Abstract
WC1+ γδ T cells of Mycobacterium bovis-infected cattle are highly responsive to M. bovis sonic extract (MBSE). In mycobacterial infections of other species, γδ T cells have been shown to respond to protein and nonprotein antigens, but the bovine WC1+ γδ T-cell antigenic targets within MBSE require further definition in terms of the dominance of protein versus nonprotein components. The present study sought to characterize the WC1+ γδ T-cell antigenic targets, together with the role of interleukin-2 (IL-2), in the context of M. bovis infection. This was achieved by testing crude and defined antigens to assess protein versus nonprotein recognition by WC1+ γδ T cells in comparison with CD4+ αβ T cells. Both cell types proliferated strongly in response to MBSE, with CD4+ T cells being the major producers of gamma interferon (IFN-γ). However, enzymatic digestion of the protein in MBSE removed its ability to stimulate CD4+ T-cell responses, whereas some WC1+ γδ T-cell proliferation remained. The most antigenic protein inducing proliferation and IFN-γ secretion in WC1+ γδ T-cell cultures was found to be ESAT-6, which is a potential novel diagnostic reagent and vaccine candidate. In addition, WC1+ γδ T-cell proliferation was observed in response to stimulation with prenyl pyrophosphate antigens (isopentenyl pyrophosphate and monomethyl phosphate). High levels of cellular activation (CD25 expression) resulted from MBSE stimulation of WC1+ γδ T cells from infected animals. A similar degree of activation was induced by IL-2 alone, but for WC1+ γδ T-cell division IL-2 was found to act only as a costimulatory signal, enhancing antigen-driven responses. Overall, the data indicate that protein antigens are important stimulators of WC1+ γδ T-cell proliferation and IFN-γ secretion in M. bovis infection, with nonprotein antigens inducing significant proliferation. These findings have important implications for diagnostic and vaccine development.
Despite increasing knowledge of antigenic targets within mycobacterial diseases, further information is needed on the antigenic recognition of individual T-cell subsets to facilitate development of diagnostic tests and vaccines. To date, much attention has focused on the role of αβ T cells, but there is increasing evidence for the involvement of γδ T cells in mycobacterial disease (5). Human and murine tuberculosis studies have revealed that γδ T cells display potent activation (CD25 expression), proliferation, and gamma interferon (IFN-γ) secretion after mycobacterial antigen stimulation (2, 15, 50). Anti-inflammatory, regulatory, and protective roles have also been ascribed to this T-cell population in tuberculosis infections (4, 14, 21, 24). However, despite these observations, the exact role of γδ T cells in tuberculosis is poorly understood.
Studies on bovine immune responses have also demonstrated a significant involvement of γδ T cells in Mycobacterium bovis infections. After experimental infection, kinetic changes in the WC1+ γδ T-cell population were observed early postchallenge (42). Such dynamic changes may correlate with the appearance of these cells in association with developing early stage M. bovis lesions (8). From the lesions they may also be involved in the recruitment of other cells to sites of M. bovis infection (46). More recently, we have shown that WC1+ γδ T cells from M. bovis-infected animals become highly activated, proliferate strongly, and release low levels of IFN-γ after in vitro stimulation with mycobacterial antigens (47). Subsequently, it was found that in vivo the WC1+ γδ T cells also became highly activated (CD25+) in the peripheral circulation at 3 to 4 weeks after M. bovis challenge (22). In vivo depletion of WC1+ γδ T cells in M. bovis challenged cattle suggested a role for WC1+ γδ T cells in the Th1 bias of developing antimycobacterial immunity, possibly via innate production of IFN-γ (22).
Although some functional responses and postulated roles for WC1+ γδ T cells in M bovis infection have been described, the antigenic targets of these cells are not defined. In other species, γδ T cells have been described as having a wide antigenic recognition (18, 20). In their contribution to human tuberculosis immunity, γδ T cells respond to protein and nonprotein antigens, including low-molecular-weight phosphate compounds (4, 12, 18, 32, 36, 49). Different forms of antigens may lead to different patterns of cell-mediated immune (CMI) responses. For example, live bacilli, killed bacilli, and soluble mycobacterial antigens stimulate different proliferative and IFN-γ responses by γδ T cells from M. tuberculosis-infected patients (15).
Antigen-dependent alterations in γδ T-cell responses could have major effects on CMI responses in tuberculosis infection, particularly as they may influence the responses of other lymphoid cells (4, 22, 44, 45). It is likely that γδ T cells play a significant role in bovine CMI responses (41), particularly as ruminant γδ T cells constitute a large proportion of lymphocytes in the peripheral circulation (19, 28). The majority of the γδ T cells also express the WC1 molecule (10). The highly activated status of WC1+ γδ T cells in M. bovis infection and their involvement in the Th1 bias of immune responses (22) suggest that they have an influential role on developing antimycobacterial immune responses.
CMI responses of WC1+ γδ T cells toward crude and defined protein and nonprotein mycobacterial antigens have not previously been investigated. The present study sought to determine the nature of antigens recognized by circulating WC1+ γδ T cells from M. bovis-infected animals and to determine their responses in terms of activation (CD25 expression), proliferation, and IFN-γ production. The role of interleukin-2 (IL-2) as an important cytokine in the function of WC1+ γδ T cells was also investigated in relation to antigenic responses during M. bovis infection.
MATERIALS AND METHODS
Experimental infections.
Friesian-cross, castrated male calves (6 months of age) from herds with no history of M. bovis infection for at least 5 years were screened in proliferation and IFN-γ assays against M. bovis and M. avium purified protein derivative antigens (Central Veterinary Laboratories, Weybridge, United Kingdom) to confirm disease-free status. Four animals were inoculated (106 CFU) intranasally with a field isolate of M. bovis (T/91/1378) (31). Age-matched uninfected animals were kept as controls (n = 2). Samples were taken at 4 to 12 weeks postinfection, and animals were confirmed as infected postmortem (i.e., at 16 months postinfection) and by M. bovis culture (9).
