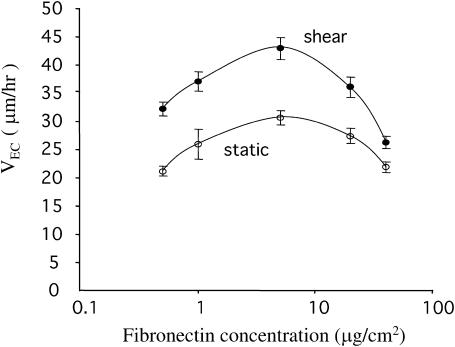
First we characterized the migration speed of the ECs on FN-coated surfaces under static and flow conditions. Because it has been shown that growth factors may decrease or increase cell motility depending on the matrix density (Maheshwari et al., 1999; Ware et al., 1998), we examined the shear effect over a range of surface concentrations of FN ([FN]; 0.5, 1, 5, 20, and 40 μg/cm2). Subconfluent BAECs were plated on FN-coated glass slides and were then kept as static controls or subjected to shear stress at 12 dyn/cm2. Cell movement was monitored by time-lapse microscopy and the nondirectional EC migration speed (VEC) was determined as described above. Under static conditions, VEC showed a bell-shaped relationship with [FN], having a peak at 5 μg/cm2 (Fig. 1). The application of shear stress significantly increased VEC at all [FN] and shifted the bell curve upwards, with the maximal speed remaining at [FN] = 5 μg/cm2 (Fig. 1).
FIGURE 1.
Shear stress increased EC migration speed over a range of fibronectin concentrations. Subconfluent ECs were plated on glass slides coated with fibronectin at 0.5, 1, 5, 20, and 40 μg/cm2 and then subjected to shear stress at 12 dyn/cm2 or kept as static controls. The nondirectional EC migration speed (VEC) was determined as described. Data are shown as mean ± SE. Each condition had at least 60 cells from three independent experiments. At each fibronectin concentration, the p-value was <0.05 for the difference in VEC between static and shear conditions. Under static and shear conditions, the p-values were <0.05 between VEC at 5 μg/cm2 and at other fibronectin concentration.
The bell-shaped relationship between cell migration speed and substrate concentration has been found in various cell-ECM systems under static conditions (DiMilla et al., 1993; Palecek et al., 1997). This is consistent with a mathematical model predicting that, under static conditions, the maximal migration speed of anchorage-dependent cells occurs at a medium substrate concentration, at which the migrating cells can form sufficient attachment with the substrate in the advancing front and yet can break the adhesion with the substrate in the trailing back (DiMilla et al., 1991). Our data show that this relation applies also to cell migration in a fluid dynamic environment. Moreover, our finding that shear stress shifted the bell-shaped VEC-[FN] curve upwards with a higher maximal speed indicates that mechanical shearing forces can superimpose on the effect of adhesion in promoting EC migration. We investigated the molecular basis by which shear stress increased VEC, with a focus on the intracellular signaling molecule Rho GTPase.
The shear-enhanced EC migration speed is associated with the activation of Rho
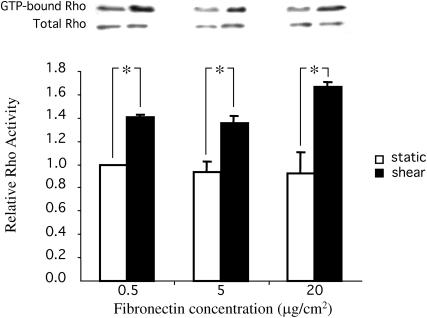
Rho GTPase has been identified as a crucial regulatory molecule in cell migration for its action on cellular contractility and actin-based cytoskeleton organization (i.e., the formation of focal adhesions and stress fibers) (Chrzanowska and Burridge, 1996; Hall and Nobes, 2000). We hypothesized that shear stress regulates VEC by modulating Rho activity. Subconfluent BAECs were plated on glass slides coated with [FN] at 0.5, 5, and 20 μg/cm2 for 2 h. These cells were then kept as static controls or subjected to shear stress at 12 dyn/cm2 for 30 min, and then the activity of Rho was determined by an affinity-precipitation assay as described above. The level of Rho activity under static conditions was independent of [FN], and it was significantly increased by shear stress at all [FN] (Fig. 2). This independence of shear-induced Rho activation on [FN] is parallel to that seen for the shear-induced increase of VEC (Fig. 1), suggesting that Rho activation may mediate the shear-induced increase of VEC. We next confirmed this hypothesis by blocking the Rho-mediated pathway.
