Abstract
A remarkable heterogeneity is often observed in the spectroscopic properties of environment-sensitive fluorescence probes in phospholipid bilayers. To explain its origin, we provided a detailed investigation of the fluorescence excitation and emission spectra of 4′-dimethylamino-3-hydroxyflavone (probe F) in bilayer vesicles with the variations of fatty acid composition, polar heads, temperature, and cholesterol content. Probe F, due to excited-state intramolecular proton transfer, exhibits two bands in emission that are differently sensitive to intermolecular interactions—thereby allowing us to distinguish universal (dipole-dipole) and specific (H-bonding) interactions within the bilayer. Spectroscopic, quenching, and anisotropy data suggest the presence of two forms of probe F at different locations in the bilayer: an H-bond free form located below sn1-carbonyls and an H-bonded form located at the polar membrane interface. We provide a quantitative analysis of the distribution of the probe between these two locations as well as the polarity of these locations, and show that both the distribution and the polarity contribute to the probe response. Moreover, analysis of literature data on other environment-sensitive probes (Prodan, Laurdan, Nile Red, NBD lipids, etc.) in lipid bilayers allows us to suggest that the bimodal distribution in the lipid bilayer is probably a general feature of low-polar molecules with polar groups capable of H-bonding interactions.
INTRODUCTION
Among the fluorescence probes commonly used in the studies of biomembranes and their phospholipid models are the so-called environment-sensitive (or site-sensitive) probes that respond to changes of their environment by shifts of fluorescence emission and/or excitation spectra (Loew, 1988; Reichardt, 1994; Valeur, 2002). The most popular membrane probes of this type are 6-propionyl-2-dimethylaminonaphthalene (Prodan), 6-lauroyl-2-dimethylaminonaphthalene (Laurdan), Nile Red, and 7-nitro-2,1,3-benzoxadiazol-4-yl (NBD-), anthroyl-, and anthroyloxy- derivatives, that are neutral hydrophobic fluorophores with high affinity to lipid bilayers. A typical example is Prodan (Fig. 1) (Weber and Farris, 1979), which is characterized by solvent-dependent shifts of fluorescence spectra due to the charge-transfer of its excited state and specific hydrogen-bonding (H-bonding) interactions (Catalan et al., 1991; Cerezo et al., 2001). Prodan is highly sensitive to different structural changes in the bilayer. For instance, a strong red shift of its emission is observed on transition from liquid crystalline phase to gel phase (Krasnowska et al., 1998). Strong changes in the emission color are also observed on addition of cholesterol (Massey 1998; Bondar and Rowe, 1999), and under the influence of other factors, such as high pressure and the addition of local anesthetics and alcohols (Chong, 1988; Zeng and Chong, 1995; Bondar and Rowe, 1999). A similar response to phase transition and the presence of cholesterol is reported for the more hydrophobic Prodan analog, Laurdan (Parasassi et al., 1990, 1994). However, the inhomogeneous nature of the lipid bilayer resulting from strong gradients of polarity and hydration (Kurad et al., 2003; Bartucci et al., 2003) and from localization of the phospholipids functional groups at different bilayer depths (Wiener and White, 1992) may not allow these neutral probes to occupy a single well-determined location and orientation. Furthermore, most of these environment-sensitive probes contain groups (carbonyl, carboxyl, nitro) that may participate in specific interactions with water molecules at different depths inside the bilayer. Therefore, the presence of different forms of the probe, either H-bonded or non-H-bonded with water, is an additional factor that may contribute to the heterogeneity of probe location and render the fluorescence profile of the probes quite heterogeneous. In this respect, it was recently shown that fluorophores containing polar groups may occupy at least two locations in the bilayer (Asuncion-Punzalan et al., 1998; Kaiser and London, 1998). Moreover, time-resolved fluorescence data on Nile Red (Krishna, 1999; Ira et al., 2003) and steady-state data on Prodan (Chong, 1988) and ketocyanine dyes (Doroshenko et al., 2002) also show that these probes may be located simultaneously in both polar and apolar regions of the membrane.
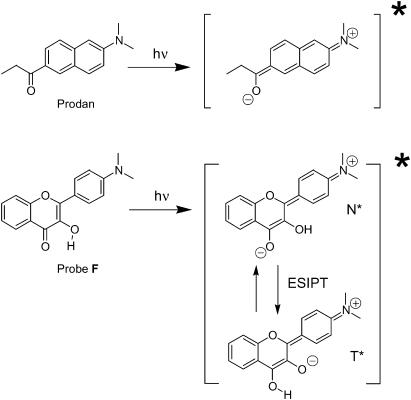
FIGURE 1.
Chemical structures of the ground and excited states of Prodan and probe F.
As a consequence, important questions have to be considered. What are the neutral environment-sensitive probes actually sensing in the bilayer? Are they sensing the properties of a particular location or the probe distribution between several locations with different properties? In the case of Prodan and its analog Laurdan, Parasassi et al. (1990, 1994) and Krasnowska et al. (1998) have shown that on phase transition and with addition of cholesterol the probes sense the change in solvent relaxation of the lipid bilayer. Meantime, Chong and co-workers have shown that the redistribution between polar and apolar sites of the bilayer may contribute to the response of Prodan to a number of factors changing the properties of the bilayer: phase transition (Chong, 1988), hydrostatic pressure (Chong, 1988), and interdigitation of the bilayer (Zeng and Chong, 1995). In line with the latter, Bondar and Rowe (1999) demonstrated that cholesterol may cause relocation of Prodan in the bilayer to a more apolar environment. In addition, the fluorescent response of lipid acyl chain linked NBD to phase transition was also attributed to redistribution of the fluorescent moiety between the hydrophobic core of the membrane and its interface (Alakoskela and Kinnunen, 2001).
Another problem that complicates the interpretation of the fluorescence response of such probes is that even for a particular location in the lipid bilayer, their fluorescence properties are sensitive to a number of parameters, such as polarity, hydration, viscosity, and so on (Valeur, 2002). In this respect, it is a very difficult or even unfeasible task to discriminate all these factors using only the information based on the spectral shift of a single fluorescence band. This discrimination could be achieved only in the cases where the number of independent environment-sensitive spectroscopic parameters is large enough to characterize various types of intermolecular interactions and, in particular, to distinguish between the effects of polarity and of hydrogen-bonding with water.
Recent studies demonstrate that such a possibility of multiparametric analysis from a single type of probe molecules can be provided by 4′-dialkylamino-3-hydroxyflavone dyes (Fig. 1) (Klymchenko and Demchenko, 2003). Their attractive feature is the presence of two bands in fluorescence emission, which makes their fluorescence spectra much more informative than that of common probes. Their first band at shorter wavelength belongs to the initially excited normal (N*) form (Fig. 1), which is similar to the emissive form of Prodan, exhibiting a strong solvatochromism (Klymchenko and Demchenko, 2003). The second band at longer wavelengths results from an excited-state intramolecular proton transfer reaction and belongs to the tautomer (T*) form (Sengupta and Kasha, 1979). The two emission bands respond differently to interactions with their environment. In addition, the intensity ratio of these bands, IN*/IT*, is highly sensitive not only to polarity, but also to specific interactions such as hydrogen-bonding with the 4-carbonyl group (Klymchenko and Demchenko, 2003, Klymchenko et al., 2003). Based on these properties, we developed an algorithm for multiparametric analysis of the probe environment allowing us to discriminate between the effects of solvent polarity and H-bond donor ability (acidicity) (Klymchenko and Demchenko, 2003). This methodology may provide us a possibility to distinguish the effects of probe redistribution in the bilayer and the changes in the local properties at particular locations.
The present work is an attempt to analyze simultaneously, by fluorescence spectroscopy, the different possible locations of a neutral probe in a lipid bilayer and the physicochemical properties of these locations. For this study we selected 4′-dimethylamino-3-hydroxyflavone, F (Fig. 1), which is similar to Prodan by its size, polarity, and the presence of dimethylamino and carbonyl groups (since the 3-hydroxyl group is intramolecularly H-bonded to the 4-carbonyl group, the former does not significantly contribute to the polarity of this molecule). We studied the fluorescence response of probe F to structural modifications of the lipid bilayer, and compared our data with those previously published concerning Prodan and some other related probes. We show that probe F is distributed between two major sites—a polar site at the bilayer interface where the probe is H-bonded to water and an apolar site without any H-bonding to water. We show that both the changes of the site properties and the redistribution between the two sites are the key mechanisms that govern the response of this probe to different structural changes in the lipid membranes. Moreover, a careful analysis of the literature allowed us to conclude that such a behavior in lipid bilayers is probably a general feature of low-polar compounds with carbonyl and other H-bond acceptor groups.
