Abstract
Myosin VI is a two-headed molecular motor that moves along an actin filament in the direction opposite to most other myosins. Previously, a single myosin VI molecule has been shown to proceed with steps that are large compared to its neck size: either it walks by somehow extending its neck or one head slides along actin for a long distance before the other head lands. To inquire into these and other possible mechanism of motility, we suspended an actin filament between two plastic beads, and let a single myosin VI molecule carrying a bead duplex move along the actin. This configuration, unlike previous studies, allows unconstrained rotation of myosin VI around the right-handed double helix of actin. Myosin VI moved almost straight or as a right-handed spiral with a pitch of several micrometers, indicating that the molecule walks with strides slightly longer than the actin helical repeat of 36 nm. The large steps without much rotation suggest kinesin-type walking with extended and flexible necks, but how to move forward with flexible necks, even under a backward load, is not clear. As an answer, we propose that a conformational change in the lifted head would facilitate landing on a forward, rather than backward, site. This mechanism may underlie stepping of all two-headed molecular motors including kinesin and myosin V.
INTRODUCTION
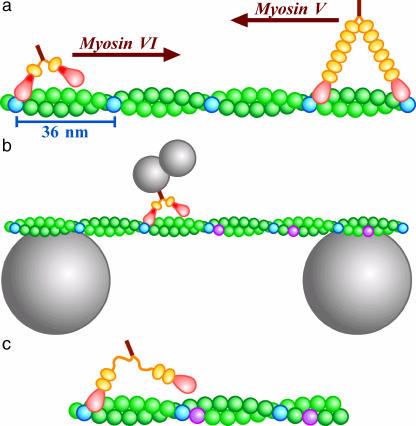
Myosin VI is a member of the myosin superfamily that consists of adenosine 5′-triphosphate (ATP)-driven molecular motors that track along an actin filament. It is believed that myosin VI plays a role in intracellular vesicle and organelle transport (Mermall et al., 1994). Myosin VI is distinct from other members in that it moves in the opposite direction on an actin filament toward the pointed, or minus, end (Wells et al., 1999; Homma et al., 2001). The movement is stepwise, and processive in that a single molecule of myosin VI moves for many steps without detaching from actin (Rock et al., 2001; Nishikawa et al., 2002). Another class of myosin, myosin V that moves toward the barbed, or plus, end, has also been shown to be a processive stepper (Mehta et al., 1999; Rief et al., 2000; Tanaka et al., 2002; Ali et al., 2002). Both myosin VI and V, like most other myosins, have two globular motor domains, usually called heads. The heads are connected through a neck-like structure, reinforced with light chains, to a central stalk (Fig. 1 a). A head of myosin binds to actin and hydrolyzes ATP to produce a mechanical step, possibly by tilting the neck forward as a lever (Huxley, 1969; Walker et al., 2000). The neck of myosin V indeed appears to tilt (Walker et al., 2000; Burgess et al., 2002; Forkey et al., 2003), and tilting of the long neck can account for the observed step size (Mehta et al., 1999; Rief et al., 2000; Tanaka et al., 2002; Ali et al., 2002) of ∼36 nm (Fig. 1 a). However, myosin VI (Rock et al., 2001; Nishikawa et al., 2002) and a truncation mutant of myosin V (Tanaka et al., 2002), both of which are short necked, also showed similar step sizes, casting doubt on simple walking.
FIGURE 1.
Experimental design. (a) Movement of myosin VI and V along an actin filament. Heads of myosins are in pink, and necks in orange, orange balls representing a light chain or calmodulin that binds to the neck. Myosin VI contains an extra insert in the head (red). Every 13th monomers of actin (green), counting both strands, are shown in blue. The barbed (fast growing) end of the filament is on the left. (b) Motility assay system (not to scale). An actin bridge was made on two beads, either 4.5 or 6 μm in diameter. A duplex of smaller beads carrying a myosin VI molecule was allowed to move freely along and around the filament. (c) Recent work indicates that the extra insert in myosin VI is a second calmodulin binding domain and that a flexible region connects it to the stalk (see text).
