Abstract
We investigate lateral organization of lipid domains in vesicles versus supported membranes and monolayers. The lipid mixtures used are predominantly DOPC/DPPC/Chol and DOPC/BSM/Chol, which have been previously shown to produce coexisting liquid phases in vesicles and monolayers. In a monolayer at an air-water interface, these lipids have miscibility transition pressures of ∼12–15 mN/m, which can rise to 32 mN/m if the monolayer is exposed to air. Lipid monolayers can be transferred by Langmuir-Schäfer deposition onto either silanized glass or existing Langmuir-Blodgett supported monolayers. Micron-scale domains are present in the transferred lipids only if they were present in the original monolayer before deposition. This result is valid for transfers at 32 mN/m and also at lower pressures. Domains transferred to glass supports differ from liquid domains in vesicles because they are static, do not align in registration across leaflets, and do not reappear after temperature is cycled. Similar static domains are found for vesicles ruptured onto glass surfaces. Although supported membranes on glass capture some aspects of vesicles in equilibrium (e.g., gel-liquid transition temperatures and diffusion rates of individual lipids), the collective behavior of lipids in large liquid domains is poorly reproduced.
INTRODUCTION
Recently, fluorescence microscopy has been used to directly observe micron-scale liquid domains in giant unilamellar vesicles made from model mixtures of phospholipids and cholesterol (Dietrich et al., 2001; Samsonov et al., 2001; Veatch and Keller, 2003). Much of the current excitement about these results stems from the possibility that the observed domains resemble rafts, at least in the outer leaflet of the plasma membrane (Wang and Silvius, 2001). Rafts are submicron regions in cell membranes that are enriched in particular lipids and proteins, and are thought to be important for signaling, adhesion, endocytosis, apoptosis, protein organization, and lipid regulation (Thomas et al., 1994; de Jong et al., 1997; Simons and Ikonen, 1997; Edidin, 2001; Anderson and Jacobson, 2002).
This article describes work toward patterning the same liquid domains seen in vesicles on solid substrates. Supported bilayers have found uses in sensors (Cornell et al., 1997) and there have been recent advances both in patterning bilayers and in targeting proteins to particular membrane regions (Groves et al., 1997; Wagner and Tamm, 2000; Kam and Boxer, 2003; Khan et al., 2003). An advantage of supported membranes is that they can be assembled to mimic the lipid asymmetry between leaflets of natural membranes. It has been shown that features such as reasonable diffusion constants and the transition from gel to liquid crystalline phases can be retained in supported membranes (Tamm and McConnell, 1985; Linseisen et al., 1997). Supported lipid bilayers are most often assembled on glass substrates because of the ease of vesicle deposition (Rädler et al., 1995) and some work has already been accomplished in imaging coexisting domains on glass supports. For example, Dietrich et al. (2001) reported that depositing monolayers of phospholipids and cholesterol mixtures on alkylated glass substrates resulted in domains similar to liquid domains in vesicles. In that work the deposited lipids recovered after photobleaching, indicating lipids in both phases are mobile.
We are interested in how miscibility behavior is altered when lipid membranes are in close proximity to a solid support rather than in a free-floating vesicle. We already know that vesicles exhibit reversible miscibility transitions. Furthermore, liquid domains in vesicles are in registration between both bilayer leaflets, diffuse on the surface of the vesicle, collide with other domains, and coalesce (Veatch and Keller, 2003). Here we investigate the feasibility of creating micron-scale liquid domains by depositing lipids from monolayers and vesicles on existing layers of either silanes or lipids on glass. We also study the ability of domains in one leaflet to affect domains in the opposite leaflet. We have chosen to work primarily with the mixtures DOPC/DPPC/Chol and DOPC/Brain sphingomyelin/Chol because their vesicle behavior has been previously examined (Veatch and Keller, 2003) and they have been employed as models of raft-forming lipids (Dietrich et al., 2001). As observed by fluorescence microscopy of giant unilamellar vesicles, mixtures of these lipids in ratios of 1:1:1 and 2:2:1 fall within a large region of two coexisting liquid phases, far from a phase boundary. We will demonstrate that monolayers of these same compositions deposited directly on solid glass supports poorly capture the rich miscibility phase behavior seen in vesicles.
MATERIALS AND METHODS
Phospholipids and cholesterol (Chol) were obtained from Avanti Polar Lipids (Alabaster, AL). Lipids were used without further purification and stock solutions were stored in chloroform at −20°C. The dye Texas Red dipalmitoyl-phosphatidylethanolamine (TR-DPPE, Molecular Probes, Eugene, OR) was used to provide contrast between lipid phases. This contrast is due to unequal partitioning of the dye in each separated phase. Probe behavior is altered by local lipid environment. Dye concentration ranged from 0.5 to 1 mol %. Varying dye from 0.2 to 2 mol % does not significantly change bilayer phase behavior (Veatch and Keller, 2003) or monolayer phase behavior. Mixtures of DOPC/DPPC/Chol and DOPC/BSM/Chol were both prepared in ratios of 1:1:1 and 2:2:1. Vesicle spreading solution was made of 100 mM NaCl, 10 mM Tris at pH 8.
Glass coverslips were cleaned by two procedures:
Coverslips were cleaned in No-Chromix (Godax Laboratories, Takoma Park, MD) and sulfuric acid, rinsed in pure water of resistivity >18 MΩ cm (Barnstead, Dubuque, IA), cleaned in an argon plasma for 5 min (Harrick Scientific, Ossining, NY), and used within 24 h.
