Abstract
Pharaonis phoborhodopsin (ppR, or pharaonis sensory rhodopsin II, NpsRII) is a sensor for the negative phototaxis of Natronomonas (Natronobacterium) pharaonis. Arginine 72 of ppR corresponds to Arg-82 of bacteriorhodopsin, which is a highly conserved residue among microbial rhodopsins. Using various Arg-72 ppR mutants, we obtained the following results: 1), Arg-72ppR together possibly with Asp-193 influenced the pKa of the counterion of the protonated Schiff base. 2), The M-rise became approximately four times faster than the wild-type. 3), Illumination causes proton uptake and release, and the pH profiles of the sequence of these two proton movements were different between R72A mutant and the wild-type; it is inferred that Arg-72 connects the proton transfer events occurring at both the Schiff base and an extracellular proton-releasing residue (Asp-193). 4), The M-decays of Arg-72 mutants were faster (∼8–27 folds at pH 8 depending on mutants) than the wild-type, implying that the guanidinium prevents the proton transfer from the extracellular space to the deprotonated Schiff base. 5), The proton-pumping activities were decreased for mutants having increased M-decay rates, but the extent of the decrease was smaller than expected. The role of Arg-72 of ppR on the photochemistry was discussed.
INTRODUCTION
Halobacterium salinarum has four retinal membrane proteins: bacteriorhodopsin, i.e., bR (Oesterhelt and Stoeckenius, 1971; Lanyi and Luecke, 2001; Haupts et al., 1999); halorhodopsin, i.e., hR (Varo, 2000; Mukohata et al., 1999); sensory rhodopsin, i.e., sR or sRI (Hoff et al., 1997); and phoborhodopsin, i.e., pR; also called sensory rhodopsin II, or sRII (Kamo et al., 2001; Sasaki and Spudich, 2000; Takahashi et al., 1985). The first two work as light-driven ion pumps—bR as an outward proton pump, and hR as an inward Cl− pump. The second two work as photosensors that relay light signals to their cognate transducer proteins, which initiates a phosphorylation cascade that regulates the flagella motors to control phototaxis. The ground state of sRI (absorption maximum, i.e., λmax, is equal to 587 nm) mediates positive phototaxis whereas its long-lived photointermediate (λmax = 373 nm) is a sensor for negative phototaxis (Spudich and Bogomolni, 1984). Phoborhodopsin or sRII (λmax = 480 nm) is also a sensor for negative phototaxis (Takahashi et al., 1985). Thus, this bacterium is attracted toward light longer than 520 nm where the two ion pumps can work (Tomioka et al., 1986), and repelled from the shorter wavelength light that contains harmful UV light. Pharaonis phoborhodopsin (ppR) or pharaonis sensory rhodopsin II (NpsRII) is a photosensor in Natronomonas (Natronobacterium) pharaonis corresponding to pR (or sRII) of H. salinarum (Hirayama et al., 1992; Lüttenberg et al., 1998). ppR (NpsRII) is more stable than pR (sRII), especially in dilute salt solutions (Scharf et al., 1992). Success in the functional expression of ppR in Escherichia coli cell membranes allowed a simple preparation of the protein in large amounts, which permitted more detailed investigations (Shimono et al., 1997).
The amino acid sequence has been determined for all four kinds of pigments, and the sequences aligned (Zhang et al., 1999; Shimono et al., 1998; Ihara et al., 1999). Three-dimensional structures are now available for all archaeal rhodopsins with the exception of sRI: the structure of bR was reviewed by Lanyi and Luecke (2001) and Neutze et al. (2002); hR was by Kolbe et al. (2000); and NpsRII (or ppR) was by two groups, Luecke et al. (2001) and Royant et al (2001). The general features of the structure are quite similar with seven transmembrane helices (A–G) situated almost perpendicular to the membrane. The chromophore (all-trans-retinal) binds to a lysine residue located on helix G via a protonated Schiff base. The structures of the membrane helices of ppR and bR are very similar to each other; the root mean-square deviation value between bR and ppR is 0.95 Å2 when the transmembrane helices are confined, and this value is reduced to 0.77 Å2 with only C–G helices being confined (Pebay-Peyroula et al., 2002). On excitation by light, the chromophore of each of these four pigments undergoes an all-trans → 13-cis isomerization (Imamoto et al., 1992a), which is followed by thermal relaxations to the original state through a set of photochemical intermediates (Miyazaki et al., 1992; Chizhov et al., 1998). This sequence is called photocycling. Differences between ion-pumping and photosensor rhodopsins are that 1), the photocycling rate of the photosensor is much slower (seconds) than that of the ion pumps (∼10 ms) (Imamoto et al., 1992b; Scharf et al., 1992), and 2), the photosensor of sR and pR is associated with a cognate transducer within the membrane called HtrI and HtrII (pHtrII for ppR), respectively (Yao and Spudich, 1992, 2001; Hoff et al., 1997; Spudich, 1998; Zhang et al., 1999). The x-ray structure of the complex between NpsRII (ppR) and its truncated pHtrII was solved by Gordeliy et al. (2002).
Alignment of primary amino acid sequences identifies Arg-82bR or Arg-72ppR as a highly conserved residue among archaeal rhodopsins (Ihara et al., 1999; Spudich et al., 2000; Brown, 2001), and hence this residue is considered a very important residue. This Arg residue is also found in proteorhodopsin from sea bacteria (Beja et al., 2001), NOP-1 from Neurospore crassa (Bieszke et al., 1999), Chlamydomonas reinhardtii sensory rhodopsin (Sineshchekov et al., 2002), and Anabaena sensory rhodopsins (Jung et al., 2003). The role of Arg-82bR has been investigated in bR: this residue controls the proton release to the extracellular space; the pKa value of Asp-85bR that is a counterion of the protonated Schiff base; and the rate of retinal thermal isomerization (Balashov et al., 1993; 1995; Govindjee et al., 1996).
The crystal structure of ppR (NpsRII) shows that the guanidinium group of Arg-72ppR is oriented toward the extracellular side of the membrane compared to that of Arg-82bR (Luecke et al., 2001; Royant et al., 2002), meaning that the distance of each guanidinium nitrogen atom of Arg-72 from the Schiff base is ∼11 Å, whereas the distance of that in bR is ∼8 Å. This difference is considered to cause the interaction between Arg-72ppR and Asp-75ppR, as counterion of the protonated Schiff base, to become weak in comparison with that in bR. This difference may give rise to pKa differences of functionally important amino acids of ppR from those of bR.
The aim of this article is to clarify the role of Arg-72ppR on the photochemistry of ppR, and for this end, we investigated the photocycling and the proton transport of various Arg-72ppR mutants. The M-decays of Arg-72ppR mutants were faster (∼8–27 folds at pH 8, depending on mutants) than the wild-type, which is interpreted as the positive charge of the guanidinium preventing the proton transfer through the extracellular channel (EC) to the unprotonated Schiff base during M-decay. In addition, this Arg-72ppR controls the pKa value of Asp-75ppR, the counterion of the protonated Schiff base. The timing of the light-induced proton release and uptake of Arg-72ppR was found to be different from that of the wild-type. The preliminary results were presented at the 1st Asian Conference on Photobiology at Awaji Island, Hyogo, Japan (Ikeura et al., 2002).