Most of these previous studies did not allow free rotation of myosin around an actin filament, and thus myosin may have been forced to step between binding sites that are 36 nm apart along one side of the actin filament. Unconstrained motion of myosin V on an actin bridge (Fig. 1 b) has been shown to be a long-pitch (2.2 μm) left-handed spiral (Ali et al., 2002), indicating an average step size of 34.8 nm that is slightly shorter than the actin helical repeat. Short-necked myosin VI would spiral with a shorter pitch (<2 μm) to the left or spiral with the 72-nm actin pitch to the right if the step size is <18 nm. To see if, or how, myosin VI spirals, we constructed the system in Fig. 1 b. The result was an unexpected one, a long-pitch right-handed spiral, which is difficult to explain by the lever action alone. We propose that a conformational change in a lifted head is an important mechanism that assures these and other linear molecular motors to proceed in the correct direction even under a backward load.
MATERIALS AND METHODS
Proteins and beads
A recombinant, full-length myosin VI (M6WT) was expressed and purified as described (Nishikawa et al., 2002). Actin was biotinylated and stained with phalloidin-tetramethylrhodamine (Ali et al., 2002). A quantity of 1-μm polystyrene beads (F-8814, Molecular Probes, Eugene, OR), or a mixture of 1- and 0.45-μm beads, were treated (Ali et al., 2002) in buffer A (10 mM imidazole (pH 7.6), 150 mM KCl, 1 mM MgCl2, 1 mM EGTA, 10 mM dithiothreitol (DTT)) containing 10 mg/ml bovine serum albumin (BSA) to form duplexes (∼10% of all beads). The beads were coated with myosin VI by mixing at myosin/bead molar ratio c in buffer A containing 10 mg/ml bovine serum albumin, as described (Rief et al., 2000). The coating efficiency was estimated (Ali et al., 2002) by challenging a single (not duplex) bead against an actin filament to see if it bound and moved in buffer A containing 5 mM ATP. The fractions of beads that bound to actin, pb, and that bound and moved, pm, could be fitted with p = 1 − exp(−λc), which represents the theoretical probability (based on Poisson statistics) that a bead carries one or more active motors (Block et al., 1990); λb was 0.028, and λm was 0.010. Motility assay was performed at c = 1 (pm = 0.01 and pb = 0.03), c = 20 (pm = 0.18 and pb = 0.43), or c = 1000 (p = 1).
Motility assay
Actin bridges between 4.5- or 6.0-μm carboxylated polystyrene beads (Polyscience, Warrington, PA) were formed in a flow chamber (Ali et al., 2002). Then, beads decorated with myosin VI in buffer A containing ATP, 6 mg/ml glucose, 0.2 mg/ml glucose oxidase, 0.02 mg/ml catalase, and 0.2% β-mercaptoethanol were infused. To minimize Brownian motion, we selected a tightly suspended actin filament, and positioned a bead duplex onto the filament using optical tweezers (Suzuki et al., 1996). We moved the filament by moving the microscope stage until it bound the duplex, and turned off the optical trap to let the duplex move along the actin filament. Bright-field images showing bead movement and fluorescence images showing actin filaments were simultaneously recorded with video cameras (Suzuki et al., 1996). Positions and orientations of bead duplexes were analyzed by eye to the precision of ±1 pixel (0.13 μm) and ±0.2 revolutions (Ali et al., 2002). Observations were made at 26°C.
RESULTS AND DISCUSSION
Spiral motion of myosin VI
After confirming the formation of a fluorescently stained actin bridge between large beads (Fig. 1 b), we selected a bead duplex carrying myosin VI with optical tweezers and manipulated it around the actin bridge, in the presence of ATP, until the duplex started to move along actin. Most duplexes did not move at the myosin/bead ratio c = 1 or 20 (Table 1), indicating, on the assumption of Poisson statistics (Block et al., 1990), that >98% (c = 1) or >90% (c = 20) of duplexes that moved carried only one active myosin molecule (also see Materials and Methods above); most duplexes moved at c = 1000. At 5 mM ATP, the average velocity was 230 nm/s, consistent with previous studies (Rock et al., 2001; Nishikawa et al., 2002).
TABLE 1.
