Abstract
The conductance of the BK channel was evaluated in reconstituted bilayers made of POPE/POPS (3.3:1), or POPE/POPS with an added 20% of either SPM (3.3:1:1), CER (3.3:1:1), or CHL (3.3:1:1). The presence of SPM, which is known to increase bilayer thickness, significantly reduced the conductance of the BK channel. To directly test the role of membrane thickness, the conductance of the BK channel was measured in bilayers formed from PCs with acyl chains of increasing length (C14:1–C24:1), all in the absence of SPM. Slope conductance was maximal at a chain length of (C18:1) and much reduced for both thinner (C14:1) and thicker (C24:1) bilayers, indicating that membrane thickness alone can modify slope conductance. Further, in a simplified binary mixture of DOPE/SPM that forms a confined, phase-separated bilayer, the measured conductance of BK channels shows a clear bimodal distribution. In contrast, the addition of CER, which has an acyl chain structure similar to SPM but without its bulky polar head group to POPE/POPS, was without effect, as was the addition of CHL. The surface structure of membranes made from these same lipid mixtures was examined with AFM. Incorporation of both SPM and CER resulted in the formation of microdomains in POPE/POPS monolayers, but only SPM promoted a substantial increase in the amount of the high phase observed for the corresponding bilayers. The addition of CHL to POPE/POPS eliminated the phase separation observed in the POPE/POPS bilayer. The decrease in channel conductance observed with the incorporation of SPM into POPE/POPS membranes was, therefore, attributed to larger SPM-rich domains that appear thicker than the neighboring bilayer.
INTRODUCTION
We previously determined that clinically relevant doses of ethanol modulate the activity of the BK channel in cell membranes (Dopico et al., 1998), or when they are inserted into artificial bilayers made of POPE/POPS (Crowley et al., 2000, 2003). POPE/POPS bilayers are common starting points for model studies of protein function in artificial membranes but vastly underestimate the diversity of lipids normally found in nerve membranes (Coronado et al., 1984). We used this simple binary system in our initial studies of alcohol's direct effects on BK channel function (Chu et al., 1998). However, natural neuronal membranes contain a number of other lipid constituents including SPM, CER, and CHL (Sprong et al., 2001). There is ample evidence from other systems that changes in lipid composition can have robust effects on the material properties of bilayers and the local structure of membranes and the proteins they contain (Mobashery et al., 1997). The importance of the targeting, positioning, and functioning of integral membrane proteins as found in particular lipid regions has gradually been appreciated (Brown and London, 1998). As part of our ongoing studies of the BK channel we next sought to evaluate the consequences of including these natural lipid components into our model membranes. We were initially surprised to find that making the lipid environment more neuronal by adding individual lipid components (SPM, CER, or CHL) normally found in neurons, had significant effects on the function of the BK channel. Here we report the effects of changes in lipid composition on BK channel conductance.
The fluid mosaic model for the organization of a cell membrane proposed that a lipid bilayer functions as a neutral two-dimensional solvent that has little influence on integral membrane proteins (Singer and Nicolson, 1972). In this view, lipid bilayers were assumed to be passive substrates. The properties of the plasma membrane of a cell were thought to be mainly dictated by the structure and function of the proteins they contain. However, more recent studies suggest that the lipid bilayer that forms a cell's plasma membrane is not a homogeneous fluid bilayer. It is now thought to contain some liquid-ordered microdomains (lipid rafts) that are typically enriched in SPM and CHL floating in the fluid bilayer (Simons and Ikonen, 1997; Brown and London, 1998: Anderson and Jacobson, 2002; Dietrich et al., 2001; Yuan et al., 2002). Some membrane proteins appear to be dynamically targeted to particular lipid microdomains that are important in initiating and regulating signaling pathways. It has been proposed (Bretscher and Munro, 1993) that membrane thickness plays an important role in this process. Although the hydrophobic thickness of the bilayer is clearly an important factor in determining the energetics of mismatch with the hydrophobic region of a transmembrane protein, it has also been suggested that other bilayer physical properties such as surface tension, deformability, curvature, and flexibility may also be important for a particular protein (Lundbaek and Andersen, 1994; Lundbaek et al., 2003; Perozo et al., 2002). There is abundant evidence to indicate that membrane lipid composition may affect the function of some integral proteins, such as pumps (Lee, 1998), enzymes (Starling et al., 1995; Saslowsky et al., 2002; Bayburt and Sligar, 2002), and carriers (Eisenman et al., 1975). In most of these cases there is an optimum in function at a particular bilayer thickness. Where this has been investigated, lipid chain lengths near C18, as observed here for the BK channel, are often most favorable for function. It is still poorly understood just how the lateral organization of a natural lipid bilayer forms microdomains (Sprong et al., 2001) and, further, how the physical properties within a lipid domain (e.g., surface charge, lateral stress, curvature, and bilayer thickness (Martinac and Hamill, 2002; Mobashery et al., 1997)) modulate the function of the integral membrane channel proteins they contain (Maxfield, 2002; Lee, 2003).
Studies of pore-forming proteins like gramicidin have shown quite clearly that the hydrophobic mismatch that arises between the hydrophobic hydrocarbon core of the bilayer and the hydrophobic regions of the transmembrane portion of the channel are sufficiently energetic to give rise to alterations in the structure and function of the protein and may also locally deform the structure of the bilayer (Nielsen et al., 1998; Nielsen and Andersen, 2000; Chiu et al., 1999; Goforth et al., 2003; Lundbaek and Andersen, 1994). In fact, studies on gramicidin in bilayers provide a proof of principle that membrane thickness has a direct effect on channel function (Mobashery et al., 1997; Martinac and Hamill, 2002). It is not clear how bilayer thickness would affect other channel-forming proteins, especially ones like BK, where the transmembrane portion of each monomer is a single helical structure. However, despite the special circumstances of pore formation in the gramicidin channel, which requires the dimerization of two protein monomers, one in each leaflet, the energy associated with any hydrophobic mismatch must still exist for all transmembrane proteins.
Large-conductance Ca2+-activated voltage-gated K+ channels (BK) represent a functional subtype of K+ channels with unique features. They display a large unitary conductance and can be activated by both membrane depolarization and an elevation of intracellular Ca2+ (Vergara et al., 1998). These aspects of function can be altered by changes in the binding of particular accessory β-subunits to the pore forming α-subunit (Xia et al., 1999, 2000; Petkov et al., 2001). To the extent that recent structural observations (Doyle et al., 1998) of the bacterial KcsA (LeMasurier et al., 2001; Heginbotham et al., 1999) and KvAP K+ channels (Jiang et al., 2003a,b) are prototypical for the large family of related K+ channels (Yellen, 2002) including BK, we can assume that the selectivity and altered conductance of the BK channel for K+ arises largely from changes in the staging of K+ ions in the central cavity of the channel. In addition, permeation depends on the focusing properties of the α-pore helices (Roux and MacKinnon, 1999), and the specialized structure of the selectivity pore, including its diameter, length, and the spacing of the charged groups that line the pore (Doyle et al., 1998).
The effects of membrane glycerophospholipids on the function of the BK channel have been described. Most of them are thought to arise from the indirect effects of changes in surface charge that result from the inclusion of negatively charged lipids into the membrane. The effect of this change in charge is to alter the local concentrations of cations which are then sensed by the pore of the channel (Bell and Miller, 1984). The lateral organization of the lipids in these bilayers and their direct impact on the function of BK channels, however, has not been explored. It was assumed that, except for differences in surface charge, the molecular organization was uniform in POPE/POPS bilayers or bilayers of PE/PC or PE/PI (Moczydlowski et al., 1985; Turnheim et al., 1999; Bell and Miller, 1984). In fact, recent studies show that PC and PS are nonideally mixed in the bilayer, so that a PC/PS mixture forms both PC-rich and PS-rich domains (Ross et al., 2001). On the other hand, SPM alone can form thicker (Maulik et al., 1986) lateral microdomains with or without the presence of CHL in glycerophospholipid membranes (Lee, 2001; Yuan and Johnston, 2002). The effects of SPM and the lateral organization of the lipid bilayer on the function of BK channel have not, to our knowledge, been studied. We start by assuming that the local lipid environment surrounding a BK channel and the channel itself form cooperative parts of an intricate signaling unit (Mobashery et al., 1997). The lipids are considered to be crucial in affecting the detailed properties of the signaling machine from cell to cell. We also assume that individual lipid components may in turn be determinants of the large-scale properties of the whole bilayer (such as thickness, surface charge, lateral stress, etc.) (Lee, 2003).
In this study, we measured slope conductance of BK channels in native HEK cell membranes and investigated the effects of SPM, CER, and CHL, each incorporated into POPE/POPS bilayers containing decane, on the function of human BK channel α-subunits (hSlo) isolated from the same stably transfected HEK 293 cells (Ahring et al., 1997). SPM has the same phosphatidylcholine head group as PC, and is zwitterionic at neutral pH. CER is the product of the hydrolysis of sphingomyelin and has the same acyl chain structure as SPM but with a small hydroxyl head group like CHL. Bilayer thickness was also manipulated independent of SPM content by forming bilayers from a series of PCs with increasing chain lengths. BK channels in both thicker and thinner bilayers had reduced conductance. The lateral organization of acyl chains and polar head groups of solvent-free, supported POPE/POPS membranes with or without SPM, or CER, or CHL was also examined by atomic force microscopy. Recent advances in AFM enable one to detect submicron-sized domains in these membranes under physiological conditions (Dufrene and Lee, 2000; Epand et al., 2001; Reviakine et al., 2000; Rinia et al., 2002; Karrasch et al., 1994). We conclude that the attenuation of the conductance of BK channels observed upon the incorporation of SPM into POPE/POPS bilayers is mainly due to a substantial increase in lateral domain formation that alters bilayer thickness. We assumed that different bilayer thicknesses would alter the forces on the channel protein in ways that could cause physical changes in the tilt of the α-pore helices or in the dimensions of the selectivity pore regions of the BK channel (Roux and MacKinnon, 1999; Devaux and Zachowski, 1994; Park et al., 2003; Williamson et al., 2002). We also conclude that the changes in conduction we observed arise from physical alterations in the channel protein and are not the result of secondary electrostatic effects induced by changes in surface charge (Sonnleitner et al., 2002; Park et al., 2003). A preliminary report of some of these findings has been made in abstract form (Yuan et al., 2003).