Coverslips were boiled in dilute soap solution (7X from ICN Biomedicals, Aurora, OH), plasma-cleaned, and used within 24 h. The best results were obtained with this cleaning method.
Supported bilayers were assembled on glass coverslips either by rupturing vesicles, or by transferring two monolayers from an air-water interface. All experiments were conducted at room temperature (22.5 ± 1°C) unless otherwise noted. Lipid monolayers and supported lipid layers were imaged with a Nikon microscope (Y-FL or ME600, Melville, NY) equipped with a Photometrics Coolsnap FX charge-coupled device camera (Roper, Princeton, NJ).
Giant unilamellar vesicles (GUVs) were prepared as in Veatch and Keller (2003) in either 100 mM sucrose or pure water, diluted with spreading solution or pure water, allowed to spontaneously rupture onto the glass surface, then viewed on a temperature-controlled stage. Large unilamellar vesicles (LUVs) were prepared by extrusion as follows. Mixtures of lipids in chloroform were dried under nitrogen in test tubes and then placed under vacuum. Lipids were then hydrated with pure water to ∼5 mg/ml, warmed to 60°C (well above the miscibility transition temperature), and extruded through 100-nm pores in polycarbonate membranes (Avanti Polar Lipids). We have previously shown that LUVs extruded by this method retain the same lipid composition as originally mixed (Bezzine et al., 2002). The resultant LUV solution was mixed with an equal volume of spreading solution, placed in contact with coverslips cleaned by the 7X method, and the excess vesicles were rinsed away.
Lipid monolayers were assembled in a home-built Langmuir trough in which surface pressure was monitored with a Wilhelmy plate (Riegler & Kirstein, Berlin, Germany). To prevent oxidation, monolayers were held under argon. For experiments in air, oxidation was minimized by exposing the monolayer to air for <10 min. Monolayers that were exposed to air for intentional oxidation were typically deposited on the trough at ∼20 mN/m and allowed to equilibrate for 15–20 min. The surface pressure was then decreased to ∼1 mN/m for 10 min and slowly increased to 32 mN/m over ∼15 min. To form a supported bilayer, the first lipid monolayer was deposited from a Langmuir trough to a clean glass coverslip by the Langmuir-Blodgett technique. The second layer was deposited by the Langmuir-Schäfer technique. Unless specifically noted all depositions were performed at room temperature at a surface pressure of 32 ± 2 mN/m. Monolayers were also deposited on silanized coverslips by the Langmuir-Schäfer technique at 32 ± 2 mN/m. Clean coverslips were silanized by placing them with a vial of newly opened silanes under partial vacuum for 15 min and used the same day of preparation.
RESULTS AND DISCUSSION
Monolayers at the air-water interface
We observe immiscible liquid phases in monolayers containing the mixtures DOPC/DPPC/Chol and DOPC/BSM/Chol. The lipids separate into a bright phase containing the dye and most of the DOPC, plus a dark phase containing most of the saturated lipid and cholesterol (Veatch and Keller, 2002). As surface pressure is increased in the monolayer, the lipids pass through a miscibility transition and mix into one uniform liquid phase. We find that transition pressures are 12 ± 2 mN/m for 1:1:1 DOPC/BSM/Chol and 14.5 ± 2 mN/m for 2:2:1 DOPC/BSM/Chol. Transition pressures are similar for ratios of 1:1:1 and 2:2:1 of DOPC/DPPC/Chol. It is known that unsaturated lipids oxidize in contact with air (Kim and LaBella, 1987; Benvegnu and McConnell, 1993; Hagen and McConnell, 1997). This oxidation alters the miscibility transition pressure of lipids in monolayers (Benvegnu and McConnell, 1993). Oxidation can be avoided by enclosing the Langmuir trough in a glove bag filled with either argon or nitrogen (Hagen and McConnell, 1997). We find that miscibility transition pressures are stable to within 2 mN/m per h when the monolayer is under argon, but increase dramatically when the lipids are exposed to air. For example, transition pressures of monolayers that contain unsaturated lipids and are exposed to air can rise above 32 mN/m in as little as 20 min, and easily within 1 h.
In other words, domains are not initially observed in monolayers of our lipid mixtures at 32 mN/m. If a monolayer is left exposed to air, the immiscibility transition pressure will rise and domains can ultimately be observed at pressures of 32 mN/m—far above the initial transition pressure of the monolayer. When we intentionally expose our monolayers to air for long periods of time, we believe we are oxidizing the unsaturated lipids, which increases the miscibility transition pressure. Monolayer domains after oxidation at 32 mN/m appear almost indistinguishable from their unoxidized counterparts at low pressure except that movement of domains due to Brownian motion is slower, as is expected due to the higher surface pressure.
LB-LS supported bilayers
Our studies test how well lipid layers supported on glass mimic bilayers in vesicles. We conducted our experiments at 32 mN/m because lipids in monolayers are thought to most closely approximate lipids in bilayers at that surface pressure (Demel et al., 1975). Using the LB-LS method, we create a supported bilayer by transferring two lipid monolayers at 32 mN/m to a coverslip. The first lipid monolayer is deposited by the Langmuir-Blodgett (LB) method and the second layer by the Langmuir-Schäfer (LS) method. Table 1 summarizes all of the experimental trials with LB-LS supported bilayers, which fall into three categories:
Domain capture.
Overlap.
Possible transbilayer coupling.
TABLE 1.