Summary of bead-duplex experiments
| Myosin/bead (c) | 1 | 20 | 1000 |
|---|---|---|---|
| Total number tested* | 694 | 729 | 31 |
| Duplexes bound to actin | 40 | 243 | 30 |
| Moved for >0.5 μm | 24 | 143 | 25 |
| Rotated for >0.5 turn† | 5 | 18 | 7 |
| Pitch (μm/turn) | 2.2 ± 1.1 | 2.5 ± 1.3 | 5.6 ± 2.9 |
| Run length (μm) | 1.0 ± 0.4 | 1.8 ± 0.9 | 3.0 ± 1.1 |
| Moved straight | 19 | 125 | 18 |
| Run length (μm) | 1.2 ± 0.5 | 1.3 ± 1.3 | 3.6 ± 1.4 |
ATP concentrations were 100 μM, 400 μM, or 5 mM. The average velocity estimated on randomly chosen data was 230 ± 91 nm/s at 5 mM (n = 18), 48 ± 30 nm/s at 400 μM (n = 40), and 18 ± 11 nm/s at 100 μM (n = 20); no significant dependence on c. All ranges are standard deviations.
A duplex was manipulated for ∼30 s from various directions toward actin. This maneuver was repeated at least 5 times (10 at c = 1) until binding occurred.
All rotations were right handed except for one at c = 1000, which was left handed with a pitch of 3.3 μm/turn and run length of 5 μm. Pitch and run length in this table do not include this datum.
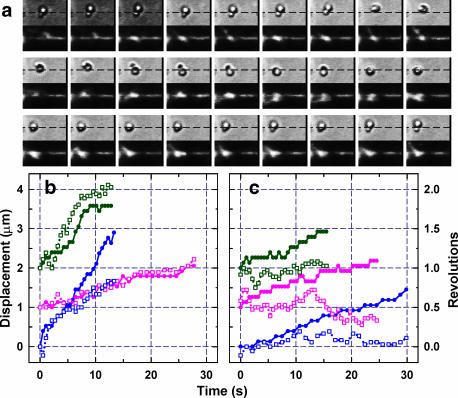
Fig. 2 a shows sequential images of a bead duplex (upper rows) and actin filament (lower rows) at 0.4-s intervals. The dashed lines in the upper images show the position of the actin filament deduced from the lower images. The focus for the upper images was set such that a bead appears white when it is closer to the observer and black when it is away. The upper images thus show that the bead duplex moved as a right-handed spiral around the actin filament. In 10.8 s, the duplex moved 1.4 μm while making 1.0 turn, indicating a spiral pitch of 1.4 μm. Fig. 2, b and c, compile six time courses, of which three show right-handed spiraling and the others straight movement without significant rotation. Except for one left-handed spiral at c = 1000, we observed only these two patterns, and we observed mostly straight motion as shown in Table 1. Also, as with kinesin (Hua et al., 2002) and myosin V (Ali et al., 2002), we did not observe clear 180° rotation of myosin VI around its own axis that is expected to accompany every step if a motor with two identical heads attains the same posture after each step (Howard, 1996). The absence of 180° rotation is accounted for if a free joint(s) exists in the necks to give the motor the freedom of adopting different postures after even and odd steps. For myosins (including VI and V) with necks reinforced with light chains, a free joint must exist to orient both heads in the same direction, breaking their twofold symmetry, when the two heads attach to actin during processive walking. Electron micrographs of myosin V (Walker et al., 2000) indeed indicate the presence of a free joint near the neck-stalk junction. The absence of extensive rotation, either spiraling or the 180° rotation, could be due to the attachment of a bead duplex that would impede fast rotation. However, slowing down the overall motion by a factor of >10, by reducing the ATP concentration down to 100 μM, did not change the motional patterns (Table 1).
FIGURE 2.
Examples of duplex movement. (a) Sequential images at 0.4-s intervals. Upper and lower panels show bright-field and corresponding fluorescence images; width of each panel, 7.4 μm. (Dashed lines) Position of the actin filament deduced from lower panels. This bead duplex (1 and 0.45 μm) carried a short actin filament that also rotated. (b and c) Time courses for spiral (b) and straight (c) movements at c = 1 (green), 20 (magenta), and 1000 (blue). (Closed symbols) Displacement along actin; (open symbols) rotation. ATP concentration was 400 μM, except for the blue curve in b (5 mM). For clarity, curves are shifted vertically. Images in a correspond to part of green curves in b. Also see movies in Supplementary Material.