MATERIALS AND METHODS
Materials
POPE, POPS, DPPE, brain SPM and CER (these two latter are mixtures in which >70% of the compounds have acyl chains longer than 18:0), the monounsturated 1,2-diacyl-sn-glycero-3-phosphocholine (PC) series with increasing acyl chain lengths (C14:1–C24:1), and CHL were obtained from Avanti Polar Lipids (Alabaster, AL). They were used without further purification. Decane and salts were from Aldrich Chemical (St. Louis, MO). All aqueous solutions were prepared with 18.3 MΩ.cm Milli-Q or Omnisolv water.
Membrane preparation
The cDNA encoding hSlo α channels kindly provided by Dr. P. Ahring, NeuroSearch A/S (Copenhagen, Denmark) was overexpressed in HEK 293 cells. The cells were grown in DMEM supplemented with 10% fetal bovine serum, 50 units/ml penicillin, 50 mg/ml streptomycin, and 2.5 mM HEPES (Gibco) at 37°C in a humidified 5% CO2/95% air incubator. We used cell lines stably expressing hSlo α, (HEK/α-1.2) (Ahring et al., 1997). Membrane fragments were prepared using a protocol developed for COS cells (Sun et al., 1994) with some slight modifications described elsewhere (Crowley et al., 2003).
Electrophysiology
Bilayer recordings
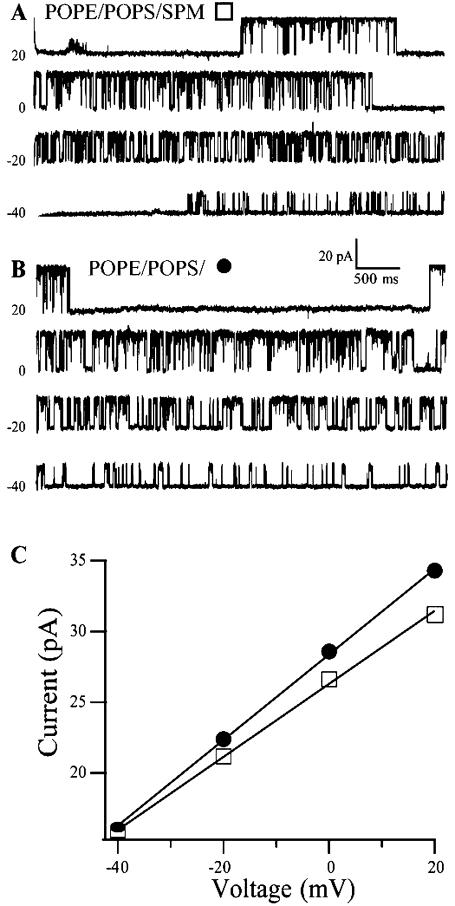
Single channel recordings were carried out with standard planar bilayer technology (Alvarez, 1986; Baba et al., 1999). The lipids evaluated in the bilayer recordings included POPE, a mixture of POPE/POPS (3.3:1), DOPE/SPM (3:2), and the POPE/POPS mixture with 20% (molar ratio) of either SPM, CER or CHL, all initially dissolved in chloroform. The solvent was removed by evaporation with a N2 stream and the dried lipid film was resuspended in decane to form a final total lipid concentration of 25 mg/ml. Mixtures containing CER were less soluble in decane and were used at a lower total concentration (10 mg/ml). The bilayer was formed by painting the lipid solution over a 100-μm hole (Wonderlin et al., 1990) formed in a horizontal plastic coverslip sealed with tackiwax to a Teflon partition separating an upper and lower chamber. Bilayer capacitance was monitored by noting the current across the bilayer in response to a triangle wave (10 mV/25 ms). Membrane suspensions (0.2–0.5 μl) containing crude membrane fragments were added to the upper cis chamber with a micropipette centered on the hole in the partition. In most bilayers, this caused the membrane to rupture. Channel incorporation was typically achieved within a few minutes after “brushing” the membrane fraction across the annulus with fresh lipids to form a new bilayer. A silver-silver chloride wire electrode was placed in each chamber for voltage clamping and current detection. The trans chamber was connected to ground and the cis chamber was connected to the positive input of a patch-clamp amplifier (EPC-9, HEKA Elektronik, Lambrecht, Germany) (Gillis, 2000). Single-channel events were sampled at 5 kHz with Pulse software (Gillis, 2000). The data were filtered at 3 kHz for analysis, and 500 Hz for display. All experiments were done at room temperature (22°C). Data were analyzed using TAC and TAC-fit programs (Bruxton Corp., Seattle, WA) and Igor Pro (WaveMetrics, Lake Oswego, OR). Slope conductance (Fig. 1) was calculated from a linear current amplitude (I)-voltage relationship (I/V plot) in which the voltage ranged from −40 to 40 mV in 20-mV steps. (Chu et al., 1998).
FIGURE 1.
Single-channel current records of a BK channel in a POPE/POPS/SPM (3.1:1:1, w/w) bilayer (A) and another in a POPE/POPS (3.1:1) bilayer (B). The current-voltage relationship of the open channels in these same recordings is shown for the POPE/POPS bilayer (•, 307 pS) and the POPE/POPS/SPM bilayer (□, 256 pS) in C. The holding voltages are indicated on each record.
HEK 293 cell recordings
HEK 293 cells stably expressing hSlo α channels were cultured in 35-mm culture dishes until 45–60% confluence was reached. Cells were washed for 30 min in high calcium (2.2 μM) bath solution followed by 10-min wash in intracellular recording solution (1 μM calcium) prior to recording. The recording conditions were modified from those used in the bilayer experiments to obtain stable patch recordings. Single-channel recordings were performed in excised inside-out membrane patches using standard patch-clamp techniques (Hamill et al., 1981). All recordings were made under symmetric K+ conditions where the potassium concentration was the same in the bath solution and recording solution. Electrodes were fabricated from glass pipettes (Drummond Scientific, Broomall, PA), pulled using a Model P-97 Flaming/Brown micropipette puller (Sutter Instrument, Novato, CA), and coated with sylgard (Dow Corning, Midland, MI) to reduce capacitance and noise. The tips were fire polished using a microforge (Narishige, Kyoto, Japan) to yield electrodes between 10 and 15 mΩ when filled with high K+ extracellular recording solution. An agar bridge containing an Ag/AgCl pellet and 3% agar in buffer solution was used as a ground.
Single-channel currents were recorded using an EPC-7 (List Electronics, Darmstadt, Germany) patch-clamp amplifier at a bandwidth of 3 kHz and were low-pass filtered at 1 kHz using an eight pole Bessel filter (model 902LPF, Frequency Devices, Haverhill, MA) and sampled at 200 kHz using PCLAMP 8 (Axon Instruments, Union City, CA). Data were acquired and stored using an A/D converter and a Dell Computer.
Single-channel conductances were obtained from I/V plots. Each patch was recorded at a given voltage from −40 to +60 for a minimum of 10–30 s.
Solutions
All solutions used in the patch recordings were made with Omnisolv water. The high-calcium bath solution contained (in mM) 135 Na+ gluconate, 5 K+ gluconate, 2.2 CaCl2, 1 MgCl2, and 15 HEPES. The buffered calcium solution for the agar bridges contained (in mM) 135 Na+ gluconate, 5 K+ gluconate, 4.5 CaCl2, 5 EGTA, 1 MgCl2, and 15 HEPES. The high-K+ extracellular recording solution contained (in mM) 140 K+ gluconate, 2.2 CaCl2, 4 EGTA, 4 HEDTA, 1 MgCl2, and 15 HEPES. The 1-μM calcium intracellular recording solution contained (in mM) 140 K+ gluconate, 5 Na+ gluconate, 0.43 CaCl2, 2 HEDTA, 1 MgCl2, and 15 HEPES. Solutions were brought to pH 7.35 with KOH or NaOH. These conditions were optimized for seal formation and patch stability.
For bilayer recordings the cytoplasmic cis solution contained 300 mM KCl, 1.05 mM CaCl2, 1.25 mM HEDTA, and 10 mM HEPES, pH 7.2. The free Ca2+ was estimated to be ∼25 μM using the WinMaxC32 program, V2.40, with updated constants (Bers et al., 1994). The extracellular (trans) solution in the lower chamber contained 30 mM KCl, 0.1 mM HEDTA, and 10 mM HEPES, pH 7.2. These bilayer conditions were optimized for obtaining channel fusion and insertion.
Microscopy
AFM analysis of supported membranes made with Langmuir-Blodgett transfer
The monolayer samples of POPE/POPS (3.3:1), or POPE/POPS with 20% SPM or CER or CHL were compressed and transferred at a surface pressure of 30 mN/m to mica in a LB trough (NIMA 611, Coventry, UK) using Milli-Q water as the subphase. The solvent-free hybrid bilayers were prepared as previously described (Yuan and Johnston, 2001; Yuan et al., 2002). Briefly, a uniform flat monolayer was first formed from DPPE and transferred at a surface pressure of 45 mN/m to a freshly cleaved mica surface (Yuan and Johnston, 2001). Then the DPPE-coated mica was used as a support for a second layer of the lipid of interest at a surface pressure of 30 mN/m. The resulting bilayers were kept under the subphase till they were transferred under water to the AFM liquid cell (Molecular Imaging, Phoenix, AZ) for imaging.