Supported bilayers of different lipid compositions formed by Langmuir-Blodgett/Langmuir-Schäfer (LB-LS) deposition at 32 mN/m onto glass substrates
| LB-LS Bilayers | 1st layer (LB) composition | Domains in 1st monolayer? | 2nd layer (LS) composition | 2nd layer (LS) condition | Domains in 2nd monolayer? | Bilayer domains? | Fig. |
|---|---|---|---|---|---|---|---|
| 1. Domain capture | eggPC | No | 1:1:1 and 2:2:1DOPC/DPPC/Chol | No oxidationOxidation | NoYes | NoYes | |
| 1:1:1 and 2:2:1 | No oxidation | No | No | ||||
| DOPC/BSM/Chol | Oxidation | Yes | Yes | 1, 2, 3 | |||
| 2. Overlap | 2:2:1 DOPC/BSM/Chol oxidation | Yes | 2:2:1 DOPC/BSM/Chol | Oxidation | Yes | Yes | 4 |
| 3. Possible translayer coupling | 2:2:1 DOPC/BSM/Chol no dye, oxidation | Yes (no dye) | eggPCPOPCDOPC | No oxidationNo oxidationNo oxidation | NoNoNo | YesYesYes | 7 D |
| 70:30 DOPC/Chol | No oxidation | No | Yes | 7 C | |||
| 1:1:1 DOPC/BSM/Chol | No oxidation | No | Yes | 7 B | |||
| 2:2:1 DOPC/BSM/Chol | Oxidation | Yes | Yes | 7 A |
“Oxidation” denotes that the monolayer lipid miscibility transition pressure rose above 32 mN/m due to exposure to air. It is noted whether domains were observed in the two monolayers before deposition (Domains in monolayer?) and in the bilayer after deposition (Bilayer domains?). Additional experiments were performed with depositions at pressures below 32 mN/m, and are described in the text.
In domain capture, cholesterol-rich domains are transferred directly from a monolayer at the air-water interface to a surface. As an example, Fig. 1 shows domains in an oxidized DOPC/BSM/Chol monolayer both before and after LS deposition at 32 mN/m on an LB layer of EggPC. Domains are not observed in the EggPC layer either before or after deposition. Domains in LB-LS bilayers exhibit the same contrast and distribution of domain sizes as in the oxidized monolayer at the air-water interface (Fig. 1). For monolayers of 1:1:1 DOPC/BSM/Chol, bright spots appear on a dark background; this transfer can be observed in Figs. 1–3. Monolayers of 2:2:1 DOPC/BSM/Chol have reversed contrast and this is observed in the supported bilayers imaged in Figs. 4 and 7. Our results apply equally well to mixtures of DOPC/DPPC/Chol (Table 1).
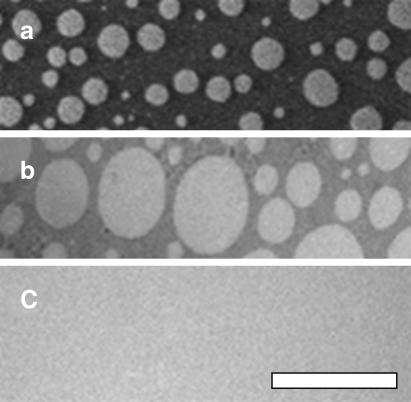
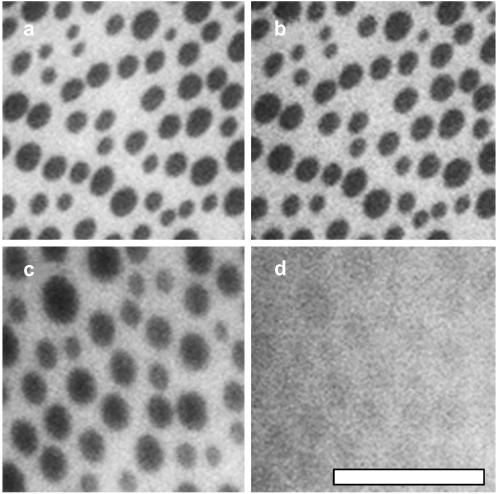
FIGURE 1.
A mixture of 1:1:1 DOPC/BSM/Chol (a) oxidized at free air-water interface at ∼30 mN/m, (b) deposited after oxidation on a coverslip previously coated with a monolayer of eggPC, or (c) deposited on a coverslip previously coated with a monolayer of eggPC and exposed to as little oxidation as possible. The difference in domain sizes between the two images is insignificant; similar regions were found in both systems. Similar results are observed for 1:1:1 DOPC/DPPC/Chol. The scale bar is 50 μm.
FIGURE 3.
(a–d) Temperature cycle of 1:1:1 DOPC/BSM/Chol monolayer deposited on a layer of eggPC on an acid-cleaned glass coverslip. The temperature series is 22°C, 39°C, 51°C, and 22°C, respectively. Micrographs were taken at ∼0, 7, 10, and 15 min. The scale bar is 50 μm.
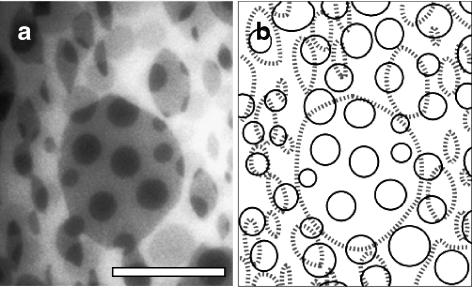
FIGURE 4.
Domains are not in registration in the top and bottom leaflets of a supported LB-LS bilayer. (a) LB-LS bilayer of 2:2:1 DOPC/BSM/Chol deposited on a soap-cleaned glass coverslip. (b) Sketch of domains in each leaflet to aid visualization. Thin solid lines trace domains in the top layer and dashed thick lines trace domains in the bottom layer. The scale bar is 20 μm.