The straight movement of myosin VI on one side of the actin filaments implies an average step size coincident with the actin helical repeat of 36 nm (blue subunits in Fig. 1). The right-handed spiral indicates larger steps (purple in Fig. 1 b). The pitch of 2.4 μm (mean at c ≤ 20) implies (Ali et al., 2002) a step size of 36 nm × (2400 nm + 72 nm)/(2400 nm) = 37.1 nm. These step sizes are slightly longer than those of myosin V or of myosin XI (Tominaga et al., 2003), which are both long necked: to walk with such long strides, myosin VI has to somehow extend its necks. Another possibility is the sliding of an attached head along one strand of actin, whereby short-necked myosin VI could produce an apparently large step size (Nishikawa et al., 2002; Tanaka et al., 2002). The essentially straight motion observed here could be explained if the short-pitch (72 nm) spiraling expected to accompany the sliding is precisely cancelled by crossing, with the other head, onto the other strand. Here we focus on the simpler mechanism of neck extension.
Myosin VI may move like kinesin
The idea of neck extension is not unprecedented. Conventional kinesin, which proceeds along a microtubule with 8-nm steps (Svoboda et al., 1993), has two rather short necks that are not reinforced with light chains and are considered flexible. In a likely scenario (Vale and Milligan, 2000), an unattached head makes a diffusional search for a next binding site. The sites are distributed 8-nm apart, and reaching the site 8-nm ahead requires almost full extension of the flexible necks. To bias the diffusion of the unattached head in the forward direction, the attached head docks a part of its neck, called neck-linker, such that the docked part is oriented forward. It is undocked again to extend the neck when the associate head is to be thrown forward. For myosin VI, two possibilities have been suggested for its neck extension: undocking or unfolding of the extra insert in the head (red parts in Fig. 1) (Rock et al., 2001), or unzippering of part of the stalk coiled coil (Nishikawa et al., 2002). An electron micrograph indeed showed necks of myosin VI that were somehow extended (Nishikawa et al., 2002). More recent work indicates that the extra insert in myosin VI is actually a second calmodulin binding domain (Bahloul et al., 2004), and that a flexible region follows this part before the two necks join to form a coiled coil (B. R. Rami and J. A. Spudich, Stanford University, and H. L. Sweeney and C. Franzini-Armstrong, Pennsylvania University, personal communications, 2004). Thus, myosin VI seems to be able to span the observed ∼36-nm step size by extending the flexible portion of its two necks (Fig. 1 c). The probably stiff calmodulin-binding region could serve as a lever, but the lever is too short (∼8 nm) to carry a lifted head 36 nm forward. The head must reach the forward binding site by diffusion. Myosin VI likely walks in a way similar to kinesin (biased diffusional search).
Biasing of diffusion, however, is not trivial in the presence of an opposing external force. In kinesin, the free energy difference between the docked and undocked states is small (Rice et al., 2003), implying that docking would fail when the stalk is pulled back by a load. For myosin V, rotating a landed neck forward as a lever (Moore et al., 2001; Veigel et al., 2002; Burgess et al., 2002) could serve the purpose of biasing the diffusion of the lifted head. For forward bias, however, the pivot near the neck-stalk junction (Fig. 3 a) has to pass the attached head by moving forward by >≈18 nm (Fig. 1 a). But the pivot would be pulled back by 10 nm at 2 pN of backward load where myosin V still moves forward (Rief et al., 2000), given an estimated neck stiffness (Veigel et al., 2002) of 0.2 pN/nm. Also, rotating the attached neck, ∼23 nm long, against the backward load of 2 pN requires a torque of more than 40 pN·nm, the torque of a powerful rotary motor F1-ATPase (Yasuda et al., 1998). For myosin V, too, simple biasing seems difficult at a high load. This is more serious with myosin VI for which the lever is short and the rest of the neck is flexible: when the stalk is pulled backward, the unattached head would tend to diffuse backward rather than forward. Nevertheless, myosin VI moves forward under a backward load up to ∼2 pN (Rock et al., 2001; Nishikawa et al., 2002).