Bilayers from vesicle fusion
Vesicle fusion was also used to prepare symmetric solvent-free bilayers without compression. Save the presence of the solvent, these should be similar to the structure of the uncompressed bilayer used in the bilayer recordings. POPE/POPS (3.3:1) mixture or POPE/POPS (3.3:1) mixture with 20% brain SPM was dissolved in chloroform (1 mg/ml) in a small vial and then dried under a stream of nitrogen and held under high vacuum overnight to form lipid films. Multilamellar vesicles were prepared by swelling the lipid films with water. The resulting multilamellar vesicles were then sonicated for 2 min with a probe sonicator to obtain a clear solution of unilamellar vesicles. Vesicle solution (100–200 μl) was used to form a supported bilayer on a freshly cleaved mica coverslip clamped in a liquid cell (Molecular Imaging) after pretreatment with 100 μl phosphate-buffered saline solution (PBS buffer, 15 mM 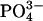 , 15 mM Na+, K+). The incubation time before viewing was varied between 45 min and 2 h. The bilayers were extensively rinsed with water to remove unattached vesicles before imaging.
, 15 mM Na+, K+). The incubation time before viewing was varied between 45 min and 2 h. The bilayers were extensively rinsed with water to remove unattached vesicles before imaging.
AFM measurements
AFM measurements for monolayer samples were carried out on a multimode nanoscope III atomic force microscope (Digital Instruments, Santa Barbara, CA) in repulsive mode in air. Bilayer samples were imaged under water with a MAC-mode Picoscan AFM (Molecular Imaging). The acyl chains are imaged in the monolayers whereas the polar head groups of the lipid are in contact with the tip in the bilayers. Samples from LB transfer were imaged in contact mode, and samples from vesicle fusion were imaged in MAC mode as described elsewhere (Yuan and Johnston, 2001).
Statistics
Statistical comparisons between the conductances observed in different conditions employed standard ANOVA and Tukey honest significant difference tests for independent samples with unequal N's as found in the basic statistics module of Statistica (V5.5, Statsoft, Tulsa, OK) (McCallum, 1999). Deviations from a normal distribution of conductances were evaluated with the Shapiro-Wilk's W test of normality (Shapiro and Wilk, 1965). Smaller values of p (given in Fig. 3) indicate more variability and larger deviations away from normality. Statistical significance for all of these tests was set at p ≤ 0.05. The bimodal fit to the data illustrated in Fig. 4 was generated by the sigmoidal fit tool in Origin 7 (OriginLab, Northampton, MA).
FIGURE 3.
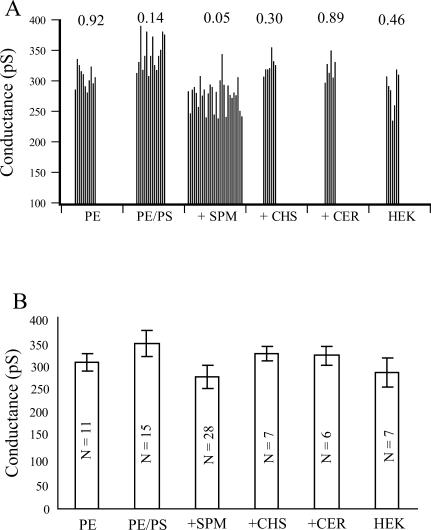
(A) Single-channel conductance of BK channels measured in bilayers of different lipid compositions and from HEK cells. Each bar represents a single-channel conductance measured in an individual experiment. The value above each composition is the probability that the set of scores observed deviates from normality as determined by the Shapiro-Wilk test of normality (Shapiro and Wilk, 1965). (B) Average slope conductance ± SD for each of the six lipid compositions. Probability values for each pairwise comparison are indicated in Table 1. In every case, significant values are assumed when p ≤ 0.05. N = number of recordings in each mean.
FIGURE 4.
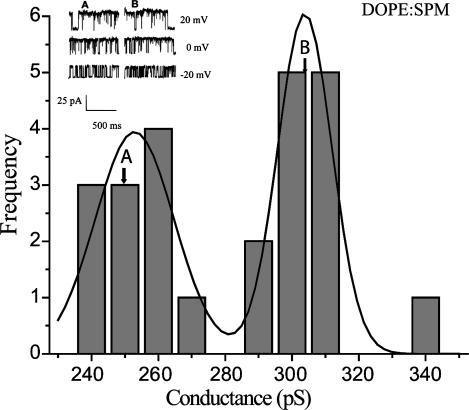
Single-channel conductances of 18 individual BK channels measured in bilayers composed of DOPE and SPM (3:2). The W value calculated by the Shapiro-Wilk test (W = 0.88890, p < 0.0128) indicates that the observed sample deviates significantly from normality. This is further illustrated by the bimodal fit to this sample generated by the sigmoidal fit tool in Origin. This sample has at least two modes, one at 259.07 pS and a second at 307.71 pS. The conductance values indicated by the lettered arrows are the first (a) and second (b) measurements, respectively, from one of the channels. They are separated from each other by 2 min of recording time. The recordings shown in the insert are from these same two measurements at each of the indicated holding voltages.
RESULTS
The conductance of the BK channel was measured by recording single-channel currents at various applied voltages and plotting the current versus voltage (I/V) curve to obtain the slope conductance. Fig. 1, A and B, show randomly selected records of BK channel activity, first in a channel in a POPE/POPS/SPM bilayer (Fig. 1 A) and another in a POPE/POPS bilayer (Fig. 1 B) at various holding voltages under identical asymmetrical ionic conditions. The I/V curves show a linear relationship in the range of voltages studied (Fig. 1 C). We have not explored larger voltage excursions or concentration extremes where nonlinearity is likely to become evident (LeMasurier et al., 2001). The slope conductance of the BK channel is much reduced in bilayers containing SPM. We note that the inclusion of SPM and decane into a bilayer may produce many physical changes in the structure of the bilayer, including an increase in bilayer thickness (Holthuis et al., 2001). To test whether the reduced conductance of the BK channel in bilayers containing SPM is primarily due to increased bilayer thickness rather than any of the other physical parameters of the bilayer, we next evaluated bilayers of different thickness (as set by varying acyl chain length) in the absence of SPM.
Changes in bilayer thickness directly alter BK channel conductance
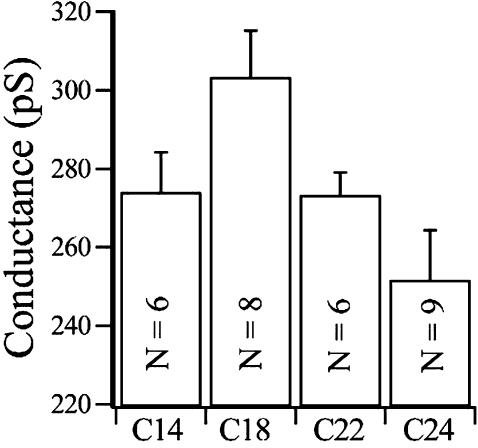
We tested the effect of changes in membrane thickness directly by forming bilayers from a homologous series of lipids with a constant head group structure but with increasing acyl chain lengths. Other studies have demonstrated that this manipulation results in bilayers with increasing thickness (Caffrey and Feigenson, 1981; Lewis and Engelman, 1983). Such a chemical series is available from Avanti (1,2-diacyl-sn-glycero-3-phosphocholines, with unsaturated chain lengths that vary from C-14:1 to C-24:1). These compounds are sufficiently soluble in decane to form stable bilayers if they are combined with DOPE. The chain length of each of the PEs used was selected to match the chain length of the particular PC employed as closely as possible. Fig. 2 summarizes the direct effects of altering bilayer thickness on BK conductance. There is a clear maximum in the measured conductance at a chain length of PC18:1. This chain length corresponds to a measured hydrophobic thickness of ∼37.5 Å (Tahara and Fujiyoshi, 1994), close to the thickness predicted for the hydrophobic region of the channel protein (Doyle et al., 1998). Both thicker (PC-24) and thinner (PC-14) bilayers show reduced conductances. Similar functional optima have been observed with other transmembrane proteins in bilayers of increasing thicknesses (Caffrey and Feigenson, 1981; Martinac and Hamill, 2002; Lee, 1998). Thus, as predicted from the studies with gramicidin (Mobashery et al., 1997), bilayer thickness directly modulates slope conductance of the BK channel. We suggest that the physical basis for this effect on BK involves small changes in the angle of the α-pore helices. This change is caused by protein deformation attendant upon the energy associated with a hydrophobic mismatch between the thickness of the hydrophobic core of the lipid and the protein. This will alter the positioning of K+ in the central ion chamber (Doyle et al., 1998; Roux and MacKinnon, 1999; Williamson et al., 2002). Alternatively, changes in bilayer thickness may alter the length and diameter of the selectivity pore in a manner analogous to the reduction in diameter caused by stretching the helix of a “Chinese handcuff.” This stretching (or compression), again driven by a hydrophobic mismatch will also change the relative spacing between the binding sites in the selectivity pore for K+ and collectively reduce ion transport through the pore (Mobashery et al., 1997).
FIGURE 2.
The mean conductance + 1 SD of BK channels incorporated into PC/PE containing bilayers with acyl chains of increasing lengths, C14:1–24:1. N = number of recordings contributing to each mean.