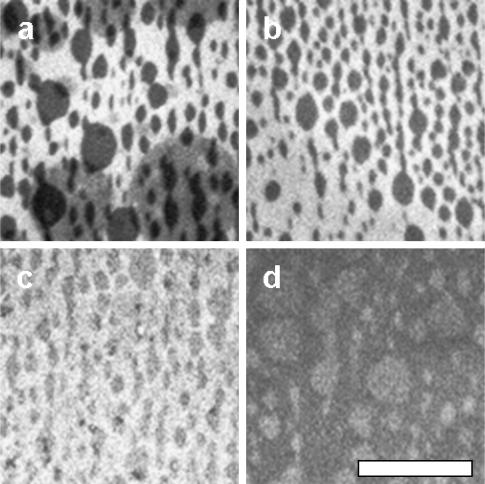
FIGURE 7.
(a) Overlap of domains is observed when the first layer contains no dye and oxidized 2:2:1 DOPC/BSM/Chol. The second layer contains the same composition, with dye. Almond-shaped domains characteristic of the first layer are observed even when there is no overlap of domains. (b–d) Supported bilayers as in (a) except the second layer contains dye and either (b) unoxidized 1:1:1 DOPC/BSM/Chol, (c) unoxidized 70:30 DOPC/Chol, or (d) unoxidized DOPC. The scale bar is 50 μm.
If the lipids are not intentionally oxidized, the monolayer is above the miscibility transition pressure and is homogenous in appearance. After deposition on the LB-LS bilayer supported on glass, no domains are observed. Oxidation is prevented as described in Materials and Methods, either by the common technique of maintaining the Langmuir trough under argon (Hagen and McConnell, 1997), or by quickly depositing the monolayer before extensive oxidation has occurred. Similar results are seen in supported membranes by both methods of preventing oxidation.
Summarizing the results above, large domains are present in the LB-LS bilayer only if they originally existed in the free monolayer at the air-water interface. Liquid domains exist in the free monolayer at 32 mN/m only if they are intentionally oxidized. We find this surprising because in vesicles of either DOPC/DPPC/Chol or DOPC/BSM/Chol, oxidation is not required to produce coexisting liquid phases. It is common to find literature examples in which monolayers are prepared for deposition at 32 mN/m by lengthy compression procedures without precautions against oxidation noted, which may result in deposited domains, e.g., Khan et al. (2003) and Lawrence et al. (2003).
Our results are not unique to deposition at 32 mN/m. If LB-LS deposition is performed at surface pressures below the miscibility transition pressure, no oxidation is necessary to produce domains in either the Langmuir monolayer or in the LB-LS bilayer. As described in the previous section, free monolayers of our lipid mixtures have low miscibility transition pressures when not oxidized and high pressures when oxidized. Unoxidized monolayers of 1:1:1 DOPC/BSM/Chol have a transition pressure of ∼12 mN/m. When monolayers are deposited below this transition pressure (9 mN/m), domains are transferred and behave similarly to domains deposited from oxidized monolayers at high pressure.
Fig. 2 demonstrates that a large fraction of the individual lipids in the second (LS) layer are able to diffuse. Diffusion is observed by photobleaching the fluorescing lipids in an area and then new fluorescing lipids diffuse into the region (FRAP). Despite diffusion of individual lipids on a LB-LS bilayer on glass, the domains are static, unlike liquid domains in a vesicle. As seen in Figs. 1 and 2, domains in the LB-LS bilayer are not circular as line tension would normally dictate for coexisting liquid phases. Although the domains are originally circular and liquid in the free monolayer at the air-water interface, they do not behave as liquids in vesicles when deposited. Noncircular domains were also noticed by Dietrich et al. (2001), who suggested that peaks in the glass substrate act as pinning sites at the boundary between the two liquid phases. Since domains do not travel in the LB-LS bilayer, they do not collide and coalesce as observed in vesicles.
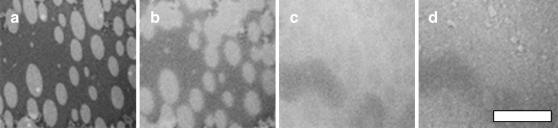
FIGURE 2.
Individual lipids diffuse in a monolayer of 1:1:1 DOPC/BSM/Chol deposited on a monolayer of eggPC on an acid-cleaned glass coverslip. (a) Supported bilayer before photobleach, (b) immediately after being photobleached for 16 min, and (c) after a recovery for another 16 min. The scale bar is 20 μm.
Liquid domains in vesicles are further distinguished from their counterparts in supported membranes by a reversible miscibility transition that occurs on the order of seconds (Veatch and Keller, 2002). In contrast, as temperature is increased in the LB-LS bilayer through the vesicle miscibility temperature (from ∼22°C in Fig. 3 a to ∼39°C in Fig. 3 b), we observe that domains slowly become rough and gradually diffuse into a uniform phase. The micrograph in Fig. 3 b is taken >7 min after temperature was raised. The original pattern is nearly gone a few minutes later at ∼51°C (Fig. 3 C). When the temperature is decreased back to 22°C, no new large domains re-form as they would have in vesicles. A faint speckle in the area of the original domains is often observed after a temperature cycle as in Fig. 3 d. The speckle may be due to the formation of domains near the resolution of our microscope. These domains may be pinned and unable to coalesce into larger domains.