MATERIALS AND METHODS
Preparation of samples
Expression and purification of histidine-tagged recombinant ppR and Arg-72ppR mutant proteins were essentially the same as described previously (Ikeura et al., 2003; Shimono et al., 1997). The proteins were reconstituted with L-α-phosphatidylcholine (PC from egg, Avanti, Alabaster, AL) with the molar ratio of 1:50, and the procedure was the same as described previously (Iwamoto et al., 2003; Kandori et al., 2001).
pH titration
Proteins reconstituted with egg-PC were suspended in 400 mM NaCl supplemented with six-mixed buffer (citric acid, MES, HEPES, MOPS, CHES, and CAPS, each at 10-mM concentration). After washing 2 or 3 times (15,000 × g for 30 min) the sample was resuspended. This six-mixed buffer has the advantage of an approximately constant buffer strength over a wide pH range (Balashov et al., 1995). The spectra at varying pHs were obtained using a U-3210 spectrophotometer (Hitachi, Tokyo, Japan) in which an end-on photomultiplier was installed to reduce the scattering artifact. The pH was adjusted to the desired value using H2SO4 or NaOH. Temperature was 20°C. Data were analyzed with a model of two interacting residues (Balashov et al., 1995) and data fitting was done using Microcal Origin software (Microcal Software, Northampton, MA).
Flash-photolysis measurements
For measurements in the time range longer than milliseconds (such as M-decay, O-decay, and the recovery of the original pigment), a Xe-flash (>540 nm, 200 μs of the duration) was used with an appropriate combination of filters (KL54/Y52, Toshiba, Tokyo, Japan). The apparatus and methods were the same as those described previously in Miyazaki et al. (1992). For the measurement of M-rise, the second harmonic of the fundamental beam of the Q-switched ND-YAG laser (532 nm, 7 ns) was employed as an actinic-light source, and data were accumulated 100 times for each run.
Light-induced proton transfer (release or uptake)
The egg-PC-reconstituted samples were washed two or three times and suspended in pure water. Fifty to one hundred microliters of this suspension (∼10 μM of ppR) was dropped on a transparent SnO2 electrode surface and dried (diameter of spot, ∼10 mm). The adhesion was so strong that the protein was not detached from the electrode surface unless washed with a detergent. This has been inferred from the fact that repeated use of the electrode is possible without any change in the signal amplitude. This SnO2 electrode was used as a working electrode and another SnO2 electrode was used as a counterelectrode. A solution of 400 mM NaCl was sandwiched by these two electrodes, and the solution was supplemented with 1 mM of the six-mixed buffer, the pH of which was adjusted with H2SO4 or NaOH to the desired value. An electronic circuit was used that was essentially the same as previously described in Iwamoto et al. (1999a). The light-generated electromotive force that arose between the two SnO2 electrodes was picked up and amplified through a 15-Hz low-cut filter (MEG-1200, Nihon-Koden, Tokyo, Japan). This filter eliminated signals due to the fluctuation of the baseline and differentiated the signals. The actinic-light pulse (duration of 4 ms) was provided by a mechanical shutter as described elsewhere (Iwamoto et al., 2003).
Light-induced proton transport
Photoinduced proton transport by the wild-type ppR or Arg-72ppR mutants was assayed using inside-out membrane vesicles that were prepared by passing the cells through a French press (500–700 kg/cm2, Ohtake, Tokyo, Japan) (Ikeura et al., 2002; Sudo et al., 2001). The photoinduced pH change in the vesicle suspension was measured with a SnO2 electrode. One hundred microliters of the vesicle suspension was confined on the surface of the SnO2 electrode using a dialysis membrane. The amounts of ppR or mutants in this vesicle suspension were adjusted to be constant at 66 μg. The other surface of this dialysis membrane is faced to 400 mM NaCl without buffer, with which another SnO2 electrode as a counterelectrode was contacted. Electric signals arising between a pair of SnO2 electrodes were measured with a DC potentiometer (Potentiostat/Galvanostat 2000, Toho Technical Research, Naruse, Machida, Japan). The constant actinic light was provided from 300 W Xe-lamp through a hot mirror, an interference filter (500 nm), and a cutoff filter (Y46 > 460 nm).
RESULTS
pKa of the counterion of the Schiff base
The pKa of the counterion of the Schiff base, Asp-75ppR, can be estimated by pH titration: when it is protonated, the maximum wavelength is red-shifted, and the color turns to pink (Chizhov et al., 1998; Shimono et al., 2000). The titration data are plotted in Fig. 1, where the ordinate represents the ratio of A541 at the respective pH to that at a sufficiently low pH where all the pigment is in the pink form. This ratio represents the fraction of the pink form. This figure shows that the order of the pKa is wild-type < R72K < R72A < R72Q, revealing that Arg-72ppR, presumably due to the positive charge of guanidinium group, decreases the pKa of the counterion.
FIGURE 1.
Spectroscopic pH titration to determine pKa of Asp-75ppR, the counterion of the protonated Schiff base. The ordinate represents the ratio of the 541-nm absorbance at the respective pH (see text). The protein (∼10 μM) was suspended in 400 mM NaCl supplemented with six-mixed buffer (see Materials and Methods), and the pH was adjusted with H2SO4 or NaOH. The titration data were analyzed with the scheme shown in the inset and pKa values were estimated with a nonlinear regression. Temperature was 20°C.
The titration curves were analyzed with the Henderson-Hasselbalch equation, and it was found that data were well fitted with an equation with a single pKa for R72AppR and R72QppR in which Arg-72ppR was replaced with a neutral amino acid. In contrast, for the mutant containing a positively charged residue (R72KppR) as well as for the wild-type, the data cannot be fitted with a single pKa equation but could be successfully fit using the schema described in an inset of Fig. 1. This scheme implies that the pKa of the counterion is affected by the ionization state of another residue (X) (compare pKa1 and pKa4), and the pKa of X is, in turn, affected by the ionization state of Asp-75ppR, the counterion (compare pKa3 and pKa4) (Balashov et al., 1995).
The pKa values of wild-type determined here are different from those previously reported (Chizhov et al., 1998; Shimono et al., 2000), but our recent results (Mizuta et al., unpublished results) show the large dependence on lipid species used.
The pKa of the protonated Schiff base may be changed by the replacement of Arg-72ppR. Unfortunately, however, the exact value cannot be determined, but seems to be a little larger than that of the wild-type in the dark (>12; S. P. Balashov, unpublished results).