FIGURE 3.
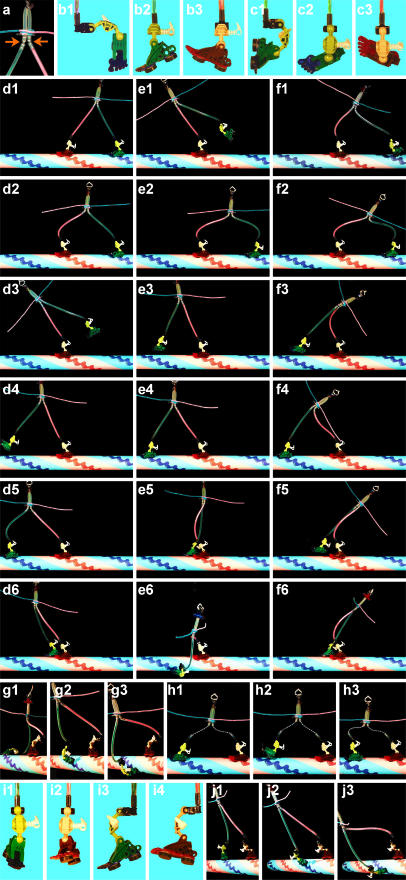
A toy model showing various walking postures. Note whether a leg is straight and strain free and whether a sole is parallel to the surface to allow rapid and stable landing. (a) Free joints likely reside near the leg (neck)-stalk junction in myosin V (Walker et al., 2000) and in other parts, too, in kinesin and myosin VI. (b) Toe down (green) and toe up (red) positions are assumed. (c) Various ankle structures are possible, such as this where the leg and sole are parallel, but toe up/down always means the toe being closer to (red)/farther from (green) the leg. (d) Walking forward with the lifted toe in the up position is difficult. (e) Walking is easy if the lifted toe is down. (f) With the lifted toe down, landing will be on a forward, rather than backward, site even in the presence of a backward force. (g) Straight toe up-down makes spiral landing difficult. (g1) On 17th subunit; (g2) on 11th; (g3) on 7th. (h) Even with flexible legs (many free joints), toe down in the lifted foot assures forward landing if landing requires full extension of the legs. (i) Ankles that work obliquely. (j) Oblique ankles make a spiral motor; compare the straight legs in j2 and the bent legs in g2.
Toe up-down mechanism
Here we propose a new concept for biasing. Because we discuss walking mechanisms in this section, we call the heads “feet” and necks “legs,” and construct a toy model as in Fig. 3. The ankle between a foot and leg is assumed to be bent forward or backward, depending on the state of bound nucleotide. This has been shown for myosin V (Walker et al., 2000; Burgess et al., 2002), and the ankle action in a landed foot is the basic idea of the lever action mechanism. The ankle action is yet to be demonstrated for myosin VI, for which forward implies the opposite direction. For kinesin, docking/undocking of the neck linker may be regarded as the ankle action. Our proposal here is that the ankle action in a lifted foot is equally, or possibly more, important: through the ankle action, the sole is correctly oriented such that landing on a forward site is favored compared to a backward site. This mechanism is distinct from the biasing of diffusion itself, whereby a lifted foot is positioned above a forward site (e.g., Woehlke and Schliwa, 2000) irrespective of whether the sole is oriented properly or not. The mechanism is also distinct from the selection of a proper prepowerstroke configuration that would ensure an efficient lever action after landing (Xu and Root, 2000).