Another feature of vesicle behavior is that domains are in registration across both leaflets of the bilayer. We observe that this behavior is not reproduced in LB-LS supported bilayers. The LB-LS bilayer in Fig. 4 is made from two oxidized DOPC/BSM/Chol monolayers. Domains in the first layer have characteristic almond shapes, and are elongated in the direction of the LB deposition across the entire coverslip. Domains in the second layer (LS) are more rounded, and are not in registration with domains in the first layer (Fig. 4). All domains are static and do not move toward registration over experimental timescales up to 2–3 h.
Supported monolayers on silanes
Domains have also been reported in lipid monolayers deposited on silanized glass (Dietrich et al., 2001). As with the LB-LS bilayers, we observe that domains are present in supported layers after deposition at 32 mN/m only if they are initially present in the oxidized monolayer. Since there is no possibility for lipid flip-flop in monolayers supported on silanized glass, our results are not due to flip-flop between lipid leaflets.
Just as in supported bilayers, in supported membranes we find by FRAP that individual lipids diffuse, but domains do not change shape, travel, or coalesce. In our laboratory, this system produced a wide range of diffusion rates, sharpness of the domain boundary, and temperature response, even between slides simultaneously produced with identical cleaning methods, lipids, and new silanes. An illustration is in Fig. 5. In some cases domains have sharp edges and do not change with temperature. In cases when temperature does affect domains, the result is similar to the LB-LS bilayers shown earlier, such that domains slowly diffuse into a uniform phase as in Fig. 5, c and d. Returning to low temperature, speckle is sometimes observed, which may be caused by the formation of small domains. In addition, the supported monolayer sometimes has a faint imprint of the initial domains. This suggests some lipid molecules are pinned and unable to diffuse. An increase in temperature above the vesicle miscibility temperature is not always required for domains to diffuse into a uniform phase, although the process is slower at room temperature, occurring on the order of hours.
FIGURE 5.
Monolayers deposited on silanized coverslips demonstrate a range of behaviors. (a and b) A mixture of 2:2:1 DOPC/BSM/Chol is raised from 22°C to 50°C. The domains appear stable. (c and d) The same mixture of 2:2:1 DOPC/BSM/Chol on a simultaneously prepared coverslip shows greater mobility of lipids at both 22°C and 50°C, respectively. The scale bar is 20 μm.
Deposited vesicles
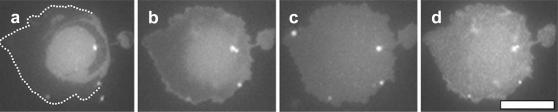
Given that supported monolayer systems poorly reproduce the behavior of free vesicles, we next investigate both large unilamellar vesicles (LUVs) and giant unilamellar vesicles (GUVs) deposited on a glass coverslip cleaned with 7X. Even in this system, the miscibility behavior of free vesicles is not reproduced. LUVs deposited on glass produce a continuous bilayer sheet without forming any large domains as in free vesicles. Of course it is possible that smaller domains, below the resolution of fluorescence microscopy, are formed as in van Duyl et al. (2003). In free GUVs in solution, large circular domains appear below the miscibility transition and disappear above it (Veatch and Keller, 2002). When a GUV is deposited on the glass surface below the transition temperature, it produces a noncircular bilayer island (a “splat”) with noncircular domains as in Fig. 6. Neither the splat shape nor the domain shapes change significantly over the course of 16 h. At high temperatures, domains in the splat disappear. When returning to low temperature, large domains do not reappear; however, speckle is common.
FIGURE 6.
A single GUV of DOPC/BSM/Chol (2:2:1) deposited on a glass coverslip cleaned with 7X. The series is at temperatures 22°C, 32°C, 42°C, and 22°C. Micrographs were taken at ∼0, 5, 6, and 18 min, respectively. Note the speckle pattern in d. White spots are small vesicles in solution that are not connected to the plane of the membrane. The scale bar is 20 μm.
Transbilayer effects
Surprisingly, the same overlap of domains is observed when the first layer contains no dye, as shown in Fig. 7 a. The round domains transferred with the second LS layer are originally the same darkness as the almond-shaped domains that are associated with the first LB layer, but quickly become lighter. In other words, the final distribution of dye is unequal between the two types of domains. The change in domain darkness could indicate a difference in mobility of individual lipids between the two leaflets. This is consistent with an NMR study in which diffusion of lipids in the leaflet closest to a silica substrate was found to be a factor-of-two slower than in the leaflet facing bulk water, leading the researchers to conclude that coupling between layers is weak compared to coupling with the surface (Hertzer et al., 1998). It should be noted that earlier work on LB-LS bilayers deposited on glass found no difference in diffusion rate between leaflets (Tamm and McConnell, 1985).
Almond-shaped domains characteristic of the first layer are observed even when there is no overlap of domains from the second layer. This is shown in Fig. 7 b for an LS layer containing unoxidized lipids of DOPC/BSM/Chol on top of an LB layer of oxidized DOPC/BSM/Chol with no dye. In this case, only the domains that are associated with the LB layer are seen. The same results are observed when the second layer contains a mixture of DOPC/Chol, which would not separate on its own in either a monolayer or vesicle. Particularly perplexing is that almond-shaped domains are still seen when the second layer contains only one component, regardless of whether it is DOPC, eggPC, or POPC, and that the contrast is reversed.
We find it frankly difficult to explain all of the results in Fig. 7. To summarize: we have observed almond-shaped domains characteristic of the first layer when the second layer contains lipids that separate in monolayers (oxidized DOPC/BSM/Chol); that separate in vesicles but not monolayers (unoxidized DOPC/BSM/Chol); that separate in neither (DOPC/Chol); and that have only one component (DOPC, eggPC, or POPC). There are four most likely explanations of our results:
Condensed, cholesterol-rich domains in the lower leaflet induce condensed domains in the upper leaflet.