MATERIALS AND METHODS
Dilauroyl-, dimyristoyl-, dipalmitoyl-, and dioleoyl-phosphatidylcholine (DLPC, DMPC, DPPC, and DOPC, respectively), egg yolk phosphatidylcholine (EYPC), egg yolk phosphatidylglycerol (EYPG), egg yolk phosphatidic acid (EYPA), dioleoyl phosphatidylglycerol (DOPG), bovine brain phosphatidylserine (BBPS), and cholesterol were purchased from Sigma-Aldrich (Lyon, France). TempoPC (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphotempocholine) 5- and 12-SLPC (l-palmitoyl-2-(5- and 12-doxyl)stearoyl-sn-glycero-3-phosphocholine) were purchased from Avanti Polar Lipids (Alabaster, AL). 4′-Dimethylamino-3-hydroxyflavone (F) was synthesized and purified as described elsewhere (Ormson et al., 1994). The sample of probe F was pure according to thin layer chromatography, 1H-NMR data, absorption, and fluorescence data in organic solvents.
Large unilamellar vesicles (LUV) were obtained by the classical extrusion method as described previously (Hope et al., 1985). LUVs were labeled by adding an aliquot (generally 2 μl) of probe stock solution (2 mM) in dimethyl sulfoxide to 2-ml solutions of vesicles. The fluorescence spectrum was recorded immediately after addition of the probe, since a very rapid binding kinetics was determined. Quenching experiments were performed as described previously (Abrams and London, 1993; Klymchenko et al., 2002). A 15 mM HEPES, pH 7.4 buffer was systematically used in all the experiments. Concentrations of probe F and lipids were 2 and 200 μM, respectively, unless indicated.
Fluorescence spectra were recorded on an SLM-Aminco 48000 (Urbana-Champaign, IL) spectrofluorometer and corrected by subtracting the baseline spectra of the corresponding blank vesicles. Fluorescence anisotropy measurements were performed on an SLM-Aminco 8000 spectrofluorometer. All the measurements were carried out at 20°C, and the excitation wavelength was systematically 400 nm, unless indicated. Deconvolution of probe F fluorescence spectra into three bands (N*, H-N*, and T*) was made by using the Siano software kindly provided by its author (Dr. A. O. Doroshenko, Kharkov, Ukraine). The program is based on an iterative nonlinear least-square method that is itself based on the Fletcher-Powell algorithm. The individual emission bands were approximated by a log-normal function (Siano and Metzler, 1969) which accounts for three parameters: position, full width at the half-maximum (FWHM) and asymmetry (P). Band asymmetry is defined by dividing the FWHM (in cm−1) of the band into blue and red parts according to the position of the band maximum and then calculating the ratio of the blue to the red part. For the iteration process, FWHM of the two short-wavelength bands (N* and H-N*) were fixed at 3000 cm−1. The other parameters, band asymmetry, positions of all the bands and FWHM of the T* band were allowed to vary in the iteration process. The location depths of probe molecules were estimated with the parallax quenching method (Abrams and London, 1993) by using TempoPC, 5-, and 12-SLPC as quenchers. The same deconvolution procedure was applied to the spectra obtained during parallax quenching experiments. The quenching efficiencies of the individual forms of probe F were estimated from the changes in the integral intensities of the corresponding deconvoluted bands.
RESULTS
Heterogeneity in the ground and excited states of probe F in EYPC vesicles
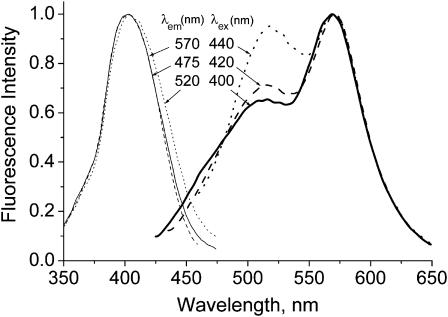
Depending on the lipid composition (different polar heads, unsaturation degree), the emission spectra of probe F in vesicles differ mostly by the relative intensities of the two bands (Duportail et al., 2001, 2002), whereas the spectral position of these bands is not strongly affected. Excitation and emission spectra of probe F in EYPC vesicles are presented in Fig. 2. The short-wavelength emission band attributed to the N* form is unusually broad and exhibits a shoulder at ∼475 nm. This behavior has never been observed in neat solvents (Klymchenko and Demchenko, 2003). Moreover, another anomaly is the small separation of the two emission bands (∼50 nm, Fig. 2). According to the extensive studies of dye F and its 4′-diethylamino analog in organic solvents, the two emission bands exhibit a much larger separation (by 80–120 nm) when the N* band is of lower intensity as compared to the T* band (Ormson et al., 1994; Klymchenko and Demchenko, 2003). As a consequence, the broad shape of the N* band and its small separation from the T* band are the first indications of its possible heterogeneity. In this respect, the short-wavelength shoulder may be attributed to the classical well-separated N* band, whereas the red part of the short-wavelength emission band (with a maximum at ∼515 nm) may correspond to another emissive species with different spectroscopic properties.
FIGURE 2.
Excitation spectra at different emission wavelengths (left) and emission spectra at different excitation wavelengths (right) of probe F in EYPC large unilamellar vesicles. The spectra are normalized at their maximum. Concentration of probe and lipids were 1 and 200 μM, respectively, in 15 mM HEPES buffer, pH 7.4.
The presence of an additional emissive species is supported by the fact that the emission spectrum of probe F in EYPC vesicles depends on the excitation wavelength (Fig. 2). Previously, this effect was interpreted as a red-edge effect (Duportail et al., 2002). However, the red-edge excitation in this case results not only in the red shift of the short-wavelength maximum but also in a dramatic decrease of the T* band and the disappearance of the shoulder at 475 nm (Fig. 2). The latter effects cannot be explained by the classical red-edge effect in rigid media (Demchenko, 2002) but suggest the presence of at least two probe species: one with dual emission and the other one selectively excited in the red-edge with a single emission band at ∼520 nm. A similar single emission band at similar spectral position has already been reported for probe F and its analogs in polar protic solvents, like ethanol or methanol (Duportail et al., 2001; Klymchenko and Demchenko, 2003). Consequently, the heterogeneity of the short-wavelength band of the emission spectrum of probe F in vesicles may tentatively be attributed to the involvement of a fraction of the probes in intermolecular hydrogen-bonding, so that the normal N* form may coexist with a hydrogen-bonded (H-N*) form. This observed heterogeneity cannot be the result of the partition of probe F between the lipid bilayer and the aqueous bulk inasmuch as the probe is hydrophobic, with low solubility in water—and because its quantum yield in water (0.3%) is two orders-of-magnitude lower than that in membranes (Klymchenko et al., 2002).
The dependence of excitation spectra on emission wavelength provides a deeper understanding of the origin of this heterogeneity. Indeed, the excitation spectra recorded at the T* band emission maximum (570 nm) and at the short-wavelength shoulder of the N* band (475 nm) were found to be the same, whereas the spectrum recorded at the N* band maximum (500 nm) was red-shifted by ∼3 nm (Fig. 2). This shift is even more clearly seen at the longer wavelengths of the excitation spectra. Such a shift has already been described for 4′-dialkylamino-substituted 3HF derivatives in organic solvents. This shift is not associated with the polarity (as a function of dielectric constant) of the environment but is due to hydrogen-bonding with protic solvents (Klymchenko and Demchenko, 2003). In addition, it has been shown that only one type of H-bonding complex between 3-hydroxyflavones and protic solvent can be detected in emission (Klymchenko et al., 2003). Therefore, the dependence of excitation spectra of probe F in vesicles on emission wavelength could be explained by the presence of only two ground-state species: one that is H-bonded and another that is non-H-bonded.
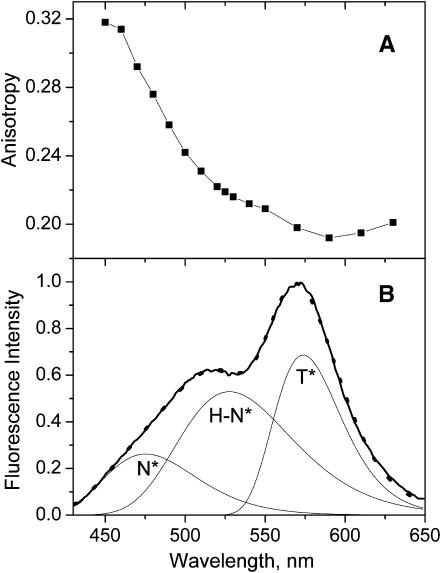
The presence of several emissive species for probe F in the lipid bilayer can be directly tested by fluorescence anisotropy. With only one emissive species the fluorescence anisotropy should not change over the emission band belonging to a particular electronic transition. In probe F, the long-wavelength absorption band is represented by a single transition and the orientations of the absorption and emission dipole moments do not differ significantly in the ground and N* excited states (Nemkovich et al., 2002). Inasmuch as the T* state emission dipole presents a different orientation (due to a changed electronic charge distribution after proton transfer), then, in a relatively restricted medium like a lipid vesicle, the emission from the N* state will be highly polarized, and that from the T* state much less. Therefore, for probe F in one particular location, changes in anisotropy would only be expected in the spectral regions where the N* and T* states overlap. In contrast to this, a clear anisotropy decrease is already seen in the whole N* band region (Fig. 3 A). By shifting from the short-wavelength shoulder to the main short-wavelength maximum (from 450 nm to 520 nm), the fluorescence anisotropy decreases strongly. A further shift from the short-wavelength maximum to the T* band maximum (from 520 nm to 570 nm) results in an additional decrease of the anisotropy that reaches a minimum value at the T* band maximum.
