Abstract
The inositol (1,4,5)-trisphosphate receptor (InsP3R) is an intracellular calcium (Ca2+) release channel that plays a crucial role in cell signaling. In Drosophila melanogaster a single InsP3R gene (itpr) encodes a protein (DmInsP3R) that is ∼60% conserved with mammalian InsP3Rs. A number of itpr mutant alleles have been identified in genetic screens and studied for their effect on development and physiology. However, the functional properties of wild-type or mutant DmInsP3Rs have never been described. Here we use the planar lipid bilayer reconstitution technique to describe single-channel properties of embryonic and adult head DmInsP3R splice variants. The three mutants chosen in this study reside in each of the three structural domains of the DmInsP3R—the amino-terminal ligand binding domain (ug3), the middle-coupling domain (wc703), and the channel-forming region (ka901). We discovered that 1), the major functional properties of DmInsP3R (conductance, gating, and sensitivity to InsP3 and Ca2+) are remarkably conserved with the mammalian InsP3R1; 2), single-channel conductance of the adult head DmInsP3R isoform is 89 pS and the embryonic DmInsP3R isoform is 70 pS; 3), ug3 mutation affects sensitivity of the DmInsP3Rs to activation by InsP3, but not their InsP3-binding properties; 4), wc703 channels have increased sensitivity to modulation by Ca2+; and 5), homomeric ka901 channels are not functional. We correlated the results obtained in planar lipid bilayer experiments with measurements of InsP3-induced Ca2+ fluxes in microsomes isolated from wild-type and heterozygous itpr mutants. Our study validates the use of D. melanogaster as an appropriate model for InsP3R structure-function studies and provides novel insights into the fundamental mechanisms of the InsP3R function.
INTRODUCTION
The inositol (1,4,5)-trisphosphate receptor (InsP3R) is an intracellular calcium (Ca2+) release channel that plays a critical role in Ca2+ signaling (Berridge, 1993). Three mammalian isoforms of the InsP3R—type I (InsP3R1), type II (InsP3R2), and type III (InsP3R3)—have been identified (Furuichi et al., 1994). All three mammalian isoforms share a common domain structure and 60–70% sequence identity (Furuichi et al., 1994). The differences in functional properties between mammalian InsP3R isoforms are beginning to be elucidated (Miyakawa et al., 1999; Thrower et al., 2001). InsP3Rs are subjected to multiple levels of regulation (Berridge, 1993; Bezprozvanny and Ehrlich, 1995; Taylor, 1998). Structural determinants responsible for InsP3R1 conductance and gating properties (Boehning et al., 2001; Ramos-Franco et al., 1999) and modulation by Ca2+ (Miyakawa et al., 2001; Nosyreva et al., 2002; Tu et al., 2003), ATP (Tu et al., 2002), and phosphorylation (Tang et al., 2003b; Wagner et al., 2003) have been uncovered in recent functional experiments with InsP3R1 wild-type and mutant channels.
A single copy of the InsP3R gene (itpr) is present in the genome of Drosophila (Hasan and Rosbash, 1992; Yoshikawa et al., 1992), a model organism, that has been used widely for genetic screens (Myers et al., 2000). Sequence analysis reveals that the Drosophila InsP3R protein (DmInsP3R) shares the same domain structure and ∼60% sequence identity with mammalian InsP3R isoforms, with the highest level of conservation in the amino-terminal ligand binding and the carboxy-terminal channel regions. Thus, it is likely that analysis of the DmInsP3R mutant isoforms, identified in genetic screens (Deshpande et al., 2000; Joshi et al., 2004; Venkatesh and Hasan, 1997), will provide important insights into fundamental mechanisms of the InsP3R function. However, in the absence of information regarding the functional properties of the wild-type DmInsP3R isoforms, the interpretation of results obtained from DmInsP3R mutants are limited.
Here we describe the functional properties of two wild-type DmInsP3R splice variants (Sinha and Hasan, 1999) and three DmInsP3R mutants (ug3, wc703, and ka901) (Deshpande et al., 2000; Joshi et al., 2004). The mutant isoforms affect residues conserved among all known InsP3Rs (Joshi et al., 2004). For functional analysis of DmInsP3R we expressed DmInsP3R wild-type and mutants in sf9 cells by baculoviral infection and analyzed the properties of resulting channels in planar lipid bilayers. This is the same approach that we used previously for structure-function analysis of the mammalian InsP3R1 (Nosyreva et al., 2002; Tang et al., 2003a, b; Tu et al., 2002, 2003). Our results indicate that the main functional properties of InsP3Rs are conserved in evolution from flies to mammals. Moreover, changes in the DmInsP3R properties induced by mutations provide novel insights into fundamental mechanisms of InsP3R function. We also demonstrate that functional properties observed in vitro correlate well with in vivo function of DmInsP3R mutants by measurement of Ca2+ fluxes from wild-type and heterozygous mutant flies.
MATERIALS AND METHODS
Ca2+ release assay
A modified protocol from Bramley et al. (1990) was used. In brief, microsomes were prepared in the presence of 200 μM free Ca2+, from either 75 wandering third-instar larvae or ∼200 adult heads, obtained from the appropriate genotypes. The Canton-S strain (CS) was used for studying properties of the wild-type InsP3R and all point mutants were generated in the background of this wild-type strain. The tissues were homogenized in TSHMo buffer (10 mM Tris pH 7.4, 0.25 M sucrose, 12 mM monothioglycerol, 10 mM sodium molybdate, 200 μM CaCl2) and a mixture of protease inhibitors (1 mM PMSF, 10 μg/ml leupeptin, and 10 μM E64) by 10–12 strokes of a 15-ml glass Teflon homogenizer. The homogenate was centrifuged immediately at 20,000 g for 20 min at 4°C. The supernatant was recentrifuged at 109,000 g on a tabletop ultracentrifuge (Rotor TLA 45 of Beckman centrifuges, Beckman Coulter, Fullerton, CA) for 1 h. The pellet was resuspended in fresh Ca2+-free TSHMo buffer and centrifuged again at 109,000 g for 20 min. The pellet was resuspended in 150 μl of Ca2+-free TSHMo buffer and used for Ca2+ release assays and for protein estimation by the Bradford's method. Microsomes were made in parallel, in Ca2+-free buffer and otherwise identical conditions, and added to the buffers used for determining the standard curve for Ca2+ for each individual experiment (see below for details of Ca2+-EGTA standard curve). The endogenous leak rate of each microsomal vesicle's preparation was checked by a standard fluorescence assay (Mathew et al., 1982, data not shown) before using them in the Ca2+-release assay. Only vesicles with minimal Ca2+ leak, measured over a period of 10 min, were used for the Ca2+ release assay. No obvious difference in the leak rates between wild-type and mutant vesicles was observed.