Antigens.
M. bovis sonic extract (MBSE) was prepared as previously described (39). Briefly, M. bovis (T/91/1378) was grown in Middlebrook 7H9 medium, harvested, washed then ultrasonicated. MBSE was then clarified by centrifugation and filtered (0.22 μm [pore size]). Proteinase K (PK; Sigma, Poole, United Kingdom)-digested MBSE (PKMBSE) was prepared by triple digestion (36). Aliquots of MBSE (1 ml at 1 mg/ml; pH 7.7) were subjected to three cycles of digestion (with PK; 25 μg of MBSE/ml) for 15 min at 37°C, followed by heating to 60°C for 5 min. Recombinant ESAT-6 was prepared in Escherichia coli K-12 as previously described (33). Isopentenyl pyrophosphate (IPP), monomethyl phosphate (MMP), heat shock protein 70, and concanavalin A were obtained from Sigma. Recombinant M. bovis protein antigens MPB59, MPB64, MPB70, and MPB83 were produced in E. coli as described previously (26, 30).
IL-2.
Recombinant human IL-2 (Invitrogen, Groningen, The Netherlands) was mainly used at 5 U/ml. One experiment also used IL-2 at 5, 100, and 500 U/ml.
Antibodies.
Hybridomas producing mouse monoclonal antibodies (MAbs) against the bovine leukocyte markers CD4 (CC8, immunoglobulin G2a [IgG2a], and CC30, IgG1) and WC1 (CC15, IgG2a) were obtained from the European Collection of Animal Cell Cultures (Porton Down, Wiltshire, United Kingdom). Hybridoma culture supernatants were used at a 1/10 dilution for flow cytometric analysis and magnetic (MACS) labeling. Anti-bovine CD25/IL-2R (IgG1) MAb CACT116A (27) (VMRD, Inc., Pullman, Wash.) was used at a 1/100 dilution for flow cytometry labeling. Primary MAbs were detected by using secondary goat anti-mouse isotype-specific conjugates (IgG2a-fluorescein isothiocyanate [FITC] and IgG1-phycoerythrin [PE]; Southern Biotechnology Associates, Inc., Birmingham, Ala.).
Preparation of PBMC, T-cell subsets, and APC.
Peripheral blood mononuclear cells (PBMC) were separated from heparinized blood samples over Ficoll-Paque as described previously (39). CD4+ and WC1+ T-cell subsets were labelled with anti-bovine MAbs CD4 (CC8) or WC1 (CC15) (1/10 dilution) and positively selected with goat anti-mouse-microbeads by using the Miltenyi Biotec (Bergisch Gladbach, Germany) MACS system as described previously (25). Purified T cells were prepared at 106 cells/ml in T-cell culture medium (RPMI 1640 supplemented with 10 mM HEPES buffer, 2 mM l-glutamine, and 5% fetal calf serum [Gibco, Paisley, United Kingdom] and 25 μg of gentamicin sulfate/ml [sigma]). Purified T-cell subsets were routinely found to be >96% pure as determined by flow cytometry and >98% viable as determined by trypan blue exclusion. Purified WC1+ cell preparations were also checked and found to be negative for contamination with CD4+ cells by using MAb CC30. Antigen-presenting cells (APC) were prepared by incubating PBMC (107 cells/ml) with mitomycin C (50 μg/ml; Sigma) at 37°C for 30 min. The APC were washed three times with phosphate-buffered saline (PBS) by centrifugation and resuspended in medium at 106 cells/ml.
Lymphocyte proliferation assay.
PBMC were prepared at 106 cells/ml (200 μl/well). CD4+ or WC1+ T cells (1.5 × 105 cells/ml) were cultured in triplicate with APC (added at 105 cells/ml; 200 μl/well) in 96-well microtiter plates (Nunc, Roskilde, Denmark). Antigens were added (25 μl/well) to a final concentration of 4 μg/ml, except for ESAT-6, IPP, and MMP, which were used at 2 μg/ml. PBS (25 μl/well) was added to control wells. In some experiments, IL-2 was added to appropriate cultures at 5 U/ml or up to 500 U/ml, as indicated. Proliferation cultures were incubated for 5 days and pulsed with 0.25 μCi of [3H]thymidine (Amersham International, Amersham, United Kingdom). Incorporated radiolabel was measured by liquid scintillation as described previously (39). The results were recorded and are reported as counts per minute (cpm). A test antigen cpm of ≥2 × the control PBS cpm was considered a positive proliferation.
IFN-γ enzyme-linked immunosorbent assay.
Cultures were set up as for proliferation assays above with M. bovis antigens or PBS controls. After 96 h of incubation, 100 μl of supernatant was aspirated from duplicate wells and assayed for IFN-γ by using the Bovigam enzyme immunoassay (CSL Ltd., Victoria, Australia). Results were expressed as the optical density at 450 nm (OD450). An OD of greater than twice the background level (i.e., in PBS wells) was considered a positive response.
Flow cytometric analysis.
Flow cytometry of PBMC in short-term cultures has been described previously (47). Briefly, 7 ml of PBMC (106 cells/ml in medium) were maintained in 25-cm2 flasks with antigen or PBS ± IL-2 (5 U/ml) for 24 h in 6% CO2 at 37°C. Primary labelling was performed with WC1 and CD25 MAbs, followed by detection with goat anti-mouse IgG2a-FITC and IgG1-PE conjugates. After labelling cells were fixed in 1% paraformaldehyde (Sigma) in PBS. Flow cytometry was performed with a FACS Vantage (Becton Dickinson, Oxford, United Kingdom). Lymphocytes were identified (25), and green (FITC) and orange (PE) log integral signals were obtained from the gated population. Cells were counted (total of 10,000), and analyses were performed by using CellQuest software (Becton Dickinson).
Statistical analysis.