Sphingomyelin decreases the conductance of the BK channel in POPE/POPS bilayers
Fig. 3 A illustrates the distribution of individual conductances obtained in each of the several mixtures examined. Also included are the values obtained with single-channel recordings from patches of HEK cell membrane. Unlike each of the bilayer compositions, the patches from HEK cells do not contain decane. The conductances found for all five of the compositions and the HEK cells were compared with a one-way ANOVA (employing weighted means to correct for unequal ns). The ANOVA was highly significant, with F (5, 68) = 18.537, p < 0.0000001, suggesting that there are several significant differences between the sample of conductances observed in the different lipid compositions. Fig. 3 B illustrates the group means and SDs for each of the conditions. All possible pairwise comparisons were then evaluated using Tukey‘s unequal N HSD test. Table 1 lists the p values obtained for each of these comparisons.
TABLE 1.
Probabilities for post hoc tests
| Group | PE 305.68 | PE/PS 344.91 | +SPM 276.16 | +CHS 324.59 | +CER 319.77 | HEK 285.73 |
|---|---|---|---|---|---|---|
| PE | 0.001575 | 0.012053 | 0.584863 | 0.856780 | 0.526395 | |
| PE/PS | 0.000129 | 0.444151 | 0.268238 | 0.000140 | ||
| +SPM | 0.000261 | 0.002021 | 0.934116 | |||
| +CHS | 0.999220 | 0.039660 | ||||
| +CER | 0.126123 | |||||
| HEK |
Unequal N HSD; variable data.
Error: between MS = 578.47, df = 68.000.
Several facts are clear from this analysis. First, there is considerable variation in the conductances observed within any one composition, as indicated by the value of W in Fig. 3 A. Further, it is clear that the slope conductances of individual BK channels measured in POPE/POPS and POPE/POPS/SPM bilayers are especially variable, as indicated by the smaller probability values obtained with the Shapiro-Wilk W test. In fact, the distribution in POPE/POPS/SPM is significantly nonmodal, p = 0.05, and there appear to be two or more modes in this three-component mixture. Many of the single-channel conductance measurements of BK in POPE/POPS bilayers were ∼320 pS. Occasionally we also observed conductances of ∼370 pS. In POPE/POPS/SPM bilayers, most of the single-channel conductances were significantly smaller, ∼280 pS, and some channels were as low as 240 pS. Secondly, although there was considerable variability in all compositions, the addition of the charged lipid PS to the PE bilayer resulted in a significant increase in conduction, as had been reported by others (Bell and Miller, 1984; Park et al., 2003). Also the presence of SPM in the POPE/POPS bilayer significantly decreased the single-channel conductance of nearly all of the channels observed in this composition. Addition of the same amount of CHL into a POPE/POPS bilayer, on the other hand, did not cause a large change in the conductance, in agreement with our earlier work (Crowley et al., 2003). Addition of CHL to bilayers is thought to significantly change the physical properties of the membrane (such as its compressibility) while only slightly increasing the bilayer thickness (Lundbaek et al., 2003). The lack of an effect on channel conductance with incorporation of CHL to POPE/POPS bilayer suggests that physical changes, other than thickness of the bilayer that may occur, are not very important. Interestingly, the incorporation of the same amount of CER, whose acyl chain structure is the same as SPM but lacks the bulky polar head, into a POPE/POPS bilayer also had little effect on single-channel conductance, which further suggests that attenuation of the conductance of the BK channel in the presence of SPM is not likely due to a particular organization of the acyl chains. We suggest, as an organizing principle, that the variability in slope conductance observed in each composition arises from the presence of a channel “painted” into bilayer domains with slightly different thicknesses. In a real sense, it appears that the BK channel is flexible enough to provide a sensitive transducer of bilayer thickness. The variability encountered also suggests that there can be a large number of different thicknesses in these three-part mixtures.
To further bolster the importance of thickness domains in these recordings we next sought a simpler, two-component mixture (DOPE/SPM), which would form a more defined, phase-separated bilayer predicted to have two distinctive thicknesses. These particular compounds were selected because the main phase transition temperature of DOPE is much lower (Tm = −16°C) than that of SPM (Tm = 45°C). Thus, at the temperature of these experiments, there should form two distinct phases with different thicknesses. Binary mixtures of DOPE and SPM were also stable enough to allow us to make several repeat measures of slope conductance in one recording. This allowed us to evaluate the stability of our basic measurement. We reasoned that if this binary mixture produced two regular-thickness domains, one for DOPE and a second for SPM, then we would expect to see a population of conductance measures which should be decidedly bimodal. Also, if the channel were sufficiently mobile within these domains or incorporated near the edge of a particular domain we might also expect to occasionally see the measured slope conductance change from one modal value to another as the channel moves from one domain to another. Fig. 4 illustrates the distribution of conductances of 18 individual BK channels measured in DOPE/SPM bilayers. Also illustrated is the bimodal curve generated by the sigmoidal fit tool in Origin 7. It is clear from the distribution that there are two modes in this set of recordings. One is at 259.07 pS and the second at 307.71 pS. As expected, the W statistic computed for this sample is significantly nonmodal (W = 0.88890, p = 0.0128). We also note that repeat measures of slope conduction separated by several minutes were not significantly different from each other, with a mean difference of 1.3 ± 2.9 pS (mean ± SD, N = 5). However, in one other recording the second slope conductance measurement was shifted by 55 pS, as indicated by the lettered arrows in Fig. 4 . This is consistent with the movement of the channel protein from one domain to the other. This suggests quite strongly that these bilayers contained domains with two different thicknesses in spite of the inclusion of decane in the bilayer. Channels “painted” into these domains could be distorted by a hydrophobic mismatch, and thus produce two different sets of slope conductances. We also note that the smaller of these modes is very close in value to the smaller values seen in the POPE/POPS/SPM distribution illustrated in Fig. 3 A, suggesting the presence of channels in SPM-rich domains in both compositions. We also note that the larger conductance mode is very near the mode observed with POPE alone, predicting that bilayers containing POPE, or DOPE, alone have similar thicknesses. We next sought further evidence for domain formation in solvent-free supported bilayers with these same compositions with AFM.
Sphingomyelin promotes phase separation in POPE/POPS monolayers and bilayers
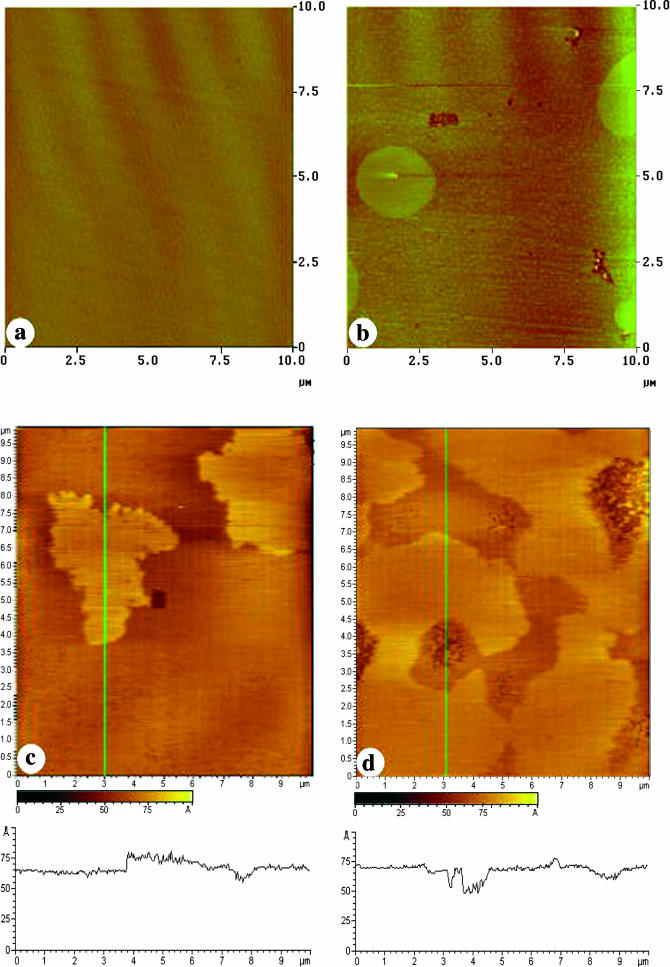
The BK channel protein is an integral protein spanning the lipid bilayer. The function of the protein is likely to be affected by the lateral organization of head groups and acyl chains in the bilayer that determines hydrophobic interactions between the lipids and the transmembrane segments of the protein. The lateral organization of the polar headgroups at the bilayer surface may also influence the surface charge, the distribution of ions, and the orientation of the protein. To study the effects of SPM on the lateral organization of POPE/POPS membranes, we transferred monolayers of the POPE/POPS mixture or POPE/POPS with SPM onto hydrophilic mica to display the acyl chain surface of the monolayer. These monolayers were also transferred to DPPE-coated mica, which forms a hybrid bilayer to image the polar head group surface of the bilayer. The supported membranes were examined with AFM. The AFM image of Fig. 5 a shows that a monolayer of POPE/POPS alone gives a uniformly flat surface, with no indication of domain formation. The AFM image of the POPE/POPS head groups on the DPPE hybrid bilayer (Fig. 5 c), surprisingly, showed clear phase separations. The height difference between the two phases is small, averaging ∼0.5 nm. Since DPPE forms a uniformly flat monolayer at 45 mN/m (Yuan and Johnston, 2001), the phase separation observed in the hybrid bilayer (Fig. 5 d) must arise from domains that are formed in the POPE/POPS monolayer in the top leaflet. The observed phase separation agrees well with previous observations for PC/PS membranes, where the PC-rich and PS-rich domains were detected with friction AFM imaging (Ross et al., 2001). It is likely that the difference between the monolayer and bilayer results reflects the fact that domains are more readily visualized when imaging the polar head groups, due to the negatively charged PS, than when imaging the acyl chain surface of the monolayers. We attempted to identify the two phases in our images by varying the molar ratio of POPE/POPS (from 9:1 to 1:1 to 1:3) in different bilayers. In each case, domain formation was observed but the amount of high phase was not proportional to the POPE /POPS ratio in these bilayers (data not shown), indicating complex nonideal miscibility between the two components.