Membrane-impermeant Ca2+-sensitive ratiometric fluorescent dye, Fura-2 (Molecular Probes, Eugene, OR) was prepared in calcium-free water. Steady-state fluorescence measurement was performed in a SPEX Fluorolog-2 spectrofluorometer (SPEX Industries, Edison, NJ) at 20°C so as to minimize nonspecific calcium leak. For each run, ∼15 μg of adult head microsomes or ∼50 μg of larval microsomal vesicles were added to 2 ml of assay buffer (20 mM Tris, pH 7.4 and 80 mM NaCl in calcium-free water) containing 5 μM Fura-2. Steady-state kinetics of Ca2+ release were measured at various concentrations of InsP3 and quantified by plotting a standard curve, with known amounts of free Ca2+ using the standard Ca2+-EGTA buffering system. To take into account the buffering capacity of the microsomes, the standard curve was obtained for each experiment in the presence of wild-type or mutant microsomes, made in parallel in Ca2+-free buffer. For heparin-mediated inhibition studies, a nonratiometric dye, Fluo-4, was used at 5 μM concentration. To examine the amount of Ca2+ trapped in microsomal vesicles of all genotypes fluorescence measurements were done before and after the addition of 0.1% Triton X-100, which was used for disrupting the microsomal vesicles, in the presence of 2 μM Fura-2.
Expression of the DmInsP3R in sf9 cells
Full-length embryonic DmInsP3R coding sequence (Sinha and Hasan, 1999) was cloned into pFastBac1 expression vector (Invitrogen, Carlsbad, CA). The 5′ UTR of the DmInsP3R clone was replaced with the Kozak sequence by polymerase chain reaction to generate the DmInsP3R-pFastBac1 construct. The Nhe1(1415)/Nhe1(3210) fragment of the DmInsP3R-pFastBac1 clone was replaced by the corresponding fragment from an adult head (AH) AH-DmInsP3R clone (Sinha and Hasan, 1999) to yield the AH-DmInsP3R-pFastBac1 construct. Recently described ka901 (G2630S), wc703 (G2117E), and ug3 (S224F) mutations (Joshi et al., 2004) were generated in the DmInsP3R-pFastBac1 construct by megaprimer polymerase chain reaction or by using the QuikChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA), and verified by sequencing. DmInsP3R, AH-DmInsP3R, ka901, wc703, and ug3 baculoviruses were generated and amplified using Bac-to-Bac system (Invitrogen) as described previously (Nosyreva et al., 2002; Tu et al., 2002). The DmInsP3R wild-type and mutants were expressed in Spodoptera frugiperda (sf9) cells as previously described (Nosyreva et al., 2002; Tu et al., 2002). Expression of the DmInsP3R in wild-type and mutant flies and sf9 cells was confirmed by Western blotting with the affinity purified anti-DmInsP3R rabbit polyclonal antibody (IB-9075) raised against KLH-conjugated peptide CEQRKQKQRLGLLNTTANSLLPFQ derived from the DmInsP3R sequence. Lysates from D. melanogaster larvae and adult heads were used as controls for the specificity of IB-9075 antibody (see Results).
Single-channel recordings and analysis of the DmInsP3R activity
Recombinant DmInsP3R wild-type and mutants expressed in sf9 cells were incorporated into the bilayer by microsomal vesicle fusion as previously described for InsP3RI (Nosyreva et al., 2002; Tu et al., 2002). The cis chamber contained 250 mM HEPES-Tris, pH 7.35 and the trans chamber, which was held at virtual ground, contained 55 mM Ba(OH)2, dissolved in 250 mM HEPES, pH 7.35. Single-channel conductance values of the DmInsP3R were determined from the slope of a linear fit to unitary current amplitude versus transmembrane voltage data in the range between +10 mV and −30 mV. For calcium-dependence experiments, free Ca2+ concentration in the cis chamber was controlled in the range of 10 nM (pCa 8) to 10 mM (pCa 2) by mixture of 1 mM EGTA, 1 mM HEDTA, and variable concentrations of CaCl2 or Ca-HEPES2. To construct InsP3- and Ca2+-dependence curves for the DmInsP3R, the determined values of Po were averaged across several independent experiments at each InsP3 or Ca2+ concentration. For InsP3-dependence experiments the averaged values of Po are presented as mean ± SE (n = number of independent experiments) and fit by equation 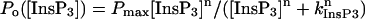 modified from Lupu et al. (1998), where Pmax is a maximal Po value, n is a Hill coefficient, and kInsP3 is the apparent affinity of the DmInsP3R for InsP3. For Ca2+-dependence experiments the averaged values of Po are presented as mean ± SE (n = number of independent experiments) and fit by the bell-shaped equation
modified from Lupu et al. (1998), where Pmax is a maximal Po value, n is a Hill coefficient, and kInsP3 is the apparent affinity of the DmInsP3R for InsP3. For Ca2+-dependence experiments the averaged values of Po are presented as mean ± SE (n = number of independent experiments) and fit by the bell-shaped equation 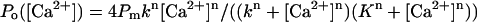 , modified from Bezprozvanny et al. (1991), where Pm is a parameter proportional to the maximal Po value, n is a Hill coefficient, k is the apparent affinity of Ca2+ activating site, and K is the apparent affinity of Ca2+ inhibitory site (Tu et al., 2003). The parameters of the optimal fits to InsP3- and Ca2+-dependence are in the text.
, modified from Bezprozvanny et al. (1991), where Pm is a parameter proportional to the maximal Po value, n is a Hill coefficient, k is the apparent affinity of Ca2+ activating site, and K is the apparent affinity of Ca2+ inhibitory site (Tu et al., 2003). The parameters of the optimal fits to InsP3- and Ca2+-dependence are in the text.
3H-InsP3 binding assay
Scatchard analysis of 3H-InsP3 binding to the DmInsP3R and ug3 microsomes was performed as previously described for InsP3RI (Kaznacheyeva et al., 1998). Briefly, microsomes (20 μg protein) were incubated on ice with 10 nM 3H-InsP3 and variable amounts of nonlabeled InsP3 (from 10 nM to 8 μM) in the binding buffer (50 mM Tris-HCl, pH 9.0, 1 mM EDTA, 1 mM DTT, 100 mM NaCl) and precipitated with 12.5% PEG and 1.2 mg/ml γ-globulin at 14,000 g. Precipitates were quickly washed with the binding buffer, dissolved in Soluene (PerkinElmer, Boston, MA) and their [3H] content was determined by liquid scintillation counting. Nonspecific counts, determined in the presence of 25 μM unlabeled InsP3, were subtracted from the total to yield specific binding. The binding affinity and the density of specific 3H-InsP3 binding sites was determined from the linear fit to the Scatchard plot. The specific activity of 3H-InsP3 used in this assay (Amersham Biosciences, Piscataway, NJ) was 45 Ci/mmol.
RESULTS
Ca2+ release properties of the DmInsP3R wild-type and mutants
The domain structure of the InsP3R (Mignery and Sudhof, 1990) is conserved between the DmInsP3R and mammalian InsP3R isoforms. Similar to mammalian InsP3Rs, the DmInsP3R can be divided into an amino-terminal ligand binding (DmInsP3R-N, M1-H650) domain, a carboxy-terminal channel-forming (DmInsP3R-C, S2360-Q2829) domain, and a middle-coupling (DmInsP3R-M, N651-W2359) domain (Fig. 1). Two alternate splice events (at positions 980 and 1390) were originally described for the embryonic and adult head (AH) forms of the DmInsP3R (Sinha and Hasan, 1999). From subsequent sequencing of genomic DNA of the itpr gene it is now clear that alternate splicing occurs only at residue 980 to give the two forms shown in Fig. 1, which differ by the nine-amino-acid insertion in the AH isoform. There is no intron near residue 1390, and consequently both forms are identical in this region and contain the sequence GVGHSV. A number of lethal DmInsP3R mutants were recently obtained by chemical mutagenesis using ethyl methane sulfonate (Deshpande et al., 2000). Among 14 lethal mutant alleles of the DmInsP3R obtained in the ethyl methane sulfonate screen, genomic DNA in the region of the open reading frame has recently been sequenced from seven alleles (Joshi et al., 2004). In this article we focus on three DmInsP3R point mutants (ug3, wc703, and ka901; see Fig. 1) in which the altered residues are conserved between all known InsP3Rs (Joshi et al., 2004).
