Abstract
The relationship between tissue inflammation and clearance of the opportunistic pathogen Pneumocystis carinii is poorly understood. We asked whether the anti-inflammatory cytokine interleukin-10 (IL-10) is released during the host response to infection with P. carinii and whether local delivery of the IL-10 gene could suppress tissue inflammatory responses without compromising clearance of infection. Control and CD4-depleted mice were inoculated with P. carinii, and at serial intervals after inoculation, lung tissue was assayed for IL-10 by enzyme-linked immunosorbent assay. We found that IL-10 was released in lung tissue in control mice and was present in higher concentrations in CD4-depleted mice with progressive infection. Control and CD4-depleted mice were then pretreated with 109 PFU of intratracheally administered adenoviral vector containing the viral IL-10 gene or the luciferase gene followed by inoculation with P. carinii. Pretreatment with viral IL-10 did not alter clearance of infection in control mice or severity of infection in CD4-depleted mice but did decrease tissue inflammation. We then asked whether gene transfer of viral IL-10 could decrease tissue inflammation during immune reconstitution. In these experiments, immunodeficient scid mice were inoculated with P. carinii and were heavily infected after 4 weeks. When these mice are immunologically reconstituted by intravenous administration of spleen cells from normal mice, a hyperinflammatory reaction developed in lung tissue, associated with high mortality. In comparison to control mice, mice treated with viral IL-10 prior to reconstitution showed significantly decreased lung wet weight, bronchoalveolar lavage fluid (BALF) lactate dehydrogenase, and BALF neutrophils. In contrast, infection intensity, as measured by PCR for P. carinii rRNA, was unchanged between the IL-10 and luciferase groups. Survival was also improved in the IL-10-treated mice. We conclude that release of IL-10 is part of the host response to infection with P. carinii and that gene therapy with viral IL-10 can lessen excessive tissue inflammation without altering pathogen clearance. In the setting of immune reconstitution and P. carinii pneumonia, pretreatment with the viral IL-10 gene decreases excessive tissue inflammation and improves survival. These results are relevant to acute respiratory failure after initiation of antibiotic treatment for human P. carinii pneumonia and to immune reconstitution syndromes in human immunodeficiency virus-positive patients started on highly active antiretroviral therapy.
Recruitment of inflammatory cells into lung tissue is an important element of host defense against infection. This is particularly true for infection with the fungal pathogen, Pneumocystis carinii, where migration of CD4+ T lymphocytes into lung tissue is required to prevent the development of pneumonia (16, 34). However, excessive tissue inflammation in response to P. carinii can also be detrimental to the host. For example, in immune reconstitution syndromes and early after initiation of antimicrobial therapy for P. carinii pneumonia, exuberant pulmonary inflammatory responses can cause pulmonary edema, impaired gas exchange, and acute respiratory failure (41, 42). Obviously, efficient host defense requires mechanisms to both initiate and then to downregulate tissue inflammation.
One mechanism to control or modulate tissue inflammation is the elaboration of anti-inflammatory cytokines. One such anti-inflammatory cytokine is interleukin-10 (IL-10). Although IL-10 has multiple effects on the inflammatory response, most of these effects may be considered anti-inflammatory (38). For example, IL-10 suppresses Th1 lymphocyte responses, activation of macrophages, and release of chemotactic factors for neutrophils. In bacterial pneumonia, neutralization of endogenous IL-10 enhances tissue inflammation and improves bacterial clearance (14, 39).
The role of IL-10 in host responses to P. carinii is unknown. The purpose of this study was to investigate release of endogenous IL-10 in normal and CD4-depleted mice challenged with P. carinii and to examine the effect of pulmonary delivery of the IL-10 gene on host responses to this pathogen.
(Data were presented in part at the May 2002 annual meeting of the American Thoracic Society in Atlanta, Ga.)
MATERIALS AND METHODS
Animals.
Specific-pathogen-free female BALB/c mice were purchased at 7 or 8 weeks of age from Hilltop Laboratories (Scottsdale, Pa.). CB-17scid mice were purchased at age 5 to 8 weeks from Taconic Farms (Germantown, N.Y.). All animals were housed in filter-topped cages in an isolation room at the animal care facility. All caging procedures and surgical manipulations were done under a laminar flow hood. Mice were fed autoclaved chow and water ad libitum. The animals were kept in the facility for at least 2 days before any treatment was begun.
Adenoviral vectors.
We used the plasmid pcDSRa-BCRFI (kind gift from Thomas Ritter), which contains the viral IL-10 (vIL-10) cDNA. This cDNA was cut out from pcDSRa-BCRFI by EcoRI and subcloned into pBS. From pBSvIL-10 it was cloned into pACCMV via KpnI-XbaI. The control AdLuc vector is identical to this but encoded firefly luciferase as described earlier (19). Both viruses were propagated on 293 cells and purified over CsCl as described earlier (18), and contained <0.01 ng of endotoxin/ml as determined by the QCL-1000 Limulus lysate assay. We have previously used this vector system to deliver the gamma interferon (IFN-γ) gene to mice infected with P. carinii (19). Instillation of AdLuc into the lungs of normal mice (without P. carinii) is associated with a modest influx of neutrophils into lavaged cells (which is gone by 1 week) and a minimal influx of lymphocytes that persists to 1 month afterward.
CD4+ depletion.
Mice received intraperitoneal injections of 0.3 mg of anti-CD4 (hybridoma GK 1.5) in 0.1 ml of phosphate-buffered saline (PBS) each week, which results in a sustained and profound depletion of CD4+ lymphocytes from the blood and spleen (34).