FIGURE 2.
Shear stress induced the activation of Rho GTPase over a range of fibronectin concentrations. Subconfluent ECs were plated on glass slides coated with fibronectin at 0.5, 5, and 20 μg/cm2 and then subjected to shear stress at 12 dyn/cm2 for 30 min or kept as static controls. The activities of Rho were assessed by affinity-precipitation. Representative Western blots show the activation of Rho by shear stress. Values in the bar graph are the means of three independent experiments; error bars are SE. (*) p-value < 0.05 between sheared samples and static controls at the same fibronectin concentration.
The shear-enhanced EC migration speed is dependent on p160ROCK
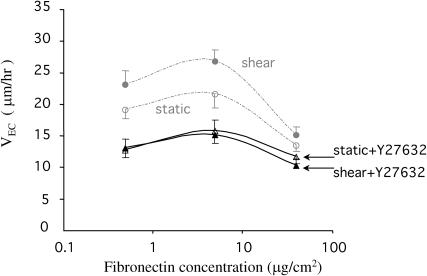
The inactivation of Rho by Rho inhibitors (e.g., C3 exoenzyme) or by dominant-negative mutants can abolish many Rho-regulated functions in cells and often results in dramatic morphological changes with a complete loss of motility (Ishizaki et al., 2000; Miura et al., 1993; Nobes and Hall, 1999; Paterson et al., 1990). Therefore, we chose to block a specific Rho effecter relevant to cell migration instead of Rho directly. It has been shown that the activation of Rho increases cellular contractility through p160ROCK, a Rho associated serine/threonin kinase that increases the phosphorylation of myosin light chain, which subsequently increases the actin-myosin contractility, enhancing the formation of tension-dependent structures such as stress fibers (Amano et al., 1997; Hall and Nobes, 2000; Van and D'Souza, 1997). Y27632 is a specific inhibitor of p160ROCK that disrupts stress fibers and has been used extensively to assess the role of the Rho-p160ROCK pathway in regulating cell functions (Ishizaki et al., 2000; Uehata et al., 1997). Previously it was shown that Y27632 at 10 μM abolished stress fibers but had little effect on the cell cycle and cytokinesis (Ishizaki et al., 2000). Pretreatment of BAECs with 10 μM Y27632 for 30 min significantly decreased VEC under both static and shear conditions (Fig. 3), shifted the VEC-[FN] curves downwards, and abolished the difference of VEC between static and shear conditions. These results indicate that the Rho-p160ROCK pathway mediates the shear-induced increase in VEC. Next we correlated the biochemical pathway with the biophysical force necessary for powering cell movement.
FIGURE 3.
The effect of Rho kinase inhibitor on the shear-enhanced EC migration speed. BAECs were treated with Y27632, a specific inhibitor of Rho-associated protein kinase p160ROCK, at 10 μM for 30 min before the experiments. Y27632 decreased the nondirectional EC migration speed (VEC) for both static and flow conditions to almost identical levels. Data are shown as mean ± SE. Each condition had at least 60 cells from three independent experiments.
