Abstract
Activation of store-operated Ca2+ entry (SOCE) into the cytoplasm requires retrograde signaling from the intracellular Ca2+ release machinery, a process that involves an intimate interaction between protein components on the intracellular and cell surface membranes. The cellular machinery that governs the Ca2+ movement in muscle cells is developmentally regulated, reflecting maturation of the junctional membrane structure as well as coordinated expression of related Ca2+ signaling molecules. Here we demonstrate the existence of SOCE in freshly isolated skeletal muscle cells obtained from embryonic days 15 and 16 of the mouse embryo, a critical stage of muscle development. SOCE in the fetal muscle deactivates incrementally with the uptake of Ca2+ into the sarcoplasmic reticulum (SR). A novel Ca2+-dependent facilitation of SOCE is observed in cells transiently exposed to high cytosolic Ca2+. Our data suggest that cytosolic Ca2+ can facilitate SOCE whereas SR luminal Ca2+ can deactivate SOCE in the fetal skeletal muscle. This cooperative mechanism of SOCE regulation by Ca2+ ions not only enables tight control of SOCE by the SR membrane, but also provides an efficient mechanism of extracellular Ca2+ entry in response to physiological demand. Such Ca2+ signaling mechanism would likely contribute to contraction and development of the fetal skeletal muscle.
INTRODUCTION
Store-operated Ca2+ entry (SOCE) represents an important mechanism that allows for refilling of a depleted intracellular Ca2+ store after sustained activation of the Ca2+ release channels located on the endoplasmic reticulum in nonmuscle cells, or sarcoplasmic reticulum (SR) in muscle cells (Putney, 1986; Parekh and Penner, 1997). This Ca2+ entry pathway provides the essential link between extracellular Ca2+ reservoir and intracellular Ca2+ storage, and serves important roles in a variety of cell signaling processes, including proliferation, apoptosis, and motility (Birnbaumer et al., 2000). Research into the molecular and cellular function of SOCE has been carried out primarily in nonexcitable cells, and to some extent in smooth muscle cells (Parekh and Penner, 1997; Birnbaumer et al., 2000; McFadzean and Gibson, 2002).
As a molecular signal that initiates the contractile events of skeletal muscle, a precise spatial and temporal encoding of Ca2+ signal is achieved through cross talk between voltage sensors on the plasma membrane (PM) and ryanodine receptors (RyR)/Ca2+ release channels on the SR membrane, a cascade of coordinated events that often involves both orthograde and retrograde protein-protein interactions (Dirksen, 2002; Ma and Pan, 2003). The voltage-gated Ca2+ channel located on the PM of skeletal muscle has slow activation kinetics, and does not support Ca2+ influx in response to single action potential stimulation (Brum et al., 1988). The twitch force in skeletal muscle is therefore triggered mainly by the acute release of Ca2+ from the SR, primarily via voltage-induced Ca2+ release (VICR), and secondarily amplified by Ca2+-induced Ca2+ release (CICR) through activation of neighboring RyRs not directly coupled to the voltage sensor (Yang et al., 2001). A unique property of the skeletal muscle is the presence of an anatomical structure named the triad junction, where the transverse tubular (t-tubular) invaginations of PM face the terminal cisternae of the SR (Takekura et al., 2001). This triadic junction enables efficient operation of VICR and CICR in adult skeletal muscle.
The fetal skeletal muscle, however, does not have a well-developed t-tubular network and a well-coordinated triad junction structure, which sets a hindrance to the operation of VICR and CICR. In addition, the Ca2+ handling properties of the SR are less efficient in maintaining a high intracellular Ca2+ storage in the developing fetal muscle, as compared with that in the mature adult skeletal muscle (Flucher et al., 1993; Froemming and Ohlendieck, 1998). Thus, alternative pathways other than the voltage-gated Ca2+ entry should exist in fetal skeletal muscle to support the Ca2+ signaling required for muscle-specific gene expression, cell differentiation, and myofilament contraction. For such non-voltage-dependent Ca2+ entry to participate in physiological function, it must be adaptable, i.e., function incrementally in response to the cellular demand.
Recent studies have demonstrated the existence of SOCE in adult muscle cells (Kurebayashi and Ogawa, 2001) and cultured skeletal myotubes (Hopf et al., 1996; Pan et al., 2002; Shin et al., 2003). These studies show that the activation of SOCE is likely coupled to conformational changes of the RyR or IP3 receptors and is sensitive to changes in the triad junction structure (Pan et al., 2002; Launikonis et al., 2003). However, the function of SOCE in fetal muscle development and the underlying mechanism of SOCE activation have not been defined. Here we provide the first evidence to support the existence of SOCE in freshly isolated multinucleated fetal skeletal muscle cells. We also identify a graded deactivation of SOCE by Ca2+ storage in the SR, and a unique facilitation of SOCE by the transient elevation of cytosolic Ca2+. The SOCE pathway may not only prevent store depletion but also supply for Ca2+ involved in the myogenesis and maturation of the fetal skeletal muscle.
MATERIALS AND METHODS
Intercostal muscles were obtained from Swiss White mouse fetuses at embryonic days 15 and 16 (E15 and E16). Individual muscle cells were obtained by enzymatic dissociation of the dissected fetus ribcage, using the procedure described in Strube et al. (1992). The cells were plated onto polylysine-coated glass-bottomed petri dishes, and incubated in a Ca2+-free balanced salt solution (BSS) for 1 h to allow for passive depletion of intracellular Ca2+ storage. Cells were loaded with 5 μM Fura-2-AM in Ca2+-free BSS, for measurement of changes in intracellular [Ca2+]i, as well as changes in the rate of Mn2+-quenching of Fura-2 as indicator of SOCE, following the procedure of Pan et al. (2002). All experiments were performed at room temperature (20–22°C).