P. carinii inoculation.
P. carinii for inoculation was prepared as described earlier (34) using lung homogenates from chronically infected scid mice (Taconic Farms). In brief, scid mice with chronic P. carinii infection were injected with a lethal dose of pentobarbital and their lungs were aseptically removed and frozen in 1 ml of PBS at −70°C. Frozen lungs were homogenized (Model 80 Stomacher; Tekmar Instruments, Cincinnati, Ohio) in 10 ml of PBS, were forced through a sterile 70-μm-pore-size nylon cell strainer (Falcon 2350; Becton Dickinson Labware, Franklin Lakes, N.J.), and pelleted at 500 × g for 10 min at 4°C. The pellet was resuspended in PBS. The pellet was diluted 1:4 with PBS and smeared on a microscope slide. The slide was stained with modified Giemsa stain (Diff-Quik; Baxter, McGraw Park, Ill.). The number of P. carinii cysts was quantified microscopically, and the inoculum concentration was adjusted to 2 × 106 cysts/ml. Recipient BALB/c mice were anesthetized intraperitoneally with pentobarbital (75 mg/kg of body weight) and injected intratracheally with 2 × 105 P. carinii cysts.
Immune reconstitution.
In some experiments, P. carinii-infected scid mice were immunologically reconstituted by intravenous injection of 5 × 107 spleen cells from normal BALB/c mice or BALB/c mice immunized with 2 × 105 P. carinii cysts intratracheally 2 weeks earlier (42).
Bronchoalveolar lavage.
After a lethal dose of pentobarbital (400 mg/kg), mice were exsanguinated by aortic transection. The trachea was exposed through a midline incision and cannulated with a polyethylene catheter. The lungs were lavaged with 0.5-ml aliquots of sterile, calcium- and magnesium-free PBS (GIBCO/BRL, Gaithersburg, Md.) containing 0.6 mM EDTA up to a total of 11 ml.
Lavage cell counts and differential.
Lavage cells were collected by centrifugation at 500 × g for 10 min. The cells were then washed with PBS, centrifuged onto glass slides at 20 × g for 5 min, and stained with Diff-Quik (Baxter) for differential cell counting.
Lung digest.
In selected experiments, lung digest was prepared for analysis of cytokine concentrations in lung tissue. The right lung was removed aseptically and forced through a 70-μm-pore-size nylon cell strainer (Falcon) in 0.5 ml of PBS. The resultant fluid was cleared of debris and cells by centrifugation and frozen at −70°C for later analysis.
RNA isolation/TaqMan probes and primers for P. carinii rRNA.
Total RNA was isolated from the right lung by using TRIZOL reagent (catalog no. 15596-026; Life Technologies, Rockville, Md.). As a standard for the assay, a portion of P. carinii subsp. muris rRNA (43) (GenBank accession no. AF257179) was cloned into PCR 2.1 Vector (Invitrogen) and P. carinii rRNA was produced by in vitro transcription using T7 TNA polymerase (catalog no. P 1300; Promega). The pair of TaqMan PCR primers for mouse P. carinii rRNA are 5′-ATG AGG TGA AAA GTC GAA AGG G-3′ and 5′-TGA TTG TCT CAG ATG AAA AAC CTC TT-3′. The probe was labeled with a reporter fluorescent dye, 6-carboxyfluorescein (6-FAM), and the sequence was 6-FAM-AACAGCCCAGAATAATGAATAAAGTTCCTCAATTGTTAC-TAMRA. Real-time reverse transcriptase (RT)-PCR was done using a two-step method. Reverse transcription reactions were done in a volume of 25 μl containing 5 μl of RNA sample, 1× TaqMan RT buffer, 5.5 mM magnesium chloride, a 500 μM concentration of each deoxynucleoside triphosphate, 2.5 μM random hexamer, 0.4 U of RNase inhibitor/μl, and 1.25 U of MultiScribe RT (PE Biosystems N 808-0234)/μl. Samples were incubated at 25°C for 10 min, reverse transcribed at 48°C for 30 min, and RT inactivated at 95°C for 5 min. PCRs were done in a volume of 50 μl containing 5 μl of cDNA, 1× TaqMan universal PCR master mix (PE Biosystems), primer, and probe. An initial 2-min incubation was done at 50°C for uracil-n-glycosylase activity to prevent carryover reaction. The reaction was terminated by heating at 95°C for 5 min. The PCR amplification was performed for 40 cycles with each cycle at 94°C for 20 s and 60°C for 1 min. All reaction were carried out in duplicate using the ABI prism 7700 SDS. Data were converted to rRNA copy number using a standard curve of known-copy-number P. carinii rRNA.
LDH activity.
Lactate dehydrogenase (LDH) activity in bronochoalveolar lavage fluid was assayed spectrophotometrically (340 nm) using LD-L reagent from Sigma (catalog no. 228-500P).
ELISA for endogenous and vIL-10.
The first 1 ml of cell-free lung lavage fluids was stored at −80°C and assayed for vIL-10 using a standard sandwich-type assay with two immunological steps. The first step leads to the capture of IL-10 by a monoclonal anti-IL-10 antibody bound to the well of a 96-well plate. In the second step, a second monoclonal anti-IL-10 antibody, which is biotinylated, was added together with streptavidin peroxidase conjugate. After incubation, the binding of the streptavidin-peroxidase via biotin was detected by the addition of a chromogenic substrate for peroxidase. All incubations were performed on a shaker platform at room temperature. The plates were washed three times with wash buffer between all steps. The intensity of the coloration produced is proportional to the IL-10 concentration in the sample or standard (IL-10 enzyme-linked immunosorbent assay [ELISA] kit, part no. 1 M1987; Immunotech, Marseille, France). Endogenous IL-10 was detected using a mouse IL-10 immunoassay kit (catalog no. M 1000; R&D Systems) similar to the ELISA for vIL-10.
Histologic examination of lung tissue.
