Abstract
Previous studies have demonstrated that the carboxyl terminus of the gap junction protein Cx43 (Cx43CT) can act as an independent, regulatory domain that modulates intercellular communication in response to appropriate chemical stimuli. Here, we have used NMR, chemical cross-linking, and analytical ultracentrifugation to further characterize the biochemical and biophysical properties of the Connexin43 carboxyl terminal domain (S255-I382). NMR-diffusion experiments at pH 5.8 suggested that the Connexin43 carboxyl terminus (CX43CT) may have a molecular weight greater than that of a monomer. Sedimentation equilibrium and cross-linking data demonstrated a predominantly dimeric state for the Cx43CT at pH 5.8 and 6.5, with limited dimer formation at a more neutral pH. NMR-filtered nuclear Overhauser effect studies confirmed these observations and identified specific areas of parallel orientation within Cx43CT, likely corresponding to dimerization domains. These regions included a portion of the SH3 binding domain, as well as two fragments previously found to organize in α-helical structures. Together, these data show that acidification causes Cx43CT dimer formation in vitro. Whether dimer formation is an important structural component of the regulation of Connexin43 channels remains to be determined. Dimerization may alter the affinity of Cx43CT regions for specific molecular partners, thus modifying the regulation of gap junction channels.
INTRODUCTION
Gap junctions allow for the direct exchange of ions and other small molecules (<1kDa) between neighboring cells. These intercellular channels are formed by oligomerization of connexin proteins. Six connexins form a hemichannel, or connexon. Two connexons, one provided by each apposing cell, bind across the extracellular space to form a gap junction channel. Recent studies show that not only the presence, but also the regulation of gap junctions is essential for normal tissue function. Our laboratories have been interested in understanding the changes in high-order structure that associate with the regulation of the major cardiac gap junction protein, Connexin43 (Cx43)1 (Duffy et al., 2002,2004; Sorgen et al., 2002).
One intracellular stimulus that has long been known to regulate gap junctions is pH. Acidification of the intracellular space leads to a loss of intercellular communication (Spray and Bennett, 1985; Francis et al., 1999; Stergiopoulos et al., 1999). This phenomenon, commonly known as “pH gating”, is proposed to act as a substrate for the development of malignant ventricular arrhythmias consequent to myocardial ischemia (Kleber et al., 1987; Janse and Wit, 1989; Cascio et al., 1995; Peters et al., 1997). Work preformed in Xenopus oocytes showed that truncation of the carboxyl terminal region of Cx43 (Cx43CT) prevented acidification-induced channel closure (Morley et al., 1996). Coexpression with the carboxyl terminal (CT) partially recovered pH gating. This result led to the hypothesis that pH gating results from a “ball-and-chain” type mechanism in which a gating particle (the CT) binds to a separate region of the protein (a “receptor”) to close the channel. This model also applies to the regulation of Cx43 channels by other factors (Homma et al., 1998; Zhou et al., 1999; Moreno et al., 2002). In general, the CT region of Cx43 is seen as a regulatory domain that can interact with the cytoplasmic microenvironment and modify intercellular communication accordingly.
A number of studies show that the CT domain remains functional even if it is not fused with the pore-forming region of the connexin protein. Indeed, the isolated CT domain retains its ability to regulate Cx43 channels in response to pH (Morley et al., 1996), insulin and insulin-like growth factor (Homma et al., 1998), c-Src (Zhou et al., 1999), and transjunctional voltage (Moreno et al., 2002). Moreover, several studies show that the Cx43CT fragment acts in vitro as a substrate for a number of kinases thought to phosphorylate Cx43 channels (Swenson et al., 1990; Goldberg and Lau, 1993; Saez et al., 1993; Moreno et al., 1994; Loo et al., 1995; Cooper et al., 2000; Lampe and Lau, 2000), and it can bind to known molecular partners of Cx43 such as zonula ocludens-1 (Giepmans and Moolenaar, 1998; Toyofuku et al., 2001), tubulin (Giepmans et al., 2001), and the SH3 domain of Src (Kanemitsu et al., 1997; Duffy et al., 2004). These results suggest that the CT domain retains at least some of its functional properties when in isolation. In vitro studies on the biophysical and biochemical properties of the CT domain are therefore expected to shed light on the molecular mechanisms regulating Cx43 gap junctions.