For our fluorescence setup, the Ca2+-insensitive isosbestic excitation wavelength of Fura-2 was determined to be λ = 357 nm in the muscle cell preparation. For each measurement, Fura-2 was dually excited at wavelengths of 357 nm and 380 nm and emitted fluorescence was measured at 510 nm. Changes in [Ca2+]i were expressed as changes of the fluorescence ratio of F357/F380. The rate of Mn2+ entry was inferred from the rate of decrease of Fura-2 fluorescence measured at F357. The cells tested were continuously superfused with the given extracellular solutions using a gravity-driven thin polyethylene capillary perfusion system.
Standard balanced salt solution (BSS) contained (in mM): 137 NaCl, 5.4 KCl, 2 CaCl2, 1.2 MgCl2, 20 D-(+)-Glucose, 1 NaH2PO4, and 20 HEPES, adjusted to pH 7.4 with NaOH. In the Ca2+-free BSS, Ca2+ was replaced by 2 MgCl2 plus 0.5 EGTA. The Mn2+-quenching solution contained 0.5 MnCl2 in Ca2+-free BSS (MgCl2 adjusted to 1.5). The high-K+ solution contained 140 KCl with equimolar decrease of NaCl in Ca2+-free BSS.
Data were given as mean ± SE. Least-squares fits were performed using a Marquardt-Levenberg algorithm. Statistical significance was determined using paired Student's t-tests.
RESULTS
SR depletion and Ca2+ reuptake in fetal skeletal muscle
The Ca2+ signaling process in skeletal muscle undergoes major changes during fetal development, in particular from E14 to E18, when the cells switch from an extracellular Ca2+-dependent Ca2+ release to a process independent from extracellular Ca2+ entry (Strube et al., 1992). Along with fetal development, both SR volume and SR Ca2+ content increase, underlying the hallmark of myogenesis (Flucher et al., 1993; Froemming and Ohlendieck, 1998). Accompanying the maturation of the SR Ca2+ handling functions, the cellular membrane structures also undergo major morphological changes. The initial stage of peripheral coupling between SR and PM starts at E5.5, followed by the formation of an extended t-tubular network and t-tubule/SR junctions. The later stage starts abruptly between E15 and E16. After this event, there is a prolonged period of maturation, up to 4 weeks when stable triad junctions are formed and acquire their proper location at the A–I junction (Takekura et al., 2001).
Realizing the critical nature of muscle development between E14 and E18, we therefore focused our Ca2+ measurement studies on the intercostal muscle cells isolated from mice at the fetal developmental stage of E15 and E16. Individual muscle cells were enzymatically dissociated from the intercostal muscles and loaded with the Fura-2-AM Ca2+ indicator. These freshly isolated muscle cells were found to invariably undergo spontaneous depletion of their SR Ca2+ stores, after incubation in a Ca2+-free bath solution. Within the typical 2-h period spent in a Ca2+-free solution (which is necessary for dissociation of single muscle cells as well as the adhesion of cells to the petri dish and sufficient intracellular loading of the Fura-2 indicator), the cells undergo a profound decrease in the SR Ca2+ content, as demonstrated by the lack of depolarization-induced Ca2+ release, or caffeine-induced Ca2+ release (Fig. 1 A, top trace).
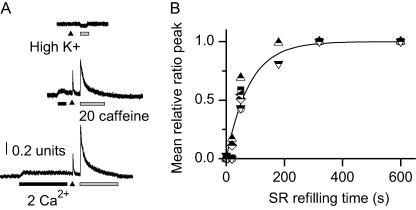
FIGURE 1.
Time course of SR Ca2+ refilling in fetal skeletal muscle. (A) An E16 muscle cell was incubated with the Ca2+-free BSS for ∼2 h. High K+ (arrow) or caffeine (20 mM, shaded horizontal bar) failed to elicit changes in cytosolic Fura-2 fluorescence, indicating complete SR Ca2+ depletion. The artifactual decrease in Fura-2 signal is due to the known nonspecific effect of caffeine on Fura-2 (upper trace). Fifty seconds of exposure to 2 mM extracellular Ca2+ led to elevation of cytosolic [Ca2+]i, and increased response to high-K+ and caffeine-induced Ca2+ transient, indicating SR Ca2+ refilling (middle trace). Three-hundred-and-twenty seconds of exposure to 2 mM Ca2+ led to further increase in caffeine-induced Ca2+ transient (lower trace). (B) Peak of caffeine-induced Ca2+ transient plotted as a function of the durations the cells were exposed to 2 mM Ca2+. Data from individual experiments were fitted separately with a single exponential function, from which the individual time constant was derived. The mean time constant of SR refilling, 79 ± 12 s, was obtained from six individual experiments. The solid curve represents the exponential function of y = 1–exp(−t/79).