Proton release or uptake at an earlier stage of the photocycling
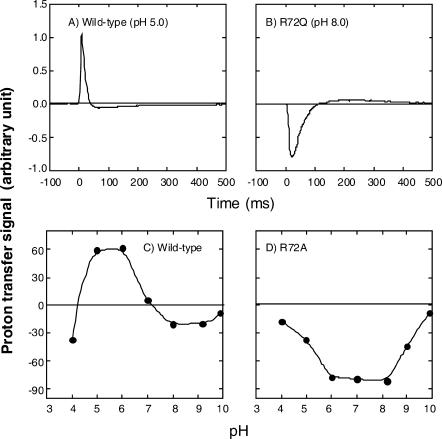
During the photocycling of ppR, proton release and uptake occur (Iwamoto et al., 1999a). Fig. 2 A shows light-induced proton transfer for the wild-type (400 mM NaCl, pH 5.0). Note that the signal has been differentiated with a 15-Hz low-cut filter. The positive signal indicates proton release from ppR. This figure reveals that the proton release occurs first when illumination was applied, followed by the uptake. This observation is different from that reported previously (Iwamoto et al., 1999a). We note that the present sample is reconstituted with egg-PC, and that for solubilized ppR, proton uptake and release coincide with the O-formation and O-decay, respectively, as was reported previously (Iwamoto et al., 1999a). R72QppR shows first proton uptake followed by release, as shown in Fig. 2 B. The medium contained 400 mM NaCl at pH 8.0.
FIGURE 2.
Flash-induced proton transfer reaction of the wild-type (A and C) and Arg-72ppR mutant (B and D). R72Q was used in B and R72A was used in D. A and B show the proton transfer data measured by SnO2 electrode. The potential difference between a pair of SnO2 electrodes was measured using a 15-Hz low-cut filter, which gave the differentiated signals. For the wild-type (A), the light pulse (4-ms duration) led first to proton release, followed by the uptake. B shows the opposite sequence for R72QppR. The magnitudes of the first photoresponses are plotted against pH in C and D for the wild-type and R72AppR. The medium for all experiments was 400 mM NaCl. The pH values in A and B were 5.0 and 8.0, respectively. For detailed experimental procedures, see Materials and Methods.
In Fig. 2, C and D, the magnitudes of the first events observed immediately after the light stimuli are plotted against the pH of the medium. The wild-type (Fig. 2 C) and R72AppR (Fig. 2 D) data are quite different: the mutant never shows positive values, meaning that proton uptake precedes release over the whole pH range examined. The results for R72QppR were the same as R72AppR (data not shown). In contrast, the wild-type shows positive (pH ∼3–7) and negative (pH ∼7–10) signals. This figure clearly shows that the Arg-72ppR affects the timing of the proton release, similar to the effected Arg-82bR (Balashov et al., 1993; Govindjee et al., 1996).
Effect on the rise of M-intermediate
The rise of M-intermediate was measured after a laser flash: in the presence of 400 mM NaCl (pH 8.0), this process was analyzed well by a single exponential equation and the time constants observed were 0.05 and 0.22 μs−1 for the wild-type and R72AppR, respectively. The rate for the mutant is approximately four times larger than that of the wild-type. The disappearance of the positive charge of guanidinium facilitates proton transfer from the Schiff base to the deprotonated counterion, Asp-75ppR. Similar increases in the M-rise time constant were observed for Arg-82 mutants of bR (e.g., an approximately 10-times increase of R82AbR) (Balashov et al., 1993; Hatanaka et al., 1996).
Effect on the M-decay rate
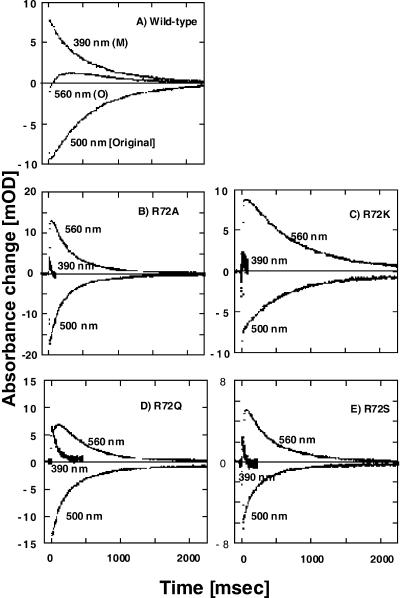
Fig. 3 shows the traces of flash-photolysis data (in 400 mM NaCl, pH 8.0) at selected wavelengths for the wild-type and various Arg-72 mutants of ppR. Clearly, Arg-72 mutants of ppR show much faster M-decay than the wild-type depending on mutants. The M-decay was analyzed by a single exponential equation, and the rate constants for various mutants of ppR were 1.7, 45.3, 14.3, 10.4, and 40.8 s−1 for the wild-type, R72A, R72K, R72Q, and R72S, respectively. As a result of the fast M-decay, the O-intermediate shows a faster rise with a larger amplitude, but the decay rate of the O-intermediate or the recovery rate of the original pigment is scarcely changed.
FIGURE 3.
Flash-photolysis data of selected wavelengths for the wild-type (A), R72AppR (B), R72KppR (C), R72QppR (D), and R72SppR (E). All samples were reconstituted with egg-PC. Absorbance changes in 390, 500, and 560 nm monitor the concentration of M-intermediate, ppR, and O-intermediate, respectively. The medium was 400 mM NaCl buffered with 10 mM of MES plus CHES at pH 8.0. Temperature was 20°C.
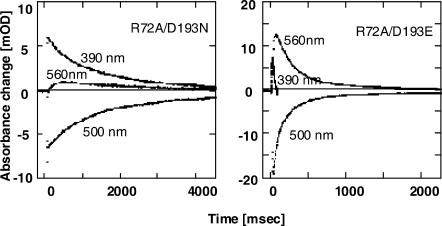
R72A/D193N double mutant shows slow M-decay
Fig. 4 shows flash-photolysis data at typical wavelengths for the R72A/D193NppR (left) and R72A/D193EppR double mutants (right). It is very interesting that in contrast to the R72AppR single mutant, R72A/D193NppR shows that M-decay is approximately two times slower than that of the wild-type (Fig. 3 A). On the other hand, R72A/D193EppR shows very fast M-decay, which is the same as for the R72A single mutant.
FIGURE 4.
Flash-photolysis data of selected wavelengths for R72A/D193N (left) and R72A/D193E (right) ppR mutants. Experimental conditions were the same as in Fig. 3.
M-decay rate of mutants-mutants under varying pH
The M-decay rates of various Arg-72ppR mutants of ppR were measured under varying pH. The results are plotted in Fig. 5 where the ordinate represents the logarithm of the M-decay rate constant, k1 (s−1). The mutants are R72A (▪), R72K (⧫), R72Q (▴), R72S (▾), R72A/D193N (□) and R72A/D193E ( ), and the data for the wild-type are shown by ○. This figure reveals that log k1 is approximately linear with pH for all Arg-72ppR mutants except for R72A/D193N and the wild-type. The slopes of these Arg mutants are 0.74 (R72A), 0.96 (R72K), 0.88 (R72Q), 0.58 (R72S), and 0.79 (R72A/D193E), indicating that the slope is close to unity except for R72S. For this mutant, relatively larger values are obtained in the pH range from 8.8 to 9.5; the reason for this is not known. If these are omitted for the regression, the slope will become larger.