For scoring of infection and inflammation, the lungs were fully inflated with 10% formalin. The trachea was tied off, and the lungs were fixed in 10% neutral formalin. Paraffin-embedded sections were stained with hematoxylin and eosin or Gomori-methenamine silver and scored blindly for intensity of infection and for alveolar and perivascular inflammation as previously described (5, 11, 24).
Statistics.
Data are reported as mean ± standard deviation (SD). Scalar comparisons were made by analysis of variance or by t test. Survival curves were compared using the log-rank test. Statistical significance was accepted when P was less than 0.05.
RESULTS
Release of endogenous IL-10 after inoculation with P. carinii.
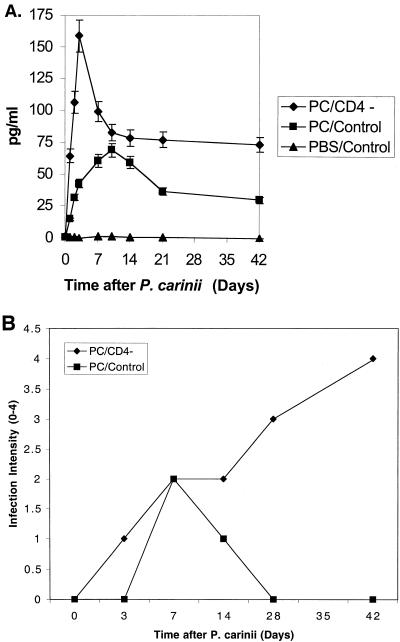
In our first set of experiments, we asked whether IL-10 is released in lung tissue in response to inoculation of P. carinii. IL-10 was assayed by ELISA in lung tissue from control and CD4-depleted mice at serial intervals after inoculation with P. carinii (Fig. 1A). An additional control included mice inoculated with an equivalent volume of PBS alone. Concentrations of IL-10 in mice inoculated with P. carinii were significantly different (P < 0.01) from those in mice inoculated with PBS alone at all time points. IL-10 was barely detectable in control mice inoculated with PBS. This was also true for CD4-depleted mice inoculated with PBS (data not shown). Comparing CD4-depleted mice to control mice inoculated with P. carinii, IL-10 in lung tissue was significantly higher (P < 0.01) in the CD4-depleted mice at all times except 10 days. CD4-depleted mice showed an earlier peak concentration of IL-10 at 3 days after inoculation with P. carinii than did control mice, where IL-10 peaked at 10 days (Fig. 1A). IL-10 in lung tissue remained higher in CD4-depleted mice with progressive infection out to 42 days after P. carinii. Additional experiments using an RNase protection assay showed parallel results for IL-10 mRNA (data not shown). Lung tissue was also scored for intensity of infection with P. carinii at similar time intervals (Fig. 1B). Control mice successfully cleared the infection, while CD4-depleted mice showed a progressive increase in intensity of infection. Comparing Fig. 1A to Fig. 1B, it can be seen that IL-10 in lung tissue roughly paralleled clearance of infection in control mice, while IL-10 remained persistently elevated in CD4-depleted mice with progressive infection.
FIG. 1.
Concentration of endogenous IL-10 in lung tissue after P. carinii (PC) challenge. (A) Control and CD4-depleted mice were inoculated with P. carinii, and lung tissue was assayed for IL-10 at serial intervals. An additional control included mice inoculated with PBS. Each data point represents mean ± SD for at least five animals. (B) Fixed lung tissue was also examined and scored microscopically for intensity of infection. Data represent the mean results for at least three animals.
Time course of vIL-10 expression after intratracheal injection.
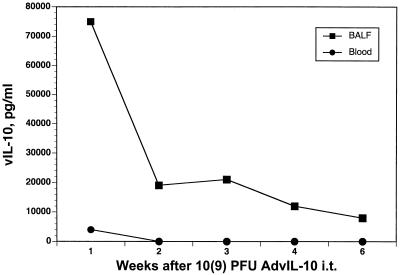
As a prelude to using adenoviral IL-10 (AdvIL-10) in P. carinii infection, we examined the kinetics of expression of vIL-10 after pulmonary gene transfer. Figure 2 shows the time course of vIL-10 protein expression in bronchoalveolar lavage fluid and in blood after intratracheal injection of 109 PFU of AdvIL-10. High levels of vIL-10 were detectable in bronchoalveolar lavage fluid with a peak at 1 week after gene transfer and persistence to 6 weeks. Minimal vIL-10 was observed in the blood compartment at 1 week and was undetectable thereafter.
FIG. 2.
Expression of the vIL-10 transgene after intrapulmonary injection. A given amount (109 PFU) of AdvIL-10 was injected intratracheally (i.t.), and vIL-10 was assayed by ELISA in bronchoalveolar lavage fluid and in serum at serial intervals. Each data point represents the mean of three animals.
Effect of pretreatment with AdvIL-10 or AdLuc on clearance or severity of infection with P. carinii.
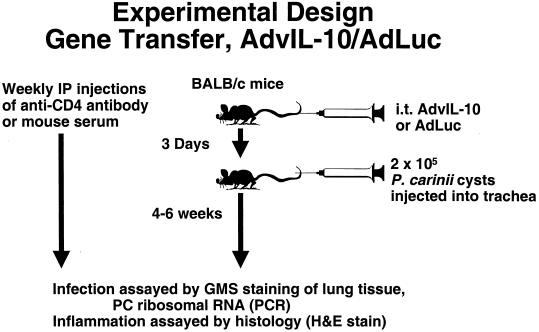
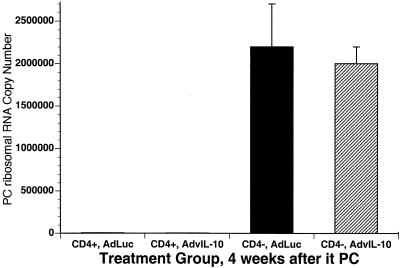
We next examined the effect of pretreatment with AdvIL-10 on clearance of infection. The experimental design for these experiments is shown in Fig. 3. Animals were sacrificed at 4 and 6 weeks after inoculation with P. carinii, and the intensity of infection in lung tissue was assayed by real-time PCR for P. carinii rRNA. The results at the 4-week time point are shown in Fig. 4. Pretreatment with AdvIL-10 did not alter clearance of infection in control mice (CD4+) in comparison to control mice receiving AdLuc. Similarly, in CD4-depleted mice (CD4−), pretreatment with AdvIL-10 did not change the severity of chronic P. carinii infection in comparison to that in CD4-depleted mice receiving AdLuc. Similar observations were found when CD4-depleted mice were sacrificed at 6 weeks after P. carinii inoculation (data not shown).