FIGURE 1.
Schematic of the DmInsP3R showing point mutants used in this study. The amino-terminal ligand-binding domain (DmInsP3R-N, M1-H650) consists of an inhibitory domain (ID, M1–E231) and the core-binding domain (CD, H232–H650). The middle-coupling domain (DmInsP3R-M, N651–W2359) contains a site with a nine-amino-acid insertion in the adult head (AH) isoform in position E980 (Sinha and Hasan, 1999) and a putative Ca2+-sensor region (Cas, G1986–S2354). The carboxy-terminal channel-forming domain (DmInsP3R-C, S2360–Q2829) consists of M1–M6 transmembrane domains and a pore-forming region (P). Also shown are positions of three DmInsP3R point mutants identified in an ethyl methane sulfonate screen (Joshi et al., 2004), i.e., ug3 (S224F), wc703 (G2117E), and ka901 (G2630S).
To determine consequences of identified mutations for the DmInsP3R function, a series of InsP3-induced Ca2+ release experiments were performed with microsomes prepared from adult head and larval homogenates of wild-type and mutant flies. For generation of Ca2+-loaded microsomal vesicles the buffer contained known amounts of free Ca2+ (Materials and Methods). Mutant vesicles were obtained from heterozygous flies, due to the fact that all homozygous mutant combinations are lethal. Since the functional InsP3 receptor is a tetramer, in which interactions between monomers are known to affect Ca2+ release, it was reasoned that heterozygotes might exhibit mutant properties. In these experiments DmInsP3R function was quantified by measuring the InsP3 concentration required to achieve half-maximal Ca2+-release (Km, nM InsP3) and maximal amount of released Ca2+ (Rmax, nmol Ca2+/mg microsomal protein) values. As seen in Fig. 2 A, increasing amounts of InsP3 showed an increase in fluorescence which was converted to the amount of Ca2+ released based on a calibration curve generated using a standard Ca-EGTA buffering system. The amount of Ca2+ released with increasing amounts of InsP3 was seen to follow typical Michaelis-Menten kinetics (Fig. 2 B). We found that in wild-type organisms, the Km values are similar in microsomes from larvae (405 ± 8 nM InsP3) and adult heads (398 ± 14 nM InsP3) (Fig. 2 B). In contrast, the Rmax value is much higher in microsomes from adult heads (635 ± 42 nmol Ca2+/mg protein) than from larvae (319 ± 22 nmol Ca2+/mg protein; see Fig. 2 B). The Km and Rmax values were calculated as the average from at least three independent experiments. The graphs on Fig. 2 B show a single fit to the averaged data points.
FIGURE 2.
Ca2+ fluxes supported by the DmInsP3R mutants. (A) Representative traces of Ca2+ release assay in microsomes isolated from the wild-type adult heads in the absence of InsP3 (trace a) and in the presence of 20 nM (trace b), 200 nM (trace c), and 2 μM (trace d) InsP3. The fluorescence values at 340 and 380 nm were utilized to calculate the amount of Ca2+ released (see Materials and Methods). Further, the free Ca2+ present without addition of InsP3 (trace a) was subtracted from those with InsP3 additions. (B) InsP3-dependent Ca2+ fluxes in microsomal vesicles isolated from whole larvae (○) and adult heads (•) of the wild-type Canton-S (CS) strain. (C) Inhibition of InsP3-mediated Ca2+ − release by heparin. Arrows indicate the time of addition of various reagents to microsomal vesicles in a buffer containing the fluorescent Ca2+ indicator dye Fluo-4. The two traces shown in black (− heparin) and gray (+ heparin) were obtained with the same batch of microsomes made from heads of the wild-type CS strain. The blips observed close to each arrow occur due to interference of the light beam by the syringe used to add reagents to the cuvette. Similar results have been observed independently with two other batches of microsomal vesicles. (D–F) InsP3-dependent Ca2+ fluxes in microsomes isolated from adult heads (•) of ug3/+ (D), wc703/+ (E), and ka901/+ (F) flies. Wild-type adult head data from B are shown for comparison by the thin line in each graph. The difference in Km between the wild-type and ug3/+ heterozygotes in D is statistically significant (P < 0.001). The concentrations of InsP3 required for half-maximal Ca2+ release (Km) are shown by arrowheads for wild-type and lines for mutants in B and D–F. All data in B and D–F are shown as the mean ± SD from at least three independent batches of microsomes. The data were fit to an exponential rise using SigmaPlot5 (SPSS, Chicago, IL).
The analysis of DmInsP3R mutants was carried out using AH microsomes, which release more Ca2+ (twofold higher Rmax) than the larval microsomes (Fig. 2 B). Qualitatively similar results were obtained in experiments with microsomes from mutant larvae (data not shown). The specificity of the InsP3R-mediated release in this assay was verified by using heparin, a competitive inhibitor of the InsP3R. For this purpose, a nonratiometric dye, Fluo-4, was used. As seen in Fig. 2 C, InsP3-mediated Ca2+ release is completely inhibited in the presence of 50 μg/ml of heparin. Microsomes isolated from the ug3/+ adult heads show a dominant phenotype of lowered Km for InsP3 (Km = 274 ± 34 nM InsP3) without affecting the Rmax value (Fig. 2 D). Microsomes from adult heads of wc703/+ heterozygotes show an increased Rmax (923 ± 29 nmol Ca2+/mg protein; Fig. 2 E) but an unchanged Km compared to wild-type (Fig. 2 E). Microsomes obtained from ka901/+ adult heads released over twice as much Ca2+ (Rmax = 1678 ± 124 nmol Ca2+/mg protein), with a significantly lower Km (Km = 308 ± 63 nM), compared to wild-type microsomes (Fig. 2 F).
From these experiments we concluded that each tested mutant allele has distinct Ca2+-release properties in the Ca2+ flux assay. The observed changes in Rmax could be due to different levels of the DmInsP3R protein among mutants or different amounts of Ca2+ trapped in vesicles from different genotypes. By Western blotting the levels of DmInsP3R protein were found to be equivalent in the wild-type and all mutant microsomes (Fig. 3 A). The amount of Ca2+ trapped in vesicles of all the genotypes was also found to be equivalent as determined by measuring Ca2+ release on addition of 0.1% Triton X-100 (Fig. 3 B). However, as expected from Fig. 2, E and F, the amount of InsP3-releasable Ca2+ was found to be higher in vesicles from itpwc703 and itprka901 heterozygotes (Fig. 3 B). Therefore, the observed changes in Rmax and Km values most likely reflect changes in DmInsP3R functional properties induced by each mutation. To test this idea conclusively an alternate approach was adopted.
FIGURE 3.