Cholesterol alone flips from the lower layer to the upper layer.
Dye alone from the upper leaflet flips to the lower leaflet.
The DOPC, BSM, and cholesterol all mix completely by flip-flop between layers.
We will discuss each explanation separately:
Fig. 7, a–c, are consistent with a model in which domains in the lower leaflet induce condensed domains in the upper leaflet. However, Fig. 7 d has a reversed contrast and we would expect condensed domains of DOPC, eggPC, or POPC to exclude dye. As noted by a reviewer, this difficulty would disappear if the system in Fig. 7 d produced completely new behavior in the probe. The possibility that there is coupling between lipid leaflets is particularly interesting because although there is evidence that raft domains span the entire bilayer in plasma membranes, to date, domains have been observed only in model membranes that mimic the outer leaflet composition (Wang and Silvius, 2001).
It is unlikely that the flip-flop of cholesterol alone would explain our results. In Fig. 7 a, the composition of each layer is the same and would not drive a large net transfer between leaflets. In Fig. 7 d, we would not expect to see demixing if cholesterol alone flipped to the upper leaflet because DOPC does not normally phase-separate from cholesterol in either monolayers at 32mN/m or in vesicle bilayers. Nevertheless, if there were phase separation, we would expect the contrast to be reversed in Fig. 7 d. Again, this last difficulty would disappear if a completely new behavior of the probe were discovered.
Dye flipping to the lower leaflet is unlikely because Texas Red is a large hydrophilic group, and because some evidence exists that if Texas Red DPPE transfers at all between leaflets in supported membranes, it does so unidirectionally from the lower leaflet to the upper leaflet (A. Parikh, 2003, personal communication). In addition, the contrast in Fig. 7 d is still wrong.
Lastly, it is possible that all of the lipids (DOPC, BSM, and cholesterol) mix completely by flip-flop between bilayer leaflets. Reported flip-flop rates of membrane phospholipids are on the order of hours to days (Kornberg and McConnell, 1971; Devaux et al., 2002). This suggests that the supported bilayer is not in compositional equilibrium, although reported cholesterol flip-flop rates are faster (Steck et al., 2002).
As with option 1, complete mixing between layers would explain Fig. 7, a–c, but cannot explain the reversed contrast in Fig. 7 d unless a new behavior of the probe is invoked. This is because a complete mixture of the leaflets in Fig. 7 d creates a composition of 7:2:1 DOPC/BSM/Chol, which produces few small dark domains on a bright background in both Langmuir monolayers and vesicles (results not shown).
Membrane mobility in literature
In addition to the work cited above, other studies have documented immobile lipid domains on solid supports. Ratto and Longo (2002) found that 100–200-nm solid bilayer domains of distearoylPC moved <100 nm/h in a background of fluid dilauroyl phosphatidylcholine (DLPC) on a mica surface. The researchers concluded that the low mobility of domains was due to the presence of solid phases that were too large to be moved by thermally excited fluid lipid molecules. Tokumasu et al. (2003) studied supported bilayers of DLPC/DPPC/Chol and concluded that the mica support quantitatively altered the phase behavior in a region containing a gel-like DPPC-rich phase and a fluid-like DLPC-rich phase. Muresan and Lee (2001) also studied lipids on mica and found that the mobility of bilayer islands (“splats”) depended on which type of mica was used. For example, on high-grade mica, they found that adjacent bilayer splats from 130-nm eggPC vesicles became nearly circular over ∼10 min, although larger domains changed shape more slowly. Using muscovite mica they found that some splats were immobilized, and concluded that interactions of the bilayer with the substrate made a large contribution to the dissipation force. In other words, the immobility of lipids on some surfaces is not merely due to the confinement of the thin water layer between the lipids and the surface, which can increase the effective viscosity of the water layer an order-of-magnitude higher than the bulk (Israelachvilli et al., 1990). In our system, the water layer between the deposited lipid bilayer and the glass substrate is ∼1 nm (Sonnleitner et al., 1999; Kiessling and Tamm, 2003). Rädler et al. (1995) have discussed how the sliding of lipid bilayers on a rough glass surface is reduced by pinning sites at a surface density of ∼103/μm2. They note that the density of pinning sites can increase after argon sputtering.
By examining diffusion constants of individual lipids, we would not necessarily have predicted the low mobility of both the domains and vesicle splats we observe on glass surfaces. Dietrich et al. (2001) also found reasonable rates of diffusion for lipids in a mixture of 1:1:1 DOPC/BSM/Chol on silanized glass: 1.1 μm2/s in the bright phase and 0.38 μm2/s in the dark phase (Dietrich et al., 2001). These values are close to 0.41 μm2/s reported for fluorescently labeled DPPE in cell membranes of murine fibroblasts (Dietrich et al., 2002). FRAP on a similar system showed lateral mobility of individual lipids, but not of domains (Dietrich et al., 2001; Khan et al., 2003).
For a sensor to incorporate a supported bilayer that reproduces the behavior of cell membranes and free vesicles, the interaction between the bilayer and the substrate must be made smaller than in the supported membranes in this study. Improvements have been seen by introducing a modest separation of the bilayer from the surface. For example, when two bilayer membranes are consecutively deposited on a glass surface, the membrane furthest from the surface produces miscibility transitions closer to those observed in free vesicles (Kaizuka and Groves, 2004). Using polymers or tethers as a soft cushion between a bilayer and a surface has been shown to aid in the incorporation of large proteins in supported bilayers (Sackmann and Tanaka, 2000; Kiessling and Tamm, 2003). It may also aid in reproducing equilibrium miscibility phase behavior in the supported bilayer system, provided that the tether density is kept low enough that there are not many immobile obstacles to diffusion. Diffusion rates in model membranes without cholesterol are as high as 17.7 μm2/s in bilayers attached to a support via a polymer tether (Naumann et al., 2002), and climb to 20.6 ± 0.9 μm2/s in black lipid membranes (Sonnleitner et al., 1999).