FIGURE 3.
Fluorescence studies of probe F in EYPC vesicles. (A) Spectrum of emission anisotropy. (B) Emission spectrum (solid line) and its deconvolution into N*, H-N*, and T* spectral components. The sum of deconvoluted bands (dots) matches closely the experimental spectrum. Concentration of probe and lipids were 1 and 200 μM, respectively, in 15 mM HEPES buffer, pH 7.4. Excitation wavelengths were 400 nm.
These results show that the N* band is heterogeneous and suggest the presence of at least two N* forms in emission: the N* form giving a short-wavelength band with a maximum ∼460–480 nm and characterized by a high fluorescence anisotropy; and a H-N* form giving a long-wavelength band ∼520–530 nm with a lower anisotropy. It should be noted that the strong decrease of the anisotropy in the 450–530-nm range cannot be due to some overlap of the N* band with the T* band since in this range the contribution of the narrow (∼1500 cm−1) and highly asymmetric T* band is negligible (see below). The observed lower fluorescence anisotropy of the H-N* form as compared to the N* form may result from the significantly higher fluorescence lifetime of the former, since it is known that the lifetime of probe F in protic solvents is much longer than that in aprotic media (Ormson et al., 1994).
Thus, according to the analysis of the fluorescence spectra and anisotropy, two probe species contribute to the fluorescence properties of F in EYPC vesicles: a non-H-bonded species exhibiting dual emission with maxima ∼475 nm and 570 nm, and an H-bonded species with a single band ∼520 nm.
Deconvolution of probe F fluorescence spectra into three individual components
To analyze the contribution of each form to the emission spectrum of probe F in EYPC vesicles, we deconvoluted this spectrum into three bands corresponding to N*, H-N*, and T* forms by using log-normal functions (Siano and Metzler, 1969). These functions were previously applied in the deconvolution of fluorescence spectra of environment-sensitive probes (Emel'ianenko et al., 2000) and have the main advantage to describe the whole shape of the spectra, since they take into account not only the position and width (FWHM) of the different bands but also their asymmetry. In our previous studies on 3HF derivatives, we demonstrated that the dual-emission spectra can be adequately described by the superposition of two log-normal functions (Klymchenko and Demchenko, 2003; Klymchenko et al., 2003). In the present case, to provide the most accurate and physically justified deconvolution, we have to select optimal parameters (band maximum, FWHM, and asymmetry) for each of the log-normal functions. According to our data in neat solvents (Klymchenko and Demchenko, 2003), the shape of the N* band observed in aprotic solvents does not differ significantly from that in protic solvents, so that their asymmetry and FWHM are approximately the same, at ∼0.9 and 2500–3000 cm−1, respectively. In contrast, the T* band exhibits a much higher asymmetry at ∼0.75, and an almost two-times-smaller FWHM at ∼1500 cm−1. In this respect, to deconvolute probe F fluorescence spectrum in EYPC vesicles, we fixed the FWHM of the N* and H-N* bands at 3000 cm−1. The other parameters, i.e., asymmetry of the three bands, FWHM of the T* band, and positions of the N* (475 nm), T* (570 nm), and H-N* (520 nm) bands, were allowed to vary during the iteration process.
The results of deconvolution show that the probe F fluorescence spectrum in EYPC vesicles is well described by the superposition of three components, assigned to the N*, H-N*, and T* forms of the probe (Fig. 3 B), with a correlation parameter r2 = 0.9996. To validate this approach to other lipid systems, we analyzed the fluorescence spectra of probe F in lipid vesicles differing in phase state, surface charge, fatty acid composition, and the presence of cholesterol. Some of the spectroscopic data were obtained in our previous studies (Duportail et al., 2001, 2002; Klymchenko et al., 2002). The results are presented in Table 1. For all the studied phospholipid vesicles, we obtained an excellent deconvolution of the fluorescence spectra into three bands with a correlation parameter r2 ≥ 0.999. The FWHM and position of the T* band as well as the asymmetries of the three bands were found to be very close for all the studied lipid vesicles (Table 1). Since the data in neat solvents also show that these parameters are rather constant, the invariance of the obtained values validates our deconvolution approach. Meantime, we observe some changes in the positions of the N* and H-N* bands (Table 1), which are known to be solvent-dependent (Klymchenko and Demchenko, 2003). The most significant variation is observed for the relative intensities of the three bands (Table 1).
TABLE 1.
Spectroscopic parameters obtained from the deconvolution into three bands, N*, H–N*, and T*, of the spectra of probe F in phospholipid vesicles
| Sample* | λN* nm | λH–N* nm | λT* nm | PN* | PH–N* | PT* | FWHMT* cm−1 | IN* | IH–N* | IT* |
|---|---|---|---|---|---|---|---|---|---|---|
| EYPC | 475 | 528 | 574 | 0.896 | 0.892 | 0.805 | 1500 | 0.262 | 0.529 | 0.686 |
| DOPC | 473 | 527 | 573 | 0.901 | 0.939 | 0.776 | 1500 | 0.283 | 0.777 | 0.563 |
| DOPC (1/25) | 473 | 527 | 574 | 0.900 | 0.905 | 0.765 | 1500 | 0.240 | 0.786 | 0.560 |
| DMPC, 14°C | 474 | 521 | 574 | 0.883 | 0.891 | 0.843 | 1580 | 0.107 | 0.312 | 0.830 |
| DMPC, 38°C | 476 | 527 | 577 | 0.899 | 0.909 | 0.819 | 1470 | 0.171 | 0.451 | 0.744 |
| EYPC-Chol | 471 | 523 | 574 | 0.882 | 0.894 | 0.841 | 1630 | 0.195 | 0.246 | 0.870 |
| DPPC | 474 | 521 | 574 | 0.911 | 0.912 | 0.826 | 1590 | 0.103 | 0.275 | 0.851 |
| DLPC | 475 | 529 | 575 | 0.905 | 0.900 | 0.783 | 1530 | 0.177 | 0.422 | 0.749 |
| EYPG | 475 | 527 | 570 | 0.862 | 0.912 | 0.766 | 1510 | 0.505 | 0.650 | 0.571 |
| DOPG | 481 | 531 | 571 | 0.826 | 0.900 | 0.809 | 1380 | 0.562 | 0.728 | 0.349 |
| BBPS | 477 | 527 | 570 | 0.886 | 0.854 | 0.765 | 1460 | 0.605 | 0.575 | 0.598 |
| EYPA | 479 | 525 | 569 | 0.900 | 0.900 | 0.770 | 1550 | 0.641 | 0.398 | 0.716 |
λN*, λH–N*, and λT*, positions of the maximum of the N*, H–N*, and T* bands, respectively; PN*, PH–N*, and PT*, asymmetries of the corresponding bands; FWHMT*, full width at half-maximum of the T* band; and IN*, IH–N*, and IT*, peak intensities of the corresponding bands, obtained from the normalized emission spectra.
LUVs were studied at 20°C with [Probe]/[Lipid] = 1/100, unless indicated; DOPC (1/25) corresponds to [Probe]/[Lipid] = 1/25; EYPC-Chol corresponds to 30% (mol wt) of cholesterol in EYPC.
Site polarity and location of the probe in the bilayer
The question arises, then, what are the precise locations of the H-bonded and non-H-bonded forms in a lipid bilayer? The two forms may either stay at the same location or alternatively, at two locations differing in depth and polarity. Based on the unique solvent-dependent spectroscopic properties of 3-hydroxyflavone dyes, it is possible to discriminate between these two possibilities. If we suppose that the two different forms of probe F are at identical locations, then it would not be possible to explain the substantial separation between the N* and H-N* bands observed with all types of lipid vesicles, which is as large as 50 nm (2000 cm−1) (see Table 1). Indeed, from the comparison of the spectral properties of probe F in protic and aprotic solvents of the same polarity, we showed that H-bonding leads only to a relatively small red shift of the N* band, of 15–20 nm (600 cm−1) (Klymchenko and Demchenko, 2003). Thus, it follows that the 50-nm shift observed in lipid vesicles probably reflects the differences in polarity of surrounding of the H-bonded and non-H-bonded species, so that the former are located in a more polar site of the bilayer.
Based on the emission spectra of probe F (Ormson et al., 1994; Duportail et al., 2001) and its 4′-diethylamino-analog (Klymchenko and Demchenko, 2003) in organic solvents, the position of the N* band (Table 1) and the intensity ratio IN*/IT* (Table 2) observed for probe F in EYPC vesicles can be expressed as a function of the dielectric constant ɛ (within the continuous dielectric model) or as a function of the empirical solvent polarity index, ET(30) (Reichardt, 1994). Using both of these scales we estimate the polarity of this probe-F location between that of ethyl acetate (ɛ = 6.02, ET(30) = 38.1) and dichloromethane (ɛ = 9.08, ET(30) = 40.7), whereas the position of the H-N* band indicates a polarity close to that of ethanol (ɛ = 24.85, ET(30) = 53.7). Therefore, it can be inferred that probe F mainly binds to the membrane at two different locations. One is of high polarity, probably at the membrane interface, where the probe forms H-bonds with water molecules, and the other is hydrophobic, deeper in the bilayer, where it does not form any intermolecular H-bonds.