), and the data for the wild-type are shown by ○. This figure reveals that log k1 is approximately linear with pH for all Arg-72ppR mutants except for R72A/D193N and the wild-type. The slopes of these Arg mutants are 0.74 (R72A), 0.96 (R72K), 0.88 (R72Q), 0.58 (R72S), and 0.79 (R72A/D193E), indicating that the slope is close to unity except for R72S. For this mutant, relatively larger values are obtained in the pH range from 8.8 to 9.5; the reason for this is not known. If these are omitted for the regression, the slope will become larger.
FIGURE 5.
The logarithm of k1, the rate constant of the M-decay, is plotted against pH in the medium. The mutants are R72A (▪), R72K (♦), R72Q (▴), R72S (▾), R72A/D193N (□), and R72A/D193E ( ), and data of the wild-type are shown by ○. Experimental conditions were the same as in Fig. 3.
), and data of the wild-type are shown by ○. Experimental conditions were the same as in Fig. 3.
In Fig. 1, the pH-titration profile of R72KppR is different from that of the other Arg-mutants whereas the pH profiles of mutants shown in Fig. 5 are all similar. Perhaps this is because the lysine residue is in its neutral form under the condition of Fig. 5 (pH 7.5–10), but bears a positive charge under the conditions of Fig. 1 (pH < 7).
Photoinduced proton pumping activity of Arg-72ppR mutants
The process of M-decay involves the protonation of the deprotonated Schiff base. The proton-donating residue of bR, Asp-96, is replaced with Phe-86 in ppR (Seidel et al., 1995); the cytoplasmic channel (CP) of ppR, then, is very hydrophobic—suggesting that the proton conductivity through the CP is very small. Nevertheless, light-induced proton pumping is observed for ppR alone, implying that proton transfer through CP occurs during M-decay. On the other hand, complex formation of ppR with pHtrII stops the light-induced proton pumping with little or no change in the M-decay rate (Sudo et al., 2001; Schmies et al., 2001; Hippler-Mreyen et al., 2003). This has been interpreted as follows: Association with pHtrII results in the closure of the CP channel, and hence, for the complex, the proton should come to the deprotonated Schiff base solely through EC, implying no proton pumping activity of the complex. For ppR alone, the ratio of the proton conductivity of EC/CP might be large and then the M-decay rate of ppR might be unchanged by its association with pHtrII despite the CP-channel closure. The proton is released to EC and taken up both from EC and CP during photocycling. Therefore, the proton uptake via EC is futile for the photoinduced proton pumping of ppR, and the photoinduced proton pumping activity of ppR alone is a rough measure for the fraction of the proton entry from CP to the deprotonated Schiff base.
Results of light-induced proton pumping experiments with inside-out membrane vesicles containing various Arg-72ppR mutants are shown in Fig. 6. The amount of pigment was kept constant for all mutants (66 μg). No light-induced proton movements were observed for the membrane vesicles derived from cells containing the vector alone, which did not express ppR (data not shown). In Fig. 6, two corrections have been made to account for two distortions of the data. The first correction is for the fraction of the bathochromic pink form that is unable to pump protons due to the protonation of the counterion of Schiff base, Asp-75ppR, in the dark. This fraction was estimated from the pKa values shown in Fig. 1. The second correction is for the turnover rate; the amounts of protons transported per unit time should be proportional to the rate, which was estimated from the half-time of the recovery to the original pigment. The activity of R72A/D193NppR is almost equal to that of the wild-type (∼80%), and R72AppR and R72A/D193EppR show ∼30% of the activity of the wild-type. It is interesting that the mutants having the faster M-decay have lower activity than those having the slower M-decay.
FIGURE 6.
Photoinduced proton-pumping through inside-out membrane vesicles derived from E. coli cells expressing ppR or mutants. Data were corrected by two factors (for details, see Results). •, the wild-type; ○, R72A; ▴, R72A/D193E; and ▵, R72A/D193N. The medium contained 400 mM NaCl at pH 6.5 without buffer.
DISCUSSION
Assignment of the residue X
Results of Fig. 1 reveal that the positive charge at the Arg-72ppR position decreases the pKa of Asp-75ppR, the counterion of the Schiff base. Similar observations have been reported for bR. The pKa of the counterion for R82AbR and R82QbR was increased to ∼7 whereas that of the wild-type was 2.5, and the replacement by the positively charged residue (R82KbR) kept the pKa at a relatively low value of 3.5 (Balashov et al., 1995). The magnitudes of pKa changes in ppR caused by the replacement with the neutral amino acids are smaller than those of bR, however. This may be because of the difference in the direction of the guanidinium pointing toward the Schiff base in bR or pointing away from the Schiff base in ppR, which increases the distance between the positive charge and the counterion in bR (Luecke et al., 2001; Royant et al., 2001).
As described in Results, the residue X is required for quantitative explanations for Fig. 1 (see the inset). What is the residue X? Since Asp-75ppR is deprotonated in the dark at neutral pH, the pKa of the residue X may be 5.7, that is, the pKa2 value of the wild-type. The residue X might be Asp-193ppR. Reasons for this assignment: it has been shown that pKa of the counterion (Asp-85bR) in bR is affected by the protonation state of Glu-204bR (Richter et al., 1996; Govindjee et al., 1996) that corresponds to Asp-193ppR. In addition, we showed previously that the electric charge of residue 193 of ppR affects the pKa of the Schiff base (Iwamoto et al., 2002a), indicating that Asp-75ppR, Arg-72ppR, and Asp-193ppR are connected via hydrogen-bonding through water molecules as is shown in x-ray crystal structure (Pebay-Peyroula et al., 2002).
Light-induced proton transfer reactions on the wild-type and Arg-72ppR
Fig. 2 shows the difference between the order of the flash-induced proton release and uptake between the wild-type and Arg-72ppR. Iwamoto et al. (2004) showed that light-induced proton release at the earlier stage of the photocycling was not observed for D193NppR under the present conditions, suggesting strongly that Asp-193ppR is a key residue involved in proton release. Note that Asp-193ppR corresponds to Glu-204bR, which is one of the members of the proton-releasing complex in bR (Balashov et al., 1997; Dioumaev et al., 1998; Koyama et al., 1998).
In bR, the movement of the orientation of the side chain of Arg-82bR during photocycling is considered to cause a pKa change of the proton-releasing complex consisting of Glu-194bR and Glu-204bR (Tanio et al., 1999; Petkova et al., 1999; Royant et al., 2000; Luecke et al., 1999; Luecke, 2000; Neutze et al., 2002). Therefore, the pKa change in a group analogous to Glu-204bR, the proton-releasing Asp-193ppR, and the role of Arg-72ppR in wild-type ppR, are very interesting. So far we have no information concerning the possible movement of Arg-72ppR during photocycle.