First, we deal with the case of elastic legs that are connected to the stalk through a free joint (Fig. 3 a). Legs of myosin V are presumably semirigid and elastic. The case of flexible legs, expected for kinesin, will be considered later, followed by discussion of myosin VI. We assume that bending an elastic leg requires a considerable amount of energy. Key features to note in Fig. 3 are whether a leg is straight and thus is relaxed and whether a sole is parallel to the surface to allow rapid and stable landing. 1), When both feet land on actin, the posture with least strain (bending) in the legs is the one in which the forward toe (red) is down and rear toe (green) is up (Fig. 3 d1). 2), Bending of the red ankle into toe up position, e.g., upon phosphate release, pulls the green foot and brings it up, e.g., by promoting adenosine 5′-diphosphate (ADP) release and subsequent ATP binding in the rear foot (Fig. 3, d2 and d3). This is the lever action. 3), The red leg leans forward and biases the diffusion of the green leg forward. 4), If the green toe remains up, however, its landing on a distant forward site would be difficult because the sole is not in the correct orientation (Fig. 3 d4), and forced landing would result in leg bending (Fig. 3 d5); natural landing would be on a site close to the red foot (Fig. 3 d6). 5), If the green toe goes down upon lifting (Fig. 3 e1), e.g., in response to ATP binding or subsequent hydrolysis, its natural landing site will be a distant forward site (Fig. 3, e3 and e4), whereas other sites would induce leg bending (Fig. 3, e2, e5, and e6). Thus, toe up-down in the lifted foot correctly selects a distant forward site for landing, independent of biased diffusion.
The biased landing by toe up-down operates even if the body (stalk) of the motor is pulled back by an external load. If the leg-stalk junction is pulled back beyond the red foot, simple biasing through leg fluctuation around the junction would fail and tend to promote backward landing (Fig. 3 f1). With the green toe down, however, the green foot still tends to land on a distant forward site (Fig. 3, f3 and f4) and not on a backward site (Fig. 3 f2), although landing near the red foot would also be allowed if the external force is very high (Fig. 3 f6).
The biased landing by toe up-down can operate even if legs are completely flexible, as in kinesin, as long as the landing sites are far apart and require full extension of legs (Fig. 3, h1 and h3). This is because the orientation of the sole on a fully extended leg is restricted, and the sole orientation in the extended leg is dependent on the bend of the ankle (compare Fig. 3, h1 and h2).
Legs of myosin VI are presumably semirigid in the lower half and flexible in the upper half (Fig. 1 c). Unless the ankle action occurs obliquely (see below), the stiff lower legs would make landing at intermediate distances difficult, because that would require an extremely bowlegged posture (Fig. 3, g2 and g3). This accounts for the observed long strides. Because the long strides require almost full extension of the flexible part, landing on a forward site will be warranted, as with kinesin (Fig. 3 h). Unlike a microtubule, however, an actin filament offers landing sites close to a landed foot. If the upper leg is flexible over a sizable length, landing on a nearby forward site (Fig. 3 f6) will not add much strain, particularly in the presence of a backward force. Thus, with an increase in the backward load, we expect to observe frequent short steps in myosin VI, resulting in a smaller average step size. In this regard, the right-handed spiral that we observe here in the absence of a load could also be due to occasional landing on a nearby site (Fig. 3 e5), although most of the steps must still be made onto a site ∼36 nm forward.
Because both myosin VI and V move essentially straight along actin, the ankle actions in Fig. 3 are all assumed to be along the axis of actin. One could, in principle, design a spiral motor using oblique ankles (Fig. 3, i and j). Nature, though, would not adopt such a design unless she finds a merit in extensive spiraling. One might think that, starting from Fig. 3 j2, the red foot could move straight forward and land on the blue site 36 nm ahead. If the green foot then also moves straight forward and lands on the red site 36 nm ahead, the result would be a straight motion of the whole motor without spiraling, with the average step size of 18 nm, not 36 nm. This, however, is extremely unlikely, because the red ankle would alternate between forward-left (Fig. 3 j2) and backward-left configurations, whereas the green ankle would alternate between forward right and backward right (Fig. 3 j2): the two ankles, which are basically identical, would undergo completely different series of conformational changes to power the motor. Oblique ankles are thus destined to make a spiral motion, through the alternation of forward left and backward right (or forward right and backward left).