Levels of the DmInsP3R protein and Ca2+ in wild-type and mutant vesicles. (A) Western blot of wild-type and mutant adult head microsomes with anti-DmInsP3R polyclonal antibody. Lane 1, CS adult head lysate; lanes 2–5, microsomal vesicles from adult heads of the genotypes as indicated. Five micrograms of total protein was loaded in each lane. The blot was stripped and reprobed with antiserum to α-spectrin (278 kDa) as a loading control. When levels of the DmInsP3R were compared with the levels of the loading control by densitometry analysis, no significant changes were observed between the various genotypes. The representative blot of three independent experiments is shown. (B) Fraction of maximal Ca2+ released from microsomes in response to 2 μM InsP3 (black bars) as a fraction of total vesicular Ca2+ (estimated by lysing the microsomes with 0.1% Triton X-100, open bars). The fraction of InsP3-releasable Ca2+ was significantly higher in itprwc703/+ and itprka901/+ (P < 0.001 for both) microsomes than in wild-type (CS).
Single-channel properties of the DmInsP3R embryonic and adult head isoforms
Single-channel recordings offer by far the most detailed level of characterization of functional properties of channels. To obtain single-channel recordings of the DmInsP3R, we adopted a previously described approach developed for the structure-functional analysis of the mammalian InsP3R1 (Nosyreva et al., 2002; Tang et al., 2003a, b; Tu et al., 2002, 2003). The DmInsP3R (embryonic form) and AH-DmInsP3R (adult head form) were expressed in sf9 cells by baculovirus infection and the expression was confirmed by Western blotting (Fig. 4) using affinity-purified anti-DmInsP3R rabbit polyclonal antibodies. The apparent molecular weight of DmInsP3R and AH-DmInsP3R expressed in sf9 cells (280 kDa) was identical to endogenous DmInsP3R detected by the same antibodies in Drosophila larval and adult head lysates (Fig. 4). No signal was observed in samples prepared from noninfected sf9 cells (Fig. 4).
FIGURE 4.
Expression of the DmInsP3R in sf9 cells. Western blot of Drosophila larval (LL) and adult head (AHL) lysates and microsomes isolated from noninfected sf9 cells (sf9) and sf9 cells infected with DmInsP3R, AH-DmInsP3R, ug3, wc703, and ka901 baculoviruses as indicated. The DmInsP3R was detected using an affinity-purified anti-DmInsP3R polyclonal antibody. For the larval lysate 30 μg of total protein was loaded whereas 20 μg was loaded in the lane containing the adult head lysate. For each microsomal preparation, 100 ng of total microsomal protein was loaded on the gel except for ug3, where 300 ng total microsomal protein was loaded. The representative blot of three independent experiments is shown.
Recombinant DmInsP3R and AH-DmInsP3R expressed in sf9 cells were incorporated into the bilayer by microsomal vesicle fusion as described previously for the wild-type and mutant mammalian InsP3R1 (Nosyreva et al., 2002; Tang et al., 2003a, b; Tu et al., 2002, 2003). InsP3-gated currents supported by the DmInsP3R and the AH-DmInsP3R channels were recorded using 50 mM Ba2+ as the charge carrier at 0-mV holding potential (Fig. 5, A and B). InsP3-gated channels in bilayers were observed in 45 of 50 experiments with DmInsP3R microsomes and in 40 of 50 experiments with AH-DmInsP3R microsomes, but were never (n = 10) observed in experiments with microsomes from noninfected sf9 cells. Single-channel analysis revealed that the mean current amplitude at 0-mV holding potential is equal to 1.80 ± 0.04 pA for the DmInsP3R (Fig. 5 A) and 1.86 ± 0.01 pA for the AH-DmInsP3R (Fig. 5 B); the mean open dwell time is equal to 4.3 ± 0.3 ms for the DmInsP3R (Fig. 5 A) and 3.6 ± 0.1 ms for the AH-DmInsP3R (Fig. 5 B) (Table 1).
FIGURE 5.
Functional properties of the wild-type isoforms of the DmInsP3R. Representative channel activity of the DmInsP3R (A) and the AH-DmInsP3R (B) recorded in the presence of 0.2 μM Ca2+, 0.5 mM Na2ATP, and 2 μM InsP3 (standard recording conditions). Current records are shown at compressed and expanded timescales as indicated. Unitary current amplitude histograms and open dwell time distributions from the same experiments are shown below the current records. Unitary currents were fitted with a Gaussian function that was centered at 1.83 pA for the DmInsP3R and at 1.86 pA for the AH-DmInsP3R. Open time distributions were fit with a single exponential function (curve) that yielded a τ0 of 4.0 ms for the DmInsP3R and 3.98 ms for the AH-DmInsP3R. Similar analysis was performed for at least five independent experiments with the DmInsP3R and the AH-DmInsP3R microsomes.
TABLE 1.
Comparison of main functional properties of DmInsP3R and mammalian InsP3R1 (Tang et al., 2003b; Tu et al., 2002)
| Conductance properties
|
Gating properties
|
InsP3-dependence
|
Bell-shaped Ca2+ dependence
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| InsP3R isoform | Splice variant | i, pA | γ, pS | τo, ms | Po max % | kd, nM | nHill | nHill | Kact, μM | Kinh, μM | Peak (pCa) |
| InsP3R1 | SII(+) | 1.9 pA | 81 pS | 4.7 ms | 19% | 190 nM | 1.47 | 1.3 | 0.22 μM | 0.20 μM | 6.6 |
| DmInsP3R | emb | 1.8 pA | 70 pS | 4.3 ms | 15% | 100 nM | 1.13 | 0.91 | 0.28 μM | 0.53 μM | 6.4 |
| DmInsP3R | AH | 1.9 pA | 89 pS | 3.6 ms | 17% | ND | Similar to embryonic | ||||
| wc703 | emb | 1.75 pA | 70 pS | 4.5 ms | 21% | ND | 1.96 | 0.17 μM | 0.18 μM | 6.8 | |
| ug3 | emb | 1.72 pA | 71 pS | 6.4 ms | 17% | ND | Similar to embryonic | ||||
To generate a current-voltage relationship, the transmembrane potential was varied between +10 mV and −30 mV during current recordings. A linear fit to the obtained results (Fig. 6) yielded single-channel conductances of 70.0 ± 0.1 pS for the DmInsP3R and 89.0 ± 0.1 pS for the AH-DmInsP3R. From previous molecular analysis of these isoforms it is known that Drosophila larvae express both splice variants (predominantly the embryonic isoform) whereas only the AH form is present in adult heads. The higher conductance of the AH-DmInsP3R is thus consistent with the differences in Rmax values observed between larval and adult head microsomes in the Ca2+-release assay (Fig. 2 B).
FIGURE 6.
Current-voltage relationship of the DmInsP3R isoforms. Current-voltage relationship of the DmInsP3R (•) and the AH-DmInsP3R (○) channels measured at transmembrane voltages between +10 mV and −30 mV. Average data from at least four independent experiments with DmInsP3R and AH-DmInsP3R are shown as the mean ± SE at each voltage. The linear fit to the data (lines) yielded single-channel conductance of 70.0 pS for the DmInsP3R and 89 pS for the AH-DmInsP3R.