TABLE 2.
Deconvolution and TBS analysis data on intensity ratio of the N* and T* emission bands, IN*/IT*, and the redistribution parameter, FB/FF, of probe F in phospholipid vesicles
| Sample
|
||||
|---|---|---|---|---|
| Deconvolution
|
TBS analysis
|
|||
| IN*/IT* | FB/FF | IN*/IT* | FB/FF | |
| EYPC | 0.382 | 0.874 | 0.334 | 0.875 |
| DOPC | 0.503 | 1.370 | 0.476 | 1.300 |
| DOPC(1/25) | 0.430 | 1.510 | 0.398 | 1.528 |
| DMPC, 14°C | 0.129 | 0.598 | 0.146 | 0.570 |
| DMPC, 38°C | 0.230 | 0.831 | 0.211 | 0.810 |
| EYPC-Chol | 0.224 | 0.390 | 0.225 | 0.409 |
| DPPC | 0.121 | 0.520 | 0.151 | 0.482 |
| DLPC | 0.236 | 0.765 | 0.198 | 0.764 |
| EYPG | 0.884 | 0.822 | 0.822 | 0.804 |
| DOPG | 1.610 | 0.988 | 1.171 | 1.168 |
| BBPS | 1.012 | 0.636 | 0.875 | 0.675 |
| EYPA | 0.895 | 0.398 | 0.854 | 0.468 |
The samples and experimental conditions correspond to those in Table 1.
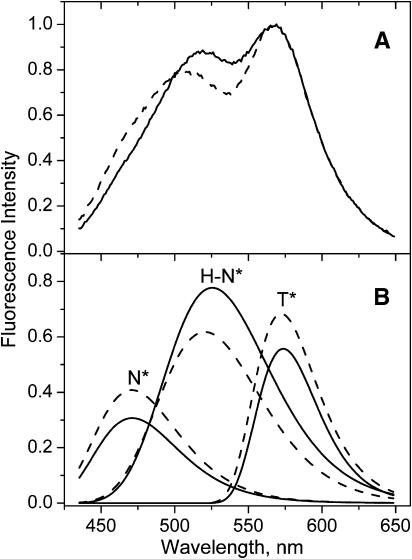
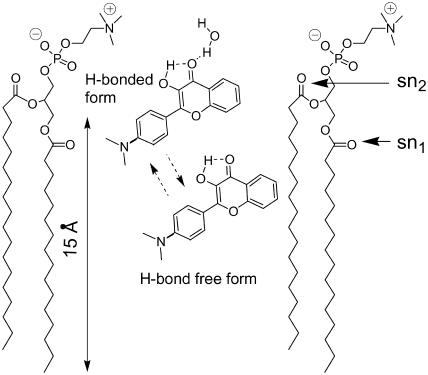
To provide a direct demonstration of the distribution of probe F between the surface and deeper sites of the bilayer, we performed fluorescence quenching experiments in DOPC vesicles using the shallow located TempoPC quencher (Abrams and London, 1993). In the presence of 15% of the quencher, the fluorescence spectrum of probe F changes significantly: the main maximum of the short-wavelength band decreases in relative intensity and shifts to the blue, whereas the short-wavelength shoulder (∼475 nm) becomes more pronounced (Fig. 4 A). This shows that in the presence of the quencher the contribution of the H-N* band with the maximum ∼525–530 nm decreases significantly. This decrease in the relative contribution of the H-N* band can be further deduced from the deconvolution data (Fig. 4 B). Thus, a shallow quencher provides a selective quenching of the H-bonded form of the probe located at the bilayer interface decreasing its relative emission intensity with respect to the more deeply located non-H-bonded form. According to our previous parallax quenching measurements, with the application of shallow, medium, and deep quenchers, TempoPC, 5-, and 12-SLPC, respectively, the average location of the probe F in DOPC membranes is at 16 Å from the bilayer center (Klymchenko et al., 2002). These data can be reanalyzed separately for H-bonded and H-bond free forms using the deconvolution procedure described above (Table 3). The parallax analysis (Abrams and London, 1993) applied to the quenching data of these two forms shows that the H-bonded form is located at 16.5 Å from the bilayer center, which corresponds to a location between sn2-carbonyl groups and the phospholipid polar heads (Fig. 5). Meanwhile, the H-bond free form is located much deeper, at 9 Å from the bilayer center (Table 3), at a level between sn1-carbonyls and first hydrocarbon groups (Fig. 5). The somewhat higher values of quenching observed for the H-bonded form compared to the H-bond free form (Table 3) are probably related to the longer fluorescence lifetime of the former, in line with the data in organic solvents (Ormson et al., 1994). Thus our spectroscopic and quenching data provide a clear evidence for the distribution of probe F between a highly polar location at the membrane interface and a deep hydrophobic location in the lipid bilayer.
FIGURE 4.
Quenching of probe F emission in DOPC vesicles with TempoPC lipid. Fluorescence spectra (A) and corresponding deconvolution data (B) of probe F in DOPC vesicles in the absence (solid lines) and in the presence of 15% of TempoPC (dashed lines). Spectra are normalized at the T* band maximum. Experimental conditions are as in Fig. 3.
TABLE 3.
Parallax quenching data of H-bonded and H-bond free forms of probe F in DOPC vesicles
| Probe F form | FTC/F0 | F5/F0 | F12/F0 | Zcf,* Å |
|---|---|---|---|---|
| H-bonded | 0.37 | 0.42 | 0.45 | 16.5 |
| H-bond free | 0.63 | 0.59 | 0.58 | 9 |
FTC/F0, F5/F0, and F12/F0 are the values of fluorescence quenching ratios of DOPC vesicles containing 15 mol % TempoPC, 5-SLPC, or 12-SLPC, respectively, to DOPC vesicles lacking nitroxide-labeled lipid. The values were obtained by deconvolution of the fluorescence quenching data obtained previously (Klymchenko et al., 2002). The obtained integral intensities of the H-N* form and a sum of integral intensities of N* and T* forms were used to calculate the quenching for H-bonded and H-bond free forms of probe F.
Zcf is the distance between the middle of the bilayer and the chromophore center calculated from the parallax equation (Abrams and London, 1993).
FIGURE 5.
Estimated location of probe F in PC lipid bilayer. Location of phospholipid functional groups is based on the study of Wiener and White (1992). Positions of sn1 and sn2 carbonyls are indicated by horizontal arrows. Dashed arrows indicate the possibility of redistribution of probe F between the two locations.
The distribution of probe F between polar and apolar locations in the lipid bilayer could be further analyzed by calculating the relative integral intensities of H-N*, N*, and T* bands. Since the FWHMs of N* and H-N* bands are close to each other and are twice as wide as that of the T* band (see Table 1), it is possible to calculate the approximate partition of probe F between these locations from the following equation:
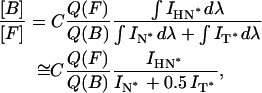 |
(1) |
where [B] and [F] are the concentrations of the probe F in H-bonded and H-bond free forms, respectively, C is a constant corresponding to the ratio of molecular absorptivities of the H-bonded and H-bond free forms of the dye at the excitation wavelength, and Q(F) and Q(B) are their respective fluorescence quantum yields. In this work, the [B]/[F] ratio could not be calculated straightforwardly since the ratio of quantum yields, Q(F)/Q(B), is unknown and cannot be easily determined. This quantum yield ratio cannot be derived from the data in neat solvents, since the non-emissive deactivation rates in solvents and membranes may be different. However, if we assume that the ratio of quantum yields does not change substantially from one type of lipid vesicles to another (unless specific quenching effects are involved), the intensity ratio FB/FF = IH-N*/(IN* + 0.5 IT*) could be useful to estimate the changes in the partition of probe F between the two locations.
It is worthwhile to note that at the low concentration ratio [probe]/[lipid] = 1/100 applied in the present study the emission spectrum is concentration-independent (Klymchenko et al., 2002). This could be expected, inasmuch as both binding sites are not saturated, and the probe distribution is determined by the site relative affinities. We observe an increase in the FB/FF ratio only at [probe]/[DOPC] = 1/25 and higher (Table 2). This may be the consequence of the saturation of the hydrophobic binding sites or the changes in the bilayer properties at high probe concentration.
Thus, the analysis of the fluorescence properties of probe F in phospholipid vesicles allows us to estimate the distribution of the probe between two major locations as well as the polarity of these locations. Some estimations could be based on the positions (referred by the wavelength of band maximum λ) of the three deconvoluted bands. Indeed, λN* indicates the polarity of the hydrophobic site and λHN* indicates the polarity of the polar site. These estimates are rather rough, since the positions of the bands do not exhibit strong variations. For more sensitive and convenient estimates, two parameters based on ratiometric measurements of fluorescence intensity, IN*/IT* and FB/FF, may be used. The former was already introduced as a polarity indicator (Klymchenko and Demchenko, 2003), and thus can characterize the polarity of the low polar location in phospholipid vesicles. The latter characterizes the distribution of probe F between its two locations.