Statistical analyses were performed by using analysis of variance on GENSTAT statistical software (Clarendon Press, Oxford, United Kingdom). Proliferation cpm data was log transformed prior to statistical analysis. Flow cytometric data was also analyzed by Kolmogorov-Smirnov (KS) statistics within the CellQuest program.
RESULTS
Proliferation and IFN-γ responses toward MBSE and PKMBSE.
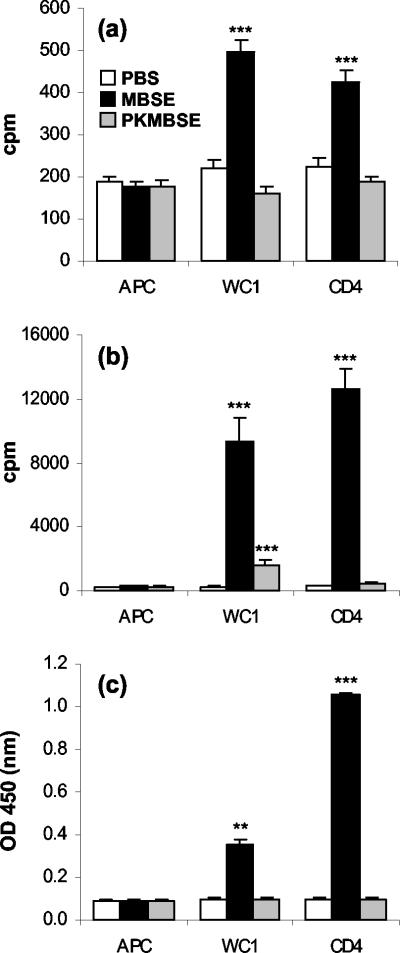
After inoculation with M. bovis, all four animals developed strong CMI responses toward MBSE antigen by 28 days postinfection (group mean = a preinfection cpm value of 634 ± 150 and a postinfection cpm value 28,501 ± 4,461 for MBSE). Preliminary experiments showed that PK treatment of MBSE did not result in a material that inhibited PBMC concanavalin A mitogen-stimulated proliferation responses (data not shown). In control animals, MBSE induced low levels of proliferation in CD4+ and WC1+ T-cell cultures (P <0.001) (Fig. 1a) that were altered by PK treatment of the MBSE. These responses in control animals highlight the lack of specificity observed with crude mycobacterial antigens. In infected animals, the strong proliferation responses to MBSE seen in both T-cell populations were significantly reduced in PKMBSE-stimulated cultures (Fig. 1b) (P <0.001). The PK digestion of protein in MBSE removed its ability to stimulate CD4+ T-cell proliferation, whereas significant WC1+ T-cell proliferation was still observed toward the nonprotein components of MBSE in all infected animals (Fig. 1b and Fig. 2). The WC1+ γδ T cells released much lower levels of IFN-γ in response to MBSE antigen than the CD4+ T cells (P <0.001) (Fig. 1c). PK treatment of MBSE also impaired its ability to induce IFN-γ release from either CD4+ or WC1+ T cells (Fig. 1c).
FIG. 1.
Lymphocyte proliferation responses of T-cell subsets from control uninfected animals (a) and proliferation (b) and IFN-γ release (c) by T-cell subsets from M. bovis-infected animals in response to mycobacterial antigen preparations of MBSE and PKMBSE. WC1+ γδ and CD4+ T cells were magnetically (MACS) purified and cultured with antigen in the presence of autologous mitomycin C-treated PBMC as a source of APC. APC alone were included as controls. Lymphocyte proliferation data are presented as cpm. A proliferation response was considered positive if the test antigen cpm value was ≥2 × the PBS control cpm value. IFN-γ ELISA results are expressed as the OD450; an OD value of 2 × the background (PBS) value was considered a positive response. The results presented show the mean group cpm or the mean group OD450 plus the standard error of the mean for six repeated experiments. Statistically significant differences between control PBS-treated and antigen-stimulated cells are indicated as follows: ✽✽✽, P <0.001; ✽✽, P < 0.01.
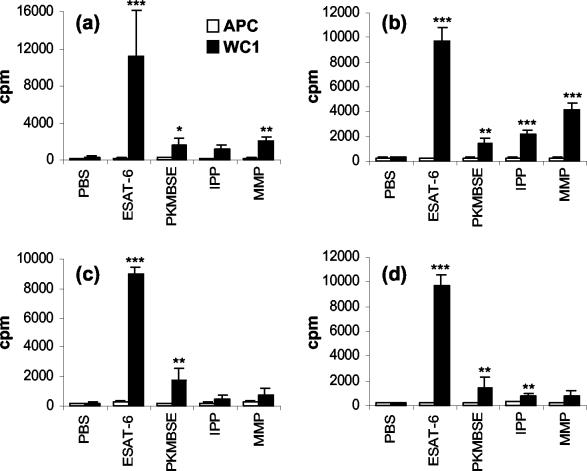
FIG. 2.
WC1+ γδ T-cell proliferation responses of four M. bovis-infected animals to PKMBSE and defined protein and nonprotein mycobacterial antigens in animals 01 (a), 02 (b), 03 (c), and 04 (b). MACS-purified WC1+ γδ T cells were cultured in the presence of autologous mitomycin C-treated PBMC as a source of APC. The results for individual animals are expressed as the cpm or the OD450 (plus the standard error of the mean), as described in Fig. 1. Significant differences between control PBS-treated and antigen-stimulated cells are indicated as follows: ✽✽✽, P <0.001; ✽✽, P <0.01; ✽, P <0.05.
Proliferation and IFN-γ responses to defined antigens.