To further characterize functional properties of the DmInsP3R, its open probability was measured in a range of Ca2+ and InsP3 concentrations. The analysis of the DmInsP3R InsP3-sensitivity (Fig. 7) yielded an apparent affinity for InsP3 equal to 100 nM. We found that similar to mammalian InsP3R1 (Table 1), DmInsP3R displays a bell-shaped Ca2+ dependence with the peak at pCa 6.4 (Fig. 8). On an average, the single-channel open probability of the DmInsP3R channels at pCa 6.4 was equal to 0.15 ± 0.015. A fit to the averaged Ca2+-dependence data (Fig. 8 B) using a modified bell-shaped equation from Bezprozvanny et al. (1991) yielded the affinities of activating and inhibitory sites of the DmInsP3R equal to 0.28 μM Ca2+ and 0.53 μM Ca2+, respectively. Although the affinity of the activating site is similar to mammalian InsP3R1 (Nosyreva et al., 2002; Tu et al., 2002, 2003), the affinity for the inhibitory site is lower in the DmInsP3R (Table 1), indicating that higher concentrations of cytoplasmic Ca2+ are required for inhibition of the InsP3R in Drosophila. Similar results were obtained for the AH-DmInsP3R (data not shown).
FIGURE 7.
InsP3 dependence of the DmInsP3R. The DmInsP3R Po measured in three independent experiments were averaged together at each InsP3 concentration as described in Materials and Methods and shown as mean ± SE (•). The averaged data were fitted by the equation modified from Lupu et al. (1998) as explained in Materials and Methods. The parameters of optimal fit (smooth curve) yielded kInsP3 = 100 nM, nHill = 1.13, and Pmax = 0.078.
FIGURE 8.
Calcium dependence of the DmInsP3R. (A) Current recordings of the DmInsP3R from the same experiment at different Ca2+ concentration as indicated. (B) Bell-shaped Ca2+ dependence of DmInsP3R. The Po measured in five independent experiments with DmInsP3R were averaged together at each Ca2+ concentration as described in Materials and Methods and shown as mean ± SE (•). The averaged data were fitted by the bell-shaped equation modified from Bezprozvanny et al. (1991) as explained in Materials and Methods. The optimal fit (smooth curve) was obtained with nHill = 0.91, Pm =0.13, kact = 0.28 μM Ca2+, and Kinh = 0.53 μM Ca2+.
Functional properties of the ug3 mutant
To characterize single-channel properties of the ug3 mutant (embryonic form) it was expressed in sf9 cells by baculovirus infection. The expression levels of ug3 mutant channels in sf9 cells were lower than expression levels of the wild-type channels (in Fig. 4, a three-times excess of ug3 microsomes was loaded). When microsomes isolated from ug3-infected sf9 cells were fused to planar lipid bilayers, InsP3-gated channels were observed less frequently than in experiments with wild-type microsomes (in 20 of 34 experiments; Fig. 9). The mean current amplitude of ug3 channels at 0-mV holding potential is 1.7 ± 0.32 pA (Fig. 9), with a mean open dwell time of 6.4 ± 0.8 ms (Fig. 9), and single-channel conductance of 71.0 ± 0.2 pS (n = 3, data not shown). Thus, ug3 channels display conductance properties similar to the wild-type DmInsP3R (embryonic form), but have 47% longer mean open time.
FIGURE 9.
Functional properties of ug3 channels. Representative channel activity of ug3 channels recorded in the standard recording conditions. Current records are shown at compressed and expanded timescales as indicated. Unitary current amplitude histograms and open dwell time distributions from the same experiments are shown below the current records. Unitary current distribution was fit with a Gaussian function that was centered at 1.71 pA. Open time distribution was fit with a single exponential function (curve) that yielded a τ0 of 5.6 ms. Similar analysis was performed for at least three independent experiments with ug3 microsomes.
The amino-terminal region of the InsP3R forms a ligand-binding domain (Fig. 1). Biochemical and structural studies of mammalian InsP3R1 indicate that the InsP3R ligand-binding domain can be subdivided into a core InsP3 binding domain and an inhibitory domain (Bosanac et al., 2002; Yoshikawa et al., 1999, 1996; see also Fig. 1, this article). Sequences corresponding to the core and inhibitory domains are highly conserved between DmInsP3R and InsP3R1, and the ug3 mutation (S224F) is interestingly located at the interface between the predicted inhibitory and core domains of the DmInsP3R (Fig. 1). From the position of the ug3 mutation in the ligand-binding domain it was expected that ug3 may have an effect on the binding affinity of the DmInsP3R for InsP3. Binding of 3H-InsP3 to microsomes isolated from the DmInsP3R- and ug3-infected sf9 cells yielded affinity values of 152 nM InsP3 for the wild-type DmInsP3R (embryonic form) and 214 nM InsP3 for the ug3 mutant (Fig. 10). The observed difference in affinity was found to be insignificant upon repetition of the experiment (data not shown). Notably, the affinity for InsP3 is either the same or slightly lower in ug3 when compared with wild-type DmInsP3R in 3H-InsP3 binding experiments (Fig. 10). This conclusion contrasts with higher sensitivity of ug3/+ microsomes to InsP3 in the Ca2+ flux assay as compared to the wild-type microsomes (Fig. 2 D).
FIGURE 10.
3H-InsP3 binding studies with the DmInsP3R and ug3 microsomes. Scatchard analysis of 3H-InsP3 binding to the DmInsP3R (•) and ug3 (○) microsomes. Linear fit to the data (lines) yielded kd = 152 nM InsP3 and Bmax = 180 pmol/mg for the DmInsP3R and kd = 214 nM InsP3 and Bmax = 124 pmol/mg for the ug3 mutant. The experiment was repeated in duplicate with similar results.
To understand this discrepancy, the sensitivity of ug3 channels to activation by low InsP3 concentrations was investigated in bilayer experiments. We discovered that ug3 channels displayed maximum open probability (Po) at InsP3 concentrations as low as 50 nM (Fig. 11, A and B). Much higher concentrations of InsP3 are required to fully activate the wild-type DmInsP3R (Fig. 11, C and D, and Fig. 7). The ug3 channels are expressed in sf9 cells at a much lower level than DmInsP3R. This was evident from the Bmax values (180 pmol/mg for DmInsP3R versus 124 pmol/mg for ug3 mutant) and the significantly lower abundance of the InsP3R positive band in ug3 microsomes, as seen in a quantitative Western blot (Fig. 4, data not shown). Because of reduced expression levels of ug3 mutant and resulting difficulties with channel recordings, we have not been able to obtain a complete InsP3-dependence curve for this mutant in planar lipid bilayers. However, it is clear from Fig. 11 that, unlike the DmInsP3R, the open probability for ug3 is similar at 50 nM and 2 μM InsP3, indicating a shift in apparent sensitivity to activation by InsP3.
FIGURE 11.
InsP3 sensitivity of ug3 channels. (A) Activity of ug3 channels in bilayers in the presence of 50 nM InsP3 and 2 μM InsP3 is shown. Each current trace corresponds to 10 s of current recording from the same experiment. (B) The average open probability (Po) of ug3 channels is calculated for a 5-s window of time and plotted for the duration of an experiment. The times of 50 nM and 2 μM InsP3 additions to the bilayer are shown above the Po plot. Data from the same experiment are shown in A and B. Similar results were obtained in three independent experiments. (C–D) Same data as in A and B for the wild-type DmInsP3R channels. Similar results were obtained in three independent experiments.