FIGURE 5.
AFM images of a POPE/POPS monolayer transferred onto mica (a); a POPE/POPS bilayer on DPPE-coated mica (c); a POPE/POPS/SPM monolayer transferred onto mica (b); and a POPE/POPS/SPM bilayer on DPPE-coated mica (d). All transfers occurred at a surface pressure of 30 mN/m. The z-scale is 10 nm for images in c and d. Section analysis traces for the lines across these images (in green) are shown under their respective images.
The addition of 20% SPM to the standard POPE/POPS mixture resulted in the appearance of a few large domains and numerous smaller domains in the monolayer (Fig. 5 b). These were attributed to the formation of SPM-rich domains in the POPE/POPS monolayer that were ∼0.4–0.5 nm thicker than the POPE/POPS monolayer (low phase). The same monolayer composition was also transferred onto DPPE-coated mica to form hybrid bilayers and imaged with AFM (Fig. 5 d), which displays the molecular organization of the polar head groups of POPE/POPS/SPM. The phase separation observed in the POPE/POPS bilayer was still visible, but the bilayer was covered with much more high phase in the POPE/POPS/SPM bilayer (Fig. 5 d) than in the POPE/POPS bilayer (Fig. 5 c). Here the height difference was larger, averaging ∼0.6–0.7 nm.
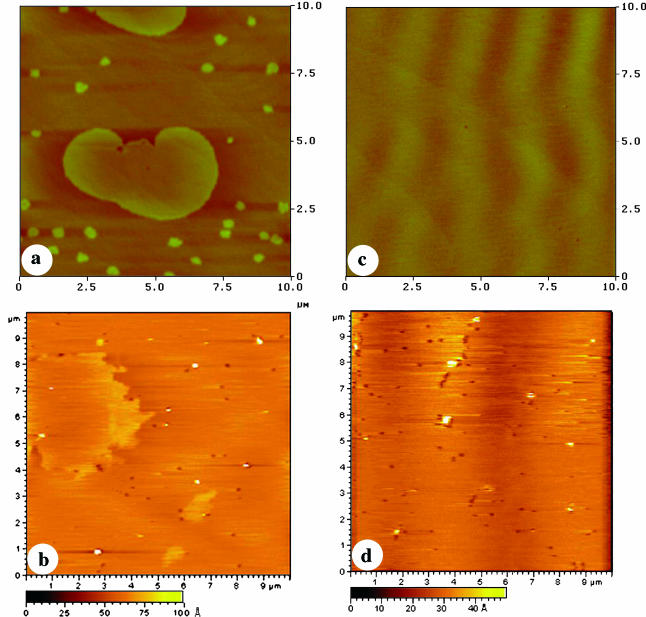
For comparison, the monolayers of POPE/POPS with 20% CER or CHL were also transferred onto mica or DPPE-coated mica and imaged with AFM (Fig. 6). The addition of CER to the POPE/POPS mixture resulted in a similar formation of a few large domains and many small domains in the monolayer (Fig. 6 a). In the bilayer, the phase separation observed for the POPE/POPS bilayer still occurred (Fig. 6 b), but the fraction of the higher phase was much lower than for a POPE/POPS/SPM bilayer (Fig. 5 d). Domain formation was not observed for a POPE/POPS monolayer containing 20% CHL (Fig. 6 c). Consistent with this, the addition of 20% CHL eliminated the phase separation observed in the POPE/POPS bilayer (Fig. 6 d).
FIGURE 6.
AFM images for a POPE/POPS/CER monolayer transferred onto mica (a) and DPPE-coated mica (b) and a POPE/POPS/CHL monolayer transferred onto mica (c) and DPPE-coated mica (d) at a surface pressure of 30 mN/m. The z-scale is 10 nm for images b and d.
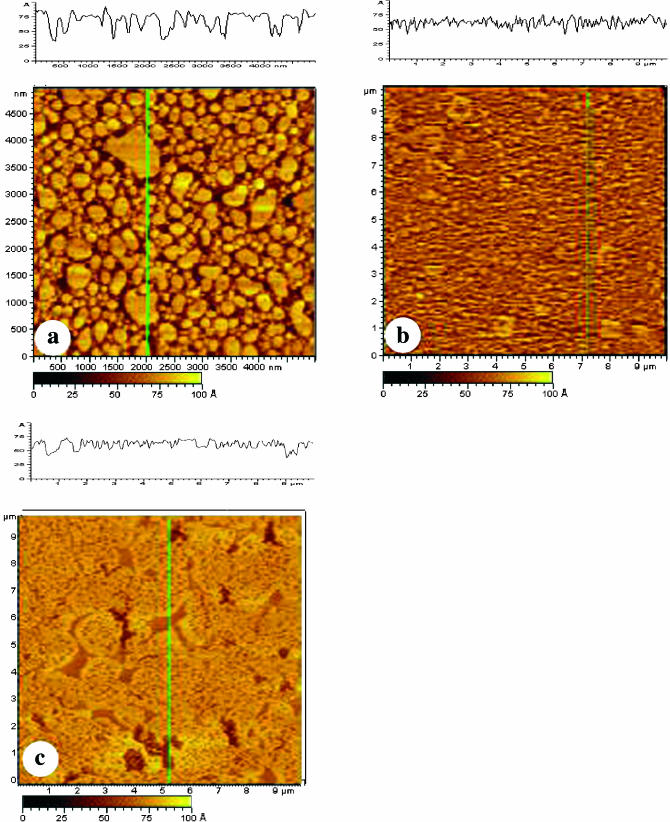
To determine if the phase separation observed in POPE/POPS and POPE/POPS/SPM membranes prepared by LB compression and asymmetrical transfer was not forced by compression, bilayers of POPE/POPS and POPE/POPS/SPM were also prepared with a vesicle fusion technique. This gives an uncompressed and symmetrical bilayer whose structure is likely to be closer to that occurring in the bilayers used in the single-channel recordings, save the absence of solvent. Fig. 7 a shows an incompletely covered POPE/POPS bilayer prepared by using a small vesicle concentration and a short incubation time. Many individual bilayer patches were observed in Fig. 7 a, which enable us to obtain an estimate of the total bilayer thickness. An average bilayer thickness of ∼3.5 nm was obtained for the POPE/POPS bilayer, which is slightly larger than the thickness measured earlier for egg PC (3.0 nm) (Yuan and Johnston, 2001), but is in good agreement with the bilayer thickness measured for 1-palmitoyl-2-oleoylphosphatidylcholine (POPC) (Nezil and Bloom, 1992). It is interesting to note that even in these small bilayer patches, the heterogeneous organization of the POPE/POPS bilayer is visible, in comparison with the homogeneous bilayer observed previously with egg PC (Yuan and Johnston, 2001). Some dark lower regions can be seen in the center of lighter (higher) bilayer patches. Complete coverage forming a single bilayer was achieved by adding more vesicle solution and using a longer incubation time (Fig. 7 b). Again, compared with the uniformly flat bilayer seen for egg PC, the POPE/POPS bilayer is more heterogeneous. There are at least two phases with an average height difference of 1.1 nm. The presence of SPM in the POPE/POPS mixture makes it difficult to prepare a bilayer by vesicle fusion using the same conditions as for the POPE/POPS mixture. Instead, a longer incubation time and slight heating of the sample were required to give complete bilayer coverage. The effect of this common treatment on domain formation is unknown. Fig. 7 c shows a POPE/POPS/SPM bilayer prepared this way. Clear phase separation can be seen in the bilayer. The height difference between the two phases is in the range of ∼1.3–1.4 nm and the amount of the higher phase has increased compared to the POPE/POPS bilayer (Fig. 7 b).
FIGURE 7.
AFM images of POPE/POPS bilayers (a and b) and a POPE/POPS/SPM bilayer (c) prepared by vesicle fusion deposition. (a) A POPE/POPS bilayer prepared by incubating 100 μl of the POPE/POPS vesicle solution on mica for 45 min and then imaged immediately after rinsing with water. (b) A POPE/POPS bilayer prepared by incubating 200 μl of the POPE/POPS vesicle solution on mica for 90 min and then imaged immediately after rinsing with water. (c) A POPE/POPS/SPM bilayer prepared by incubating 200 μl of a POPE/POPS/SPM vesicle solution on mica for 2 h at 45°C. The bilayer was then rinsed with water, kept under water overnight, and finally imaged with AFM.
DISCUSSION
The totality of our results can best be understood by assuming a direct effect of bilayer thickness on the conformation and conducting properties of the embedded BK channel protein. We report that the single-channel conductance of the voltage-clamped BK channel is significantly attenuated when it is incorporated into a POPE/POPS bilayer containing SPM. We also demonstrate that bilayers containing SPM have a larger fraction of higher phase (i.e., a thicker bilayer) than that observed for a POPE/POPS mixture, in AFM studies. Further, altering bilayer thickness by other direct means (increasing chain length) also results in alterations in conduction. In addition, we confirm that the addition of a similar fraction of CHL to a POPE/POPS bilayer does not change its conductance (Crowley et al., 2003), eliminating surface charge dilution as the responsible effector.
Membrane lipid composition has been reported to affect the conductance of BK channels obtained from different tissues (Moczydlowski et al., 1985; Turnheim et al., 1999; Bell and Miller, 1984; Park et al., 2003). Although we do not yet know the lipid composition of HEK cells, it seems clear that BK channel conductance as measured in those membranes is within the range expected from our reconstitution studies. It further demonstrates that the presence of decane in the bilayer recordings is not a determining factor in setting conductance levels, as the HEK cell recordings completely lack decane.