Subsequent perfusion of the cell with a Ca2+-containing solution resulted in rapid entry of Ca2+ into the cytoplasm, and progressive reuptake of Ca2+ into the SR. As shown in Fig. 1 A (middle trace), a 50-s exposure of the cell to 2 mM Ca2+ led to significant elevation of the resting cytosolic [Ca2+]i. The Fura-2 fluorescence signal (F357/F380) increased from 0.53 ± 0.05 in a Ca2+-free solution to 0.63 ± 0.05 in a solution containing 2 mM Ca2+ (n = 6, p < 0.001). Furthermore, the elevation of [Ca2+]i appeared to be sustained in the presence of 2 mM Ca2+ (see also Fig. 2 A). This suggests an efficient Ca2+ entry mechanism and also an important role of extracellular Ca2+ entry in maintaining the resting [Ca2+]i in the fetal skeletal muscle.
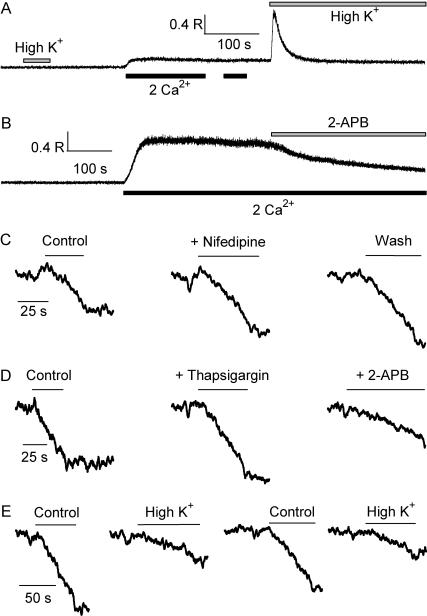
FIGURE 2.
Store-operated Ca2+ entry in fetal skeletal muscle cells. (A) High-K+ bath solution did not trigger intracellular Ca2+ release in an E16 cell preincubated in 0 Ca2+ for ∼2 h. Perfusion of 2 mM Ca2+ led to sustained elevation of cytosolic [Ca2+]i. No further increase was observed when [Ca2+]o was applied for the second time. Second exposure to high-K+ triggered transient elevation of [Ca2+]i, indicative of SR Ca2+ refilling. (B) The cell was pretreated with thapsigargin (10 μM) for 10 min. Switching the bath solution from 0 Ca2+ to 2 mM Ca2+ led to elevation of [Ca2+]i via SOCE, which was inhibited by 2-APB. (C) Quenching of Fura-2 fluorescence by 0.5 mM Mn2+ in a cell with passively depleted SR Ca2+ content. 5 μM nifedipine did not affect the rate of Fura-2 quenching. (D) Mn2+ quenching of Fura-2 measured in the same cell, in the control condition with passively depleted SR Ca2+ content, after addition of 10 μM thapsigargin, and after addition of 20 μM 2-APB. (E) Mn2+ entry rate was reversibly and reproducibly decreased when the bath solution was changed from control to a high-K+ solution.
With accumulated exposure to extracellular Ca2+, the cells started to gradually refill their SR, as indicated by the increased Ca2+ release in response to caffeine and high K+. After a 320-s exposure to 2 mM Ca2+, the SR appeared to be completely refilled, as indicated by the maximum caffeine-induced Ca2+ release (Fig. 1 A, bottom trace). The peak value of caffeine-induced Ca2+ release was used to estimate the degree of SR Ca2+ refilling, and plotted versus duration of exposure to extracellular Ca2+ (Fig. 1 B). By fitting the data from individual cells with an exponential function, a mean time constant of 79 ± 12 s (n = 6) for SR Ca2+ refilling was obtained at the E16 developmental stage.
Cells that were passively depleted of their SR Ca2+ content responded to the first addition of [Ca2+]o (for a duration of 140 s, in Fig. 2 A), but not to the second addition of [Ca2+]o—presumably because their SR have already been filled with Ca2+ (Fig. 2 A). Such phenomenon is consistent with the concept of SOCE in the fetal skeletal muscle. The maintenance of a constant and elevated [Ca2+]i likely represents the balance of SERCA-mediated SR Ca2+ uptake and voltage-independent entry of extracellular Ca2+; the latter process must be deactivated as a result of SR Ca2+ refilling.
Store-operated Ca2+ entry in skeletal muscle
The following experiments further substantiate the existence of SOCE in the fetal skeletal muscle. Thapsigargin (TG), a specific inhibitor of SERCA, was used to induce depletion of the SR Ca2+ content. As shown in Fig. 2 B, upon switching the bath solution from 0 mM Ca2+ to 2 mM Ca2+, an E16-muscle cell pretreated with TG responded with significant increase in [Ca2+]i, to a degree that is substantially greater than cells with passively depleted SR Ca2+ store (without TG, Fig. 2 A). Indeed, TG inhibits the uptake of cytosolic Ca2+ into the SR, and therefore reduces the buffering capacity for [Ca2+]i. Notice that the sustained [Ca2+]i elevation could be inhibited by 2-aminoetoxyphenoxyl borate (2-APB, 20 μM), a known blocker of the store-operated Ca2+ channel (SOC).
The net change in [Ca2+]i is likely the result of a summation of competing processes—SR Ca2+ uptake and release, and surface membrane Ca2+ extrusion and influx. To measure the net influx of SOC-mediated Ca2+ entry, we used the method of Mn2+-quenching of Fura-2 (Pan et al., 2002). Upon perfusion of a bath solution containing 0.5 mM Mn2+ and 0 mM Ca2+, cells that were passively depleted of their SR Ca2+ content responded with rapid quenching of the Fura-2 fluorescence due to the entry of Mn2+ through SOC (Fig. 2 C). Nifedipine, a blocker of the L-type Ca2+ channel, had no effect on the rate of Mn2+ entry at a concentration of 5 μM (n = 3). The rate of Mn2+ entry into cells that were passively depleted of their SR Ca2+ content was not affected by the addition of TG (Fig. 2 D). In paired experiments, the presence of TG did not induce significant changes in the Mn2+-entry rate measured in Ca2+-free BSS (fold of change = 0.98 ± 0.06, n = 7, difference not significant, p = 0.73). This result confirms that the spontaneous intracellular Ca2+ depletion process was complete in the fetal muscle cell after extended incubation in the Ca2+-free solution. Moreover, the Mn2+ entry through SOC could be blocked by 2-APB (Fig. 2 D).