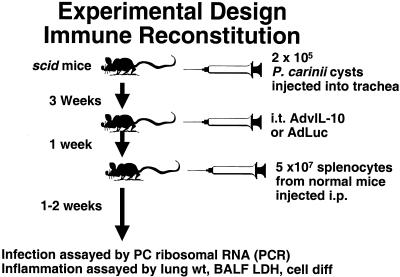
FIG. 3.
Experimental design. Gene transfer of AdvIL-10 or AdLuc. i.t., intratracheal; H&E, hematoxylin and eosin; PC, P. carinii; and GMS, Gomori-methenamine silver; IP, intrapulmonary.
FIG. 4.
Lung tissue burden of P. carinii (PC) in mice pretreated with AdvIL-10 or AdLuc. Data are from mice sacrificed 4 weeks after inoculation with P. carinii. The two leftmost columns represent control (CD4+) mice, and the two rightmost columns represent CD4-depleted (CD4−) mice. Each data point represents mean ± SD for at least 10 animals. it, intratracheal.
Effect of pretreatment with AdvIL-10 or AdLuc on tissue inflammation following inoculation with P. carinii.
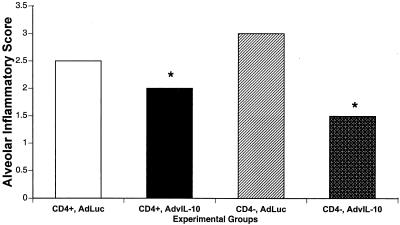
Because IL-10 has anti-inflammatory properties, we examined microscopic inflammatory scores in mice pretreated with AdvIL-10. Control and CD4-depleted mice were pretreated with AdvIL-10 or AdLuc and were then inoculated with P. carinii. Four weeks after inoculation, alveolar inflammation was scored by blinded microscopic examination of formalin-fixed, hematoxylin- and eosin-stained lung tissue. Normal lung tissue has an alveolar inflammatory score of 0, while lung tissue with an inflammatory score of 4 shows severe, confluent consolidation of alveolar parenchyma with infiltration of mononuclear cells and neutrophils and proteinaceous exudates. The results are shown in Fig. 5. Both control and CD4-depleted mice pretreated with AdvIL-10 showed significantly less alveolar inflammation than similar mice pretreated with AdLuc.
FIG. 5.
Effect of pretreatment with AdvIL-10 or AdLuc on tissue inflammation. Data are from mice sacrificed 4 weeks after inoculation with P. carinii. The two leftmost columns represent control mice, while the two rightmost columns represent CD4-depleted mice. Alveolar inflammatory scores were calculated by microscopic examination of hematoxylin- and eosin-stained lung tissue. Each data point represents mean ± SD for at least six animals. *, P < 0.05 in comparison to AdLuc.
Immune reconstitution in mice infected with P. carinii.
Because local delivery of IL-10 decreased tissue inflammation without altering clearance of infection, we then asked whether exogenous IL-10 could be used to suppress excess tissue inflammation during immune reconstitution. Immune reconstitution involves adoptive transfer of normal spleen cells into P. carinii-infected scid mice. As the reconstituted immune system responds to P. carinii in lung tissue, there are excess tissue inflammation, impaired gas exchange, and high mortality (42). The experimental design for these experiments is shown in Fig. 6. Consistent with our earlier results (Fig. 4), administration of AdvIL-10 did not alter the intensity of infection at the time of reconstitution compared to mice administered AdLuc (AdvIL-10, 1.2 × 107 ± 0.15 × 107; AdLuc, 1.4 × 107 ± 0.2 × 107 P. carinii rRNA copies/lung; P = not statistically significant).
FIG. 6.
Experimental design. Immune reconstitution during P. carinii (PC) infection. i.p., intrapulmonary; BALF, bronchoalveolar lavage fluid.
Effect of local delivery of AdvIL-10 or AdLuc on tissue inflammation during immune reconstitution in mice infected with P. carinii.