Three-band superposition (TBS) analysis
Since both above-mentioned parameters are ratiometric and rely only on IN*, IH-N*, and IT* values, we made an attempt to simplify the analysis. We observed that the band asymmetry, the FWHM and the positions of the N*, H-N*, and T* bands are relatively constant. Therefore, the changes of the relative intensities of the bands in response to structural modifications in the bilayer can be analyzed by keeping invariant the parameters describing band shapes and positions. This simplification allows us to develop a simple method of calculating the relative intensities of the different bands directly from the experimental spectra without any deconvolution.
We assume that the asymmetries, FWHMs, and positions of each band have the corresponding constant values of 0.90, 3000 cm−1, and 475 nm for the N* band; 0.90, 3000 cm−1, and 525 nm for the H-N* band; and 0.80, 1500 cm−1, and 573 nm for the T* band. Moreover, we consider the experimental spectrum as a sum of these three bands represented by their log-normal functions. It follows that we have to take into account only the overlap between the neighboring bands. In addition, due to the narrowness and asymmetry of the T* band, its contribution to the peak intensity of the H-N* band can be neglected.
In the case of two overlapping bands, the observed intensity at the band maximum of one band can be described by the sum of the peak intensity of this band with the contribution of the overlapping band at this wavelength. Thus the band intensities for the N*, H-N*, and T* forms can be obtained as
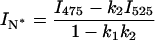 |
(2) |
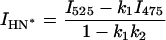 |
(3) |
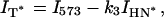 |
(4) |
where I475, I525, and I575 are the observed intensities at the corresponding wavelengths and k1 = 0.335, k2 = 0.230, and k3 = 0.500 are the coefficients determined as mean contributions of the overlapping band (H-N*, N*, and H-N*, respectively) at the corresponding wavelengths. The coefficients were calculated from the intensities of the normalized log-normal functions of H-N*, N*, and H-N* bands at 475, 525, and 570 nm, respectively.
We call this method the three-band superposition (TBS) analysis, which, in contrast to the common ratiometric analysis, takes into account the overlap between the spectral bands. The values of the IN*/IT* and FB/FF ratios obtained from the TBS analysis with respect to the deconvolution results are presented in Table 2. Both sets of results are very close, allowing us to conclude that in the present conditions, the heterogeneous fluorescence spectrum of probe F in phospholipid vesicles can be analyzed by measuring the emission intensities at only three wavelengths. It should be stressed that this analysis is limited to the cases where the shifts of these three bands are much smaller than their corresponding FWHMs (<10% of FWHMs, i.e., <5 nm for the N* band, according to our data).
Both deconvolution and TBS methodology were applied to analyze the probe response to different modifications of the bilayer structure, and these results are presented below.
Response of probe F to bilayer phase transitions
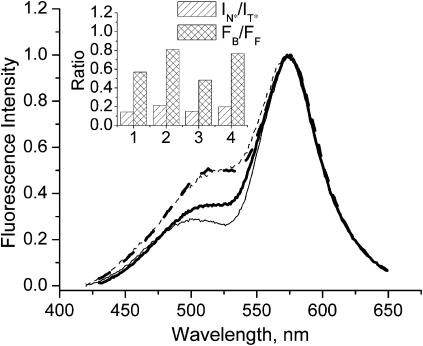
On the temperature-dependent transition from gel to liquid crystalline phase in DMPC vesicles, the probe F shows an increase of intensity of the short-wavelength emission band (Fig. 6). This effect has already been reported for the same probe in DPPC vesicles (Bondar et al., 1998), and it was concluded that in the liquid crystalline phase the probe environment becomes more polar. Quite similar effects are observed by comparing at 20°C the emission spectra in the gel phase of DPPC vesicles and in the liquid crystalline phase of DLPC vesicles (Fig. 6).
FIGURE 6.
Effect of phase transition on fluorescence spectra of probe F. Solid lines correspond to membranes in the gel phase composed of DMPC at 14°C (thin line, No. 1 of inset) and DPPC (thick line, No. 3 of inset); dashed lines correspond to membranes in the liquid crystalline phase composed of DMPC at 38°C (thin line, No. 2 of inset) and DLPC (thick line, No. 4 of inset). The spectra are normalized at the T* band maximum. Experimental conditions are as in Fig. 3. (Inset) Results of TBS analysis.
The deconvolution and TBS analysis developed in this article allowed us to characterize the phase transition in further detail (Tables 1 and 2). In DMPC and DPPC vesicles in the gel phase, probe F exhibits similar FB/FF values, whereas in DMPC and DLPC vesicles in the liquid crystalline phase, the FB/FF value is significantly larger (Fig. 6, inset), reaching a value close to that in EYPC vesicles. This indicates that on phase transition the probe molecules redistribute from the hydrophobic region of the bilayer to the membrane interface.
Moreover, the intensity ratio IN*/IT* of the two emission bands allows us also to estimate the polarity changes in the deep hydrophobic region of the lipid bilayer during the phase transition. The IN*/IT* value was found to increase, indicating an increase in the polarity in the hydrophobic region of the bilayer. This increase is accompanied by a 2-nm red shift of the N* band (Table 1), which is in line with the increase of polarity. A more pronounced red shift is observed for the H-N* band (∼7 nm), indicating a strong increase of the polarity also at the membrane interface.
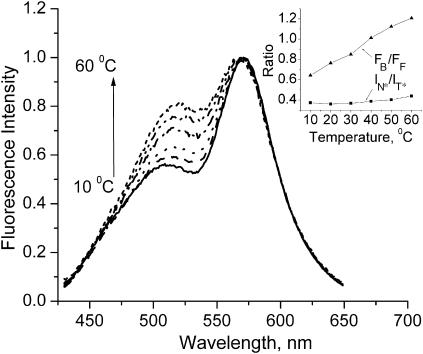
Effects of temperature in the liquid-crystalline phase
We also studied the effect of temperature on the fluorescence spectra of probe F in the liquid-crystalline phase of the EYPC bilayer. Our data show that the increase of temperature increases strongly the intensity of the short-wavelength band with respect to the T* band (Fig. 7). This observation is apparently similar to the effect of the phase transition described above. Meantime, the TBS analysis (Fig. 7 inset) further reveals some peculiarities of the temperature effect. It shows that an increase in temperature results in a strong linear increase of the FB/FF ratio, whereas the IN*/IT* ratio does not change significantly. These results suggest that the increase in temperature induces probe redistribution in favor of the membrane interface but does not affect the polarity of the hydrophobic location of the probe.
FIGURE 7.
Temperature effect on the fluorescence spectra of probe F in EYPC vesicles. The spectra are normalized at the T* band maximum. Experimental conditions are as in Fig. 3. (Inset) Results of TBS analysis. The redistribution parameter FB/FF and the polarity parameter IN*/IT* are plotted as a function of the temperature.
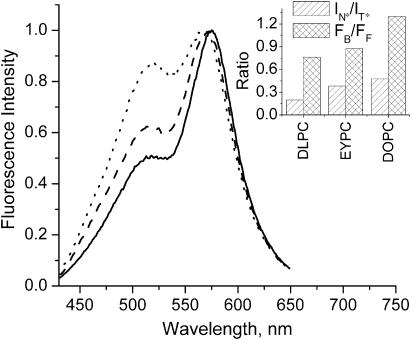
Effects of fatty acid composition
Data obtained with DOPC vesicles composed of identical unsaturated lipid species, and EYPC vesicles composed of a natural mixture of mainly unsaturated lipid species, can be compared with the data obtained with vesicles composed of saturated phospholipids like DLPC and DMPC. In the liquid crystalline state (above the phase transition temperature), the former shows significantly higher IN*/IT* values (Table 2, Fig. 8) suggesting that the hydrophobic region is significantly more polar in bilayers of unsaturated lipids than in bilayers of saturated lipids. Meantime, in this phase the redistribution parameter FB/FF is nearly the same for EYPC vesicles and lipid vesicles with saturated chains (Table 2, Fig. 8), thus demonstrating similar ability of their hydrophobic region of the bilayer to bind probe F (the polar interface being the same for all these PC vesicles).
FIGURE 8.
Fluorescence spectra of probe F in LUV vesicles composed of lipids with various fatty acid compositions: DLPC (solid), EYPC (dash), and DOPC (dot). The spectra are normalized at the T* band maximum. Experimental conditions are as in Fig. 3. (Inset) Results of the TBS analysis.
Remarkable was the observation of a difference between EYPC and DOPC vesicles (Klymchenko et al., 2002). In DOPC vesicles, probe F shows a significantly higher relative intensity of the short-wavelength emission band as compared to EYPC vesicles (Fig. 8). According to the deconvolution and TBS analysis of these spectra, the FB/FF ratio is significantly higher in DOPC than in EYPC vesicles (Table 2, Fig. 8 inset). This indicates that in DOPC vesicles, probe F has a stronger tendency to relocate to the membrane interface. This result can be explained by considering that the hydrophobic regions in EYPC vesicles are structurally more heterogeneous than in DOPC vesicles (composed of a single type of lipids), allowing a larger variety of probe binding sites. This variety of hydrophobic sites in EYPC membranes probably allows the probe to settle in the most structurally appropriate site(s) of the bilayer, which should be of higher affinity than the uniform sites of DOPC membranes. This idea is supported by the fact that charged 3HF derivatives with a more precise location in the bilayer do not sense any difference between DOPC and EYPC bilayers (Klymchenko et al., 2002). As expected, the comparison of the IN*/IT* ratios suggests that the polarity at the probe location in membrane hydrophobic region is not very different (Table 2) in the two bilayers.