PK treatment of MBSE indicated the importance of protein antigen in WC1+ T-cell proliferation and IFN-γ secretion and a significant role for nonprotein mycobacterial antigens in MBSE. Antigen recognition was further investigated by screening a selection of purified recombinant mycobacterial proteins, together with two nonprotein pyrophosphate antigens. Figure 2 shows the test-positive WC1+ T-cell proliferation responses of individual M. bovis-infected animals for the defined antigens, along with PKMBSE as a comparison for the nonprotein responses. ESAT-6 was found to be the most dominant protein antigen inducing high levels of proliferation in the WC1+ γδ T-cell cultures compared to those of PBS and APC-only control cultures (P <0.001) (Fig. 2). In some experiments it was observed that ESAT-6 alone could induce stronger proliferation responses of WC1+ γδ T cells than did MBSE antigen (compare Fig. 1b and 2; see also Fig. 3b). No responses (test negative) were observed by the WC1+ γδ T cells toward the other recombinant protein antigens tested. On average the group response toward nonprotein antigens was weak compared to ESAT-6 (Fig. 2). However, all M. bovis-infected animals had significant WC1+ γδ T cells proliferation responses to PKMBSE (P <0.05 to P <0.01). One animal (animal 02) displayed particularly strong proliferative responses to IPP and MMP compared to the PBS and APC controls (P <0.001) (Fig. 2b). Overall, three of four animals had significant proliferative responses to a least one of the pyrophosphate antigens (P <0.01 to P <0.001). (Fig. 2a, b, and d). The mitomycin C-treated APC did not proliferate in response to any of the antigens tested (Fig. 2).
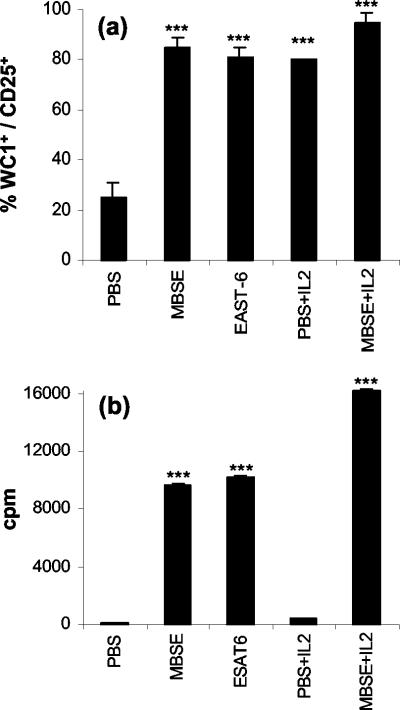
FIG. 3.
(a) Flow cytometric analysis of WC1+ γδ T-cell activation (CD25 expression) in short-term cultures of PBMC from M. bovis-infected animals after incubation with PBS, mycobacterial antigens, and/or IL-2. Flow cytometry data are presented as the group mean percentage (plus the standard error of the mean) of WC1+ γδ T cells within PBMC cultures expressing CD25 (i.e., percent WC1+ CD25+ cells/percent WC1+ cells) for four repeated experiments. (b) Proliferation responses of WC1+ γδ T cells from M. bovis-infected animals after culture with PBS, mycobacterial antigens, and/or IL-2. MACS-purified WC1+ γδ T cells were cultured in the presence of autologous mitomycin C-treated PBMC as a source of APC. The results are expressed as the group mean cpm (plus the standard error of the mean) for six repeated experiments. Statistically significant differences between control PBS-treated and antigen-stimulated cells are indicated as follows: ✽✽✽, P <0.001.
As observed for the proliferation of WC1+ γδ T cells, ESAT-6 induced significant release of IFN-γ in these cultures (P <0.01) compared to the PBS or APC controls (group mean = an ESAT-6 OD of 0.382 ± 0.027 and a PBS OD of 0.100 ± 0.0045). The other defined mycobacterial antigens tested failed to stimulate WC1+ γδ T-cell production of IFN-γ. The APC controls did not show any IFN-γ release in response to antigens tested in comparison with the PBS control. No specific proliferation or IFN-γ secretion was observed in WC1+ γδ T-cell cultures in response to ESAT-6, IPP, or MMP in uninfected control animals (data not shown).
Role of IL-2 in antigen-stimulated WC1+ γδ T-cell activation and proliferation.
In view of the dominance of protein antigens in inducing strong WC1+ γδ T-cell proliferation (Fig. 1b and 2), it was of interest to determine the role of IL-2 in the activation (CD25 expression) and proliferation of WC1+ γδ T cells. Figure 3a shows flow cytometry results of PBMC 24-h short-term cultures labeled for WC1+ and CD25 expression. MBSE and ESAT-6 (single defined antigen) induced strong CD25 expression in the majority (80 to 90%) of WC1+ γδ T cells from M. bovis-infected animals. Incubation with IL-2 alone also induced the majority of WC1+ γδ T cells to express CD25, which was similar to antigen activation with MBSE or ESAT-6 (Fig. 3a). In comparison to the PBS control short-term culture, MBSE, ESAT-6, or IL-2 alone caused highly significant activation of the WC1+ γδ T cells (P <0.001). CD25 expression on WC1+ γδ T cells was further enhanced when short-term cultures were incubated with both antigen (MBSE) and IL-2 compared to either MBSE or IL-2 alone (P <0.01). Analysis of CD25 expression on overlaid histograms for WC1+ T cells in short-term culture by using KS statistics revealed that IL-2 alone could induce a higher density of cell membrane CD25 expression on WC1+ γδ T cells compared to stimulation with MBSE (D value of up to 12%; P <0.001). The combination of MBSE and IL-2 resulted in the greatest percentage of CD25+ WC1+ T cells and also the highest density of cell surface CD25 expression compared to MBSE alone (D value of up to 35%; P <0.001) (data not shown).
The potent WC1+ γδ T-cell activation induced by MBSE and ESAT-6 antigens was paralleled by their ability to drive strong and significant proliferation of WC1+ γδ T cells compared to PBS controls (P <0.001) (Fig. 3b). Although IL-2 induced high levels of CD25 expression, alone it was not sufficient to initiate WC1+ γδ T-cell proliferation. The costimulatory role of IL-2 after antigen stimulation is seen in Fig. 3b, where IL-2 significantly (P <0.01) augmented antigen (MBSE)-driven proliferation. In a separate experiment WC1+ γδ T cells were incubated with 5, 100, and 500 U/ml of IL-2. The data confirmed that IL-2 alone could not deliver the necessary signals for WC1+ γδ T-cell proliferation (data not shown). No proliferation with antigen or IL-2 was observed in APC-only cultures.