The movement of Mn2+ through SOC could be influenced by the resting membrane potential of the fetal muscle cells. Changing the K+ concentration in the extracellular solution from 5.4 mM to 140 mM resulted in significant reduction in the rate of Mn2+ entry (Fig. 2 E). The reduced Mn2+ entry rate merely reflected the decrease in electrical driving force imposed on the Mn2+ ions (from −80 mV in control to ∼0 mV in high-K+ solution). Similar reduction of Mn2+ entry rate was observed in TG-treated cells, after switching the bath solution from the normal Ca2+-free BSS to high-K+ solution (not shown). Such phenomenon is different from the traditional voltage-gated Ca2+ entry, and suggests that activation of SOCE in the fetal muscle cells is voltage-independent. Indeed, the voltage-gated Ca2+ channels are expected to be either closed or inactivated at steady-state membrane potential, and moreover, depolarizing the membrane potential would result in activation of the Ca2+ channels and greater Mn2+ influx (in contrary to the observation). Together, our data provide conclusive evidence supporting the existence of SOCE in fetal skeletal muscle.
Graded deactivation of SOCE by SR Ca2+ storage
To further characterize the activation property of SOCE in the fetal skeletal muscle, systematic studies were performed to define the graded changes of SOCE as a function of the SR Ca2+ refilling status. As controls, a series of experiments was conducted to determine the in situ isosbestic wavelength of Fura-2 (λ = 357 nm), to ensure that the measured Mn2+ quenching would not be affected by potential changes in [Ca2+]i. In addition, careful studies were performed to explore the linear range of Fura-2 quenching by Mn2+. As shown in Fig. 3 A, repetitive perfusion of the cell with Mn2+ resulted in reproducible quenching of the Fura-2 fluorescence with similar entry rate, when the bath solution was devoid of Ca2+. In addition, the signal appeared to be linear in a wide range of fluorescence intensity. All subsequent measurements were limited to this linear Mn2+-quenchable range of Fura-2 fluorescence.
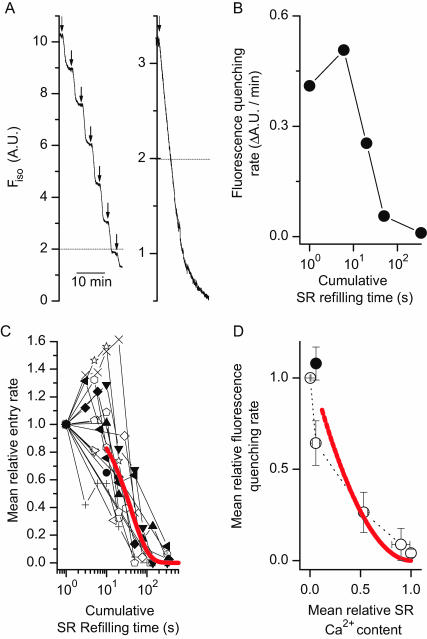
FIGURE 3.
Graded deactivation of SOCE as a function of SR Ca2+ refilling. (A) Mn2+ quenching of Fura-2 measured at the isosbestic excitation wavelength (Fiso, λ = 357 nm) in an individual fetal skeletal muscle with passively depleted SR Ca2+ content. Repetitive application of Mn2+ (0.5 mM) resulted in a reproducible and constant rate of Fura-2 quenching by Mn2+ (left). The fluorescence quenching rate remained linear above Fiso = 2 A.U., as measured in another cell (right). (B) The changes in the Mn2+ quenching rate of Fura-2 was measured in an E16 cell, after accumulative exposure to 2 mM [Ca2+]o, for durations of 0, 6, 20, 50, and 350 s, respectively. The changes were biphasic, with initial enhancement at 6 s followed by progressive decrease for longer exposures. (C) Data from individual experiments were normalized to the initial value of Mn2+-quenching rate, and plotted separately. A total of 18 complete experiments from E15 and E16 cells were shown. Out of the 18 experiments, 10 contain measurements of Mn2+-quenching at <10 s, and seven show apparent facilitation of Mn2+-quenching rate. The superimposed curve is the result of fitting an exponential function to the data collected after 10 s, to deal with the deactivation property of SOCE. The best-fit time constant was 40 ± 8 s. (D) The Mn-quenching data from 6 of the 18 experiments shown in C were averaged, and plotted as a function of SR Ca2+ content (data derived from Fig 1 B). These six experiments contain a complete set of matching time points with the SR Ca refilling measurements shown in Fig. 1 B. The relationship between SOCE availability and SR Ca2+ refilling is nonlinear, suggesting a cooperative feature of SOCE deactivation. The solid line represents the theoretical plot of x = 1–exp(−t/79) vs. y = exp(−t/40). The solid circle indicates the initial facilitation of SOCE when brief Ca2+ perfusion was applied to the bath solution (t = 4.9 s, averaged value from the 10 experiments shown in C). This initial facilitation is not significant, due to the fact that two of the six experiments lack apparent facilitation at t < 10 s.