Response to the surface charge
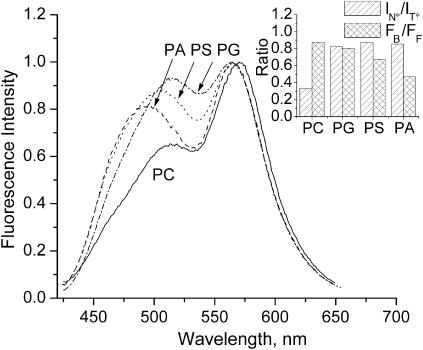
Fluorescence spectra of probe F are sensitive to the surface charge of bilayers, as we previously reported by comparing the probe behavior in neutral EYPC and anionic EYPG vesicles (Duportail et al., 2001). In the present study, these experiments were extended to other anionic vesicles made from EYPA and BBPS. As shown in Fig. 9, all anionic vesicles are presenting a short-wavelength emission band with a significantly higher relative intensity than in neutral EYPC vesicles. The same effect is observed when DOPG vesicles are compared to DOPC vesicles (Klymchenko et al., 2002). Moreover, the nature of the anionic polar head significantly affects this emission band since in PS and PA vesicles this band is blue-shifted by ∼10 and 20 nm, respectively, as compared to PG vesicles (Fig. 9). These spectroscopic effects were analyzed with the deconvolution and TBS methodology. The two-times-higher values of the IN*/IT* ratio for all four types of anionic vesicles as compared to the corresponding neutral PC vesicles (Table 2, Fig. 9 inset) indicate an important increase of the polarity of their hydrophobic region. This polarity increase is also in line with the observed red shifts of the N* band (Table 1). In contrast, the position of the H-N* band does not show any shift for the different anionic vesicles, indicating the absence of correlation between the polarity of the interface and the nature of the polar heads. Comparison of the FB/FF values suggests a significant redistribution of the probe in favor of the apolar region in the case of BBPS and EYPA vesicles (Table 2). This may contribute to the apparent blue shift of the short-wavelength band due to interplay of the overlapping N* and HN* bands (Fig. 9). In the case of EYPG vesicles, we did not observe any change of the FB/FF value in respect with EYPC vesicles, suggesting that in this case, the response of the dye to the surface charge is not connected with probe redistribution, in line with our previous conclusions (Klymchenko et al., 2002).
FIGURE 9.
Fluorescence spectra of probe F in EYPC (PC), EYPG (PG), BBPS (PS), and EYPA (PA) vesicles. The spectra are normalized at the T* band maximum. Experimental conditions are as in Fig. 3. (Inset) Results of TBS analysis.
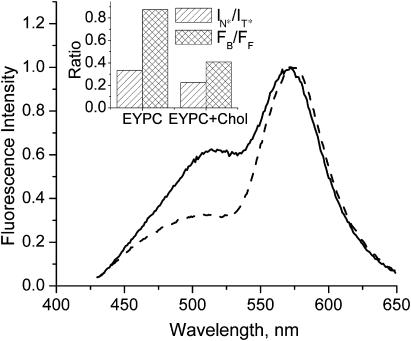
Response to the addition of cholesterol
We also reproduced and analyzed previous experiments on the effects of the inclusion of cholesterol into PC vesicles (Bondar et al., 1998). Addition of cholesterol in EYPC vesicles strongly decreases the relative contribution of the short-wavelength emission band, whereas the short-wavelength shoulder at 475 nm become more resolved (Fig. 10). According to the deconvolution and TBS analysis, the addition of cholesterol to EYPC vesicles induces a significant decrease of the IN*/IT* ratio as well as a 4-nm blue shift of the N* band, indicating a decrease in the polarity of the hydrophobic region of the bilayer (Table 2, Fig. 10 inset). Moreover, a blue shift of ∼4.5 nm of the H-N* band is also observed, indicating a decrease in the polarity at the bilayer interface. Finally, we observe a pronounced decrease in FB/FF, suggesting that addition of cholesterol induces a strong redistribution of the dye from the membrane surface to the hydrophobic region of the bilayer.
FIGURE 10.
Fluorescence response of probe F to the presence of 30% of cholesterol in LUV lipid vesicles. (Solid line) EYPC lipids; (dashed line) EYPC + 30% cholesterol. The spectra are normalized at the T* band maximum. Experimental conditions are as in Fig. 3. (Inset) Results of TBS analysis.
DISCUSSION
The present fluorescence study of neutral probe F in lipid vesicles provides direct evidence that the probe is distributed between two major locations that are characterized by different polarity and hydration. Moreover, we have shown that different factors like phase state, temperature, lipid composition and unsaturation degree, surface charge, and the presence of cholesterol influence dramatically not only the properties of the probe binding sites but also the distribution of the probe between these sites. These results obtained on probe F can be used to further understand the behavior of small neutral molecules of similar polarity.
It is commonly accepted that the polarity in the lipid bilayer decreases from the surface to the acyl chain region (Marsh, 2001; Kurad et al., 2003). We have then to understand why we observe two types of location in the bilayer for the neutral dye F. Is it a result of nonspecific heterogeneity with a broad distribution of locations or an indication of specific interaction of the probe with the bilayer? The detailed spectroscopic analysis of the excitation and emission spectra of probe F allows us to differentiate conclusively two probe locations and connect them with the ability of the probe to participate in intermolecular H-bonding. It is also evident that these two locations are characterized by different polarity (according to spectroscopic data) and depths of location (as shown by quenching data). It can be considered that the probe F being H-bonded with water exists as a complex of higher polarity than the free probe. In this respect, mainly two locations are energetically favorable for probe F in the bilayer: a deep location due to hydrophobic interactions and a surface location due to H-bonding (Fig. 5). Thus, the presence of two probe locations is thought to originate from the discrete nature of H-bonding interaction.
Such a bimodal distribution may be of more general significance and can be observed with other similar neutral fluorescence probes. A support to this hypothesis can be found in the literature on the probes containing proton-acceptor groups for H-bonding and exhibiting an apparent heterogeneous emission in lipid bilayers. The best example is Prodan, which exhibits two emission bands in lipid vesicles (Chong, 1988). As originally suggested (Chong, 1988; Chong et al., 1989), the short-wavelength band ∼435 nm may correspond to the location of the dye in the hydrophobic region of the bilayer, and the long-wavelength band ∼480 nm can be attributed to a location at the interface near the polar headgroups. In line with this hypothesis, changes of the intensity ratio of the two emission bands of Prodan in response to different physicochemical parameters correlate well with the changes in the FB/FF ratio obtained with probe F in the present study. For instance, the transition from the gel to the liquid-crystalline phase, the increase in temperature in the liquid crystalline phase, and the cholesterol depletion are found to induce an increase in FB/FF, indicating a redistribution of probe F in favor of the polar bilayer interface. At the same time, Prodan responds to these changes by an increase of the relative intensity of its long-wavelength emission band (Massey, 1998; Krasnowska et al., 1998; Bondar and Rowe, 1999), suggesting that this probe also redistributes from hydrophobic regions to the polar interface.
Detailed studies of Prodan photophysics (Catalan et al., 1991; Samanta and Fessenden, 2000; Cerezo et al., 2001) show that a significant part of its strong positive solvatochromism is due to specific H-bonding interaction with protic solvents. Indeed, according to these studies, even for the least polar protic solvents (cyclohexanol and 1-octanol) the band maximum of Prodan emission spectrum cannot be shorter than 475 nm, whereas for the most polar aprotic solvents (dimethylsulfoxide, dimethylformamide) it cannot be longer than 465 nm. Comparison of these data with those obtained in the membranes strongly suggests that the maximum at 435 nm corresponds to a H-bond free form, whereas that at 480 nm corresponds to a H-bonded form. Finally, the observation of dual emission in the time-resolved emission spectra of Prodan in DPPC (gel phase) and DLPC (liquid-crystalline phase) vesicles (Krasnowska et al., 1998) provides an additional strong support for the bimodal distribution of the probe in the bilayer.
Thus, analysis of the literature on Prodan and comparison of its behavior with probe F provide evidence for a bimodal distribution of Prodan (and its analogs) in the lipid bilayer and we suggest that this distribution is due to the presence of H-bonded and H-bond free forms of this dye.