Moczydlowski and colleagues originally compared the conductance of BK channels from rat skeletal muscle measured in symmetric KCl solutions in both PE and PS bilayers and concluded that differences in surface potential was responsible for the shift in conductance they observed (Moczydlowski et al., 1985). The large conductance measured in a PS bilayer (337 pS) when compared with that in a PE bilayer (244 pS) was attributed to differences in the surface charge of the two bilayers and the effect of charge distribution on the local potassium and calcium concentrations. This conclusion was recently revised by these authors as a result of their investigation of barium block of the BK channel when the bilayer was formed from different lipids and exposed to a range of potassium concentrations. They concluded from the fact that barium block is little altered by changes in potassium concentration in lipids with different amounts of surface charge that the mouth of the pore is electrostatically insulated from the lipid surface and that charge is not responsible for the shift in conduction originally observed. They go on to suggest that lipid-induced “…change in bilayer mechanical properties…” could be responsible (Park et al., 2003). We suggest that changes in bilayer thickness or deformation energy could account for these observations (Lundbaek and Andersen, 1994).
The conductance of the BK channel isolated from rabbit colon epithelium has been investigated (Turnheim et al., 1999) in 1:1 PE/PC, POPE/POPS, and PE/PI bilayers. Here again the large conductance of BK channels measured in POPE/POPS (224 pS) and PE/PI (237 pS) bilayers was attributed to the presence of the negative charge imparted by the PS or PI head group on the membrane surface potential surrounding the channel mouth. However, the decrease in the conductance of the BK channel measured here in POPE/POPS/SPM bilayers in comparison to that measured in POPE/POPS bilayer cannot be attributed to the differences in surface charge of these bilayers. If charge alone was responsible for the shift, then the incorporation of the same molar ratio of CHL or CER into a POPE/POPS bilayer should cause a shift in the surface charge similar to that produced by the addition of SPM. However, the conductance measured in bilayers containing CHL or CER was not statistically different from that measured in a POPE/POPS bilayer (Table 1). Moreover, we measured the conductance of the BK channel incorporated into bilayers containing PE alone at an average of 306 ± 18 pS (Fig. 3) under identical conditions. If the decrease in conductance of the BK channel is solely the result of diluting negative surface charge induced by PS in a POPE/POPS bilayer by the presence of 20% SPM, we estimate that the conductance of a BK channel in POPE/POPS/SPM (20% PS) bilayer should fall somewhere between the conductance of 345 pS for POPE/POPS bilayer (25% PS) and 306 pS for a PE bilayer (no PS). The measured conductance in the POPE/POPS/SPM bilayer (∼276 pS) was actually much smaller. Furthermore, the nonhomogenous distribution of BK channel conductance in POPE/POPS/SPM bilayers indicates to us that there are at least two domain thicknesses in this bilayer. This suggests that the structural change in the lateral organization and thickness of POPE/POPS bilayer in the presence of SPM that we observed in the AFM studies is directly responsible for the decrease in the measured conductance. This is further confirmed by the conductances observed in bilayers made with different chain lengths (Fig. 2). Here, the incorporation of the channel into bilayers made with a fixed surface composition, except for increasing chain length, results in bilayers of increasing thickness and altered conductance, all in the absence of any sphingomyelin and any predicted change in surface potential. Caution should be exercised in considering this conclusion, as there are significant differences in the lipid conditions necessary for the AFM and electrophysiological observations, whose collective effects are unknown.
Compared to many glycerophospholipids, the brain SPM used here contains a high proportion of lipids with long acyl chains (C20–24) and with phase transition temperatures >45°C (Lee, 2001). Thus, in a mixture with glycerophospholipid at room temperature, SPM should form many more ordered gel-phase domains with raised bilayer thicknesses in a sea of fluid glycerophospholipid bilayer (Thompson et al., 1985; Holthuis et al., 2001; Yuan et al., 2002). The AFM images we present here clearly show that the incorporation of SPM into a POPE/POPS bilayer results in the formation of many large SPM-rich domains and numerous smaller SPM-rich microdomains that appear to be ∼0.4 nm higher than the POPE/POPS phase (Fig. 5 d). We also observed much larger coverage of high-phase domains in POPE/POPS/SPM bilayers (Fig. 5 d) than in POPE/POPS bilayers (Fig. 5 c).
Lipid properties
Although we have emphasized the importance of bilayer thickness in modulating the slope conductance of the BK channel protein, it must be noted that thickness alone merely establishes the magnitude of the energy associated with any mismatch between the hydrophobic portion of the BK protein and the hydrocarbon core of the bilayer (de Planque et al., 2001). The distribution and resolution of this energy between modulating the physical dimensions of the protein and altering the lipid structure depends closely on the nature of the particular interaction (Weiss et al., 2003). The insertion of the relatively bulky BK protein into a thicker bilayer may well cause a local curvature in the neighboring bilayer structure in response to the mismatch. Some fraction of the available energy may also be spent in altering the dimensions of the protein. Clearly then, the deformability of the bilayer and the flexibility of the protein will collectively determine the equilibrium state reached. Thus, it is difficult to predict which of the changes in physical properties of the bilayer are the cause and which are the effects of the initial mismatch (Lundbaek and Andersen, 1994). It seems reasonable to assume that bilayers that are more easily deformable, perhaps because of the shape of their head groups, will curve and resolve more of the mismatch-generated energy leaving less available for distortion of the embedded protein (van den Brink-van der Laan et al., 2001). However, if the BK channel behaves in bilayers like the bacterial potassium channel KcsA recently studied with fluorescence spectroscopy (Williamson et al., 2002), it is likely that bilayer deformation is small because of the flexibility of the protein. For BK then, one may estimate that much of the available mismatch energy goes into deforming the channel protein to match the dimensions of the lipid rather than the lipid distorting to match the protein (Williamson et al., 2002).
We hypothesize that a change in bilayer thickness, like that we have observed in the POPE/POPS/SPM bilayer, could result in physical shifts in the shape of the channel protein. Based on the recent model structure for the K+ channel as described by MacKinnon and colleagues (Doyle et al., 1998), we can suggest several alternatives for this. Perhaps the tilt angle between neighboring α-pore helices is reduced in a thicker bilayer (Williamson et al., 2002). The consequence of this change would be to move the K+ ion staged in the central cavity further away from the entrance to the selectivity pore, as considered by Berneche and Roux (2000, 2001, 2002). This will increase the path-length to be traveled by a permeant K+ ion. It may also increase the number of water molecules between adjacent K+ ions which, in turn, will alter the spacing between those ions and the charged groups that line the selectivity pore. This, too, should cause a reduction in the throughput of ions through the selectivity channel.
Similar arguments may be advanced for the mechanism in which a thinner bilayer reduces the slope conductance of the channel as seen experimentally in Fig. 2. Clearly, moving the tilt angle of the α-pore helices away from their optimum position in either direction should cause comparable alterations in ion staging and throughput. That is, increasing the tilt angle between neighboring α-pore helices may occur in a thinner bilayer. This will move the staged K+ ion closer to the pore opening and again may alter the spacing between water and permeant ions. This will also produce a mismatch with the charged groups that line the selectivity pore.
Our finding that the conductance of a BK channel can be significantly altered simply by placing it in a different lipid composition gives considerable weight to the notion that lipids exert powerful direct effects on channel structure and function (Park et al., 2003). It is now recognized that cell plasma membranes contain a mosaic of different lipid domains (Maxfield, 2002). The localization of some ion channels in these lipid rafts has been observed (Bravo-Zehnder et al., 2000; Martens et al., 2000, 2001). The BK channel itself may be selectively targeted to particular kinds of domains in certain cells (Bravo-Zehnder et al., 2000). This may, in turn, finely tune the channel properties in each raft composition. Further studies will be necessary to elucidate the functional role of lipid raft composition and distribution on the natural neuronal signal properties of BK and other types of channels.
Although the thickness hypothesis we have advanced is consistent with all of the data we have observed, it must be said that we do not yet have a direct and independent method to determine exactly where a particular protein molecule might be in a bilayer. Thus, the relationship we have seen between channel conductance and bilayer thickness remains correlative. Since there is a great deal of isomorphism among the various physical properties of a bilayer it may ultimately prove, when techniques arise to manipulate them, that variations in some other set of mechanical parameters, beyond thickness, are responsible for our observations. Similar cautions should be considered when comparing the patch and bilayer recordings with the AFM analysis. At the moment, the differences that may arise from the presence of a solvent, symmetrical lipids, or the absence of a support in the different recording procedures cannot be evaluated. Thus, caution should be employed when the recordings are compared to each other and to the conditions required by the present AFM method.
Acknowledgments
We thank John Crowley for helpful discussions and assistance with the preparation of BK channel containing membrane fragments from HEK cells. We also thank Dr. David Stevens of Clark University for statistical consultations and Dr. Alejandro M. Dopico of the University of Tennessee for helpful comments on the manuscript.
Funds were provided by National Institutes of Health grant AA12054 to S.N.T.
Abbreviations used: BK channel, large conductance Ca2+-activated K+ channel; AFM, atomic force microscopy; POPE (16:0;18:1), 1-palmitoyl-2-oleoylphosphatidylethanolamine; POPS (16:0;18:1), 1-palmitoyl-2-oleoylphosphatidylserine; DPPE, dipalmitoylphosphatidylethanolamine; HEK, human embryonic kidney; DOPE (18:1),1,2-dioleoylphosphatidylethanolamine; PC (14:1–24:1), phosphatidylcholine; PE, phosphatidylethanolamine; PS, phosphatidylserine; PI, phosphatidylinositol; SPM (18:0–24:0), brain sphingomyelin; CER (18:0), brain ceramide; CHL, cholesterol; LB, Langmuir-Blodgett; HSO, honest significant difference.