A typical experiment performed in an E16 cell is presented in Fig. 3 B. At the beginning, passive depletion of the SR Ca2+ content resulted in near-maximum activation of SOCE and near-maximum rate of Mn2+ entry. After successive perfusion with a bath solution containing 2 mM Ca2+, the Mn2+ entry rate changed accordingly. Overall, with the accumulated uptake of Ca2+ into the SR, a graded deactivation of SOCE was observed. Typically, after a 50-s exposure to 2 mM Ca2+, >50% reduction of Mn2+-entry rate was observed in the E16 muscle. For the purpose of statistical analysis, individual datum points from separate experiments were normalized to the initial value of Mn2+-quenching rate, and plotted in Fig. 3 C as a function of the accumulated times when the cells were exposed to 2 mM extracellular Ca2+. Overall, SOCE in the E15 and E16 skeletal muscle appears to have a biphasic response after the addition of Ca2+ to the extracellular solution, i.e., initial and brief exposure to [Ca2+]o (t < 10 s) led to enhancement of SOCE whereas longer and sustained exposure to [Ca2+]o resulted in gradual reduction of SOCE. The solid line in Fig. 3 C represents the best-fit exponential decay function with data points obtained at t ≥ 10 s, having a time constant of 40 ± 8 for the fetal cells.
From the data shown in Fig. 1, one can derive the refilling status of the SR achieved after each successive perfusion with Ca2+. By plotting the changes in the Mn2+-quenching rate of Fura-2 as a function of SR Ca2+ content, one can derive the correlation between the graded-deactivation of SOCE and the SR Ca2+ content in the fetal skeletal muscle. The averaged data shown in Fig. 3 D represents a subset of those presented in Fig. 3 C, due to the fact only limited matching time points were performed with the SR Ca2+ refilling studies. Clearly, one can see that the relationship between SOCE deactivation and SR Ca2+ refilling is nonlinear (Fig. 3 D), which suggests that the Ca2+-dependent deactivation of SOCE is likely to be cooperative.
Facilitation of SOCE by cytosolic Ca2+
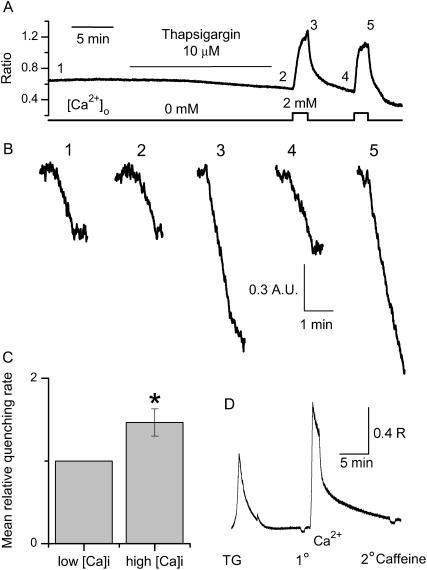
The facilitation of SOCE shown in Fig. 3 C was observed in a significant portion of the experiments with the E15 and E16 cells (7/10 total experiments), when brief perfusion of 2 mM Ca2+ was applied to the fetal muscle cells (t < 10 s). This represents a potential Ca2+-mediated facilitation of SOCE, and appears to be a unique property of SOCE in the fetal skeletal muscle. To further study the Ca2+-dependent facilitation of SOCE, we introduced TG into the bath solution (Fig. 4). After treatment with TG, perfusion with 2 mM Ca2+ would lead to elevation of cytosolic [Ca2+]i without uptake of Ca2+ into the SR (Fig. 4 A). Mn2+-quenching was measured before (Fig. 4 B, traces labeled 1, 2, and 4) or right after Ca2+ perfusion (traces labeled 3 and 5). Clearly, the rate of Mn2+ entry is significantly higher when the cytosolic Ca2+ is transiently elevated (compare traces 3 and 5 where [Ca2+]i is high, with traces 1, 2, and 4 where [Ca2+]i is low). The Ca2+-mediated enhancement of Mn2+ entry was reversible. On average, with the elevation of [Ca2+]i, a 46 ± 16% (n = 4) increase in the Mn2+ entry rate was observed in cells pretreated with TG (Fig. 4 C). The observed Ca2+-dependent facilitation of SOCE is most likely due to the changes of cytosolic Ca2+, rather than the uptake of Ca2+ into the SR, because the extensive TG treatment resulted in complete depletion of Ca2+ from the SR (Fig. 4 D). The TG-treated E16 muscle cells not only lacked initial caffeine-induced Ca2+ release, but also showed no detectable amount of Ca2+ uptake into the SR after Ca2+ entry into the cytosol through activation of SOC.
FIGURE 4.
Cytosolic Ca2+-dependent facilitation of SOCE in fetal skeletal muscle cells. (A) Measurement of changes in intracellular [Ca2+]i in an E16 cell, bathed in 0 [Ca2+]o, after 12-min incubation with 10 μM thapsigargin (TG), and after brief exposure to 2 mM extracellular Ca2+. (B) Incubation of thapsigargin in a Ca2+-free solution did not affect the rate of Mn2+ entry through SOC (compare traces 1 and 2). Transient elevation of cytosolic [Ca2+]i led to significant enhancement in the rate of Mn2+ entry through SOC (compare traces 2 and 3). The Ca2+-mediated facilitation of Mn2+ entry was reversible and reproducible (compare traces 4 and 5). (C) On average, the rate of Mn2+ quenching of Fura-2 was 1.46 ± 0.16 (n = 5)-fold higher of the control, when measurement was measured at transiently elevated [Ca2+]I compared with that at the low resting [Ca2+]i. The change was significant with a p-value of 0.06 in paired Student's t-test. (D) No detectable amount of caffeine-induced Ca2+ release was measured in an E16 cell after treatment with TG (1° addition of caffeine). Moreover, TG treatment appeared to completely prevent the Ca2+ uptake into the SR, since uptake of Ca2+ into the cytosol did not result in caffeine-induced Ca2+ release from the SR (2° addition of caffeine).