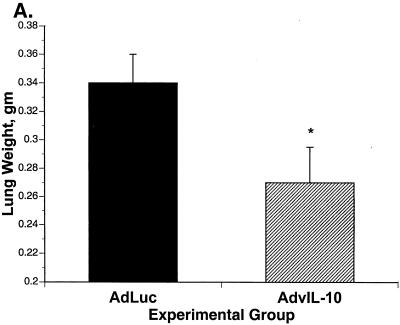
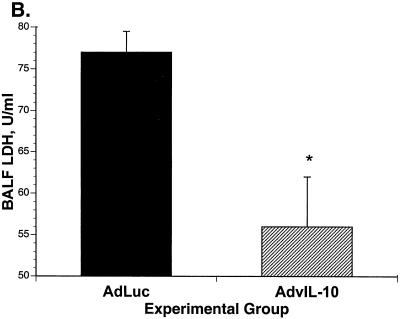
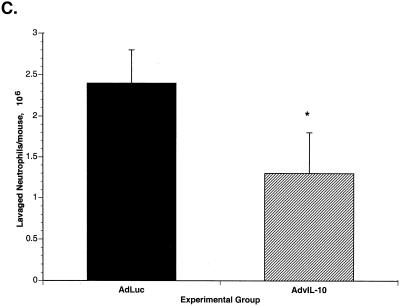
When mice were sacrificed at 2 weeks after immune reconstitution, there was significantly less tissue inflammation in the mice given AdvIL-10 than in mice given AdLuc. Tissue inflammation was assayed as lung wet weight, lavage fluid LDH, and the number of neutrophils in lavaged cells. Lung wet weight was significantly decreased in AdvIL-10 mice compared to that in AdLuc mice (Fig. 7A). LDH concentrations in bronchoalveolar lavage fluid were also significantly decreased in mice given AdvIL-10 compared to those in mice given AdLuc (Fig. 7B). Concentrations of lavaged polymorphonuclear leukocytes are shown in Fig. 7C. The total number of lavaged neutrophils per mouse was significantly decreased in mice given AdvIL-10 compared to to that in mice given AdLuc (1.3 × 106 ± 0.4 × 106/mouse for AdvIL-10; 2.4 × 106 ± 0.5 × 106/mouse for AdLuc; P < 0.05). Numbers of lavaged alveolar macrophages and lymphocytes were not significantly different in mice given AdvIL-10 from those in mice given AdLuc.
FIG. 7.
Effect of AdvIL-10 or AdLuc on parameters of tissue inflammation during immune reconstitution. (A) Lung wet weights 2 weeks after immune reconstitution in mice treated with AdvIL-10 or AdLuc. *, P < 0.05 in comparison to AdLuc. (B) Concentrations of LDH in bronchoalveolar lavage fluid 2 weeks after immune reconstitution in mice treated with AdvIL-10 or AdLuc. *, P < 0.01 in comparison to AdLuc. (C) Lavaged neutrophils 2 weeks after immune reconstitution in mice treated with AdvIL-10 or AdLuc. *, P < 0.05 in comparison to AdLuc.
Effect of local delivery of AdvIL-10 or AdLuc on survival following immune reconstitution in mice infected with P. carinii.
Finally, we asked whether treatment with AdvIL-10 could improve survival during the hyperinflammatory response associated with immune reconstitution. P. carinii-infected scid mice were administered AdvIL-10 or AdLuc and were then immune reconstituted as shown in Fig. 6. These mice were then monitored for survival up to 25 days after reconstitution. A survival curve is shown in Fig. 8. Mice began to die at 10 days after reconstitution, and survival was 0% in the AdLuc group by 17 days after reconstitution. Mortality was also observed in the AdvIL-10 group but was delayed in comparison to that in AdLuc mice, with a final survival rate of 30% at 25 days after reconstitution (P < 0.05 in comparison to AdLuc).
FIG. 8.
Survival after immune reconstitution in mice treated with AdvIL-10 or AdLuc. Survival was significantly improved (P < 0.05) in mice receiving AdvIL-10 compared to that in mice receiving AdLuc.
DISCUSSION
Results of these experiments demonstrate for the first time (to our knowledge) in vivo release of the cytokine, IL-10, during the host response to P. carinii. Warschkau et al. previously demonstrated that P. carinii cultured in vitro with splenocytes from scid mice stimulated the release of IL-10 as well as that of tumor necrosis factor, IFN-γ, and IL-12 into culture supernatants (40). Our experiments now clearly indicate that P. carinii can also stimulate release of IL-10 in vivo, at least in mice. Interestingly, release of endogenous IL-10 in our experiments was consistently higher in CD4-depleted than in control (CD4-replete) mice. This was true even at early time points after instillation of P. carinii, when the severity of infection was comparable between the two types of mice (Fig. 1). The reasons for higher IL-10 levels in CD4-depleted mice are not clear, but our experiments would suggest that CD4+ T lymphocytes are not a major cellular source of IL-10 in response to P. carinii. In this regard, IL-10 has been shown to be released from a variety of cell types, including macrophages and monocytes (10). Indeed, Mycobacterium leprae infection in IFN-γ knockout mice is associated with increased mRNA for IL-10 in granuloma cells (1). It is possible that elevated endogenous IL-10 associated with CD4 depletion (and possibly human immunodeficiency virus [HIV] infection) reflects the loss of a major cellular source of IFN-γ and that the resultant high endogenous IL-10 content is part of the immunosuppression that then permits infection with P. carinii. In support of this idea, IFN-γ and IL-10 appear to have a reciprocal relationship in lymphocytes from HIV-infected persons, such that progressive infection is associated with decreased lymphocyte production of IFN-γ and increased production of IL-10, and this relationship is reversed with antiviral treatment (30). This could also explain the clinical observation that HIV-related P. carinii pneumonia is characterized by a longer time course before diagnosis, a higher organism tissue burden, and less tissue inflammation than in PCP that complicates other immunosuppressive states (21).
Release of IL-10 in vivo has been shown in prior studies to be involved in host defense against a variety of other infectious agents, including Klebsiella pneumoniae (14, 39), Listeria monocytogenes (7), Candida albicans (8, 33), Trypanosoma cruzi (32), and Toxoplasma gondii (29). As a general rule, experimental depletion of IL-10 through antibody neutralization or usage of knockout mice leads to enhanced host responses and decreased susceptibility to infection, while elevation of IL-10 by administration of recombinant cytokine or usage of transgenic mice leads to decreased host responses and increased susceptibility to infection. An exception may be Leishmania major (6). Thus, it is surprising that elevation of IL-10 in our experiments through local delivery and expression of vIL-10 did not compromise host defense against P. carinii. One explanation for this finding lies in the observation that endogenous IL-10 is already elevated during P. carinii infection, so that further elevation of IL-10 through gene transfer might not lead to increased susceptibility to infection. Therefore, we cannot conclude from our studies that IL-10 is not important in host defense against P. carinii but can conclude only that elevation of IL-10 does not compromise clearance of infection. To further delineate the role of IL-10 in host defense against this pathogen, additional experiments are planned using an antibody neutralization approach.