Previously suggested mechanisms, docking/undocking in kinesin and lever action in myosin, focus on the ankle action in the landed foot (red foot in Fig. 3). Here we propose that the toe up-down in the lifted foot is equally important, and that the selection of a correct binding site by this mechanism may in fact be the most essential mechanism of assuring forward stepping in all linear motors with multiple legs. Another important mechanism for forward stepping is the preferential detachment of the rear foot after, and only after, the fore foot has landed. This will be achieved by strain dependence of ATPase kinetics, as has been suggested by many researchers. For myosin, landing of the fore foot will introduce strain in the rear foot, such that ADP release is promoted in the rear foot, leading to subsequent ATP binding and detachment of the rear foot (see, e.g., Veigel et al., 2002), possibly aided by toe down (= heel up) action. The affinity of kinesin for ADP has been shown to be strain dependent (Uemura and Ishiwata, 2003).
SUPPLEMENTARY MATERIAL
An online supplement to this article can be found by visiting BJ Online at http://www.biophysj.org. Two movies are available: movie 1 corresponds to part of Fig. 2 a and movie 2 to part of magenta curve in Fig. 2 c. Both are 4× slow replay when played at 30 frames/s.
Supplementary Material
Acknowledgments
We thank S. Uemura and S. Ishiwata for valuable advices and comments, Y. Onoue, K. Yogo, N. Sakaki, and other members of Kinosita lab for help, M. Shio for optical tweezers, H. L. Sweeney, C. Franzini-Armstrong, B. R. Rami, and J. A. Spudich for communicating unpublished work, and M. Fukatsu for the toy model and lab management.
This work was supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Ali, M. Y., S. Uemura, K. Adachi, H. Itoh, K. Kinosita, Jr., and S. Ishiwata. 2002. Myosin V is a left-handed spiral motor on the right-handed actin helix. Nat. Struct. Biol. 9:464–467. [DOI] [PubMed] [Google Scholar]
- Bahloul, A., G. Chevreux, A. L. Wells, D. Martin, J. Nolt, Z. Yang, L.-Q. Chen, N. Potier, A. Van Dorsselaer, S. Rosenfeld, A. Houdusse, and H. L. Sweeney. 2004. The unique insert in myosin VI is a structural calcium-calmodulin binding site. Proc. Natl. Acad. Sci. USA. 101:4787–4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block, S. M., L. S. Goldstein, and B. J. Schnapp. 1990. Bead movement by single kinesin molecules studied with optical tweezers. Nature. 348:348–352. [DOI] [PubMed] [Google Scholar]
- Burgess, S., M. Walker, F. Wang, J. R. Sellers, H. D. White, P. J. Knight, and J. Trinick. 2002. The prepower stroke conformation of myosin V. J. Cell Biol. 159:983–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forkey, J. N., M. E. Quinlan, M. A. Shaw, J. E. T. Corrie, and Y. E. Goldman. 2003. Three-dimensional structural dynamics of myosin V by single-molecule fluorescence polarization. Nature. 422:399–404. [DOI] [PubMed] [Google Scholar]
- Homma, K., M. Yoshimura, J. Saito, R. Ikebe, and M. Ikebe. 2001. The core of the motor domain determines the direction of myosin movement. Nature. 412:831–834. [DOI] [PubMed] [Google Scholar]
- Howard, J. 1996. The movement of kinesin along microtubules. Annu. Rev. Physiol. 58:703–729. [DOI] [PubMed] [Google Scholar]
- Hua, W., J. Chung, and J. Gelles. 2002. Distinguishing inchworm and hand-over-hand processive kinesin movement by neck rotation measurements. Science. 295:844–848. [DOI] [PubMed] [Google Scholar]
- Huxley, H. E. 1969. The mechanism of muscular contraction. Science. 164:1356–1366. [PubMed] [Google Scholar]
- Mehta, A. D., R. S. Rock, M. Rief, J. A. Spudich, M. S. Mooseker, and R. E. Cheney. 1999. Myosin-V is a processive actin-based motor. Nature. 400:590–593. [DOI] [PubMed] [Google Scholar]