CONCLUSION
In summary, we present evidence that liquid domains with diameters of tens of microns can be transferred from monolayers to both supported bilayers and supported monolayers. Upon transfer to a glass substrate, the domains become immobile and do not exhibit reversible miscibility transitions. This suggests that deposited domains on glass are not an equilibrium phenomenon. Clearly, there are challenges in using supported membranes to mimic miscibility behavior of free lipid bilayers. The results above are a caveat for applications that rely on mixing of lipids on solid supports. If liquid domains are present, they may be a product of oxidation, the domain sizes may not be representative of domain sizes in vesicles, and the lipids may not mix uniformly on a timescale relevant for experiments or at an appropriate temperature. Altering lipid composition in a supported membrane by the introduction of cyclodextrin or detergent may not result in an appropriate or timely change in domain morphology.
Acknowledgments
We thank Mike Halter and Viola Vogel for use of their Langmuir-Blodgett apparatus. We also thank Rayna Matsuno for preliminary work with silanized substrates.
B.L.S. and S.L.V. were supported in part by a National Science Foundation Integrated Graduate Education and Research Training Program Fellowship from the University of Washington Center for Nanotechnology. S.L.V. was additionally supported by a National Institutes of Health predoctoral training grant in Molecular Biophysics (5T32-GM08268-14). S.L.K. acknowledges support from a National Science Foundation CAREER Award (MCB-0133484), from the Research Corporation (Research Innovation Award and Cottrell Scholar Award), and from the Petroleum Research Fund, administered by the American Chemical Society.
Abbreviations used: BSM, brain sphingomyelin; Chol, cholesterol; DOPC, dioleoylphosphatidylcholine (di(18:1)PC); DPPC, dipalmitoylphosphatidylcholine (di(16:0)PC); eggPC, egg phosphatidylcholine; FRAP, fluorescence recovery after photobleaching; GUV, giant unilamellar vesicle; LB, Langmuir-Blodgett deposition; LS, Langmuir-Schäfer deposition; LUV, large unilamellar vesicle; PC, phosphatidylcholine; POPC, palmitoyl-oleoyl phosphatidylcholine.
References
- Anderson, R. G., and K. Jacobson. 2002. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 296:1821–1825. [DOI] [PubMed] [Google Scholar]
- Benvegnu, D. J., and H. M. McConnell. 1993. Surface dipole densities in lipid monolayers. J. Phys. Chem. 97:6686–6691. [Google Scholar]
- Bezzine, S., J. G. Bollinger, A. G. Singer, S. L. Veatch, S. L. Keller, and M. H. Gelb. 2002. On the binding preference of human groups IIA and X phospholipases A2 for membranes with anionic phospholipids. J. Biol. Chem. 277:48523–48534. [DOI] [PubMed] [Google Scholar]
- Cornell, B. A., V. L. Braach-Maksvytis, L. G. King, P. D. Osman, B. Raguse, L. Wieczorek and R. J. Pace. 1997. A biosensor that uses ion-channel switches. Nature. 387:580–583. [DOI] [PubMed] [Google Scholar]
- de Jong, K., D. Geldwerth, and F. A. Kuypers. 1997. Oxidative damage does not alter membrane phospholipid asymmetry in human erythrocytes. Biochemistry. 36:6768–6776. [DOI] [PubMed] [Google Scholar]
- Demel, R. A., W. S. Geurts van Kessel, R. F. Zwaal, B. Roelofsen, and L. L. van Deenen. 1975. Relation between various phospholipase actions on human red cell membranes and interfacial phospholipid pressure in monolayers. Biochim. Biophys. Acta. 406:97–107. [DOI] [PubMed] [Google Scholar]
- Devaux, P. F., P. Fellmann, and P. Herve. 2002. Investigation on lipid asymmetry using lipid probes comparison between spin-labeled lipids and fluorescent lipids. Chem. Phys. Lipids. 116:115–134. [DOI] [PubMed] [Google Scholar]
- Dietrich, C., L. A. Bagatolli, Z. N. Volovyk, N. L. Thompson, M. Levi, K. Jacobson, and E. Gratton. 2001. Lipid rafts reconstituted in model membranes. Biophys. J. 80:1417–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich, C., B. Yang, T. Fujiwara, A. Kusumi, and K. Jacobson. 2002. Relationship of lipid rafts to transient confinement zones detected by single particle tracking. Biophys. J. 82:274–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edidin, M. 2001. Shrinking patches and slippery rafts: scales of domains in the plasma membrane. Trends Biol. 11:492–496. [DOI] [PubMed] [Google Scholar]
- Groves, J. T., N. Ulman, and S. G. Boxer. 1997. Micropatterning fluid lipid bilayers on solid supports. Science. 275:651–653. [DOI] [PubMed] [Google Scholar]
- Hagen, J. P., and H. M. McConnell. 1997. Liquid-liquid immiscibility in lipid monolayers. Biochim. Biophys. Acta. 1329:7–11. [DOI] [PubMed] [Google Scholar]
- Hertzer, M., S. Heinz, S. Grage, and T. M. Bayer. 1998. Asymmetric molecular friction in supported phospholipid bilayer revealed by NMR measurements of lipid diffusion. Langmuir. 14:982–984. [Google Scholar]
- Israelachvilli, J. N., M. L. Gee, P. McGuigann, P. Thompson, and M. Robbins. 1990. Dynamics in Small Confining Systems. J. M. Drake and R. Kopelman, editors. MRS Publications, New York.