DISCUSSION
Understanding the relative contribution of T-cell subsets and their antigenic targets in M. bovis infection will significantly improve disease control strategies. We previously reported that WC1+ γδ T cells from M. bovis-infected animals are highly responsive to crude M. bovis antigen preparations, such as MBSE (47). γδ T cells recognize a range of protein and nonprotein antigens, but the relative importance of such antigens within MBSE that stimulate WC1+ γδ T-cell responses has not been determined. Enzymatic digestion of the protein component of MBSE removed its antigenicity for CD4+ T-cell proliferation and IFN-γ production. The importance of proteins as antigens for WC1+ γδ T cells was also demonstrated by PK treatment of MBSE, which resulted in an 84% reduction in proliferation. However, significant WC1+ γδ T-cell proliferation did occur in response to PKMBSE, indicating their recognition of nonprotein mycobacterial antigens. It was also apparent that the protein component of MBSE was responsible for WC1+ γδ T-cell IFN-γ production. These results would suggest that differential responses of WC1+ γδ T cells to protein or nonprotein antigens do occur, and such antigen-dependent responses could markedly influence M. bovis CMI responses. Proteins, in particular, may have a significant involvement in antigen-dependent IFN-γ secretion by WC1+ γδ T cells, possibly enhancing their role in the development of Th1 biased antimycobacterial immunity through innate or adaptive production of IFN-γ (22). Involvement of bovine WC1+ γδ T cells in innate production of IFN-γ and IL-12 would be important functional roles, linking the innate and adaptive immune systems, as has been described for human γδ T cells (7, 17, 29).
More defined protein and nonprotein antigens were screened in WC1+ γδ T-cell assays. ESAT-6 was found to be the most antigenic protein tested, inducing significant proliferation and IFN-γ secretion in WC1+ γδ T-cell cultures. The immunodominance of ESAT-6 is particularly impressive, since the responses are comparable to MBSE, which contains multiple protein and nonprotein antigens. It is significant that WC1+ γδ T cells recognize ESAT-6, which is unique to pathogenic mycobacterial species and is recognized as an important diagnostic and vaccine candidate (1, 34). The diagnostic potential of ESAT-6 for bovine tuberculosis has been reported, with ESAT-6 inducing strong and specific IFN-γ release from PBMC, in both experimentally and naturally infected field cases of M. bovis (6, 38, 40). The MPB83, MPB70, MPB64, MPB59, and heat shock protein 70 antigens failed to stimulate notable responses, and this may reflect a low antigenicity of these proteins for WC1+ γδ T cells or a low dominance during the sampling period. Some of these antigens have also been observed to give weak responses in TcR1+ γδ T-cell cultures (44). Interestingly, comparison of the total γδ T-cell population (including WC1−/CD8+ cells) (44) proliferation and the WC1+ γδ T-cell responses in the present study highlights differences in the antigenic recognition by WC1+ and WC1− γδ T-cell populations. Differential IFN-γ responses have also been reported for WC1+ (IFN-γ +) and WC1− (IFN-γ−) γδ T-cell populations (3).
Some of the most potent nonprotein antigens, recognized by human γδ T cells, identified in mycobacterial lysates include TUBag and pyrophosphate compounds (12, 43, 49). Two pyrophosphate antigens IPP and MMP were tested in the present study, and three of four of the infected animals responded to at least one of these compounds, with animal 02 displaying particularly strong WC1+ γδ T-cell proliferation in response to both IPP and MMP. No IFN-γ secretion was detected in response to these antigens, although human γδ T cells have been reported to produce IFN-γ in response to IPP (35). WC1+ γδ T-cell responses to IPP and MMP antigens may be important in some animals or at certain stages of infection. Human tuberculosis studies have reported that increased γδ T-cell activity to phosphoantigens is dependent on constant antigenic exposure and correlates with an increased bacterial load (13, 37).
Various cytokines are involved in the function of γδ T cells, but IL-2 in particular has a significant role in WC1+ γδ T-cell responses. The constitutive low-level expression of CD25 on bovine WC1+ γδ T cells may allow a lower threshold for responding to IL-2 (11, 23). It is possible that the potent activation of WC1+ γδ T cells from infected animals after in vitro stimulation with mycobacterial antigens may have resulted either indirectly via nonspecific stimulation with IL-2 secreted by CD4+ T cells or directly via antigen stimulation (47). Investigation of the role of IL-2 in WC1+ γδ T-cell activation revealed that IL-2 alone induced the majority of WC1+ γδ T cells to express high levels of CD25 to a degree similar to that seen with MBSE or ESAT-6 antigen stimulation. Therefore, IL-2 alone can cause nonspecific activation of WC1+ γδ T cells but fails to initiate their proliferation. In agreement with previous reports for bovine γδ T cells (11, 17), IL-2 appeared to act as a costimulatory factor in antigen-dependent cell division. One of the immune mechanisms that has been suggested for the control of γδ T-cell responses is the availability of IL-2 from CD4+ T cells (51). Kinetic analysis of the in vitro activation of bovine αβ and γδ T cells from M. bovis-infected animals identified CD4+ T-cell activation in advance of the WC1+ γδ T cells, thereby supporting the requirement of CD4+ help in the functions of WC1+ γδ T cells (47). However, after experimental M. bovis infection, the in vivo data indicate that WC1+ γδ T-cell activation (CD25 expression) in the peripheral circulation occurs in advance of CD4+ T-cell activation (22). In vivo, there is the possibility that early in bacterial infections γδ T cells may preferentially use other cytokines as growth factors in advance of IL-2 availability (48).