Functional properties of the wc703 mutant
To characterize single-channel properties of the wc703 mutant, its embryonic form was expressed in sf9 cells by baculovirus infection. The expression levels of the wc703 mutant in sf9 cells were comparable to expression levels of the wild-type channels (Fig. 4). When microsomes isolated from the wc703-infected sf9 cells were fused to planar lipid bilayers, InsP3-gated channels were observed in 25 of 40 experiments (Fig. 12 A). Thus, similar to the ug3 mutant, homomeric channels formed by wc703 mutant subunits are functional. The mean current amplitude of wc703 channels at 0-mV holding potential is 1.74 ± 0.08 pA (Fig. 12 A), with a mean open dwell time of 4.5 ± 0.4 ms (Fig. 12 A), and single-channel conductance of 69.0 ± 0.1 pS (n = 3, data not shown). Thus, gating and conductance properties of wc703 channels are not significantly different from the wild-type DmInsP3R (embryonic form). To determine the effect of wc703 mutation on Ca2+ sensitivity of the DmInsP3R, we measured open probability of wc703 channels in bilayers at different Ca2+ concentrations. We discovered that, in contrast to the wild-type DmInsP3R, the maximal open probability of wc703 was achieved at pCa 6.8 (Fig. 12 B). On an average, the single-channel open probability of wc703 channels at pCa 6.8 was equal to 0.21 ± 0.013. Fit to the averaged Ca2+-dependence data (Fig. 12 C, n = 3) using a modified bell-shaped equation from Bezprozvanny et al. (1991) yielded the affinities of activating and inhibitory sites of DmInsP3R equal to 0.17 μM Ca2+ and 0.18 μM Ca2+, respectively. The observed effects on the DmInsP3R Ca2+ sensitivity are consistent with the position of the wc703 mutation (G2117E) in a Ca2+-sensor region (Tu et al., 2003) of the DmInsP3R (Fig. 1). The results suggest that the increased Rmax observed in the Ca2+-release assay with microsomes from wc703/+ flies (Fig. 2 E) can be explained by the approximately twofold increase in an apparent affinity of DmInsP3R Ca2+ sensor and 30% increase in the DmInsP3R maximal single-channel open probability.
FIGURE 12.
Functional properties of wc703 channels. (A) Representative channel activity of wc703 channels recorded in the standard recording conditions. Current records are shown at compressed and expanded timescales as indicated. Unitary current amplitude histograms and open dwell time distributions from the same experiments are shown below the current records. Unitary current distribution was fit with a Gaussian function that was centered at 1.82 pA. Open time distribution was fit with a single exponential function (curve) that yielded a τ0 of 3.8 ms. Similar analysis was performed for at least four independent experiments with wc703 microsomes. (B) The current recordings of wc703 from the same experiment at different Ca2+ concentration as indicated. (C) Bell-shaped Ca2+ dependence of wc703. The Po measured in three independent experiments with wc703 were averaged together at each Ca2+ concentration as described in Materials and Methods and shown as mean ± SE (•). The averaged data were fitted by the bell-shaped equation modified from Bezprozvanny et al. (1991) as explained in Materials and Methods. The optimal fit (thick smooth curve) was obtained with nHill = 1.9, Pm = 0.2, kact = 0.17 μM Ca2+, and Kinh = 0.18 μM Ca2+. The fit to the Ca2+ dependence of the wild-type DmInsP3R (embryonic form) fromFig. 8 is shown for the reference (thin line).
Absence of channel function in the ka901 mutant
The DmInsP3R ka901 mutant (embryonic form) was expressed in sf9 cells by baculovirus infection. As determined by Western blotting, expression levels of ka901 in sf9 cells were comparable to expression levels of wild-type channels (Fig. 4). However, InsP3-gated channel activity was not observed in bilayer experiments with microsomes from ka901-infected sf9 cells (n = 15), indicating that ka901 homomeric channels are nonfunctional. This idea is supported by the observation that ka901 mutants are equivalent to null alleles of the InsP3R in Drosophila (Joshi et al., 2004). Interestingly, however, we did measure increased Ca2+ fluxes in microsomes from ka901/+ flies (Fig. 2 F). Taken together, these observations suggest that ka901 may form functional channels when assembled with the wild-type DmInsP3R subunits, but not in the homomeric state.
DISCUSSION
Major functional properties of InsP3Rs are conserved in evolution
The major functional properties of embryonic and adult head splice variants of the Drosophila InsP3R obtained from planar lipid bilayer experiments (Figs. 5–8) have been described here. To the best of our knowledge, this is the first description of single-channel properties of Drosophila melanogaster InsP3R. On comparison with the properties of rat InsP3R1, determined in identical experimental conditions in our previous studies (Tang et al., 2003b; Tu et al., 2002), we conclude that the single-channel properties of the DmInsP3R are remarkably similar to the single-channel properties of mammalian InsP3R1, apart from a broader Ca2+-dependence curve (Table 1). This conclusion agrees with the conservation of InsP3R domain structure (Fig. 1), and the high degree of sequence identity (57%) between DmInsP3R and rat InsP3R1, and with previous analysis of InsP3-dependent Ca2+ fluxes in Drosophila-derived S2 cells (Swatton et al., 2001). Overall, our results validate the use of Drosophila as a genetic model for InsP3R structure-function analysis, as results obtained with the DmInsP3R are likely to have an implication for understanding the mechanisms of mammalian InsP3R function. Interestingly, an SIII splice variant of the human type I InsP3R, spliced in a region homologous to the Drosophila adult head isoform, is enriched in brain tissues (Nucifora et al., 1995). This SIII splice variant contains nine additional amino acids, which give rise to a putative protein kinase C phosphorylation site. The nine-amino-acid insert in the fly adult head isoform also contains a putative casein kinase II phosphorylation site. It would thus be interesting to compare the single-channel properties of the SIII isoform of human InsP3RI with the adult head isoform of DmInsP3R described here.
DmInsP3R ug3—the InsP3 efficacy mutant
The ug3 mutation (S224F) is located within the ligand-binding domain of DmInsP3R (Fig. 1). Thus the observation that ug3 affects the InsP3 sensitivity of DmInsP3R channel opening (Fig. 11, A–D), but does not increase InsP3 binding affinity (Fig. 10), is intriguing. This result suggests that the InsP3R inhibitory domain plays an important role in coupling InsP3 binding to the core domain with opening of the InsP3R channel gate, in agreement with recent biochemical (Boehning and Joseph, 2000) and functional (Uchida et al., 2003) data. Increased open dwell time in ug3 mutants (by 47%) is consistent with the conclusion that ug3 is not a binding mutant, but is, in fact, a gating mutant. These data lead us to conclude that the increased apparent affinity to InsP3 (change in Km) observed in Ca2+ flux assay with ug3/+ microsomes (Fig. 2 D) results not from an increased affinity of the DmInsP3R for InsP3 but from an increased efficacy in coupling InsP3 binding with DmInsP3R channel opening. Thus, ug3 is a gain-of-function mutant that acts by increasing sensitivity of the DmInsP3R to activation by InsP3 and also by increasing dwell open time of the channels. Since the affinity of InsP3 binding is not increased by the ug3 mutation (Fig. 10), we classified the ug3 mutant as the efficacy mutant, that is, the mutant with increased efficiency of coupling between InsP3 binding and channel opening. This conclusion is consistent with the biochemical association observed between InsP3R1 amino-terminal and carboxy-terminal ends (Boehning and Joseph, 2000) and a recent suggestion that the InsP3R1 inhibitory amino-terminal domain participates in the channel-gating process (Uchida et al., 2003).
The observation that ug3 homomeric channels are functional is consistent with the weak allelic strength of ug3 mutants. Heterozygotes of the genotype ug3/90B0 die as late second- or early third-instar larvae (Deshpande et al., 2000; Joshi et al., 2004) as compared to ka901/90B0 and 90B0/90B0 (itpr null) mutants, both of which die as early second-instar larvae (Venkatesh and Hasan, 1997).