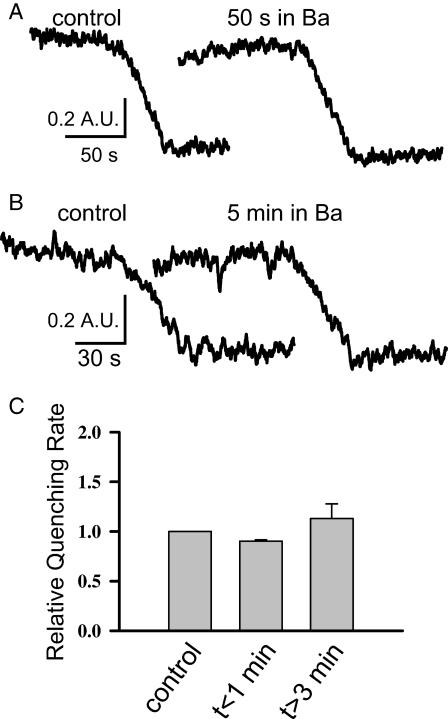
Absence of SOCE facilitation and deactivation by Ba2+
To further study the facilitation and deactivation properties of SOCE in the fetal muscle, we substituted Ba2+ for Ca2+ in the bath solution. The experiments shown in Fig. 5 clearly demonstrate that Ba2+ cannot replace Ca2+ for the initial facilitation and subsequent deactivation of SOCE in the fetal skeletal muscle. Specifically, 50 s after perfusion of 2 mM BaCl2 to the bath solution of a fetal muscle cell that had been passively depleted of its SR Ca2+ storage, the Mn-quenching rate remained unchanged compared with the control (Fig. 5 A). This shows the lack of Ba2+-dependent facilitation of SOCE. Moreover, 5 min after perfusion of 2 mM BaCl2 to the bath solution, the Mn2+-quenching rate remained essentially the same as the control (Fig. 5 B), suggesting the lack of Ba2+-dependent deactivation of SOCE. This is probably due to the fact that Ba2+ ions are not recognized by the SERCA pump. Together, our data show that the facilitation and deactivation properties of SOCE in the fetal skeletal muscle are likely to be Ca2+-specific.
FIGURE 5.
Absence of Ba2+-dependent facilitation and deactivation of SOCE in the fetal skeletal muscle. (A) Representative traces of Mn-quenching in a fetal skeletal muscle with passively depleted SR Ca2+ store (after incubation in 0 Ca2+ for 1 h). Fifty seconds after perfusion of 2 mM BaCl2 to the bath solution, the Mn2+-quenching rate remained unchanged, demonstrating the lack of Ba2+-dependent facilitation of SOCE. (B) In a separate muscle cell, 5 min after perfusion of 2 mM BaCl2 to the bath solution, the Mn2+-quenching rate remained constant as the control, suggesting the lack of Ba2+-dependent deactivation of SOCE. (C) Data from five experiments were averaged. Clearly, the Mn2+-quenching rates did not differ significantly from the control, at t < 1 min or >3 min after perfusion of Ba2+ to the bath solution.
DISCUSSION
Four conclusions can be drawn from this study:
The fetal skeletal muscle cells are susceptible to depletion of their SR Ca2+ storage in the absence of extracellular Ca2+.
SOCE exists in the fetal muscle to supply for a non-voltage-gated Ca2+ entry pathway.
Graded deactivation of SOCE enables physiological control of this important Ca2+ entry pathway in the fetal skeletal muscle.
A novel Ca2+-dependent facilitation of SOCE may provide an efficient mechanism for regulation of Ca2+ entry in the fetal skeletal muscle.
It is well known that the twitch contraction of adult skeletal muscle can be sustained in the absence of extracellular Ca2+ due to the fact that the Ca2+ entry is not necessary to initiate Ca2+ release from the SR and the SR Ca2+ storage is efficiently maintained (Brum et al., 1988). The fetal skeletal muscle, on the other hand, has weaker Ca2+ maintenance functions (our study; see also Froemming and Ohlendieck, 1998), and therefore must rely on extracellular Ca2+ entry for the myogenesis process. It has been shown that differentiation of the skeletal muscle requires an elevation of the resting cytosolic [Ca2+]i which appears to be governed by a voltage-independent Ca2+ entry mechanism (Constantin et al., 1996). The observed SOCE pathway could in principle contribute to the source of Ca2+ needed for the development and maturation of the skeletal muscle. With controlled perfusion of extracellular Ca2+, we were able to manipulate the Ca2+ refilling status of the SR. Using the Mn2+-quenching fluorescent measurement, we observed an incremental deactivation of SOCE in the fetal skeletal muscle, tightly controlled by the level of Ca2+ in the SR. Such controlled function of SOCE is of great physiological importance, as the muscle's demand for intracellular Ca2+ is expected to vary with the developmental and maturation processes.