It should also be emphasized that our experiments with gene transfer of IL-10 utilized the Epstein-Barr viral homolog of IL-10. vIL-10 is almost identical in amino acid sequences to murine IL-10 and likely represents a gene captured by the virus to facilitate interaction with the infected host (27). In terms of biological activity, vIL-10 shares many functions with endogenous IL-10 but differs in others. vIL-10 retains most of the anti-inflammatory properties of murine or human IL-10 but lacks some of the proinflammatory properties of these cytokines. For example in contrast to murine or human IL-10, vIL-10 does not upregulate major histocompatibility complex class II expression on B cells (13) or costimulate proliferation of mouse thymocytes or mast cells (23, 37). vIL-10 also differs from endogenous IL-10 as to influences on the host response to allografts (31) or tumor cells (35). These differences in biological activity may reflect differential binding to IL-10 receptor complexes (22). Additional experiments are planned to confirm our present observations using gene transfer of murine IL-10.
IL-10 may be considered an immunosuppressive cytokine and has suppressive functions for both innate and acquired immune responses. In terms of infectious diseases, IL-10 may serve to limit excess tissue inflammation and prevent tissue injury during the host inflammatory response to an infectious pathogen. In the present studies, we found that gene transfer of viral IL-10 also decreased tissue inflammation in mice inoculated with P. carinii. Furthermore, in a model of excess tissue inflammation associated with immune reconstitution, IL-10 therapy dampened pulmonary inflammation and prolonged survival. Mechanisms through which IL-10 decreases pulmonary inflammation during P. carinii challenge remain to be clarified, but our data suggest that the anti-inflammatory effect of IL-10 is mediated in part by decreased recruitment of neutrophils into lung tissue. In HIV-infected subjects with PCP, excess neutrophils in the lung lavage fluid are a marker for poor prognosis (4, 21). Numerous prior studies have shown that IL-10 inhibits the production of CXC chemokines from monocytes and macrophages (15, 20). CXC chemokines are important in the recruitment of neutrophils into infected tissue, and we propose that decreased P. carinii-induced inflammation following IL-10 gene transfer is the result of suppressed host release of CXC chemokines for neutrophils.
Local delivery of IL-10 may have therapeutic benefit in certain disease states including infectious diseases. For example, IL-10 therapy shows promise in the treatment of patients with inflammatory bowel disease (36), rheumatoid arthritis (17) and psoriasis (3) and in the prevention of endoscopic retrograde cholangiopancreatography-induced pancreatitis (9). In patients with chronic hepatitis C infection, treatment with human IL-10 reverses liver fibrosis without increasing viral titers (28). In human P. carinii pneumonia, effective antimicrobial therapy is available, but there are situations where excessive inflammation is a problem clinically. Some patients with severe P. carinii pneumonia experience respiratory failure shortly after initiation of antimicrobial therapy (12, 25). The pathogenesis of respiratory failure in this setting is felt to be tissue injury mediated through an exuberant host inflammatory response to the pathogen as it is killed by antibiotic therapy. Corticosteroids are already used as anti-inflammatory agents in these patients (2, 26), and IL-10 could conceivably be of benefit as well. Paradoxical worsening of PCP has also been reported in patients started on highly active antiretroviral therapy (41). This human immune reconstitution syndrome is analogous to the murine model of immune reconstitution used in our studies. Corticosteroids have been proposed to treat human immune reconstitution syndromes (41), and our studies suggest that IL-10 may also be a potential therapy for these cases.
In summary, the results of these experiments demonstrate that IL-10 is released in lung tissue as part of the host response to infections with P. carinii. Local gene transfer of vIL-10 decreases recruitment of neutrophils into infected lung tissue without altering clearance of infection. In the setting of immune reconstitution and P. carinii pneumonia, pretreatment with the vIL-10 gene decreases excessive tissue inflammation and decreases mortality. These results provide a rationale to study adjunctive IL-10 therapy in selected patients with P. carinii pneumonia.
Acknowledgments
This work was supported by NIH grants HL59724 (NHLBI) and AA08845 (NIAAA) and by the Louisiana Health Excellence Fund.