Shear stress increased EC traction forces
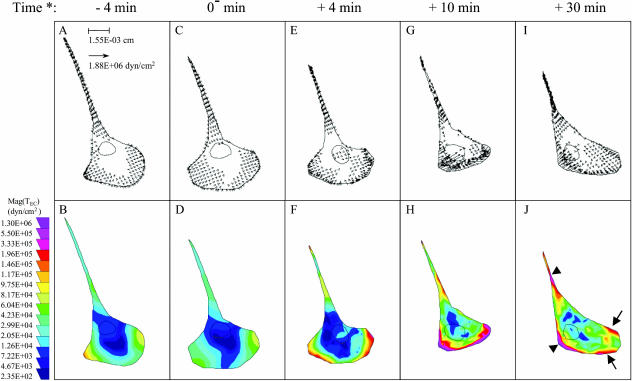
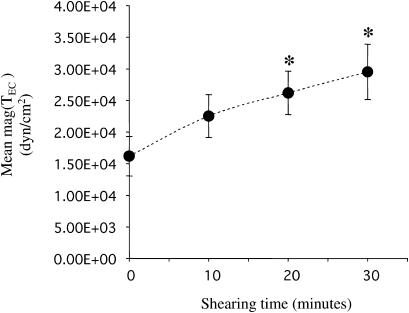
During cell migration, the contractile force generated within the cell by the actin-myosin cytoskeleton moves the cell body forward and causes tractions on the underlying substrate (Beningo and Wang, 2002; Sheetz et al., 1997). Tractions are directly related to cell contractility (Beningo et al., 2002; Beningo and Wang, 2002; DiMilla et al., 1991; Maheshwari and Lauffenburger, 1998). Since Rho was activated in ECs by fluid shear stress (Fig. 2), we investigated whether shear stress increased EC tractions (TEC) by using the traction force microscopy technique (Munevar et al., 2001a; Reinhart-King et al., 2003). Subconfluent BAECs were seeded for 2 h on FN-coated polyacrylamide substrates; these cells were then kept as static controls or exposed to shear stress at 12 dyn/cm2 while TEC was measured as described previously (Marganski et al., 2003; Munevar et al., 2001a). Fig. 4 shows the traction maps of a representative cell that was first kept under static conditions (Fig. 4, A–D) and then exposed to flow for 30 min (Fig. 4, E–J). The upper panel in Fig. 4 shows both the direction and magnitude of TEC (mag(TEC)), and the lower panel presents contour images generated by converting mag(TEC) into a color coding scheme. The time course of mean mag(TEC), i.e., the average of mag(TEC) over the entire cell area, in response to shear stress is shown in Fig. 5.
FIGURE 4.
Representative EC traction maps. Traction maps of an EC before (A–D) and after (E–J) exposure to fluid shear stress at 12 dyn/cm2. Distributions of tractions are shown as vector maps (upper panel) and as color images after converting the magnitude of tractions, mag(TEC), into color codes (lower panel) as described. Flow direction was from left to right. (*) Time relative to the start of flow. Arrows (←) and arrowheads (◂) in J point to the edge of the advancing front and the area of detachment, respectively (see text for details).
FIGURE 5.
Shear stress increased the average EC traction values. The average of traction magnitudes over the entire cell area, mean mag(TEC), was computed as described. N = 7; all seven cells had similar morphology as that in Fig 4, A–D, before flow started. Data are shown as mean ± SE. (*) p-value < 0.05 in comparison to the static controls (shearing time = 0 min).
Migrating ECs had well-defined leading lamellipodial and trailing tail regions as shown in Fig. 4. Under both static and flow conditions, the TEC vectors were organized such that the backward tractions were located in the advancing front and the forward tractions in the trailing back (Fig. 4, upper panel). This is the same as the “centripetal pattern” of tractions observed in fibroblasts without flow (Beningo et al., 2001; Galbraith and Sheetz, 1997; Munevar et al., 2001a; Pelham and Wang, 1999). Under flow, however, the cells generated a higher mean mag(TEC) over the entire cell area than under static conditions (Fig. 5). Fig. 4, E–J, shows that the largest increases in regional mag(TEC) tended to occur along the edge of the advancing front (arrows in Fig. 4 J) and the area of detachment (arrowheads in Fig. 4 J). Pretreatment of ECs with 10 μM Y27632 decreased TEC to an undetectable level in our system, which remained so for 1 h even after transfer to fresh media under both static and flow conditions (data not shown).