Muscle maturation is characterized by the expansion of SR volume, improved SERCA pump efficiency (Strube et al., 1994; Arai et al., 1992; Powell et al., 2001), and enhanced Ca2+ buffering capacity inside the SR due to increased expression of calsequestrin (Froemming and Ohlendieck, 1998). The altered expression of proteins in the junctional SR membrane could provide retrograde signals that directly regulate the function of SOCE. This was suggested by our recent study where overexpression of calsequestrin in cultured skeletal myotubes was shown to have an inhibitory effect on the operation of SOCE (Shin et al., 2003). Another process accompanying fetal muscle maturation consists of topological changes, where coupling between plasma membrane and SR evolves from primarily peripheral interactions to internal SR/t-tubule junctions, largely due to the formation of extensive t-tubular network (Takekura et al., 2001). The establishment of a close SR/t-tubule connection leads to maturation of the excitation-contraction coupling process, turning the skeletal muscle from a Ca2+ entry-dependent contraction process into a Ca2+ entry-independent contraction process (Strube et al., 1994). Presumably as a consequence of these changes, the regulatory properties of SOCE also change. Our previous study with the skeletal muscle isolated from the ryr1 and ryr3 double-knockout mice showed that the function of SOCE in skeletal muscle is compromised when ryanodine receptors are absent from the triad junction (Pan et al., 2002). However, even with the ryr1(−/−)ryr3(−/−) myotubes preparation, a residual component of SOCE was still maintained, suggesting that the ryanodine receptors cannot be the sole component that translates the signal from SR Ca2+ depletion to the SOCE activation. A recent study from Launikonis et al. (2003) provides conclusive evidence supporting the role of IP3 receptors in the activation of SOCE in adult skeletal muscle. It is known that IP3 receptors are primarily expressed in the early fetal developmental stage of the skeletal muscle and their expression level become reduced at later fetal stages, in particular from E14 to E17 (Moschella et al., 1995; Rosemblit et al., 1999). This developmental change of IP3 receptor could in principle add to another mechanism for the regulatory process of SOCE in the fetal skeletal muscle.
Our experiments revealed an important mechanism in Ca2+-dependent facilitation of SOCE in fetal skeletal muscle. Other studies have shown that SOCE can be maintained over a prolonged period of time with elevated level of cytosolic [Ca2+]i, although certain degree of inactivation of SOCE occurs with the sustained elevation of [Ca2+]i (Parekh and Penner, 1997). Our study showed that a component of SOCE in the fetal skeletal muscle could be enhanced by a transient elevation of [Ca2+]i. This was observed in a majority of the cell preparations, and clearly demonstrated by the increase in Mn2+ entry rate when strong [Ca2+]i elevation was introduced by SOCE in the presence of TG, preventing Ca2+ refilling into the SR. The enhancement of Mn2+ entry rate appears to be specific for Ca2+ ions, as the substitution of Ba2+ in the extracellular solution is ineffective. Therefore, the regulatory processes of SOCE in the fetal skeletal muscle are biphasic, with an enhancement of the SOC channel activity upon initial entry of extracellular Ca2+ followed by gradual and complete deactivation of the SOC channel function associated with the uptake of Ca2+ into the SR. The initial enhancement process could reflect 1), acute changes in the junctional membrane structure; 2), Ca2+-dependent recruitment of additional SOC into the plasma membrane; or 3), perhaps direct stimulating effect of Ca2+ via direct binding to the SOC channel or indirect stimulation of the calmodulin-dependent protein kinase. A previous work from Zweifach and Lewis (1996) reported similar Ca2+-dependent potentiation of SOCE in nonexcitable cells. Although the mechanisms of this enhancement remain to be explored in future studies, it nonetheless represents an important mechanism for rapid and efficient coupling process of Ca2+ entry.
Acknowledgments
This work is supported by grants from the National Institutes of Health, RO1-AG15556, RO1-HL69000, and RO1-CA95739 to J.M., and a Postdoctoral Fellowship from the American Heart Association to C.C.
Abbreviations used: 2-APB, 2-aminoethoxydiphenyl borate; BSS, balanced salt solution; CICR, Ca2+-induced Ca2+ release; Fura-2-AM, Fura-2-acetoxymethylester; IP3, inositol 1,4,5-trisphosphate; PM, plasma membrane; RyR, ryanodine receptor; SERCA, sarco-endoplasmic reticulum Ca2+ ATPase; SOCE, store-operated Ca2+ entry; SR, sarcoplasmic reticulum; TG, thapsigargin; VICR, voltage-induced Ca2+ release.