Editor: T. R. Kozel
REFERENCES
- 1.Adams, L., D. Scollard, N. Ray, A. Cooper, A. Frank, I. Orme, and J. Krahenbuhl. 2002. The study of Mycobacterium leprae infection in interferon-gamma gene-disrupted mice as a model to explore the immunopathologic spectrum of leprosy. J. Infect. Dis. 185:S1-S8. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 1990. Consensus statement on the use of corticosteroids as adjunctive therapy for pneumocystis pneumonia in the acquired immunodeficiency syndrome. N. Engl. J. Med. 323:1500-1504. [DOI] [PubMed] [Google Scholar]
- 3.Asadullah, K., W. Docke, M. Ebeling, M. Friedrich, G. Belbe, H. Audring, H. Volk, and W. Sterry. 1999. Interleukin-10 treatment of psoriasis: clinical results of a phase 2 trial. Arch. Dermatol. 135:187-192. [DOI] [PubMed] [Google Scholar]
- 4.Bang, D., J. Emborg, J. Elkjaer, J. Lundgren, and T. Benfield. 2001. Independent risk of mechanical ventilation for AIDS-related Pneumocystis carinii pneumonia associated with bronchoalveolar lavage neutrophilia. Respir. Med. 95:661-665. [DOI] [PubMed] [Google Scholar]
- 5.Beck, J., M. Warnock, J. Curtis, M. Sniezek, S. Arrag-Peffer, H. Kaltreider, and J. Shellito. 1991. Inflammatory responses to Pneumocystis carinii in mice selectively depleted of helper T lymphocytes. Am. J. Respir. Cell Mol. Biol. 5:186-197. [DOI] [PubMed] [Google Scholar]
- 6.Chatelain, R., S. Mauze, and R. Coffman. 1999. Experimental Leishmania major infection in mice: role of IL-10. Parasite Immunol. 21:211-218. [DOI] [PubMed] [Google Scholar]
- 7.Dai, W. J., G. Kohler, and F. Brombacher. 1997. Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10-deficient mice. J. Immunol. 158:2259-2267. [PubMed] [Google Scholar]
- 8.Del Sero, G., A. Mancacci, E. Cenci, C. d'Ostiani, C. Montagnoli, A. Bacci, P. Mosci, M. Kopf, and L. Romani. 1999. Antifungal type 1 responses are upregulated in IL-10-deficient mice. Microbes Infect. 1:1169-1180. [DOI] [PubMed] [Google Scholar]
- 9.Deviere, J., O. Le Moine, J. L. Van Laethem, P. Eisendrath, A. Ghilain, N. Severs, and M. Cohard. 2001. Interleukin 10 reduces the incidence of pancreatitis after therapeutic endoscopic retrograde cholangiopancreatography. Gastroenterology 120:498-505. [DOI] [PubMed] [Google Scholar]
- 10.de Waal Malefyt, R., and K. Moore. 1998. Interleukin-10, p. 333-364. In A. Thompson (ed.), The cytokine handbook, 3rd. ed. Academic Press, San Diego, Calif.
- 11.D'Souza, N., J. Mandujano, S. Nelson, W. Summer, and J. Shellito. 1995. Alcohol ingestion impairs host defenses predisposing otherwise healthy mice to Pneumocystis carinii infection. Alcohol Clin. Exp. Res. 19:1219-1225. [DOI] [PubMed] [Google Scholar]
- 12.Garay, S., and J. Greene. 1989. Prognostic indicators in the initial presentation of Pneumocystis carinii pneumonia. Chest 95:769-772. [DOI] [PubMed] [Google Scholar]
- 13.Go, N., B. Castel, R. Barrett, R. Kastelein, W. Dang, T. Mosmann, K. Moore, and M. Howard. 1990. Interleukin 10 (IL-10), a novel B cell stimulatory factor: unresponsiveness of X chromosome-linked immunodeficiency B cells. J. Exp. Med. 172:1625-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenberger, M. J., R. M. Strieter, S. L. Kunkel, J. M. Danforth, R. E. Goodman, and T. J. Standiford. 1995. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J. Immunol. 155:722-729. [PubMed] [Google Scholar]
- 15.Hamilton, T., Y. Ohmori, J. Tebo, and R. Kishore. 1999. Regulation of macropahge gene expression by pro- and anti-inflammatory cytokines. Pathobiology 67:241-244. [DOI] [PubMed] [Google Scholar]
- 16.Harmsen, A., and M. Stankiewicz. 1990. Requirement for CD4+ cells in resistance to Pneumocystis carinii pneumonia in mice. J. Exp. Med. 172:937-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keystone, E., J. Wherry, and P. Grint. 1998. IL-10 as a therapeutic strategy in the treatment of rheumatoid arthritis. Rheum. Dis. Clin. N. Am. 24:629-639. [DOI] [PubMed] [Google Scholar]
- 18.Kolls, J., K. Peppel, M. Silva, and B. Beutler. 1994. Prolonged and effective blockade of tumor necrosis factor activity through adenovirus-mediated gene transfer. Proc. Natl. Acad. Sci. USA 91:215-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolls, J., C. Vazquez, D. Lei, P. Schwarzenberger, P. Ye, S. Nelson, W. Summer, and J. Shellito. 1999. Interferon-gamma and CD8+ T-cells restore host defense against P. carinii in mice lacking CD4+ T-cells. J. Immunol. 162:2890-2894. [PubMed] [Google Scholar]
- 20.Kopydlowski, K., C. Salkowski, M. Cody, N. van Rooijen, J. Major, T. Hamilton, and S. Vogel. 1999. Regulation of macrophage chemokine expression by lipopolysaccharide in vitro and in vivo. J. Immunol. 163:1537-1544. [PubMed] [Google Scholar]
- 21.Limper, A., K. Offord, T. Smith, and W. I. Martin. 1989. Pneumocystis carinii pneumonia: differences in lung parasite number and inflammation in patients with and without AIDS. Am. Rev. Respir. Dis. 140:1204-1209. [DOI] [PubMed] [Google Scholar]
- 22.Liu, Y., R. de Waal Malefyt, F. Briere, C. Parham, J. M. Bridon, J. Banchereau, K. W. Moore, and J. Xu. 1997. The EBV IL-10 homologue is a selective agonist with impaired binding to IL-10 receptor. J. Immunol. 158:604-613. [PubMed] [Google Scholar]
- 23.MacNeil, I., T. Suda, K. Moore, T. Mosmann, and A. Zlotnik. 1990. IL-10, a novel cytokine growth cofactor for mature and immature T cells. J. Immunol. 145:4167-4173. [PubMed] [Google Scholar]