Our results indicate that migrating ECs under flow exert stronger tractions, which may reflect an increase in cell contractility mediated via the Rho-p160ROCK pathway. The shear-induced increase in mag(TEC) in the advancing front represents a stronger forward-pulling force, and that at the rear reflects a stronger retraction force. These results suggest that shear stress enhances both the attachment in the front and the detachment at the rear, thus enhancing EC migration.
DISCUSSION
In this study we demonstrate the shear-enhancement of EC migration speed over a range of FN density. Our data indicate that the shear-induced increase in Rho activity serves to modulate traction force generation and thus promotes both the attachment in the front and the detachment at the rear, thereby enhancing EC migration. These results provide the biochemical and biophysical bases (the Rho-p160ROCK pathway and traction force, respectively) underlying this shear effect and elucidate their interrelations.
Although shear stress has been shown to modulate EC migration as early as 1987 by Ando et al. (1987), in-depth knowledge in the molecular mechanisms involved was not available until recently. As mentioned above, persistent cell movement requires spatially and temporally coordinated events that include lamellipodial extension at the leading edge, the formation of lamellipod-substrate attachments, the contraction of cytoskeletal elements, and the release of cell-substrate attachments at the rear of the cell (Lauffenburger and Horwitz, 1996; Sheetz et al., 1997). Recent studies have shown that shear stress can modulate the asymmetric (or polarized) and dynamic cell-substrate interactions in EC migration. Studies by Li et al. (2002) demonstrated that subconfluent ECs under shear stress migrated with polarized lamellipodial protrusion and formation of focal adhesions in the direction of flow, and that these newly formed focal adhesions subsequently disassembled after the rear of the cell moved over them. Wojciak-Stothard and Ridley (2003) later found that the shear-induced asymmetric shape and directional migration of the ECs was mediated through Rho and Rac GTPases. Therefore, the following discussion on the shear modulation of EC migration is focused on the overall cell speed, not directionality, and on the role of the cytoskeletal contraction in this shear modulation.
Our results showed that shear stress increased VEC (the nondirectional migration speed as defined above) over a range of FN concentrations and that, under both static and shear conditions, VEC had a biphasic relationship with FN concentration. The adhesion between FN- or, more generally, ECM- and cell surface receptors is crucial in cell motility. A theoretical model predicted a biphasic relationship between cell speed and cell-substrate adhesive strength, with the maximal cell speed at an intermittent adhesive strength that is optimal for the cell-substrate attachment and detachment events in cell migration (DiMilla et al., 1991). The cell-substrate adhesive strength can be manipulated by several means, such as the expression level or binding affinity of the cell surface receptors and, as used in this study, the substrate concentration. Although this theoretical model describes the adhesiveness dependence of cell speed under static conditions, interestingly, our data showed that it also applies in a fluid dynamic environment. Our finding that shear stress shifted the VEC-[FN] curve upwards with a higher maximal VEC indicates that mechanical shearing forces can superimpose on the effect of adhesion in promoting EC migration. It is plausible that the effect of the mechanical shearing force is mediated through the modulation of cytoskeletal contraction.
In the physical process of cell migration, the contraction of the actin-myosin cytoskeleton drives the forward translocation of the cell body and causes traction force on the substrate (Beningo and Wang, 2002; Sheetz et al., 1997). The stimulation of actin-myosin contractility by activated Rho has been studied extensively (Chrzanowska and Burridge, 1996; Hall and Nobes, 2000). Our study showed that shear stress induced the activation of Rho and that the independence of shear-induced Rho activation on FN concentration parallels that seen for the shear-induced increase of migration speed. Furthermore, the shear-enhancement of migration speed was eliminated by inhibiting p160ROCK, the Rho effector for cellular contractility (Amano et al., 1997). These results indicate that the Rho-p160ROCK pathway mediates the shear-induced increase in cell migration speed.