References
- Arai, M., K. Otsu, D. H. MacLennan, and M. Periasamy. 1992. Regulation of sarcoplasmic reticulum gene expression during cardiac and skeletal muscle development. Am. J. Physiol. 262:614–620. [DOI] [PubMed] [Google Scholar]
- Birnbaumer, L., G. Boulay, D. Brown, M. Jiang, A. Dietrich, K. Mikoshiba, X. Zhu, and N. Qin. 2000. Mechanism of capacitative Ca2+ entry (CCE): interaction between IP3 receptor and TRP links the internal calcium storage compartment to plasma membrane CCE channels. Recent Prog. Horm. Res. 55:127–161. [PubMed] [Google Scholar]
- Brum, G., E. Rios, and E. Stefani. 1988. Effects of extracellular calcium on calcium movements of excitation-contraction coupling in frog skeletal muscle fibres. J. Physiol. 398:441–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin, B., C. Cognard, and G. Raymond. 1996. Myoblast fusion requires cytosolic calcium elevation but not activation of voltage-dependent calcium channels. Cell Calcium. 19:365–374. [DOI] [PubMed] [Google Scholar]
- Dirksen, R. T. 2002. Bi-directional coupling between dihydropyridine receptors and ryanodine receptors. Front. Biosci. 7:659–670. [DOI] [PubMed] [Google Scholar]
- Flucher, B. E., H. Takekura, and C. Franzini-Armstrong. 1993. Development of the excitation-contraction coupling apparatus in skeletal muscle: association of sarcoplasmic reticulum and transverse tubules with myofibrils. Dev. Biol. 160:135–147. [DOI] [PubMed] [Google Scholar]
- Froemming, G. R., and K. Ohlendieck. 1998. Oligomerisation of Ca2+-regulatory membrane components involved in the excitation-contraction-relaxation cycle during postnatal development of rabbit skeletal muscle. Biochim. Biophys. Acta. 1387:226–238. [DOI] [PubMed] [Google Scholar]
- Hopf, F. W., P. Reddy, J. Hong, and R. A. Steinhardt. 1996. A capacitative calcium current in cultured skeletal muscle cells is mediated by the calcium-specific leak channel and inhibited by dihydropyridine compounds. J. Biol. Chem. 271:22358–22367. [DOI] [PubMed] [Google Scholar]
- Kurebayashi, N., and Y. Ogawa. 2001. Depletion of Ca2+ in sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres. J. Physiol. 533:185–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis, B. S., M. Barnes, and D. G. Stephenson. 2003. Identification of the coupling between skeletal muscle store-operated Ca2+ entry and the inositol trisphosphate receptor. Proc. Natl. Acad. Sci. USA. 100:2941–2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, J., and Z. Pan. 2003. Junctional membrane structure and store operated calcium entry in muscle cells. Front. Biosci. 8:242–255. [DOI] [PubMed] [Google Scholar]
- McFadzean, I., and A. Gibson. 2002. The developing relationship between receptor-operated and store-operated calcium channels in smooth muscle. Br. J. Pharmacol. 135:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschella, M. C., J. Watras, T. Jayaraman, and A. R. Marks. 1995. Inositol 1,4,5-trisphosphate receptor in skeletal muscle: differential expression in myofibres. J. Muscle Res. Cell Motil. 16:390–400. [DOI] [PubMed] [Google Scholar]
- Pan, Z., D. Yang, R. Y. Nagaraj, T. A. Nosek, M. Nishi, H. Takeshima, H. Cheng, and J. Ma. 2002. Dysfunction of store-operated calcium channel in muscle cells lacking mg29. Nat. Cell Biol. 4:379–383. [DOI] [PubMed] [Google Scholar]
- Parekh, A. B., and R. Penner. 1997. Store depletion and calcium influx. Physiol. Rev. 77:901–930. [DOI] [PubMed] [Google Scholar]
- Powell, J. A., M. A. Carrasco, D. S. Adams, B. Drouet, J. Rios, M. Muller, M. Estrada, and E. Jaimovich. 2001. IP3 receptor function and localization in myotubes: an unexplored Ca2+ signaling pathway in skeletal muscle. J. Cell Sci. 114:3673–3683. [DOI] [PubMed] [Google Scholar]
- Putney, J. W., Jr. 1986. A model for receptor-regulated calcium entry. Cell Calcium. 7:1–12. [DOI] [PubMed] [Google Scholar]
- Rosemblit, N., M. C. Moschella, E. Ondriasa, D. E. Gutstein, K. Ondrias, and A. R. Marks. 1999. Intracellular calcium release channel expression during embryogenesis. Dev. Biol. 206:163–177. [DOI] [PubMed] [Google Scholar]
- Shin, D. W., Z. Pan, E. K. Kim, J. M. Lee, M. B. Bhat, J. Parness, D. H. Kim, and J. Ma. 2003. A retrograde signal from calsequestrin for the regulation of store-operated Ca2+ entry in skeletal muscle. J. Biol. Chem. 278:3286–3292. [DOI] [PubMed] [Google Scholar]
- Strube, C., R. Bournaud, I. Inoue, and T. Shimahara. 1992. Intramembrane charge movement in developing skeletal muscle cells from fetal mice. Pflugers Arch. 421:572–577. [DOI] [PubMed] [Google Scholar]
- Strube, C., M. Beurg, D. Georgescauld, R. Bournaud, and T. Shimahara. 1994. Extracellular Ca2+-dependent and independent calcium transient in fetal myotubes. Pflugers Arch. 427:517–523. [DOI] [PubMed] [Google Scholar]
- Takekura, H., B. E. Flucher, and C. Franzini-Armstrong. 2001. Sequential docking, molecular differentiation, and positioning of t-tubule/SR junctions in developing mouse skeletal muscle. Dev. Biol. 239:204–214. [DOI] [PubMed] [Google Scholar]
- Yang, D., Z. Pan, H. Takeshima, C. Wu, R. Y. Nagaraj, J. Ma, and H. Cheng. 2001. RyR3 amplifies RyR1-mediated Ca2+-induced Ca2+ release in neonatal mammalian skeletal muscle. J. Biol. Chem. 276:40210–40214. [DOI] [PubMed] [Google Scholar]
- Zweifach, A., and R. S. Lewis. 1996. Calcium-dependent potentiation of store-operated calcium channels in T lymphocytes. J. Gen. Physiol. 107:597–610. [DOI] [PMC free article] [PubMed] [Google Scholar]