At the pH where the first proton release is observed from the wild-type, Asp-193ppR may be protonated, and then proton transfer from the Schiff base to deprotonated Asp-75ppR leads to the proton release from the protonated Asp-193ppR. A proton absorbed after release is used for the protonation of the Schiff base and the deprotonated Asp-193ppR is reprotonated with the proton from Asp-75ppR during O-decay. Above pH 7, Asp-193ppR may be deprotonated, presumably for the earlier intermediates and certainly at the ppR ground state (pKa ∼5.7) so that the first proton release cannot be induced. The decrease in the signal (its absolute magnitude) above pH 9 might be caused by the slowing-down of the proton-uptake rate due to high pH in the medium. Below pH 4.5, why does the proton uptake first occur even though Asp-193ppR may be protonated? The proton concentration in the external medium might be so high that the proton cannot be released from Asp-193ppR, resulting in the first proton uptake followed by the slow release.
In contrast to the wild-type, R72AppR shows first light-induced proton uptake over the whole pH range, followed by release. Thus, for the Arg-mutant, it seems that the proton transfer from the Schiff base to Asp-75ppR cannot be “transmitted” to Asp-193ppR, a proton-releasing residue, due to the lack of the arginine residue. The proton now on Asp-75ppR may be used for the reprotonation of the Schiff base; if so, the proton that is released may be from the deprotonation of Asp-75ppR.
M-decay of Arg-72ppR
A remarkable observation from Fig. 3 is that the M-decay of Arg-72ppR mutants is faster than that of the wild-type. As postulated previously (Sudo et al., 2001), the proton that reprotonates the deprotonated Schiff base at the M-decay comes from both the CP and EC sides, and due to the hydrophobic nature of CP, the main route may be the one through the EC domain. Therefore, we conclude that Arg-72ppR, located in EC, hinders proton transfer through the EC to the deprotonated Schiff base at M-decay. This in turn prolongs the lifetime of the M-intermediate of the wild-type, one of signaling states for phototaxis (Yan et al., 1991).
Fig. 4 shows the slow M-decay of R72A/D193NppR in sharp contrast to those of R72A/D193EppR and the Arg-72ppR single mutants (Fig. 3). This result implies that, for R72AppR and presumably all Arg-72ppR mutants, proton uptake from the EC side is mediated or controlled by Asp-193ppR—which is located nearly at the end of EC, open to the external medium. We note that log k1 is approximately a linear function of pH in the region of ∼7.5–9. This may be interpreted as the passive proton transport through EC of Arg-72ppR. Another possible interpretation is that the proton transfer at M-decay of Arg-72ppR is mediated by the protonated Asp-193ppR. In this pH range, the Henderson-Hasselbalch equation predicts that the slope should be −1 when the logarithm of the fraction of protonated carboxyl group is plotted against pH, because the pKa of Asp-193ppR is probably far lower than the pH investigated (5.7 at the ppR ground state; pKa values for the intermediates are not known). A further interesting problem is whether Asp-193 of the wild-type ppR also controls the proton transfer through EC. Studies are now in progress using D193NppR.
Proton-pumping activity
Fig. 6 shows that the mutants having faster M-decay have lower light-induced proton-pumping activity compared to the wild-type. If we accept the concept that the increase in the M-decay rate of Arg-72ppR mutants is due to the increase in the proton transfer through EC and that this proton pathway does not contribute the proton-pumping, the results of Fig. 6 are qualitatively conceivable. At pH = 8, the M-decay rate constant of the wild-type is 1.7 s−1 whereas that of R72AppR is 45.3 s−1, indicating that, in the mutant, almost all protons that cause the reprotonation of the Schiff base during the M-decay are from the EC. This would predict that the proton-pumping activity might be more reduced than that observed. Therefore, we would have to consider other factors that control the light-induced proton pumping activity. One possibility is a two-photon process which was hypothesized originally to account for the unexpected strong proton-pumping of the wild-type in the presence of azide (Schmies et al., 2000), in which the O-intermediate was accumulated due to the fast M-decay, similar to the present experiment. The photoreactivity of the O-intermediate increases the turnover rate of the pigment under continuous illumination. Iwamoto et al. (2002b) verified the photoreactivity. A second possibility is an increase in the proton conductivity in the CP caused by the mutation of the arginine residue even though it is located in EC. The last possibility is that there might be two M-states, as in bR (Lanyi and Schobert, 2003), and only one contributes to the proton-pumping (M2 as is in bR). We should take into consideration the transition from M1 to M2, or the population of these two M-states being affected by the mutation. The spectroscopically separated two-M-states of the wild-type ppR was described in Chizhov et al. (1998) and Rivas et al. (2003). Further quantitative study on this respect should be necessary.
Effect on the chloride concentration in the medium
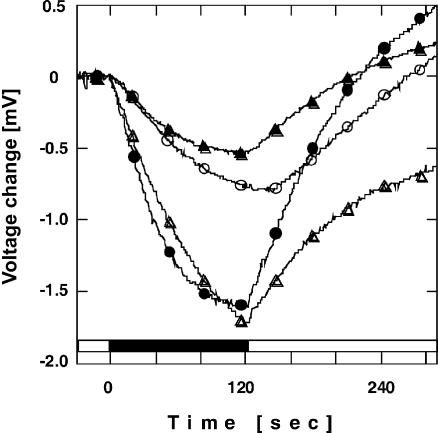
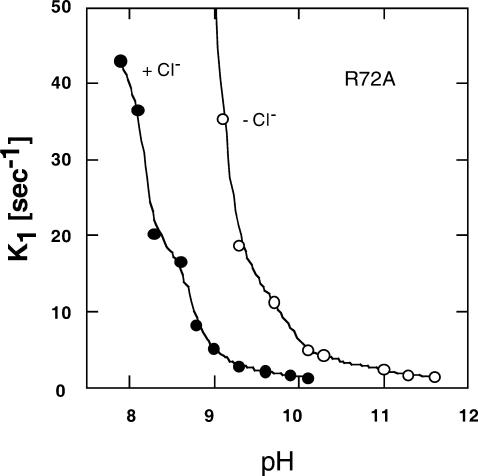
The Cl− effect on characteristics and photochemistry of ppR has been reported (Iwamoto et al., 2002a; Shimono et al., 2000). In addition, we observed here a Cl− effect on M-decay rate of R72AppR (Fig. 7). As discussed above, this result may indicate a Cl− effect on the pKa of Asp-193ppR of R72A. The Cl− concentration we used here was only 400 mM NaCl.
FIGURE 7.
Cl− concentration dependence of the M-decay rate constant of R72AppR. • shows the data in the presence of 400 mM NaCl and the ○ shows the data in the absence of Cl−. The medium was buffered with 10 mM MES plus CHES at pH 8.
CONCLUDING REMARKS
In this article, we conclude the following:
Arg-72ppR decreases the pKa of Asp-75ppR, the counterion of the protonated Schiff base.
Replacement of Arg-72ppR increases the M-decay rate, which may be the result of increased proton transfer rate through the EC. In other words, Arg-72ppR in the wild-type prevents this proton transfer, leading to the prolongation of the lifetime of M-intermediate; the effect is enhanced by lack of a proton-donating residue at the Asp-96bR position (Iwamoto et al., 1999b).