Finally, using the traction force microscopy technique, we found that migrating ECs under flow exerts stronger traction force, thus providing a link among the activation of Rho (as a mechanosensing motor), an enhancement of the transmitted contractile force (the physical force for the moving cell body), and the resulting increase in migration speed. Note that the shear-enhanced traction force occurred both at the leading edge and the rear, suggesting that shear stress enhances both the attachment in the front and the detachment at the rear, thus enhancing EC migration over the entire range of [FN] studied: the former may account for the increased VEC at a low [FN], where cell-substrate attachment is the rate-limiting step; the latter may account for the increased VEC at a high [FN], where cell-substrate detachment is the rate-limiting step (DiMilla et al., 1991; Lauffenburger and Horwitz, 1996). However, how the localized traction force is generated needs further investigation. For example, a direct assay of subcellular activity of Rho would allow us to correlate the sites of Rho activation and traction force generation at the subcellular level, and the distribution of Rho activity thus obtained would allow us to clarify whether the increases in frontal and tail tractions are both due to active events (i.e., Rho activation) or, as seen in fibroblast migration, the increase in tail tractions is simply due to passive resistance to the increase in frontal forces (Munevar et al., 2001b).
EC migration is synergistically regulated by both chemical and mechanical factors in vivo. Several recent investigations have shown that the multiple and divergent pathways by which EC motility is mediated by growth factors and ECM may converge on the cytoskeleton via Rho GTPase (see review by Shuster and Herman, 1998). Our study and others (Tzima et al., 2001; Wojciak-Stothard and Ridley, 2003) demonstrate that Rho GTPase also plays a crucial role in the shear-induced EC motility. It is worth noting, however, that the pattern of Rho activity in ECs in response to flow is different in these studies. In contrast to our results shown in Fig. 2, Rho activity in ECs was not significantly increased after exposure to flow for 30 min in these studies (Tzima et al., 2001; Wojciak-Stothard and Ridley, 2003). Such discrepancy may be due to different cell conditions, including the source of ECs (bovine versus human), the level of shear stress (arterial versus venous), and particularly the degree of confluence (confluent versus subconfluent). Indeed, it has been shown that cell junction can modulate Rho activity (Benais-Pont et al., 2003; Noren et al., 2000). Knowledge of signaling events for cell migration under flow (versus static) conditions can provide new insights into potential therapeutic interventions and tissue-engineering applications, e.g., for promoting EC migration in vascular wound repair and in designing synthetic vascular prosthesis, respectively.
Acknowledgments
We thank Suli Yuan and Phu Nguen for preparing the GST-RBD beads, Gerard Norwich for help on microscopic work, and Melissa Li for assistance in image analysis. Y27632 was a gift provided by Welfide (Osaka, Japan).
This work was supported in part by a Scientific Development grant from the American Heart Association (S. Li), National Aeronautics and Space Administration NAG2-1495 (Y. L. Wang), and National Institutes of Health Research grants HL-19454, HL-43026, and HL-64382 (S. Chien).
Yan-Ting Shiu's present address is Dept. of Bioengineering, University of Utah, Salt Lake City, UT.
Song Li's present address is Dept. of Bioengineering, University of California, Berkeley, CA.
Martin A. Schwartz's present address is Dept. of Microbiology, University of Virginia, Charlottesville, VA.