Several other examples of solvatochromic dyes containing H-bond acceptor groups and showing bimodal distribution in lipid vesicles can be found in the literature. Bimodal distribution was notably demonstrated with parallax quenching data for some dansyl-(n-(5-dimethylaminonaphthalene-1-sulfonyl) and mansyl-(n-(6-(n-methylanilino)naphthalene-2-sulfonyl)) derivatives (Asuncion-Punzalan et al., 1998). For the lipid acyl-chain-linked NBD, a bimodal distribution has been recently shown in the gel phase of DPPC vesicles using spectroscopic, anisotropy, and quenching methods (Alakoskela and Kinnunen, 2001). On phospholipid phase transition, the NBD moiety redistributes from the hydrophobic region to the lipid interface, in line with our observations for probe F. Analysis of the time-resolved solvent relaxation process of Nile Red in EYPC vesicles also shows the presence of two emissive populations (Krishna, 1999; Ira et al., 2003). Moreover, addition of cholesterol to these vesicles results in a significant growth of the short-wavelength-emitting population of this dye (Ira et al., 2003), which is again in line with the strong redistribution of probe F into the hydrophobic region of the bilayer. Direct observation of dual emission attributed to a bimodal distribution in lipid bilayers has also been reported for other dyes containing carbonyl as H-bond acceptor group, such as 4-dimethylaminochalcone (Dobretsov et al., 1976) and ketocyanines (Doroshenko et al., 2002). Moreover, the response of the former dye to the presence of cholesterol has also been reported to follow the same trends as probe F. Thus, we may conclude that the bimodal distribution in phospholipid vesicles is probably a general feature for relatively small neutral compounds containing carbonyl or other H-bond acceptor (nitro, carboxyl, sulfonyl) groups. Moreover, their distribution between the two locations is strongly determined by temperature, phase state of the membrane, presence of cholesterol, etc., and in the case of fluorescence probes, it can contribute to their response.
Deconvolution of the fluorescence spectra of probe F into discrete emissive forms allows us to analyze the polarity of the hydrophobic location of the probe separately from the effect of the redistribution of the probe between its two locations. We found that the hydrophobic location of the probe corresponds to the region below the sn1-carbonyls (Fig. 5). An increase of the unsaturation degree of the fatty acid chains as well as the phase transition from the gel to the liquid-crystalline state increases the polarity of the hydrophobic location, whereas the addition of cholesterol provides a significant decrease of this polarity. It has been already well-established by fluorescence anisotropy using DPH (1,6-diphenyl-1,3,5-hexatriene) and TMA-DPH (1-[4-(trimethylammonio)phenyl]-6-phenyl-1,3,5-hexatriene) as probes that an increase in lipids unsaturation and the transition to the liquid-crystalline phase significantly increase the fluidity of both the hydrophobic and the surface regions of the bilayer, whereas addition of cholesterol produces the opposite effect (van Blitterswijk et al., 1987; Straume and Litman, 1987). In this respect, our results demonstrate that an increase in fluidity is accompanied by an increase in polarity of the hydrophobic region of the bilayer. This can be understood by taking into account that polarity of the bilayer measured with solvatochromic probes is an integrative parameter of static and dynamic dielectric properties of the bilayer (determined by the presence and mobility of the ester groups of lipids and the imbedded water molecules; see also Gardecki et al., 1995; Parasassi et al., 1990, 1994; Valeur, 2002). Indeed, the increase in fluidity of the region corresponding to sn1-carbonyl groups may increase the mobility of both the carbonyls groups and the bound water molecules resulting in the polarity increase. The increase in fluidity due to the phase transition, the increase in unsaturation degree, and the decrease in cholesterol content are also accompanied by the increase in hydration of the bilayer (Straume and Litman, 1987; Pérochon et al., 1992; Haines, 1994; Ho et al., 1995; Binder and Gawrisch, 2001), which may also contribute to the observed polarity increase. We also show that the polarity at the interface, which can be roughly estimated from the position of the H-N* emission band, follows the same trends as the polarity in the hydrophobic region, in line with the DPH and TMA-DPH data showing similar fluidity changes in these two regions of the bilayer (Straume and Litman, 1987). The strong correlation between solvation dynamics of the bilayer and its structure observed in our data is also in line with the data obtained with other solvatochromic probes, such as Prodan and Laurdan (Parasassi et al., 1994; Massey, 1998; Krasnowska et al., 1998).
Meantime, the present data on probe F show that the phase transition and the presence of cholesterol strongly affect not only the polarity of the hydrophobic location of the probe, but also the probe distribution between the two sites. Evidently, both the polarity effects (Parasassi et al., 1990, 1994; Massey, 1998; Krasnowska et al., 1998) and the probe redistribution (Chong, 1988; Bondar and Rowe, 1999) determine the strong response of Prodan and Laurdan to structural modifications in the lipid bilayer. But since Prodan and Laurdan as well as other probes like Nile Red are single excited-state fluorophores, they do not provide sufficient information to evaluate the relative contribution of these two factors to their spectroscopic response. Moreover, the H-bonding interactions of their carbonyl groups with a protic environment result in strong red shifts of their fluorescence spectra, similar to those observed with the increase in solvent polarity. Thus, as it was shown in experiments with solvent mixtures, it is difficult with Prodan-type probes to discriminate between universal dipole-dipole and specific H-bonding interactions (Catalan et al., 1991; Cerezo et al., 2001). In this respect probe F and its analogs, being dual excited-state fluorophores, offer an essential advantage, because they can provide a simultaneous analysis of several physicochemical parameters of the surrounding (Klymchenko and Demchenko, 2003). In the case of lipid bilayers, this advantage allows us to reveal the presence and relative contribution of the hydrated (H-bonded) and nonhydrated (H-bond free) forms of the probe as well as to provide a quantitative estimation of polarity of their environment. To illustrate this we may compare the effect of temperature on the fluorescence responses of probe F and Prodan. In unsaturated-PC vesicles (in the liquid-crystalline phase) an increase in temperature induces a red shift in Prodan fluorescence spectra (Massey, 1998), which was interpreted as an increase in the polarity of the probe environment. However, our data on probe F in EYPC vesicles show that the increase in temperature induces a redistribution of the probe in favor of the membrane interface without any significant change of the polarity of the deep hydrophobic location of the probe. The latter result is in line with recent data showing that the order and hydration of bilayers composed of unsaturated lipids are poorly sensitive to temperature variation (Binder and Gawrisch, 2001).
In conclusion, our results demonstrate the advantage of a new methodology based on multiparametric analysis of the fluorescence information provided by a 3-hydroxyflavone probe. In particular, this methodology allows a simultaneous analysis of the distribution of the probe between different locations in the bilayer and of the local properties of this bilayer. This analysis can be readily performed by a simple recording of emission intensities at three wavelengths, which is not possible with other polarity-sensitive probes. The suggested model of the bimodal distribution and its quantitative description using probe F may allow understanding of the interaction with the membrane of different fluorescent and nonfluorescent low-polar molecules, containing carbonyl and other H-bond acceptor groups.
Acknowledgments
This work was supported by grants from the Centre National de la Recherche Scientifique (CNRS), Université Louis Pasteur (France), The Scientific and Technical Research Council of Turkey (TUBITAK, Turkey), and the French-Ukrainian program Dnipro. Alexander Demchenko and Andrey Klymchenko acknowledge fellowships from the Université Louis Pasteur and the Centre National de la Recherche Scientifique, respectively.