In terms of the diagnosis of M. bovis infection in cattle by CMI-based diagnostic tests, it appears that mycobacterial protein antigens (including ESAT-6) are important in stimulating strong and specific Th1 biased CMI responses by WC1+ γδ T cells. Relative to the dominance of protein antigens, the significance of nonprotein tuberculosis antigens should be given further consideration in the search for novel diagnostic reagents. Although preliminary experiments suggest that phosphoantigens may not be diagnostically useful, they may well prove beneficial for the identification of heavily diseased animals. One hypothesis is that strong WC1+ γδ T-cell recognition of phosphoantigens in M. bovis infection may correlate with an inability to contain the infection, leading to an increased risk of disease transmission.
The induction of Th1 CMI responses in WC1+ γδ T cells by protein antigens of M. bovis should be considered in vaccine development. The low levels of IFN-γ secreted by WC1+ γδ T cells may be sufficient to induce activation of macrophages and influence the Th1 bias of antimycobacterial immunity (22). A recent study of human tuberculosis suggested that γδ T-cell stimulation might be an important feature of vaccination by enhancing the induction of type 1 memory immunity (52). Since nonprotein mycobacterial antigens also induced WC1+ γδ T-cell responses, they could have a role in the control of tuberculosis. The potential of phosphate antigens as candidates for M. bovis vaccines is not known, but in humans the inclusion of such compounds may be important components of vaccines that stimulate the γδ T-cell pathway (16).
In conclusion, the data of the present study provides further evidence for the role of WC1+ γδ T-cell involvement in CMI responses to M. bovis infection. Importantly, we have shown the dominance of protein antigens at inducing a Th1 type of CMI response by WC1+ γδ T cells from infected animals. Furthermore, we identified significant responses of WC1+ γδ T cells from M. bovis-infected animals to nonprotein antigens. The antigens involved in the activation, proliferation, and release of IFN-γ by WC1+ γδ T cells may well play a role in protective immunity and, additionally, be of diagnostic benefit.
Acknowledgments
This work was supported by the Department of Agriculture and Rural Development for Northern Ireland, including a Ph.D. studentship, and by grant 81/509909 from the Biotechnology and Biological Sciences Research Council (United Kingdom).
We thank Deirdre Fitzpatrick for statistical analysis and Sydney Neill and Dermot Mackie for their assistance.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Andersen, P., M. E. Munk, J. M. Pollock, and T. M. Doherty. 2000. Specific immune-based diagnosis of tuberculosis. Lancet 356:1099-1104. [DOI] [PubMed] [Google Scholar]
- 2.Balaji, K. N., and W. H. Boom. 1998. Processing of Mycobacterium tuberculosis bacilli by human monocytes for CD4+ αβ and γδ T cells: role of particulate antigen. Infect. Immun. 66:98-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldwin, C. L., T. Sathiyaseelan, M. Rocchi, and D. McKeever. 2000. Rapid changes occur in the percentage of circulating bovine WC1+ γδ Th1 cells. Res. Vet. Sci. 69:175-180. [DOI] [PubMed] [Google Scholar]
- 4.Batoni, G., S. Esin, R. A. Harris, G. Kallenius, S. B. Svenson, R. Andersson, M. Campa, and H. Wigzell. 1998. γδ+ and CD4+ αβ+ human T-cell subset responses upon stimulation with various Mycobacterium tuberculosis soluble extracts. Clin. Exp. Immunol. 112:52-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boom, W. H. 1999. γδ T cells and Mycobacterium tuberculosis. Microbes Infect. 1:187-195. [DOI] [PubMed] [Google Scholar]
- 6.Buddle, B. M., T. J. Ryan, J. M. Pollock, P. Andersen, and G. W. de Lisle. 2001. Use of ESAT-6 in the interferon-gamma test for diagnosis of bovine tuberculosis following skin testing. Vet. Microbiol. 80:37-46. [DOI] [PubMed] [Google Scholar]
- 7.Bukowski, J. F., C. T. Morita, and M. B. Brenner. 1999. Human γδ T cells recognize alkylamines derived from microbes, edible plants, and tea: implications for innate immunity. Immunity 11:57-65. [DOI] [PubMed] [Google Scholar]
- 8.Cassidy, J. P., D. G. Bryson, M. M. G. Cancela, F. Forster, J. M. Pollock, and S. D. Neill. 2001. Lymphocyte subtypes in experimentally induced early-stage bovine tuberculous lesions. J. Comp. Pathol. 124:46-51. [DOI] [PubMed] [Google Scholar]
- 9.Cassidy, J. P., D. G. Bryson, J. M. Pollock, R. T. Evans, F. Forster, and S. D. Neill. 1998. Early lesion formation in cattle experimentally infected with Mycobacterium bovis. J. Comp. Pathol. 119:27-44. [DOI] [PubMed] [Google Scholar]
- 10.Clevers, H., N. D. MacHugh, A. Bensaid, S. Dunlap, C. L. Baldwin, A. Kaushal, K. Iams, C. J. Howard, and W. I. Morrison. 1990. Identification of a bovine surface-antigen uniquely expressed on CD4-CD8-T-cell receptor γδ+ lymphocytes-T. Eur. J. Immunol. 20:809-817. [DOI] [PubMed] [Google Scholar]
- 11.Collins, R. A., P. Sopp, K. I. Gelder, W. I. Morrison, and C. J. Howard. 1996. Bovine γδ TcR+ T lymphocytes are stimulated to proliferate by autologous Theileria annuluta-infected cells in the presence of interleukin-2. Scand. J. Immunol. 44:444-452. [DOI] [PubMed] [Google Scholar]