References
- Amano, M., K. Chihara, K. Kimura, Y. Fukata, N. Nakamura, Y. Matsuura, and K. Kaibuchi. 1997. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 275:1308–1311. [DOI] [PubMed] [Google Scholar]
- Ando, J., H. Nomura, and A. Kamiya. 1987. The effect of fluid shear stress on the migration and proliferation of cultured endothelial cells. Microvasc. Res. 33:62–70. [DOI] [PubMed] [Google Scholar]
- Benais-Pont, G., A. Punn, C. Flores-Maldonado, J. Eckert, G. Raposo, T. P. Fleming, M. Cereijido, M. S. Balda, and K. Matter. 2003. Identification of a tight junction-associated guanine nucleotide exchange factor that activates Rho and regulates paracellular permeability. J. Cell Biol. 160:729–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beningo, K. A., M. Dembo, I. Kaverina, J. V.J. V. Small, and Y. L. Wang. 2001. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J. Cell Biol. 153:881–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beningo, K. A., C. M. Lo, and Y. L. Wang. 2002. Flexible polyacrylamide substrata for the analysis of mechanical interactions at cell-substratum adhesions. Methods Cell Biol. 69:325–339. [DOI] [PubMed] [Google Scholar]
- Beningo, K. A., and Y. L. Wang. 2002. Flexible substrata for the detection of cellular traction forces. Trends Cell Biol. 12:79–84. [DOI] [PubMed] [Google Scholar]
- Chien, S., S. Li, and Y. J. Shyy. 1998. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension. 31:162–169. [DOI] [PubMed] [Google Scholar]
- Chrzanowska, W. M., and K. Burridge. 1996. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol. 133:1403–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembo, M., and Y. L. Wang. 1999. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys. J. 76:2307–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMilla, P. A., K. Barbee, and D. A. Lauffenburger. 1991. Mathematical model for the effects of adhesion and mechanics on cell migration speed. Biophys. J. 60:15–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMilla, P. A., J. A. Stone, J. A. Quinn, S. M. Albelda, and D. A. Lauffenburger. 1993. Maximal migration of human smooth muscle cells on fibronectin and type IV collagen occurs at an intermediate attachment strength. J. Cell Biol. 122:729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangos, J. A., S. G. Eskin, L. V. McIntire, and C. L. Ives. 1985. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 227:1477–1479. [DOI] [PubMed] [Google Scholar]
- Galbraith, C. G., and M. P. Sheetz. 1997. A micromachined device provides a new bend on fibroblast traction forces. Proc. Natl. Acad. Sci. USA. 94:9114–9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, A., and C. D. Nobes. 2000. Rho GTPases: molecular switches that control the organization and dynamics of the actin cytoskeleton. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355:965–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki, T., M. Uehata, I. Tamechika, J. Keel, K. Nonomura, M. Maekawa, and S. Narumiya. 2000. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol. Pharmacol. 57:976–983. [PubMed] [Google Scholar]
- Lauffenburger, D. A., and A. F. Horwitz. 1996. Cell migration: a physically integrated molecular process. Cell. 84:359–369. [DOI] [PubMed] [Google Scholar]
- Li, S., P. Butler, Y. Wang, Y. Hu, D. C. Han, S. Usami, J. L. Guan, and S. Chien. 2002. The role of the dynamics of focal adhesion kinase in the mechanotaxis of endothelial cells. Proc. Natl. Acad. Sci. USA. 99:3546–3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S., B. P. Chen, N. Azuma, Y. L. Hu, S. Z. Wu, B. E. Sumpio, J. Y. Shyy, and S. Chien. 1999. Distinct roles for the small GTPases Cdc42 and Rho in endothelial responses to shear stress. J. Clin. Invest. 103:1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshwari, G., and D. A. Lauffenburger. 1998. Deconstructing (and reconstructing) cell migration. Microsc. Res. Tech. 43:358–368. [DOI] [PubMed] [Google Scholar]