DmInsP3R wc703—the Ca2+ sensor mutant
Microsomes from wc703/+ flies display increased Ca2+ release (1.4-fold increase in Rmax) in Ca2+ flux experiments (Fig. 2 E). Although single channels formed by wc703 display wild-type conductance and gating properties (Fig. 12 A and data not shown), they also exhibit increased sensitivity to Ca2+ and a higher maximal open probability (Fig. 12, B and C, and Table 1). The peak of wc703 channel Ca2+ dependence is at pCa 6.8 (compare to pCa 6.4 for wild-type channels) and their maximal open probability is 21% (as compared to 15% for wild-type). A fit of the bell-shaped Ca2+ dependence of wc703 channels yielded affinities of activating and inhibitory Ca2+ binding sites equal to 0.17 μM Ca2+ and 0.18 μM Ca2+, respectively (as compared to 0.28 μM Ca2+ and 0.53 μM Ca2+ for the wild-type DmInsP3R). These results indicate that wc703 is also a gain-of-function mutant that acts by increasing sensitivity of DmInsP3R to activation by Ca2+. These effects are consistent with the position of wc703 mutation (G2117E) within a putative Ca2+ sensor region (Tu et al., 2003) of the DmInsP3R (Fig. 1). The wc703 homomeric channels are functional, consistent with the weak allelic strength of wc703 mutants, which is similar to that of the ug3 allele (Deshpande et al., 2000; Joshi et al., 2004).
DmInsP3R ka901—the Ca2+ channel mutant
The ka901 mutation lies in the pore-forming region of the InsP3R and RyanR families among which a putative pore-forming motif GXRXGGGI/VGD (the G2630 residue of the DmInsP3R mutated in ka901 is underlined) is highly conserved. Mutational studies of the homologous G4826 residue in RyanR2 has shown that the G4826C mutant does not form functional homomeric channels in bilayer experiments (Chen et al., 2002), similar to what we observe with ka901 homomeric channels. The absence of functional channels from ka901 homomers in bilayer experiments agrees with the genetic finding that the allelic strength of ka901 is equivalent to that of the null allele 90B0 (Joshi et al., 2004). To understand why microsomal vesicles from ka901/+ heterozygotes release greater amounts of Ca2+, co-infection with wild-type DmInsP3R and ka901 studies can be done similar to what has been done with RyanR2-G4826C to obtain hybrid channels (Chen et al., 2002; Zhao et al., 1999). These experiments are currently in progress.
CONCLUSION
Genetic and biophysical approaches to the InsP3R structure-function
In conclusion, single-channel properties of embryonic and adult head splice variants of the Drosophila InsP3R (Sinha and Hasan, 1999) and three previously isolated and sequenced Drosophila InsP3R point mutants (Deshpande et al., 2000; Joshi et al., 2004) have been characterized in our study. Our results indicate that the main functional properties of InsP3R are conserved in evolution from DmInsP3R to InsP3R1 (Table 1) and furnish further evidence for differences in function between splice variant isoforms. Changes in the DmInsP3R properties induced by random mutagenesis have helped identify residues that may not necessarily be identified easily by a targeted mutagenesis approach. Although one of these residues (ka901) in homomeric state supports the data obtained earlier from a similar mutation in the RyanR2 (Chen et al., 2002), the others provide fresh insights into the mechanism of Ca2+ dependence and the relationship between ligand binding and gating. Furthermore, the single-channel properties of the wild-type and mutant DmInsP3Rs have been correlated with characteristics of InsP3-dependent Ca2+ fluxes supported by these channels in experiments with microsomes from wild-type and mutant Drosophila. The approaches used in this work have thus allowed for the extension of genetic studies of the InsP3R in a model organism to biophysical studies on the channel.
Acknowledgments
We thank Tie-Shan Tang for assistance with biochemical experiments, and Phyllis Foley for expert administrative assistance.
S.S.'s visit to Dallas was supported by the Journal of Cell Science Traveling Fellowship. I.B. is supported by the Robert A. Welch Foundation and the National Institutes of Health (R01 NS38082). G.H. is supported by a grant from the Department of Science and Technology and core grants from the National Centre for Biological Sciences, Tata Institute of Fundamental Research, Bangalore, India.
References
- Berridge, M. J. 1993. Inositol trisphosphate and calcium signalling. Nature. 361:315–325. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny, I., and B. E. Ehrlich. 1995. The inositol 1,4,5-trisphosphate (InsP3) receptor. J. Membr. Biol. 145:205–216. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny, I., J. Watras, and B. E. Ehrlich. 1991. Bell-shaped calcium-response curves of Ins1,4,5P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 351:751–754. [DOI] [PubMed] [Google Scholar]
- Boehning, D., and S. K. Joseph. 2000. Direct association of ligand-binding and pore domains in homo- and heterotetrameric inositol 1,4,5-trisphosphate receptors. EMBO J. 19:5450–5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehning, D., D. O. Mak, J. K. Foskett, and S. K. Joseph. 2001. Molecular determinants of ion permeation and selectivity in inositol 1,4,5-trisphosphate receptor Ca2+ channels. J. Biol. Chem. 276:13509–13512. [DOI] [PubMed] [Google Scholar]
- Bosanac, I., J. R. Alattia, T. K. Mal, J. Chan, S. Talarico, F. K. Tong, K. I. Tong, F. Yoshikawa, T. Furuichi, M. Iwai, T. Michikawa, K. Mikoshiba, and M. Ikura. 2002. Structure of the inositol 1,4,5-trisphosphate receptor binding core in complex with its ligand. Nature. 420:696–700. [DOI] [PubMed] [Google Scholar]
- Bramley, T. A., G. S. Menzies, and T. Jowett. 1990. Specific binding sites for 125I-labelled human luteinizing hormone (hLH) in microsomal fractions from Drosophila. Mol. Cell Endocrinol. 73:93–104. [DOI] [PubMed] [Google Scholar]
- Chen, S. R., P. Li, M. Zhao, X. Li, and L. Zhang. 2002. Role of the proposed pore-forming segment of the Ca2+ release channel (ryanodine receptor) in ryanodine interaction. Biophys. J. 82:2436–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande, M., K. Venkatesh, V. Rodrigues, and G. Hasan. 2000. The inositol 1,4,5-trisphosphate receptor is required for maintenance of olfactory adaptation in Drosophila antennae. J. Neurobiol. 43:282–288. [DOI] [PubMed] [Google Scholar]
- Furuichi, T., K. Kohda, A. Miyawaki, and K. Mikoshiba. 1994. Intracellular channels. Curr. Opin. Neurobiol. 4:294–303. [DOI] [PubMed] [Google Scholar]
- Hasan, G., and M. Rosbash. 1992. Drosophila homologs of two mammalian intracellular Ca2+-release channels—identification and expression patterns of the inositol 1,4,5-triphosphate and the ryanodine receptor genes. Development. 116:967–975. [DOI] [PubMed] [Google Scholar]