References
- Ahring, P. K., D. Strobaek, P. Christophersen, S. P. Olesen, and T. E. Johansen. 1997. Stable expression of the human large-conductance Ca2+-activated K+ channel. FEBS Lett. 415:67–70. [DOI] [PubMed] [Google Scholar]
- Alvarez, O. 1986. How to Set Up a Bilayer System. In Ion Channel Reconstitution. C. Miller, editor. Plenum Press, New York. 115–139.
- Anderson, R. G., and K. Jacobson. 2002. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 296:1821–1825. [DOI] [PubMed] [Google Scholar]
- Baba, T., Y. Toshima, H. Minamikawa, M. Hato, K. Suzuki, and N. Kamo. 1999. Formation and characterization of planar lipid bilayer membranes from synthetic phytanyl-chained glycolipids. Biochim. Biophys. Acta. 1421:91–102. [DOI] [PubMed] [Google Scholar]
- Bayburt, T. H., and S. G. Sligar. 2002. Single-molecule height measurements on microsomal cytochrome P450 in nanometer-scale phospholipid bilayer disks. Proc. Natl. Acad. Sci. USA. 99:6725–6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, J. E., and C. Miller. 1984. Effects of phospholipid surface charge on ion conduction in the K+ channel of sarcoplasmic reticulum. Biophys. J. 45:279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berneche, S., and B. Roux. 2000. Molecular dynamics of the KcsA K+ channel in a bilayer membrane. Biophys. J. 78:2900–2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berneche, S., and B. Roux. 2001. Energetics of ion conduction through the K+ channel. Nature. 414:73–77. [DOI] [PubMed] [Google Scholar]
- Berneche, S., and B. Roux. 2002. The ionization state and the conformation of Glu-71 in the KcsA K+ channel. Biophys. J. 82:772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers, D. M., C. W. Patton, and R. Nuccitelli. 1994. A practical guide to the preparation of Ca2+ buffers. In A Practical Guide to the Study of Calcium in Living Cells. R. Nuccitelli, editor. Academic Press, New York. 3–29. [DOI] [PubMed]
- Bravo-Zehnder, M., P. Orio, A. Norambuena, M. Wallner, P. Meera, L. Toro, R. Latorre, and A. Gonzalez. 2000. Apical sorting of a voltage- and Ca2+-activated K+ channel α-subunit in Madin-Darby canine kidney cells is independent of N-glycosylation. Proc. Natl. Acad. Sci. USA. 97:13114–13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher, M. S., and S. Munro. 1993. Cholesterol and the Golgi apparatus. Science. 261:1280–1281. [DOI] [PubMed] [Google Scholar]
- Brown, D. A., and E. London. 1998. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14:111–136. [DOI] [PubMed] [Google Scholar]
- Caffrey, M., and G. W. Feigenson. 1981. Fluorescence quenching in model membranes. 3. Relationship between calcium adenosinetriphosphatase enzyme activity and the affinity of the protein for phosphatidylcholines with different acyl chain characteristics. Biochemistry. 20:1949–1961. [DOI] [PubMed] [Google Scholar]
- Chiu, S. W., S. Subramaniam, and E. Jakobsson. 1999. Simulation study of a gramicidin/lipid bilayer system in excess water and lipid. I. Structure of the molecular complex. Biophys. J. 76:1929–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, B., A. M. Dopico, J. R. Lemos, and S. N. Treistman. 1998. Ethanol potentiation of calcium-activated potassium channels reconstituted into planar lipid bilayers. Mol. Pharmacol. 54:397–406. [DOI] [PubMed] [Google Scholar]
- Coronado, R., R. Latorre, and H. G. Mautner. 1984. Single potassium channels with delayed rectifier behavior from lobster axon membranes. Biophys. J. 45:289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley, J., A. M. Dopico, and S. N. Treistman. 2000. Ethanol potentiation of cloned BK channels incorporated into planar lipid bilayers. Soc. Neurosci. Abstr. 26:1402. [Google Scholar]
- Crowley, J. J., S. N. Treistman, and A. M. Dopico. 2003. Cholesterol antagonizes ethanol potentiation of human brain BKCa channels reconstituted into phospholipid bilayers. Mol. Pharmacol. 64:365–372. [DOI] [PubMed] [Google Scholar]
- de Planque, M. R., E. Goormaghtigh, D. V. Greathouse, R. E. Koeppe, J. A. Kruijtzer, R. M. Liskamp, B. de Kruijff, and J. A. Killian. 2001. Sensitivity of single membrane-spanning α-helical peptides to hydrophobic mismatch with a lipid bilayer: effects on backbone structure, orientation, and extent of membrane incorporation. Biochemistry. 40:5000–5010. [DOI] [PubMed] [Google Scholar]
- Devaux, P. F., and A. Zachowski. 1994. Maintenance and consequences of membrane phospholipid asymmetry. Chem. Phys. Lipids. 73:107–120. [Google Scholar]
- Dietrich, C., L. A. Bagatolli, Z. N. Volovyk, N. L. Thompson, M. Levi, K. Jacobson, and E. Gratton. 2001. Lipid rafts reconstituted in model membranes. Biophys. J. 80:1417–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopico, A. M., V. Anantharam, and S. N. Treistman. 1998. Ethanol increases the activity of Ca++-dependent K+ (mslo) channels: functional interaction with cytosolic Ca++. J. Pharmacol. Exp. Ther. 284:258–268. [PubMed] [Google Scholar]
- Doyle, D. A., C. J. Morais, R. A. Pfuetzner, A. Kuo, J. M. Gulbis, S. L. Cohen, B. T. Chait, and R. MacKinnon. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 280:69–77. [DOI] [PubMed] [Google Scholar]
- Dufrene, Y. F., and G. U. Lee. 2000. Advances in the characterization of supported lipid films with the atomic force microscope. Biochim. Biophys. Acta. 1509:14–41. [DOI] [PubMed] [Google Scholar]
- Eisenman, G., S. Krasne, and S. Ciani. 1975. The kinetic and equilibrium components of selective ionic permeability mediated by nactin- and valinomycin-type carriers having systematically varied degrees of methylation. Ann. N. Y. Acad. Sci. 264:34–60. [DOI] [PubMed] [Google Scholar]
- Epand, R. F., C. M. Yip, L. V. Chernomordik, D. L. LeDuc, Y. K. Shin, and R. M. Epand. 2001. Self-assembly of influenza hemagglutinin: studies of ectodomain aggregation by in situ atomic force microscopy. Biochim. Biophys. Acta. 1513:167–175. [DOI] [PubMed] [Google Scholar]
- Gillis, K. D. 2000. Admittance-based measurement of membrane capacitance using the EPC-9 patch-clamp amplifier. Pflugers Arch. 439:655–664. [DOI] [PubMed] [Google Scholar]
- Goforth, R. L., A. K. Chi, D. V. Greathouse, L. L. Providence, R. E. Koeppe, and O. S. Andersen. 2003. Hydrophobic coupling of lipid bilayer energetics to channel function. J. Gen. Physiol. 121:477–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill, O. P., A. Marty, E. Neher, B. Sakmann, and F. J. Sigworth. 1981. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 391:85–100. [DOI] [PubMed] [Google Scholar]
- Heginbotham, L., M. LeMasurier, L. Kolmakova-Partensky, and C. Miller. 1999. Single streptomyces lividans K+ channels: functional asymmetries and sidedness of proton activation. J. Gen. Physiol. 114:551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthuis, J. C., T. Pomorski, R. J. Raggers, H. Sprong, and G. van Meer. 2001. The organizing potential of sphingolipids in intracellular membrane transport. Physiol Rev. 81:1689–1723. [DOI] [PubMed] [Google Scholar]
- Jiang, Y., A. Lee, J. Chen, V. Ruta, M. Cadene, B. T. Chait, and R. MacKinnon. 2003a. X-ray structure of a voltage-dependent K+ channel. Nature. 423:33–41. [DOI] [PubMed] [Google Scholar]
- Jiang, Y., V. Ruta, J. Chen, A. Lee, and R. MacKinnon. 2003b. The principle of gating charge movement in a voltage-dependent K+ channel. Nature. 423:42–48. [DOI] [PubMed] [Google Scholar]
- Karrasch, S., R. Hegerl, J. H. Hoh, W. Baumeister, and A. Engel. 1994. Atomic force microscopy produces faithful high-resolution images of protein surfaces in an aqueous environment. Proc. Natl. Acad. Sci. USA. 91:836–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, A. 2001. Membrane structure. Curr. Biol. 11:R811–R814. [DOI] [PubMed] [Google Scholar]
- Lee, A. G. 1998. How lipids interact with an intrinsic membrane protein: the case of the calcium pump. Biochim. Biophys. Acta. 1376:381–390. [DOI] [PubMed] [Google Scholar]
- Lee, A. G. 2003. Lipid-protein interactions in biological membranes: a structural perspective. Biochim. Biophys. Acta. 1612:1–40. [DOI] [PubMed] [Google Scholar]
- LeMasurier, M., L. Heginbotham, and C. Miller. 2001. KcsA: it‘s a potassium channel. J. Gen. Physiol. 118:303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, B. A., and D. M. Engelman. 1983. Lipid bilayer thickness varies linearly with acyl chain length in fluid phosphatidylcholine vesicles. J. Mol. Biol. 166:211–217. [DOI] [PubMed] [Google Scholar]
- Lundbaek, J. A., and O. S. Andersen. 1994. Lysophospholipids modulate channel function by altering the mechanical properties of lipid bilayers. J. Gen. Physiol. 104:645–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbaek, J. A., O. S. Andersen, T. Werge, and C. Nielsen. 2003. Cholesterol-induced protein sorting: an analysis of energetic feasibility. Biophys. J. 84:2080–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum, B. 1999. SOFTWARE: A Carnival of Stats. Science. 284:1291–1292. [Google Scholar]