- Mermall, V., J. G. McNally, and K. G. Miller. 1994. Transport of cytoplasmic particles catalysed by an unconventional myosin in living Drosophila embryos. Nature. 369:560–562. [DOI] [PubMed] [Google Scholar]
- Moore, J. R., E. B. Krementsova, K. M. Trybus, and D. M. Warshaw. 2001. Myosin V exhibits a high duty cycle and large unitary displacement. J. Cell Biol. 155:625–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa, S., K. Homma, Y. Komori, M. Iwaki, T. Wazawa, A. H. Iwane, J. Saito, R. Ikebe, E. Katayama, T. Yanagida, and M. Ikebe. 2002. Class VI myosin moves processively along actin filaments backward with large steps. Biochem. Biophys. Res. Commun. 290:311–317. [DOI] [PubMed] [Google Scholar]
- Rice, S., Y. Cui, C. Sindelar, N. Naber, M. Matuska, R. Vale, and R. Cooke. 2003. Thermodynamic properties of the kinesin neck-region docking to the catalytic core. Biophys. J. 84:1844–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rief, M., R. S. Rock, A. D. Mehta, M. S. Mooseker, R. E. Cheney, and J. A. Spudich. 2000. Myosin-V stepping kinetics: a molecular model for processivity. Proc. Natl. Acad. Sci. USA. 97:9482–9486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock, R. S., S. E. Rice, A. L. Wells, T. J. Purcell, J. A. Spudich, and H. L. Sweeney. 2001. Myosin VI is a processive motor with a large step size. Proc. Natl. Acad. Sci. USA. 98:13655–13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, N., H. Miyata, S. Ishiwata, and K. Kinosita Jr. 1996. Preparation of bead-tailed actin filaments: estimation of the torque produced by the sliding force in an in vitro motility assay. Biophys. J. 70:401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda, K., C. F. Schmidt, B. J. Schnapp, and S. M. Block. 1993. Direct observation of kinesin stepping by optical trapping interferometry. Nature. 365:721–727. [DOI] [PubMed] [Google Scholar]
- Tanaka, H., K. Homma, A. H. Iwane, E. Katayama, R. Ikebe, J. Saito, T. Yanagida, and M. Ikebe. 2002. The motor domain determines the large step of myosin-V. Nature. 415:192–195. [DOI] [PubMed] [Google Scholar]
- Tominaga, M., H. Kojima, E. Yokota, H. Orii, R. Nakamori, E. Katayama, M. Anson, T. Shimmen, and K. Oiwa. 2003. Higher plant myosin XI moves processively on actin with 35 nm steps at high velocity. EMBO J. 22:1263–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura, S., and S. Ishiwata. 2003. Loading direction regulates the affinity of ADP for kinesin. Nat. Struct. Biol. 10:308–311. [DOI] [PubMed] [Google Scholar]
- Vale, R. D., and R. A. Milligan. 2000. The way things move: looking under the hood of molecular motor proteins. Science. 288:88–95. [DOI] [PubMed] [Google Scholar]
- Veigel, C., F. Wang, M. L. Bartoo, J. R. Sellers, and J. E. Molloy. 2002. The gated gait of the processive molecular motor, myosin V. Nat. Cell Biol. 4:59–65. [DOI] [PubMed] [Google Scholar]
- Walker, M. L., S. A. Burgess, J. R. Sellers, F. Wang, J. A. Hammer, J. Trinick, and P. J. Knight. 2000. Two-headed binding of a processive myosin to F-actin. Nature. 405:804–807. [DOI] [PubMed] [Google Scholar]
- Wells, A. L., A. W. Lin, L.-Q. Chen, D. Safer, S. M. Cain, T. Hasson, B. O. Carragher, R. A. Milligan, and H. L. Sweeney. 1999. Myosin VI is an actin-based motor that moves backwards. Nature. 401:505–508. [DOI] [PubMed] [Google Scholar]
- Woehlke, G., and M. Schliwa. 2000. Directional motility of kinesin motor proteins. Biochim. Biophys. Acta. 1496:117–127. [DOI] [PubMed] [Google Scholar]
- Xu, J., and D. D. Root. 2000. Conformational selection during weak binding at the actin and myosin interface. Biophys. J. 79:1498–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda, R., H. Noji, K. Kinosita, Jr., and M. Yoshida. 1998. F1-ATPase is a highly efficient molecular motor that rotates with discrete 120° steps. Cell. 93:1117–1124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.