- 24.Mandujano, J. F., N. B. D'Souza, S. Nelson, W. R. Summer, R. C. Beckerman, and J. E. Shellito. 1995. Granulocyte-macrophage colony stimulating factor and Pneumocystis carinii pneumonia in mice. Am. J. Respir. Crit. Care Med. 151:1233-1238. [DOI] [PubMed] [Google Scholar]
- 25.Maxfield, R., B. Surkin, E. Fazzini, D. Rapaport, W. Stenson, and R. Goldring. 1986. Respiratory failure in patients with acquired immunodeficiency syndrome and Pneumocystis carinii pneumonia. Crit. Care Med. 14:443-449. [DOI] [PubMed] [Google Scholar]
- 26.McGowan, J., P. Chestney, K. Crossley, and F. LaForce. 1992. Guidelines for the use of systemic glucocorticosteroids in the management of selected infections. J. Infect. Dis. 165:1-13. [DOI] [PubMed] [Google Scholar]
- 27.Moore, K. W., P. Vieira, D. F. Fiorentino, M. L. Trounstine, T. A. Khan, and T. R. Mosmann. 1990. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science 248:1230-1234. [DOI] [PubMed] [Google Scholar]
- 28.Nelson, D. R., G. Y. Lauwers, J. Y. Lau, and G. L. Davis. 2000. Interleukin-10 treatment reduces fibrosis in patients with chronic hepatitis C: a pilot trial of interferon nonresponders. Gastroenterology 118:655-660. [DOI] [PubMed] [Google Scholar]
- 29.Neyer, L. E., G. Grunig, M. Fort, J. S. Remington, D. Rennick, and C. A. Hunter. 1997. Role of interleukin-10 in regulation of T-cell-dependent and T-cell-independent mechanisms of resistance to Toxoplasma gondii. Infect. Immun. 65:1675-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ostrowski, M., J. Gu, C. Kovacs, J. Freedman, M. Luscher, and K. MacDonald. 2001. Quantitative and qualitative assessment of human immunodeficiency virus type 1 (HIV-1)-specfic CD4+ T cell immunity to gag in HIV-1-infected individuals with differential disease progression: reciprocal interferon-gamma and interleukin-10 responses. J. Infect. Dis. 184:1268-1278. [DOI] [PubMed] [Google Scholar]
- 31.Qin, L., K. Chavin, Y. Ding, H. Tahara, J. Favaro, J. Woodward, T. Suzuki, P. Robbins, M. Lotze, and J. Bromberg. 1996. Retrovirus-mediated transfer of viral IL-10 gene prolongs murine cardiac allograft survival. J. Immunol. 156:2316-2323. [PubMed] [Google Scholar]
- 32.Reed, S., C. Brownell, D. Russo, J. Silva, K. Grabstein, and P. Morrissey. 1994. IL-10 mediates susceptibility to Trypanosoma cruzi infection. J. Immunol. 153:135-140. [PubMed] [Google Scholar]
- 33.Romani, L., P. Puccetti, A. Mencacci, E. Cenci, R. Spaccapelo, L. Tonnetti, U. Grohmann, and F. Bistoni. 1994. Neutralization of IL-10 upregulates nitric oxide production and protects susceptible mice from challenge with Candida albicans. J. Immunol. 152:3514-3521. [PubMed] [Google Scholar]
- 34.Shellito, J., V. Suzara, W. Blumenfeld, J. Beck, H. Steger, and T. Ermak. 1990. A new model of Pneumocystis carinii infection in mice selctively depleted of helper T lymphocytes. J. Clin. Investig. 85:1686-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki, T., H. Tahara, S. Narula, K. Moore, P. Robbins, and M. Lotze. 1995. Viral interleukin-10 (IL-10), the human herpes virus cellular IL-10 homologue, induces local anergy to allogeneic and syngeneic tumors. J. Exp. Med. 182:477-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Deventer, S., C. Elson, and R. Fedorak. 1997. Multiple doses of intravenous interleukin-10 in steroid-refractory Crohn's disease. Gastroenterology 113:383-389. [DOI] [PubMed] [Google Scholar]
- 37.Vieira, P., R. de Waal-Malefyt, M. N. Dang, K. E. Johnson, R. Kastelein, D. F. Fiorentino, J. E. deVries, M. G. Roncarolo, T. R. Mosmann, and K. W. Moore. 1991. Isolation and expression of human cytokine synthesis inhibitory factor cDNA clones: homology to Epstein-Barr virus open reading frame BCRFI. Proc. Natl. Acad. Sci. USA 88:1172-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wakkach, A., F. Cottrez, and H. Groux. 2000. Can interleukin-10 be used as a true immunoregulatory cytokine? Eur. Cytokine Netw. 11:153-160. [PubMed] [Google Scholar]
- 39.Wang, M., K. Jeng, and L. Ping. 1999. Exogenous cytokine modulation or neutralization of interleukin-10 enhances survival in lipopolysaccharide-hyporesponsive C3H/HeJ mice with Klebisella infection. Immunology 98:90-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warschkau, H., H. Yu, and A. F. Kiderlen. 1998. Activation and suppression of natural cellular immune functions by Pneumocystis carinii. Immunobiology 198:343-360. [DOI] [PubMed] [Google Scholar]
- 41.Wislez, M., E. Bergot, M. Antoine, A. Parrot, M. Carette, and C. Mayaud. 2001. Acute respiratory failure following HAART introduction in patients treated for Pneumocystis carinii pneumonia. Am. J. Respir. Crit. Care Med. 164:847-851. [DOI] [PubMed] [Google Scholar]
- 42.Wright, T. W., F. Gigliotti, J. N. Finkelstein, J. T. McBride, C. L. An, and A. G. Harmsen. 1999. Immune-mediated inflammation directly impairs pulmonary function, contributing to the pathogenesis of Pneumocystis carinii pneumonia. J. Clin. Investig. 104:1307-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng, M., J. E. Shellito, L. Marrero, Q. Zhong, S. Julian, P. Ye, V. Wallace, P. Schwarzenberger, and J. K. Kolls. 2001. CD4+ T cell-independent vaccination against Pneumocystis carinii in mice. J. Clin. Investig. 108:1469-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]