- Kaizuka, Y., and J. T. Groves. 2004. Structure and dynamics of supported intermembrane junctions. Biophys. J. 86:905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam, L., and S. G. Boxer. 2003. Spatially selective manipulation of supported lipid bilayers by laminar flow: steps toward biomembrane microfluidics. Langmuir. 19:1624–1631. [Google Scholar]
- Khan, T. K., B. Yang, N. L. Thompson, S. Maekawa, R. M. Epand, and K. Jacobson. 2003. Binding of NAP-22, a calmodulin-binding neuronal protein, to raft-like domains in model membranes. Biochemistry. 42:4780–4786. [DOI] [PubMed] [Google Scholar]
- Kiessling, V., and L. K. Tamm. 2003. Measuring distances in supported bilayers by fluorescence interference-contrast microscopy: polymer supports and SNARE proteins. Biophys. J. 84:408–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, R. S., and F. S. LaBella. 1987. Comparison of analytical methods for monitoring auto-oxidation profiles of authentic lipids. J. Lipid Res. 28:1110–1117. [PubMed] [Google Scholar]
- Kornberg, R. D., and H. M. McConnell. 1971. Inside-outside transitions of phospholipids in vesicle membranes. Biochemistry. 10:1111–1120. [DOI] [PubMed] [Google Scholar]
- Lawrence, J. C., D. E. Saslowsky, J. M. Edwardson, and R. M. Henderson. 2003. Real-time analysis of the effects of cholesterol on lipid raft behavior using atomic force microscopy. Biophys. J. 84:1827–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linseisen, F. M., M. Hetzer, T. Brumm, and T. M. Bayer. 1997. Differences in the physical properties of lipid monolayers and bilayers on a spherical solid support. Biophys. J. 72:1659–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muresan, A. D., and K. Y. C. Lee. 2001. Shape evolution of lipid bilayer patches adsorbed on mica: an atomic force microscopy study. J. Phys. Chem. B. 105:852–855. [Google Scholar]
- Naumann, C. A., O. Prucker, T. Lehmann, J. Ruhe, W. Knoll, and C. W. Frank. 2002. The polymer-supported phospholipid bilayer: tethering as a new approach to substrate-membrane stabilization. Biomacromolecules. 3:27–35. [DOI] [PubMed] [Google Scholar]
- Rädler, J., H. Strey, and E. Sackmann. 1995. Phenomenology and kinetics of lipid bilayer spreading on hydrophobic surfaces. Langmuir. 11:4539–4548. [Google Scholar]
- Ratto, T. V., and M. L. Longo. 2002. Obstructed diffusion in phase-separated supported lipid bilayers: a combined atomic force microscopy and fluorescence recovery after photobleaching approach. Biophys. J. 83:3380–3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackmann, E., and M. Tanaka. 2000. Supported membranes on soft polymer cushions: fabrication, characterization and applications. Trends Biotechnol. 18:58–64. [DOI] [PubMed] [Google Scholar]
- Samsonov, A. V., I. Mihalyov, and F. S. Cohen. 2001. Characterization of cholesterol-sphingomyelin domains and their dynamics in bilayer membranes. Biophys. J. 81:1486–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature. 387:569–572. [DOI] [PubMed] [Google Scholar]
- Sonnleitner, A., G. J. Schutz, and T. Schmidt. 1999. Free Brownian motion of individual lipid molecules in biomembranes. Biophys. J. 77:2638–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck, T. L., J. Ye, and Y. Lange. 2002. Probing red cell membrane cholesterol movement with cyclodextrin. Biophys. J. 83:2118–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm, L. K., and H. M. McConnell. 1985. Supported phospholipid bilayers. Biophys. J. 47:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, J. L., D. Holowka, B. Baird, and W. Webb. 1994. Large-scale co-aggregation of fluorescent lipid probes with cell surface proteins. J. Cell Biol. 125:795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokumasu, F., A. J. Jin, G. W. Feigenson, and J. A. Dvorak. 2003. Nanoscopic lipid domain dynamics revealed by atomic force microscopy. Biophys. J. 84:2609–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duyl, B. Y., D. Ganchev, V. Chupin, B. de Kruijff, and J. A. Killian. 2003. Sphingomyelin is much more effective than saturated phosphatidylcholine in excluding unsaturated phosphatidylcholine from domains formed with cholesterol. FEBS Lett. 547:101–106. [DOI] [PubMed] [Google Scholar]
- Veatch, S. L., and S. L. Keller. 2002. Organization in lipid membranes containing cholesterol. Phys. Rev. Lett. 89:268101. [DOI] [PubMed] [Google Scholar]
- Veatch, S. L., and S. L. Keller. 2003. Separation of liquid phases in giant unilamellar vesicles of ternary mixtures of phospholipids and cholesterol. Biophys. J. 85:3074–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, M. L., and L. K. Tamm. 2000. Tethered polymer-supported planar lipid bilayers for reconstitution of integral membrane proteins: silane-polyethyleneglycol-lipid as a cushion and covalent linker. Biophys. J. 79:1400–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, T. Y., and J. R. Silvius. 2001. Cholesterol does not induce segregation of liquid ordered domains in bilayers modeling the inner leaflets of the plasma membrane. Biophys. J. 81:2762–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]