- 12.Constant, P., F. Davodeau, M. A. Peyrat, Y. Poquet, G. Puzo, M. Bonneville, and J. J. Fournie. 1994. Stimulation of human γδ T cells by nonpeptidic mycobacterial ligands. Science 264:267-270. [DOI] [PubMed] [Google Scholar]
- 13.Dieli, F., G. Sireci, C. Di Sano, A. Romano, L. Titone, P. Di Carlo, J. Ivanyi, J. J. Fournie, and A. Salerno. 2000. Ligand-specific αβ and γδ T-cell responses in childhood tuberculosis. J. Infect. Dis. 181:294-301. [DOI] [PubMed] [Google Scholar]
- 14.D'Souza, C. D., A. M. Cooper, A. A. Frank, R. J. Mazzaccaro, B. R. Bloom, and I. M. Orme. 1997. An anti-inflammatory role for γδ T lymphocytes in acquired immunity to Mycobacterium tuberculosis. J. Immunol. 158:1217-1221. [PubMed] [Google Scholar]
- 15.Esin, S., G. Batoni, G. Kallenius, H. Gaines, M. Campa, S. B. Svenson, R. Andersson, and H. Wigzell. 1996. Proliferation of distinct human T-cell subsets in response to live, killed or soluble extracts of Mycobacterium tuberculosis and Mycobacterium avium. Clin. Exp. Immunol. 104:419-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Espinosa, E., C. Belmant, F. Pont, B. Luciani, R. Poupot, F. Romagne, H. Brailly, M. Bonneville, and J. J. Fournie. 2001. Chemical synthesis and biological activity of bromohydrin pyrophosphate, a potent stimulator of human γδ T cells. J. Biol. Chem. 276:18337-18344. [DOI] [PubMed] [Google Scholar]
- 17.Fikri, Y., O. Denis, P. P. Pastoret, and J. Nyabenda. 2001. Purified bovine WC1+ γδ T lymphocytes are activated by staphylococcal enterotoxins and toxic shock syndrome toxin-1 superantigens: proliferation response, TCR V gamma profile and cytokines expression. Immunol. Lett. 77:87-95. [DOI] [PubMed] [Google Scholar]
- 18.Hayday, A. C. 2000. γδ cells: A right time and a right place for a conserved third way of protection. Annu. Rev. Immunol. 18:975-1026. [DOI] [PubMed] [Google Scholar]
- 19.Hein, W. R., and C. R. Mackay. 1991. Prominence of γδ T cells in the ruminant immune-system. Immunol. Today 12:30-34. [DOI] [PubMed] [Google Scholar]
- 20.Kaufmann, S. H. E. 1996. γδ and other unconventional T lymphocytes: what do they see and what do they do? Proc. Natl. Acad. Sci. USA 93:2272-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaufmann, S. H. E., C. Blum, and S. Yamamoto. 1993. Crosstalk between αβ-T cells and γδ-T cells in vivo: activation of αβ T-cell responses after γδ T-cell modulation with monoclonal antibody GL3. Proc. Natl. Acad. Sci. USA 90:9620-9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kennedy, H. E., M. D. Welsh, D. Bryson, J. Cassidy, F. I. Forster, C. J. Howard, R. A. Collins, and J. M. Pollock. 2002. Modulation of immune responses to Mycobacterium bovis in cattle depleted of WC1+ γδ T cells. Infect. Immun. 70:1488-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirkham, P. A., H. H. Takamatsu, and R. M. E. Parkhouse. 1997. Growth arrest of γδ T cells induced by monoclonal antibody against WC1 correlates with activation of multiple tyrosine phosphatases and dephosphorylation of MAP kinase erk2. Eur. J. Immunol. 27:717-725. [DOI] [PubMed] [Google Scholar]
- 24.Ladel, C. H., C. Blum, A. Dreher, K. Reifenberg, and S. H. E. Kaufmann. 1995. Protective role of γδ T cells and αβ T cells in tuberculosis. Eur. J. Immunol. 25:2877-2881. [DOI] [PubMed] [Google Scholar]
- 25.Liebana, E., R. M. Girvin, M. Welsh, S. D. Neill, and J. M. Pollock. 1999. Generation of CD8+ T-cell responses to Mycobacterium bovis and mycobacterial antigen in experimental bovine tuberculosis. Infect. Immun. 67:1034-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lightbody, K. A., R. A. Skuce, S. D. Neill, and J. M. Pollock. 1998. Mycobacterial antigen-specific antibody responses in bovine tuberculosis: an ELISA with potential to confirm disease status. Vet. Rec. 142:295-300. [DOI] [PubMed] [Google Scholar]
- 27.MacHugh, N. D., E. L. Taracha, and P. G. Toye. 1993. Reactivity of workshop antibodies on L-cell and Cos cell transfectants expressing bovine CD antigens. Vet. Immunol. Immunopathol. 39:61-67. [DOI] [PubMed] [Google Scholar]
- 28.Mackay, C. R., J. F. Maddox, and M. R. Brandon. 1986. Three distinct subpopulations of sheep lymphocytes-T. Eur. J. Immunol. 16:19-25. [DOI] [PubMed] [Google Scholar]
- 29.Mak, T. W., and D. A. Ferrick. 1998. The γδ T-cell bridge: linking innate and acquired immunity. Nat. Med. 4:764-765. [DOI] [PubMed] [Google Scholar]
- 30.McNair, J., D. M. Corbett, R. M. Girvin, D. P. Mackie, and J. M. Pollock. 2001. Characterization of the early antibody response in bovine tuberculosis: MPB83 is an early target with diagnostic potential. Scand. J. Immunol. 53:365-371. [DOI] [PubMed] [Google Scholar]
- 31.Neill, S. D., J. Hanna, J. J. Obrien, and R. M. McCracken. 1988. Excretion of Mycobacterium bovis by experimentally infected cattle. Vet. Rec. 123:340-343. [DOI] [PubMed] [Google Scholar]