Substitution for the arginine residue at position 72 changes the timing of proton release and uptake from that of the wild-type. The lack of the arginine residue may disconnect the intramolecular proton transfer chain from the Schiff base and the extracellular part.
Further investigation on the role of Arg-72ppR on proton transfer will be of interest. One reason for its behavior may be that the orientation of the guanidinium in the dark is opposite to that of bR; we do not yet know whether this guanidinium group of Arg-72 moves to change the distance from Asp-193 during photocycling as bR.
Acknowledgments
The authors thank Sergei Balashov and Hideki Kandori for their invaluable discussions, and Takashi Kikukawa and Tsunehisa Araiso for permission to use their laser flash-photolysis apparatus.
This work was supported by Grants-in-Aid for Scientific Research from the Japanese Ministry of Education, Science, Sports and Culture.
Abbreviations used: bR, bacteriorhodopsin; hR, halorhodopsin; sR, sensory rhodopsin; pR, phoborhodopsin; ppR, pharaonis phoborhodopsin (sensory rhodopsin II); NpsRII, pharaonis sensory rhodopsin II; pHtrII, halobacterial transducer for pharaonis phoborhodopsin (sensory rhodopsin II); Arg-82bR, arginine residue at 82nd position of bR; Arg-72ppR, arginine residue at 72nd position of ppR; EC, extracellular channel; CP, cytoplasmic channel; R72AppR, mutant in which Arg-72ppR is replaced with Ala.
References
- Balashov, S. P., E. S. Imasheva, T. G. Ebrey, N. Chen, D. R. Menick, and R. K. Crouch. 1997. Glutamate 194 to cysteine mutation inhibits fast light-induced proton release in bacteriorhodopsin. Biochemistry. 36:8671–8676. [DOI] [PubMed] [Google Scholar]
- Balashov, S. P., R. Govindjee, E. Imasheva, S. Misra, T. G. Ebrey, Y. Feng, R. K. Crouch, and D. R. Menick. 1995. The two pKa of aspartate 85 and control of thermal summarization and proton release in the arginine 82 to lysine mutant of bacteriorhodopsin. Biochemistry. 34:8820–8834. [DOI] [PubMed] [Google Scholar]
- Balashov, S. P., R. Govindjee, M. Kono, E. Imasheva, E. Lukashev, T. G. Ebrey, R. K. Crouch, D. R. Menick, and Y. Feng. 1993. Effect of the arginine 82 to alanine mutation in bacteriorhodopsin on dark-adaptation, proton release, and the photochemical cycle. Biochemistry. 32:10331–10343. [DOI] [PubMed] [Google Scholar]
- Beja, O., E. N. Spudich, J. L. Spudich, M. Leclerc, and E. F. DeLong. 2001. Proteorhodopsin phototrophy in the ocean. Nature. 411:786–789. [DOI] [PubMed] [Google Scholar]
- Bieszke, J. A., E. L. Braun, L. E. Bean, S. C. Kang, D. O. Natvig, and K. A. Borkovich. 1999. The NOP-1 gene of Neurospora crassa encodes a seven-transmembrane helix retinal-binding protein homologous to archaeal rhodopsins. Proc. Natl. Acad. Sci. USA. 96:8034–8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, L. S. 2001. Proton transport mechanism of bacteriorhodopsin as revealed by site-specific mutagenesis and protein sequence variability. Biochem. Moscow. 66:1249–1255. [DOI] [PubMed] [Google Scholar]
- Chizhov, I., G. Schmies, R. Seidel, J. R. Sydor, B. Luttenberg, and M. Engelhard. 1998. The photophobic receptor from Natronobacterium pharaonis: temperature and pH dependencies of the photocycle of sensory rhodopsin II. Biophys. J. 75:999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dioumaev, A. K., H. T. Richter, L. S. Brown, M. Tanio, S. Tuzi, H. Saito, Y. Kimura, R. Needleman, and J. K. Lanyi. 1998. Existence of a proton transfer chain in bacteriorhodopsin: participation of Glu-194 in the release of protons to the extracellular surface. Biochemistry. 37:2496–2509. [DOI] [PubMed] [Google Scholar]
- Gordeliy, V. I., J. Labahn, R. Moukhametzianov, R. Efremov, J. Granzin, R. Schlesinger, G. Büldt, T. Savopol, A. J. Scheidig, J. P. Klare, and M. Engelhard. 2002. Molecular basis of transmembrane signaling by sensory rhodopsin II-transducer complex. Nature. 419:484–487. [DOI] [PubMed] [Google Scholar]
- Govindjee, R., S. Misra, S. P. Balashov, T. G. Ebrey, R. K. Crouch, and D. R. Menick. 1996. Arginine 82 regulates the pKa of the group responsible for the light-driven proton release in bacteriorhodopsin. Biophys. J. 71:1011–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupts, U., J. Tittor, and D. Oesterhelt. 1999. Closing in on bacteriorhodopsin: progress in understanding the molecule. Annu. Rev. Biophys. Biomol. Struct. 28:367–399. [DOI] [PubMed] [Google Scholar]
- Hatanaka, M., J. Sasaki, H. Kandori, T. G. Ebrey, R. Needleman, J. K. Lanyi, and A. Maeda. 1996. Effects of arginine 82 on the interactions of internal water molecules in bacteriorhodopsin. Biochemistry. 35:6308–6312. [DOI] [PubMed] [Google Scholar]
- Hippler-Mreyen, S., J. P. Klare, A. A. Wegener, R. Seidel, C. Herrmann, G. Schmies, G. Nagel, G. E. Bamberg, and M. Engelhard. 2003. Probing the sensory rhodopsin II binding domain of its cognate transducer by calorimetry and electrophysiology. J. Mol. Biol. 330:1203–1213. [DOI] [PubMed] [Google Scholar]
- Hirayama, J., Y. Imamoto, Y. Shichida, N. Kamo, H. Tomioka, and T. Yoshizawa. 1992. A photocycle of phoborhodopsin from haloalkaliphilic bacterium (Natronobacterium pharaonis) studied by low-temperature spectrophotometry. Biochemistry. 31:2093–2098. [DOI] [PubMed] [Google Scholar]
- Hoff, W. D., K.-H. Jung, and J. L. Spudich. 1997. Molecular mechanism of photosignaling by archaeal sensory rhodopsins. Annu. Rev. Biophys. Biomol. Struct. 26:223–258. [DOI] [PubMed] [Google Scholar]
- Ihara, K., T. Umemura, I. Katagiri, T. Kitagima-Ihara, Y. Sugiyama, Y. Kimura, and Y. Mukohata. 1999. Evolution of the archaeal rhodopsins: evolution rate changes by gene duplication and functional differentiation. J. Mol. Biol. 285:163–174. [DOI] [PubMed] [Google Scholar]
- Ikeura, Y., K. Shimono, M. Iwamoto, Y. Sudo, and N. Kamo. 2003. Arg-72 of pharaonis phoborhodopsin (sensory rhodopsin II) is important for the maintenance of the protein structure in the solubilized state. Photochem. Photobiol. 77:96–100. [DOI] [PubMed] [Google Scholar]