References
- Abrams, F. S., and E. London. 1993. Extension of the parallax analysis of membrane penetration depth to the polar region of model membranes: use of fluorescence quenching by a spin-label attached to the phospholipid polar headgroup. Biochemistry. 32:10826–10831. [DOI] [PubMed] [Google Scholar]
- Alakoskela, J.-M. I., and P. K. J. Kinnunen. 2001. Probing phospholipid main phase transition by fluorescence spectroscopy and a surface redox reaction. J. Phys. Chem. B. 105:11294–11301. [Google Scholar]
- Asuncion-Punzalan, E., K. Kachel, and E. London. 1998. Groups with polar characteristics can locate at both shallow and deep locations in membranes: the behavior of dansyl and related probes. Biochemistry. 37:4603–4611. [DOI] [PubMed] [Google Scholar]
- Bartucci, R., R. Guzzi, D. Marsh, and L. Sportelli. 2003. Intramembrane polarity by electron spin echo spectroscopy of labeled lipids. Biophys. J. 84:1025–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder, H., and K. Gawrisch. 2001. Effect of unsaturated lipid chains on dimensions, molecular order and hydration of membranes. J. Phys. Chem. B. 105:12378–12390. [Google Scholar]
- Van Blitterswijk, W. J., B. W. Van der Meer, and H. Hilkmann. 1987. Quantitative contributions of cholesterol and the individual classes of phospholipids and their degree of fatty acyl (un)saturation to membrane fluidity measured by fluorescence polarization. Biochemistry. 26:1746–1756. [DOI] [PubMed] [Google Scholar]
- Bondar, O. P., V. G. Pivovarenko, and E. S. Rowe. 1998. Flavonols—new fluorescent membrane probes for studying the interdigitation of lipid bilayers. Biochim. Biophys. Acta. 1369:119–130. [DOI] [PubMed] [Google Scholar]
- Bondar, O. P., and E. S. Rowe. 1999. Preferential interactions of fluorescent probe Prodan with cholesterol. Biophys. J. 76:956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalan, J., P. Perez, J. Laynez, and F. G. Blanco. 1991. Analysis of the solvent effect on the photophysics properties of 6-propionyl-2-(dimethylamino)naphthalene (PRODAN). J. Fluorescence. 1:215–223. [DOI] [PubMed] [Google Scholar]
- Cerezo, F. M., S. C. Rocafort, P. S. Sierra, F. Garcia-Blanco, C. D. Oliva, and J. C. Sierra. 2001. Photophysical study of the probes acrylodan (1-[6-(dimethylamino)naphthalen-2-yl]prop-2-en-1-one), ANS (8-anilinonaphthalene-1-sulfonate) and Prodan (1-[6-(dimethylamino)naphthalen-2-yl]propan-1-1) in aqueous mixtures of various alcohols. Helv. Chim. Acta. 84:3306–3312. [Google Scholar]
- Chong, P. L. 1988. Effects of hydrostatic pressure on the location of Prodan in lipid bilayers and cellular membranes. Biochemistry. 27:399–404. [DOI] [PubMed] [Google Scholar]
- Chong, P. L., S. Capes, and P. T. T. Wong. 1989. Effect of hydrostatic pressure on the location of Prodan in lipid bilayers: a FT-IR study. Biochemistry. 28:8358–8363. [DOI] [PubMed] [Google Scholar]
- Demchenko, A. P. 2002. The red-edge effects: 30 years of exploration. Luminescence. 17:19–42. [DOI] [PubMed] [Google Scholar]
- Dobretsov, G. E., V. A. Petrov, A. I. Deev, and I. A. Vladimirov. 1976. Distribution of small hydrophobic molecules in membranes. II. Heterogeneity of binding centers on a phospholipid bilayer surface. Biofizika. 21:459–462. [PubMed] [Google Scholar]
- Doroshenko, A. O., L. B. Sychevskaya, A. V. Grygorovych, and V. G. Pivovarenko. 2002. Fluorescence probing of cell membranes with azacrown-substituted ketocyanine dyes. J. Fluorescence. 12:455–464. [Google Scholar]
- Duportail, G., A. Klymchenko, Y. Mely, and A. Demchenko. 2001. Neutral fluorescence probe with strong ratiometric response to surface charge of phospholipid membranes. FEBS Lett. 508:196–200. [DOI] [PubMed] [Google Scholar]
- Duportail, G., A. Klymchenko, Y. Mely, and A. Demchenko. 2002. On the coupling between surface charge and hydration in biomembranes. Experiments with 3-hydroxyflavone probes. J. Fluorescence. 12:181–185. [Google Scholar]
- Emel'ianenko, V. I., I. K. Reshetniak, O. A. Andreev, and E. A. Burshtein. 2000. Analysis of log-normal components of fluorescence spectra of Prodan and Acrylodan bound to proteins. Biofizika. 45:207–219. [PubMed] [Google Scholar]
- Gardecki, J., M. L. Horng, A. Papazyan, and M. Maroncelli. 1995. Ultrafast measurements of the dynamics of solvation in polar and non-dipolar solvents. J. Mol. Liq. 65–66:49–57. [Google Scholar]
- Haines, T. H. 1994. Water transport across biological membranes. FEBS Lett. 346:115–122. [DOI] [PubMed] [Google Scholar]
- Ho, C., S. J. Slater, and C. D. Stubbs. 1995. Hydration and order in lipid bilayers. Biochemistry. 34:6188–6195. [DOI] [PubMed] [Google Scholar]
- Hope, M. J., M. B. Bally, G. Webb, and P. R. Cullis. 1985. Production of large unilamellar vesicles by a rapid extrusion procedure. Characterization of size distribution, trapped volume and ability to maintain a membrane potential. Biochim. Biophys. Acta. 812:55–65. [DOI] [PubMed] [Google Scholar]
- Ira, A. S. R. Koti, G. Krishnamoorthy, and N. Periasamy. 2003. TRANES spectra of fluorescence probes in lipid bilayer membranes: an assessment of population heterogeneity and dynamics. J. Fluorescence. 13:95–103. [Google Scholar]
- Kaiser, R. D., and E. London. 1998. Determination of the depth of BODIPY probes in model membranes by parallax analysis of fluorescence quenching. Biochim. Biophys. Acta. 1375:13–22. [DOI] [PubMed] [Google Scholar]
- Klymchenko, A. S., G. Duportail, T. Ozturk, V. G. Pivovarenko, Y. Mély, and A. P. Demchenko. 2002. Novel two-band ratiometric fluorescence probes with different location and orientation in phospholipid membranes. Chem. Biol. 9:1199–1208. [DOI] [PubMed] [Google Scholar]
- Klymchenko, A. S., and A. P. Demchenko. 2003. Multiparametric probing of intermolecular interactions with fluorescent dye exhibiting excited state intramolecular proton transfer. Phys. Chem. Chem. Phys. 5:461–468. [Google Scholar]
- Klymchenko, A. S., V. G. Pivovarenko, and A. P. Demchenko. 2003. Elimination of hydrogen-bonding effect on solvatochromism of 3-hydroxyflavones. J. Phys. Chem. A. 107:4211–4216. [Google Scholar]
- Krasnowska, E. K., E. Gratton, and T. Parasassi. 1998. Prodan as a membrane surface fluorescence probe: partitioning between water and phospholipid phases. Biophys. J. 74:1984–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna, M. M. G. 1999. Excited-state kinetics of the hydrophobic probe Nile red in membranes and micelles. J. Phys. Chem A. 103:3589–3595. [Google Scholar]
- Kurad, D., G. Jeschke, and D. Marsh. 2003. Lipid membrane polarity profiles by high-field EPR. Biophys. J. 85:1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loew, L. M. 1988. Spectroscopic Membrane Probes. Vol. 1. CRC Press, Boca Raton, FL.
- Marsh, D. 2001. Polarity and permeation profiles in lipid membranes. Proc. Natl. Acad. Sci. USA. 98:7777–7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey, J. B. 1998. Effect of cholesterol hemisuccinate on the interfacial properties of phosphatidylcholine bilayers. Biochim. Biophys. Acta. 1415:193–204. [DOI] [PubMed] [Google Scholar]
- Nemkovich, N. A., W. Baumann, and V. G. Pivovarenko. 2002. Dipole moments of 4′-aminoflavonols determined using electro-optical absorption measurements or molecular Stark-effect spectroscopy. J. Photochem. Photobiol. A. 153:19–24. [Google Scholar]
- Ormson, S. M., R. G. Brown, F. Vollmer, and W. Rettig. 1994. Switching between charge- and proton-transfer emission in the excited state of a substituted 3-hydroxyflavone. J. Photochem. Photobiol. A. 81:65–72. [Google Scholar]
- Parasassi, T., G. De Stasio, A. d'Ubaldo, and E. Gratton. 1990. Phase fluctuation in phospholipid membranes revealed by Laurdan fluorescence. Biophys. J. 57:1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasassi, T., M. Di Stefano, M. Loiero, G. Ravagnan, and E. Gratton. 1994. Cholesterol modifies water concentration and dynamics in phospholipid bilayers: a fluorescence study using Laurdan probe. Biophys. J. 66:763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérochon, E., A. Lopez, and J. F. Tocanne. 1992. Polarity of lipid bilayers. A fluorescence investigation. Biochemistry. 31:7672–7682. [DOI] [PubMed] [Google Scholar]
- Reichardt, C. 1994. Solvatochromic dyes as solvent polarity indicators. Chem. Rev. 94:2319–2358. [Google Scholar]
- Samanta, A., and R. W. Fessenden. 2000. Excited state dipole moment of PRODAN as determined from transient dielectric loss measurements. J. Phys. Chem. A. 104:8972–8975. [Google Scholar]
- Sengupta, P. K., and M. Kasha. 1979. Excited state proton-transfer spectroscopy of 3-hydroxyflavone and quercetin. Chem. Phys. Lett. 68:382–385. [Google Scholar]
- Siano, D. B., and D. E. Metzler. 1969. Band shapes of the electronic spectra of complex molecules. J. Chem. Phys. 51:1856–1861. [Google Scholar]
- Straume, M., and B. J. Litman. 1987. Influence of cholesterol on equilibrium and dynamic bilayer structure of unsaturated acyl chain phosphatidylcholine vesicles as determined from higher order analysis of fluorescence anisotropy decay. Biochemistry. 26:5121–5126. [DOI] [PubMed] [Google Scholar]
- Valeur, B. 2002. Molecular Fluorescence. Wiley VCH, Weinheim, Germany.
- Weber, G., and F. J. Farris. 1979. Synthesis and spectral properties of a hydrophobic fluorescent probe: 6-propionyl-2-(dimethylamino)naphthalene. Biochemistry. 18:3075–3078. [DOI] [PubMed] [Google Scholar]
- Wiener, M. C., and S. H. White. 1992. Structure of a fluid dioleoylphosphatidylcholine bilayer determined by joint refinement of x-ray and neutron diffraction data. III. Complete structure. Biophys. J. 61:437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, J., and P. L. Chong. 1995. Effect of ethanol-induced lipid interdigitation on the membrane solubility of Prodan, Acdan and Laurdan. Biophys. J. 68:567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]