- 32.O'Brien, R. L., Y. X. Fu, R. Cranfill, A. Dallas, C. Ellis, C. Reardon, J. Lang, S. R. Carding, R. Kubo, and W. Born. 1992. Heat-shock protein HSP60-reactive γδ cells: a large, diversified lymphocyte-T subset with highly focused specificity. Proc. Natl. Acad. Sci. USA 89:4348-4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oettinger, T., A. Holm, I. M. Mtoni, A. B. Andersen, and K. Haslov. 1995. Mapping of the delayed-type hypersensitivity-inducing epitope of secreted protein MPT64 from Mycobacterium tuberculosis. Infect. Immun. 63:4613-4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olsen, A. W., L. A. H. van Pinxteren, L. M. Okkels, P. B. Rasmussen, and P. Andersen. 2001. Protection of mice with a tuberculosis subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Infect. Immun. 69:2773-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ottones, F., J. Liautard, A. Gross, F. Rabenoelina, J. P. Liautard, and J. Favero. 2000. Activation of human V gamma 9V delta 2 T cells by a Brucella suis non-peptidic fraction impairs bacterial intracellular multiplication in monocytic infected cells. Immunology 100:252-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfeffer, K., B. Schoel, H. Gulle, S. H. E. Kaufmann, and H. Wagner. 1990. Primary responses of human T cells to mycobacteria: a frequent set of γδ T cells are stimulated by protease-resistant ligands. Eur. J. Immunol. 20:1175-1179. [DOI] [PubMed] [Google Scholar]
- 37.Poccia, F., M. Malkovsky, A. Pollak, V. Colizzi, G. Sireci, A. Salerno, and F. Dieli. 1999. In vivo γδ T-cell priming to mycobacterial antigens by primary Mycobacterium tuberculosis infection and exposure to nonpeptidic ligands. Mol. Med. 5:471-476. [PMC free article] [PubMed] [Google Scholar]
- 38.Pollock, J. M., and P. Andersen. 1997. Predominant recognition of the ESAT-6 protein in the first phase of infection with Mycobacterium bovis in cattle. Infect. Immun. 65:2587-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pollock, J. M., A. J. Douglas, D. P. Mackie, and S. D. Neill. 1994. Identification of bovine T-cell epitopes for 3 Mycobacterium bovis antigens MPB70, 19,000-MW and MPB57. Immunology 82:9-15. [PMC free article] [PubMed] [Google Scholar]
- 40.Pollock, J. M., R. M. Girvin, K. A. Lightbody, R. A. Clements, S. D. Neill, B. M. Buddle, and P. Andersen. 2000. Assessment of defined antigens for the diagnosis of bovine tuberculosis in skin test-reactor cattle. Vet. Rec. 146:659-665. [DOI] [PubMed] [Google Scholar]
- 41.Pollock, J. M., J. McNair, M. D. Welsh, R. M. Girvin, H. E. Kennedy, D. P. Mackie, and S. D. Neill. 2001. Immune responses in bovine tuberculosis. Tuberculosis 81:103-107. [DOI] [PubMed] [Google Scholar]
- 42.Pollock, J. M., D. A. Pollock, D. G. Campbell, R. M. Girvin, A. D. Crockard, S. D. Neill, and D. P. Mackie. 1996. Dynamic changes in circulating and antigen-responsive T-cell subpopulations post-Mycobacterium bovis infection in cattle. Immunology 87:236-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poquet, Y., P. Constant, F. Halary, M. A. Peyrat, M. Gilleron, F. Davodeau, M. Bonneville, and J. J. Fournie. 1996. A novel nucleotide-containing antigen for human blood γδ T lymphocytes. Eur. J. Immunol. 26:2344-2349. [DOI] [PubMed] [Google Scholar]
- 44.Rhodes, S. G., R. G. Hewinson, and H. M. Vordermeier. 2001. Antigen recognition and immunomodulation by γδ T cells in bovine tuberculosis. J. Immunol. 166:5604-5610. [DOI] [PubMed] [Google Scholar]
- 45.Smith, A. L., and A. C. Hayday. 2000. An αβ T-cell-independent immunoprotective response towards gut coccidia is supported by γδ cells. Immunology 101:325-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith, R. A., J. M. Kreeger, A. J. Alvarez, J. C. Goin, W. C. Davis, D. L. Whipple, and D. M. Estes. 1999. Role of CD8+ and WC-1+ γδ T cells in resistance to Mycobacterium bovis infection in the SCID-bo mouse. J. Leukoc. Biol. 65:28-34. [DOI] [PubMed] [Google Scholar]
- 47.Smyth, A. J., M. D. Welsh, R. M. Girvin, and J. M. Pollock. 2001. In vitro responsiveness of γδ T cells from Mycobacterium bovis-infected cattle to mycobacterial antigens: predominant involvement of WC1+ cells. Infect. Immun. 69:89-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takano, M., H. Nishimura, Y. Kimura, Y. Mokuno, J. Washizu, S. Itohara, Y. Nimura, and Y. Yoshikai. 1998. Protective roles of γδ T cells and interleukin-15 in Escherichia coli infection in mice. Infect. Immun. 66:3270-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanaka, Y., C. T. Morita, Y. Tanaka, E. Nieves, M. B. Brenner, and B. R. Bloom. 1995. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature 375:155-158. [DOI] [PubMed] [Google Scholar]
- 50.Tsukaguchi, K., K. N. Balaji, and W. H. Boom. 1995. CD4+ αβ T-cell and γδ T-cell responses to Mycobacterium tuberculosis: similarities and differences in antigen recognition, cytotoxic effector function, and cytokine production. J. Immunol. 154:1786-1796. [PubMed] [Google Scholar]
- 51.Wesch, D., S. Marx, and D. Kabelitz. 1997. Comparative analysis of αβ and γδ T-cell activation by Mycobacterium tuberculosis and isopentenyl pyrophosphate. Eur. J. Immunol. 27:952-956. [DOI] [PubMed] [Google Scholar]
- 52.Worku, S., G. J. Gorse, R. B. Belshe, and D. F. Hoft. 2001. Canarypox vaccines induce antigen-specific human γδ T cells capable of interferon-gamma production. J. Infect. Dis. 184:525-532. [DOI] [PubMed] [Google Scholar]