- Ikeura, Y., K. Shimono, M. Iwamoto, Y. Sudo, and N. Kamo. 2002. Influence of Arg72 of pharaonis phoborhodopsin on M-intermediate decay and proton pumping activity. J. Photosci. 9:311–313. [Google Scholar]
- Imamoto, Y., Y. Shichida, J. Hirayama, H. Tomioka, N. Kamo, and T. Yoshizawa. 1992a. Chromophore configuration of pharaonis phoborhodopsin and its isomerization on photon absorption. Biochemistry. 31:2523–2528. [DOI] [PubMed] [Google Scholar]
- Imamoto, Y., Y. Shichida, J. Hirayama, H. Tomioka, N. Kamo, and T. Yoshizawa. 1992b. Nanosecond laser photolysis of phoborhodopsin from Natronobacterium pharaonis: appearance of KL- and L-intermediates in the photocycle at room temperature. Photochem. Photobiol. 56:1129–1134. [Google Scholar]
- Iwamoto, M., C. Hasegawa, Y. Sudo, K. Shimono, T. Araiso, and N. Kamo. 2004. Proton release and uptake of pharaonis phoborhodopsin (sensoryrhodopsin II) reconstituted into phospholipids. Biochemistry. 43:3195–3203. [DOI] [PubMed] [Google Scholar]
- Iwamoto, M., Y. Furutani, N. Kamo, and H. Kandori. 2003. Proton transfer reactions in the F86D and F86E mutants of pharaonis phoborhodopsin (sensory rhodopsin II). Biochemistry. 42:2790–2796. [DOI] [PubMed] [Google Scholar]
- Iwamoto, M., Y. Furutani, Y. Sudo, K. Shimono, H. Kandori, and N. Kamo. 2002a. Role of Asp193 in chromophore-protein interaction of pharaonis phoborhodopsin (sensory rhodopsin II). Biophys. J. 83:1130–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto, M., Y. Sudo, K. Shimono, and N. Kamo. 2002b. Illumination accelerates the decay of the O-intermediate of pharaonis phoborhodopsin (sensory rhodopsin II). Photochem. Photobiol. 76:462–466. [DOI] [PubMed] [Google Scholar]
- Iwamoto, M., K. Shimono, M. Sumi, K. Koyama, and N. Kamo. 1999a. Light-induced proton uptake and release of pharaonis phoborhodopsin detected by a photoelectrochemical cell. J. Phys. Chem. B. 103:10311–10315. [Google Scholar]
- Iwamoto, M., K. Shimono, M. Sumi, and N. Kamo. 1999b. Positioning proton-donating residues to the Schiff base accelerates the M-decay of pharaonis phoborhodopsin expressed in Escherichia coli. Biophys. Chem. 79:187–192. [DOI] [PubMed] [Google Scholar]
- Jung, K. H., V. D. Trivedi, and J. L. Spudich. 2003. Demonstration of a sensory rhodopsin in eubacteria. Mol. Microbiol. 47:1513–1522. [DOI] [PubMed] [Google Scholar]
- Kamo, N., K. Shimono, M. Iwamoto, and Y. Sudo. 2001. Photochemistry and photoinduced proton-transfer by pharaonis phoborhodopsin. Biochem. Moscow. 66:1277–1282. [DOI] [PubMed] [Google Scholar]
- Kandori, H., K. Shimono, Y. Sudo, M. Iwamoto, Y. Shichida, and N. Kamo. 2001. Structural changes of pharaonis phoborhodopsin upon photoisomerization of the retinal chromophore: infrared spectral comparison with bacteriorhodopsin. Biochemistry. 40:9238–9246. [DOI] [PubMed] [Google Scholar]
- Kolbe, M., H. Besir, L. O. Essen, and D. Oesterhelt. 2000. Structure of the light-driven chloride pump halorhodopsin at 1.8 Ångstrom resolution. Science. 288:1390–1396. [DOI] [PubMed] [Google Scholar]
- Koyama, K., T. Miyasaka, R. Needleman, and J. K. Lanyi. 1998. Photoelectrochemical verification of proton-releasing groups in bacteriorhodopsin. Photochem. Photobiol. 68:400–406. [Google Scholar]
- Lanyi, J. K., and B. Schobert. 2003. Mechanism of proton transport in bacteriorhodopsin from crystallographic structures of the K, L, M1, M2, and M2′ intermediates of the photocycle. J. Mol. Biol. 328:439–450. [DOI] [PubMed] [Google Scholar]
- Lanyi, J. K., and H. Luecke. 2001. Bacteriorhodopsin. Curr. Opin. Struct. Biol. 11:415–419. [DOI] [PubMed] [Google Scholar]
- Luecke, H., B. Schobert, J. K. Lanyi, E. N. Spudich, and J. L. Spudich. 2001. Crystal structure of sensory rhodopsin II at 2.4 Å: insights into color tuning and transducer interaction. Science. 293:1499–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luecke, H. 2000. Atomic resolution structures of bacteriorhodopsin photocycle intermediates: the role of discrete water molecules in the function of this light-driven ion pump. Biochim. Biophys. Acta. 1460:133–156. [DOI] [PubMed] [Google Scholar]
- Luecke, H., B. Schobert, H. T. Richter, J. P. Cartailler, and J. K. Lanyi. 1999. Structural changes in bacteriorhodopsin during ion transport at 2 Ångstrom resolution. Science. 286:255–260. [DOI] [PubMed] [Google Scholar]
- Lüttenberg, B., E. K. Wolff, and M. Engelhard. 1998. Heterologous coexpression of the blue light receptor psRII and its transducer pHtrII from Natronobacterium pharaonis in the Halobacterium salinarum strain Pho81/w restores negative phototaxis. FEBS Lett. 426:117–120. [DOI] [PubMed] [Google Scholar]
- Miyazaki, M., J. Hirayama, M. Hayakawa, and N. Kamo. 1992. Flash photolysis study on pharaonis phoborhodopsin from a haloalkaliphilic bacterium (Natronobacterium pharaonis). Biochim. Biophys. Acta. 1140:22–29. [Google Scholar]
- Mukohata, Y., K. Ihara, T. Tamura, and Y. Sugiyama. 1999. Halobacterial rhodopsins. J. Biochem. 125:649–657. [DOI] [PubMed] [Google Scholar]
- Neutze, R., E. Pebay-Peyroula, K. Edman, A. Royant, J. Navarro, and E. M. Landau. 2002. Bacteriorhodopsin: a high-resolution structural view of vectorial proton transport. Biochim. Biophys. Acta. 1565:144–167. [DOI] [PubMed] [Google Scholar]
- Pebay-Peyroula, E., A. Royant, E. M. Landau, and J. Navarro. 2002. Structural basis for sensory rhodopsin function. Biochim. Biophys. Acta. 1565:196–205. [DOI] [PubMed] [Google Scholar]
- Oesterhelt, D., and W. Stoeckenius. 1971. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat. New Biol. 233:149–152. [DOI] [PubMed] [Google Scholar]
- Petkova, A. T., J. G. G. Hu, M. Bizounok, M. Simpson, R. G. Griffin, and J. Herzfeld. 1999. Arginine activity in the proton-motive photocycle of bacteriorhodopsin: solid-state NMR studies of the wild-type and D85N proteins. Biochemistry. 38:1562–1572. [DOI] [PubMed] [Google Scholar]