- Joshi, R., K. Venkatesh, R. Srinivas, S. Nair, and G. Hasan. 2004. Genetic dissection of Itpr gene function reveals a vital requirement in aminergic cells of Drosophila larvae. Genetics. 166:225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaznacheyeva, E., V. D. Lupu, and I. Bezprozvanny. 1998. Single-channel properties of inositol (1,4,5)-trisphosphate receptor heterologously expressed in HEK-293 cells. J. Gen. Physiol. 111:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupu, V. D., E. Kaznacheyeva, U. M. Krishna, J. R. Falck, and I. Bezprozvanny. 1998. Functional coupling of phosphatidylinositol 4,5-bisphosphate to inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 273:14067–14070. [DOI] [PubMed] [Google Scholar]
- Mathew, M. K., R. Nagaraj, and P. Balaram. 1982. Ionophore-mediated transmembrane movement of divalent cations in small unilamellar liposomes: an evaluation of the chlortetracycline fluorescence technique and correlations with black lipid membrane studies. J. Membr. Biol. 65:13–7. [DOI] [PubMed] [Google Scholar]
- Mignery, G. A., and T. C. Sudhof. 1990. The ligand binding site and transduction mechanism in the inositol-1,4,5-triphosphate receptor. EMBO J. 9:3893–3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa, T., A. Maeda, T. Yamazawa, K. Hirose, T. Kurosaki, and M. Iino. 1999. Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. EMBO J. 18:1303–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa, T., A. Mizushima, K. Hirose, T. Yamazawa, I. Bezprozvanny, T. Kurosaki, and M. Iino. 2001. Ca2+-sensor region of IP3 receptor controls intracellular Ca2+ signaling. EMBO J. 20:1674–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers, E. W., G. G. Sutton, A. L. Delcher, I. M. Dew, D. P. Fasulo, M. J. Flanigan, S. A. Kravitz, C. M. Mobarry, K. H. Reinert, K. A. Remington, E. L. Anson, R. A. Bolanos, H. H. Chou, C. M. Jordan, A. L. Halpern, S. Lonardi, E. M. Beasley, R. C. Brandon, L. Chen, P. J. Dunn, Z. Lai, Y. Liang, D. R. Nusskern, M. Zhan, Q. Zhang, X. Zheng, G. M. Rubin, M. D. Adams, and J. C. Venter.2000. A whole-genome assembly of Drosophila. Science. 287:2196–2204. [DOI] [PubMed] [Google Scholar]
- Nosyreva, E., T. Miyakawa, Z. Wang, L. Glouchankova, A. Mizushima, M. Iino, and I. Bezprozvanny. 2002. The high affinity calcium-calmodulin-binding site does not play a role in modulation of type 1 inositol (1,4,5)-trisphosphate receptor function by calcium and calmodulin. Biochem. J. 365:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucifora, F. C., Jr., S. H. Li, S. Danoff, A. Ullrich, and C. A. Ross. 1995. Molecular cloning of a cDNA for the human inositol 1,4,5-trisphosphate receptor type 1, and the identification of a third alternatively spliced variant. Brain Res. Mol. Brain Res. 32:291–296. [DOI] [PubMed] [Google Scholar]
- Ramos-Franco, J., D. Galvan, G. A. Mignery, and M. Fill. 1999. Location of the permeation pathway in the recombinant type 1 inositol 1,4,5-trisphosphate receptor. J. Gen. Physiol. 114:243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha, M., and G. Hasan. 1999. Sequencing and exon mapping of the inositol 1,4,5-trisphosphate receptor cDNA from Drosophila embryos suggests the presence of differentially regulated forms of RNA and protein. Gene. 233:271–276. [DOI] [PubMed] [Google Scholar]
- Swatton, J. E., S. A. Morris, F. Wissing, and C. W. Taylor. 2001. Functional properties of Drosophila inositol trisphosphate receptors. Biochem. J. 359:435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, T.-S., H. Tu, E. Y. Chan, A. Maximov, Z. Wang, C. L. Wellington, M. R. Hayden, and I. Bezprozvanny. 2003a. Huntingtin and huntingtin-associated protein 1 influence neuronal calcium signaling mediated by inositol-(1,4,5) triphosphate receptor type 1. Neuron. 39:227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, T. S., H. Tu, Z. Wang, and I. Bezprozvanny. 2003b. Modulation of type 1 inositol (1,4,5)-trisphosphate receptor function by protein kinase A and protein phosphatase 1α. J. Neurosci. 23:403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, C. W. 1998. Inositol trisphosphate receptors: Ca2+-modulated intracellular Ca2+ channels. Biochim. Biophys. Acta. 1436:19–33. [DOI] [PubMed] [Google Scholar]
- Thrower, E. C., R. E. Hagar, and B. E. Ehrlich. 2001. Regulation of Ins(1,4,5)P3 receptor isoforms by endogenous modulators. Trends Pharmacol. Sci. 22:580–586. [DOI] [PubMed] [Google Scholar]
- Tu, H., T. Miyakawa, Z. Wang, L. Glouchankova, M. Iino, and I. Bezprozvanny. 2002. Functional characterization of the type 1 inositol 1,4,5-trisphosphate receptor coupling domain SII(+/−) splice variants and the opisthotonos mutant form. Biophys. J. 82:1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu, H., E. Nosyreva, T. Miyakawa, Z. Wang, A. Mizushima, M. Iino, and I. Bezprozvanny. 2003. Functional and biochemical analysis of the type 1 inositol (1,4,5)-trisphosphate receptor calcium sensor. Biophys. J. 85:290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida, K., H. Miyauchi, T. Furuichi, T. Michikawa, and K. Mikoshiba. 2003. Critical regions for activation gating of the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 278:16551–16560. [DOI] [PubMed] [Google Scholar]
- Venkatesh, K., and G. Hasan. 1997. Disruption of the IP3 receptor gene of Drosophila affects larval metamorphosis and ecdysone release. Curr. Biol. 7:500–509. [DOI] [PubMed] [Google Scholar]
- Wagner 2nd, L. E., W. H. Li, and D. I. Yule. 2003. Phosphorylation of type-1 inositol 1,4,5-trisphosphate receptors by cyclic nucleotide-dependent protein kinases: a mutational analysis of the functionally important sites in the S2+ and S2− splice variants. J. Biol. Chem. 278:45811–45817. [DOI] [PubMed] [Google Scholar]
- Yoshikawa, F., H. Iwasaki, T. Michikawa, T. Furuichi, and K. Mikoshiba. 1999. Cooperative formation of the ligand-binding site of the inositol 1,4,5-trisphosphate receptor by two separable domains. J. Biol. Chem. 274:328–334. [DOI] [PubMed] [Google Scholar]
- Yoshikawa, F., M. Morita, T. Monkawa, T. Michikawa, T. Furuichi, and K. Mikoshiba. 1996. Mutational analysis of the ligand binding site of the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 271:18277–18284. [DOI] [PubMed] [Google Scholar]
- Yoshikawa, S., T. Tanimura, A. Miyawaki, M. Nakamura, M. Yuzaki, T. Furuichi, and K. Mikoshiba. 1992. Molecular cloning and characterization of the inositol 1,4,5-trisphosphate receptor in Drosophila melanogaster. J. Biol. Chem. 267:16613–16619. [PubMed] [Google Scholar]
- Zhao, M., P. Li, X. Li, L. Zhang, R. J. Winkfein, and S. R. Chen. 1999. Molecular identification of the ryanodine receptor pore-forming segment. J. Biol. Chem. 274:25971–25974. [DOI] [PubMed] [Google Scholar]