- Maheshwari, G., A. Wells, L. G. Griffith, and D. A. Lauffenburger. 1999. Biophysical integration of effects of epidermal growth factor and fibronectin on fibroblast migration. Biophys. J. 76:2814–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marganski, W. A., M. Dembo, and Y. L. Wang. 2003. Measurement of cell-generated deformations on flexible substrata using correlation-based optical flow. Methods Enzymol. 361:197–211. [DOI] [PubMed] [Google Scholar]
- Miura, Y., A. Kikuchi, T. Musha, S. Kuroda, H. Yaku, T. Sasaki, and Y. Takai. 1993. Regulation of morphology by rho 21 and its inhibitory GDP/GTP exchange protein (rho GDI) in Swiss 3T3 cells. J. Biol. Chem. 268:510–515. [PubMed] [Google Scholar]
- Munevar, S., Y. Wang, and M. Dembo. 2001a. Traction force microscopy of migrating normal and H-ras transformed 3T3 fibroblasts. Biophys. J. 80:1744–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munevar, S., Y. L. Wang, and M. Dembo. 2001b. Distinct roles of frontal and rear cell-substrate adhesions in fibroblast migration. Mol. Biol. Cell. 12:3947–3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes, C. D., and A. Hall. 1999. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J. Cell Biol. 144:1235–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren, N. K., B. P. Liu, K. Burridge, and B. Kreft. 2000. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J. Cell Biol. 150:567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palecek, S. P., J. C. Loftus, M. H. Ginsberg, D. A. Lauffenburger, and A. F. Horwitz. 1997. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 385:537–540. [DOI] [PubMed] [Google Scholar]
- Papadaki, M., and S. G. Eskin. 1997. Effects of fluid shear stress on gene regulation of vascular cells. Biotechnol. Prog. 13:209–221. [DOI] [PubMed] [Google Scholar]
- Paterson, H., A. Self, M. Gerrett, I. Just, K. Aktories, and A. Hall. 1990. Microinjection of recombinant p21rho induces rapid changes in cell morphology. J. Cell Biol. 111:1001–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham, R. J., Jr., and Y. Wang. 1999. High resolution detection of mechanical forces exerted by locomoting fibroblasts on the substrate. Mol. Biol. Cell. 10:935–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart-King, C. A., M. Dembo, and D. A. Hammer. 2003. Endothelial cell traction forces on RGD-derivatized polyacrylamide substrata. Langmuir. 19:1573–1579. [Google Scholar]
- Ren, X. D., W. B. Kiosses, and M. A. Schwartz. 1999. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18:578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz, M. P., D. Felsenfeld, C. G. Galbraith, and D. Choquet. 1997. Cell migration as a five-step cycle. Biochem. Soc. Symp. 65:233–243. [PubMed] [Google Scholar]
- Shuster, C. B., and I. M. Herman. 1998. The mechanics of vascular cell motility. Microcirculation. 5:239–257. [PubMed] [Google Scholar]
- Tzima, E., M. A. del Pozo, S. J. Shattil, S. Chien, and M. A. Schwartz. 2001. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 20:4639–4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehata, M., T. Ishizaki, H. Satoh, T. Ono, T. Kawahara, T. Morishita, H. Tamakawa, K. Yamagami, J. Inui, M. Maekawa, and S. Narumiya. 1997. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 389:990–994. [DOI] [PubMed] [Google Scholar]
- Van, A. L., and S. C. D'Souza. 1997. Rho GTPases and signaling networks. Genes Dev. 11:2295–2322. [DOI] [PubMed] [Google Scholar]
- Ware, M. F., A. Wells, and D. A. Lauffenburger. 1998. Epidermal growth factor alters fibroblast migration speed and directional persistence reciprocally and in a matrix-dependent manner. J. Cell Sci. 111:2423–2432. [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard, B., and A. J. Ridley. 2003. Shear stress-induced endothelial cell polarization is mediated by Rho and Rac but not Cdc42 or PI 3-kinases. J. Cell Biol. 161:429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]