- Richter, H. T., L. S. Brown, R. Needleman, and J. K. Lanyi. 1996. A linkage of the pKas of Asp-85 and Glu-204 forms part of the reprotonation switch of bacteriorhodopsin. Biochemistry. 35:4054–4062.sd [DOI] [PubMed] [Google Scholar]
- Rivas, L., S. Hippler-Mreyen, M. Engelhard, and P. Hildebrandt. 2003. Electric-field dependent decays of two spectroscopically different M-states of photosensory rhodopsin II from Natronobacterium pharaonis. Biophys. J. 84:3864–3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royant, A., P. Nollert, K. Edman, R. Neutze, E. M. Landau, E. Pebay-Peyroula, and J. Navarro. 2001. X-ray structure of sensory rhodopsin II at 2.1 Å resolution. Proc. Natl. Acad. Sci. USA. 98:10131–10136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royant, A., K. Edman, T. Ursby, E. Pebay-Peyroula, E. M. Landau, and R. Neutze. 2000. Helix deformation is coupled to vectorial proton transport in the photocycle of bacteriorhodopsin. Nature. 406:645–648. [DOI] [PubMed] [Google Scholar]
- Sasaki, J., and J. K. Spudich. 2000. Proton transport by sensory rhodopsins and its modulation by transducer-binding. Biochim. Biophys. Acta. 1460:230–239. [DOI] [PubMed] [Google Scholar]
- Scharf, B., B. Pevec, B. Hess, and M. Engelhard. 1992. Biochemical and photochemical properties of the photophobic receptors from Halobacterium halobium and Natronobacterium pharaonis. Eur. J. Biochem. 206:356–366. [DOI] [PubMed] [Google Scholar]
- Schmies, G., M. Engelhard, P. G. Wood, G. Nagel, and E. Bamberg. 2001. Electrophysiological characterization of specific interactions between bacterial sensory rhodopsins and their transducers. Proc. Natl. Acad. Sci. USA. 98:1555–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmies, G., B. Luttenberg, I. Chizhov, M. Engelhard, A. Becker, and E. Banburg. 2000. Sensory rhodopsin II from the haloalkaliphilic Natronobacterium pharaonis: light-activated proton transfer reactions. Biophys. J. 78:967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel, R., B. Scharf, M. Gautel, K. Kleine, D. Oesterhelt, and M. Engelhard. 1995. The primary structure of sensory rhodopsin II: a membrane of an additional retinal protein subgroup is coexpressed with its transducer, the halobacterial transducer of rhodopsin II. Proc. Natl. Acad. Sci. USA. 92:3036–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimono, K., M. Kitami, M. Iwamoto, and N. Kamo. 2000. Involvement of two groups in reversal of the bathochromic shift of pharaonis phoborhodopsin by chloride at low pH. Biophys. Chem. 87:225–230. [DOI] [PubMed] [Google Scholar]
- Shimono, K., M. Iwamoto, M. Sumi, and N. Kamo. 1998. V108 mutant of pharaonis phoborhodopsin: substitution caused no absorption change but affected its M-state. J. Biochem. 124:404–409. [DOI] [PubMed] [Google Scholar]
- Shimono, K., M. Iwamoto, M. Sumi, and N. Kamo. 1997. Functional expression of pharaonis phoborhodopsin in Escherichia coli. FEBS Lett. 420:54–56. [DOI] [PubMed] [Google Scholar]
- Sineshchekov, O. A., K. H. Jung, and J. L. Spudich. 2002. Two rhodopsins mediate phototaxis to low- and high-intensity light in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA. 99:8689–8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich, J. L., C.-S. Yang, K.-H. Jung, and E. N. Spudich. 2000. Retinylidene proteins: structures and functions from archaea to humans. Annu. Rev. Cell Dev. Biol. 16:365–392. [DOI] [PubMed] [Google Scholar]
- Spudich, J. L. 1998. Variations on a molecular switch-transport and sensory signaling by archaeal rhodopsins. Mol. Microbiol. 28:1051–1058. [DOI] [PubMed] [Google Scholar]
- Spudich, J. L., and R. A. Bogomolni. 1984. Mechanism of colour discrimination by a bacterial sensory rhodopsin. Nature. 312:509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo, Y., M. Iwamoto, K. Shimono, M. Sumi, and N. Kamo. 2001. Photo-induced proton transport of pharaonis phoborhodopsin (sensory rhodopsin II) is ceased by association with the transducer. Biophys. J. 80:916–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, T., H. Tomioka, N. Kamo, and Y. Kobatake. 1985. A photosystem other than PS370 also mediates the negative phototaxis of Halobacterium halobium. FEMS Microbiol. Lett. 28:161–164. [Google Scholar]
- Tanio, M., S. Tuzi, S. Yamaguchi, R. Kawaminami, A. Naito, R. Needleman, J. K. Lanyi, and H. Saito. 1999. Conformational changes of bacteriorhodopsin along the proton-conduction chain as studied with C-13 NMR of [3-C-13]-Ala-labeled protein: Arg82 may function as an information mediator. Biophys. J. 77:1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka, H., T. Takahashi, N. Kamo, and Y. Kobatake. 1986. Action spectrum of the photoattractant response of Halobacterium halobium in early logarithmic growth phase and the role of sensory rhodopsin. Biochim. Biophys. Acta. 884:578–584. [Google Scholar]
- Varo, G. 2000. Analogies between halorhodopsin and bacteriorhodopsin. Biochim. Biophys. Acta. 1460:220–229. [DOI] [PubMed] [Google Scholar]
- Yan, B., T. Takahashi, R. Johnson, and J. L. Spudich. 1991. Identification of signaling states of a sensory receptor by modulation of lifetimes of stimulus-induced conformations: the case of sensory rhodopsin II. Biochemistry. 30:10686–10692. [DOI] [PubMed] [Google Scholar]
- Yang, C. S., and J. L. Spudich. 2001. Light-induced structural changes occur in the transmembrane helices of the Natronobacterium pharaonis HtrII transducer. Biochemistry. 40:14207–14214. [DOI] [PubMed] [Google Scholar]
- Yao, V. J., and J. L. Spudich. 1992. Primary structure of an archaebacterial transducer, a methyl-accepting protein associated with sensory rhodopsin I. Proc. Natl. Acad. Sci. USA. 89:11915–11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X.-N., J. Zhu, and J. L. Spudich. 1999. The specificity of interaction of archaeal transducers with their cognate sensory rhodopsin is determined by their transmembrane helices. Proc. Natl. Acad. Sci. USA. 96:857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]