- Martens, J. R., R. Navarro-Polanco, E. A. Coppock, A. Nishiyama, L. Parshley, T. D. Grobaski, and M. M. Tamkun. 2000. Differential targeting of Shaker-like potassium channels to lipid rafts. J. Biol. Chem. 275:7443–7446. [DOI] [PubMed] [Google Scholar]
- Martens, J. R., N. Sakamoto, S. A. Sullivan, T. D. Grobaski, and M. M. Tamkun. 2001. Isoform-specific localization of voltage-gated K+ channels to distinct lipid raft populations. Targeting of Kv1.5 to caveolae. J. Biol. Chem. 276:8409–8414. [DOI] [PubMed] [Google Scholar]
- Martinac, B., and O. P. Hamill. 2002. Gramicidin A channels switch between stretch activation and stretch inactivation depending on bilayer thickness. Proc. Natl. Acad. Sci. USA. 99:4308–4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maulik, P. R., D. Atkinson, and G. G. Shipley. 1986. X-ray scattering of vesicles of N-acyl sphingomyelins. Determination of bilayer thickness. Biophys. J. 50:1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield, F. R. 2002. Plasma membrane microdomains. Curr. Opin. Cell Biol. 14:483–487. [DOI] [PubMed] [Google Scholar]
- Mobashery, N., C. Nielsen, and O. S. Andersen. 1997. The conformational preference of gramicidin channels is a function of lipid bilayer thickness. FEBS Lett. 412:15–20. [DOI] [PubMed] [Google Scholar]
- Moczydlowski, E., O. Alvarez, C. Vergara, and R. Latorre. 1985. Effect of phospholipid surface charge on the conductance and gating of a Ca2+-activated K+ channel in planar lipid bilayers. J. Membr. Biol. 83:273–282. [DOI] [PubMed] [Google Scholar]
- Nezil, F. A., and M. Bloom. 1992. Combined influence of cholesterol and synthetic amphiphillic peptides upon bilayer thickness in model membranes. Biophys. J. 61:1176–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, C., and O. S. Andersen. 2000. Inclusion-induced bilayer deformations: effects of monolayer equilibrium curvature. Biophys. J. 79:2583–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, C., M. Goulian, and O. S. Andersen. 1998. Energetics of inclusion-induced bilayer deformations. Biophys. J. 74:1966–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J. B., H. J. Kim, P. D. Ryu, and E. Moczydlowski. 2003. Effect of phosphatidylserine on unitary conductance and Ba2+ block of the BK Ca2+-activated K+ channel: re-examination of the surface charge hypothesis. J. Gen. Physiol. 121:375–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perozo, E., A. Kloda, D. M. Cortes, and B. Martinac. 2002. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat. Struct. Biol. 9:696–703. [DOI] [PubMed] [Google Scholar]
- Petkov, G. V., A. D. Bonev, T. J. Heppner, R. Brenner, R. W. Aldrich, and M. T. Nelson. 2001. β1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J. Physiol. 537:443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reviakine, I., W. Bergsma-Schutter, C. Mazeres-Dubut, N. Govorukhina, and A. Brisson. 2000. Surface topography of the p3 and p6 annexin V crystal forms determined by atomic force microscopy. J. Struct. Biol. 131:234–239. [DOI] [PubMed] [Google Scholar]
- Rinia, H. A., J. W. Boots, D. T. Rijkers, R. A. Kik, M. M. Snel, R. A. Demel, J. A. Killian, J. P. van der Eerden, and B. de Kruijff. 2002. Domain formation in phosphatidylcholine bilayers containing transmembrane peptides: specific effects of flanking residues. Biochemistry. 41:2814–2824. [DOI] [PubMed] [Google Scholar]
- Ross, M., C. Steinem, H. J. Galla, and A. Janshoff. 2001. Visualization of chemical and physical properties of calcium-induced domains in DPPC/DPPS Langmuir-Blodgett layers. Langmuir. 17:2437–2445. [Google Scholar]
- Roux, B., and R. MacKinnon. 1999. The cavity and pore helices in the KcsA K+ channel: electrostatic stabilization of monovalent cations. Science. 285:100–102. [DOI] [PubMed] [Google Scholar]
- Saslowsky, D. E., J. Lawrence, X. Ren, D. A. Brown, R. M. Henderson, and J. M. Edwardson. 2002. Placental alkaline phosphatase is efficiently targeted to rafts in supported lipid bilayers. J. Biol. Chem. 277:26966–26970. [DOI] [PubMed] [Google Scholar]
- Shapiro, S. S., and M. B. Wilk. 1965. An analysis of variance test for nomality (complete samples). Biometrika. 52:591–611. [Google Scholar]
- Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature. 387:569–572. [DOI] [PubMed] [Google Scholar]
- Singer, S. J., and G. L. Nicolson. 1972. The fluid mosaic model of the structure of cell membranes. Science. 175:720–731. [DOI] [PubMed] [Google Scholar]
- Sonnleitner, A., L. M. Mannuzzu, S. Terakawa, and E. Y. Isacoff. 2002. Structural rearrangements in single ion channels detected optically in living cells. Proc. Natl. Acad. Sci. USA. 99:12759–12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprong, H., P. van der Sluijs, and G. van Meer. 2001. How proteins move lipids and lipids move proteins. Nat. Rev. Mol. Cell Biol. 2:504–513. [DOI] [PubMed] [Google Scholar]
- Starling, A. P., J. M. East, and A. G. Lee. 1995. Effects of phospholipid fatty acyl chain length on phosphorylation and dephosphorylation of the Ca2+-ATPase. Biochem. J. 310:875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, T., A. A. Naini, and C. Miller. 1994. High-level expression and functional reconstitution of Shaker K+ channels. Biochemistry. 33:9992–9999. [DOI] [PubMed] [Google Scholar]
- Tahara, Y., and Y. Fujiyoshi. 1994. A new method to measure bilayer thickness: cryo-electron microscopy of frozen hydrated liposomes and image simulation. Micron. 25:141–149. [DOI] [PubMed] [Google Scholar]
- Thompson, T. E., M. Allietta, R. E. Brown, M. L. Johnson, and T. W. Tillack. 1985. Organization of ganglioside GM1 in phosphatidylcholine bilayers. Biochim. Biophys. Acta. 817:229–237. [DOI] [PubMed] [Google Scholar]
- Turnheim, K., J. Gruber, C. Wachter, and V. Ruiz-Gutierrez. 1999. Membrane phospholipid composition affects function of potassium channels from rabbit colon epithelium. Am. J. Physiol. 277:C83–C90. [DOI] [PubMed] [Google Scholar]
- van den Brink-van der Laan, R. E. Dalbey, R. A. Demel, J. A. Killian, and B. de Kruijff. 2001. Effect of nonbilayer lipids on membrane binding and insertion of the catalytic domain of leader peptidase. Biochemistry. 40:9677–9684. [DOI] [PubMed] [Google Scholar]
- Vergara, C., R. Latorre, N. V. Marrion, and J. P. Adelman. 1998. Calcium-activated potassium channels. Curr. Opin. Neurobiol. 8:321–329. [DOI] [PubMed] [Google Scholar]
- Weiss, T. M., P. C. Van Der Wel, J. A. Killian, R. E. Koeppe, and H. W. Huang. 2003. Hydrophobic mismatch between helices and lipid bilayers. Biophys. J. 84:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson, I. M., S. J. Alvis, J. M. East, and A. G. Lee. 2002. Interactions of phospholipids with the potassium channel KcsA. Biophys. J. 83:2026–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonderlin, W. F., A. Finkel, and R. J. French. 1990. Optimizing planar lipid bilayer single-channel recordings for high resolution with rapid voltage steps. Biophys. J. 58:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, X. M., J. P. Ding, and C. J. Lingle. 1999. Molecular basis for the inactivation of Ca2+- and voltage-dependent BK channels in adrenal chromaffin cells and rat insulinoma tumor cells. J. Neurosci. 19:5255–5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, X. M., J. P. Ding, X. H. Zeng, K. L. Duan, and C. J. Lingle. 2000. Rectification and rapid activation at low Ca2+ of Ca2+-activated, voltage-dependent BK currents: consequences of rapid inactivation by a novel β subunit. J. Neurosci. 20:4890–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen, G. 2002. The voltage-gated potassium channels and their relatives. Nature. 419:35–42. [DOI] [PubMed] [Google Scholar]
- Yuan, C., J. Furlong, P. Burgos, and L. J. Johnston. 2002. The size of lipid rafts: an atomic force microscopy study of ganglioside GM1 domains in sphingomyelin/DOPC/cholesterol membranes. Biophys. J. 82:2526–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, C., and L. J. Johnston. 2001. Atomic force microscopy studies of ganglioside GM1 domains in phosphatidylcholine and phosphatidylcholine/cholesterol bilayers. Biophys. J. 81:1059–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, C., and L. J. Johnston. 2002. Phase evolution in cholesterol/DPPC monolayers: atomic force microscopy and near field scanning optical microscopy studies. J. Microsc. Oxf. 205:136–146. [DOI] [PubMed] [Google Scholar]
- Yuan, C., R. J. O'Connell, and S. N. Treistman. 2003. Sphingomyelin promotes phase separation and modulates the conductance of the BK channel in model membranes. Biophys. J. 84:542A. [DOI] [PMC free article] [PubMed] [Google Scholar]