We have initiated a characterization of the high-order structure of the Cx43CT domain, and backbone resonances have been recently assigned (Sorgen et al., 2002). Moreover, we have used NMR translational diffusion analysis to study the mobility of Cx43CT using magnetic gradients (Duffy et al., 2002). These data showed reduced diffusion velocity of the Cx43CT fragment at low pH, which could be explained by the formation of a higher molecular weight species, perhaps consequent to oligomerization of the fragment. In this study, we used cross-linking, analytical centrifugation, and NMR to show that the Cx43CT domain exists in a predominantly monomeric state at pH 7.5 and a predominantly dimeric state at pH 6.5 and 5.8. We have further identified the specific residues involved in dimerization. These include a fragment of the SH3 binding domain, as well as two regions thought to organize as α-helical structures (Sorgen et al., 2002). We hypothesize that dimerization of the CT domain may occur in functional Cx43 channels and play a role in the regulation of gap junctions in response to low pH.
MATERIALS AND METHODS
Expression and purification of recombinant GST-Cx43CT
The Cx43CT, 15N-Cx43CT, and 15N13C-Cx43CT polypeptides were expressed and purified as described previously (Duffy et al., 2002). All polypeptides were confirmed for purity and analyzed for degradation by SDS-PAGE, NMR, and mass spectroscopy. The analysis showed that Cx43CT kept at 25°C and at pH 7.2 and 7.5 was stable for up to ∼30 h; after this time, protein cleavage was detected near residue G321, possibly due to autoproteolyisis. All experiments were performed within a 24-h window.
Cross-linking
The cross-linking of Cx43CT was carried out for 1 h at room temperature using 10 mM of 1-Ethyl-3-(3-Dimethylaminopropyl) carbodiimide Hydrochloride (EDC). The reactions occurred in phosphate-buffered saline (PBS) at pH 5.8, 6.5, and 7.5 and were quenched by the addition of ethanolamine-HCL to a final concentration of 100 mM. Complete quenching was achieved by leaving the reactions standing for 10 min at room temperature followed by heating in SDS sample buffer. The products of the reaction were then run on 15% SDS-PAGE gels.
Immunoblot analysis
Complexes within the gel were electrophoretically transferred to nitrocellulose membrane (Schleicher and Schuell, Keene, NH) and probed for Cx43 as previously described (Thi et al., 2003). Briefly, membranes were blocked in 5% skim milk in phosphate-buffered saline (PBS) and probed for 1 h at room temperature using polyclonal antibodies directed against the carboxyl terminal domain of Cx43 (Zymed, South San Francisco, CA, and 181A, gift from Elliot Hertzberg, AECOM, Bronx, NY) diluted in 5% skim milk in PBS. After rinses in PBS with 0.05% Tween20 (PBST) membranes were incubated with horseradish peroxidase-conjugated rabbit secondary IgGs (Santa Cruz Biotech, Santa Cruz, CA). Protein bands were detected using Amersham ECL detection kit (Amersham Biosciences, Piscataway, NJ) and exposed on Fuji X-Ray film.
Analytical centrifugation
Sedimentation equilibrium experiments were performed using a Beckman Optima XL-I analytical ultracentrifuge and an AN-60Ti rotor. The Cx43CT was analyzed at 25°C in PBS buffer (pH 5.8, 6.5, 7.2, and 7.5). For each condition, data were collected at three concentrations (A280 = 0.3, 0.5, and 0.9) and two rotor speeds (18,000 rpm and 26,000 rpm). Absorbance scans at 280 nm were taken after 22 h and 24 h at each speed; it was assumed that equilibrium was reached if the scans were unchanged. Data analysis was performed using the Beckman XL-A/XL-I software package within Microcal, ORIGIN v4 using values of the buffer density and protein partial specific volume determined as described below. Each analysis consisted of the six-absorbance scans taken at three different nominal concentrations and at each of the two rotor speeds.
Buffer densities were determined using a Mettler DE40 density meter operated at the experimental temperature and data were analyzed with the program Sednterp v1.03. Partial specific volume was determined from amino acid residue composition and calculated in Sednterp.
Nuclear magnetic resonance
NMR data were acquired at 7°C using a Bruker DRX-600 spectrometer fitted with a triple resonance probe and triple axis gradients. All experimental data to determine backbone sequential assignments have been described (Sorgen et al., 2002). Intermolecular nuclear Overhauser effects (NOEs) were observed in a 3D 13C F1-edited, F3-13C/15N-filtered NOE spectrum (Lee et al., 1994), with a mixing time of 125 ms. 13C15N-Cx43CT alone was used as the control for leakage through the filter. 13C15N-Cx43CT was titrated with unlabeled Cx43CT to a 1:1 molar ratio at pH 5.8. NMR spectra were processed using NMRPipe (Delaglio et al., 1995) and analyzed using NMRView (Johnson and Blevins, 1994).
RESULTS
Cx43CT chemical cross-linking
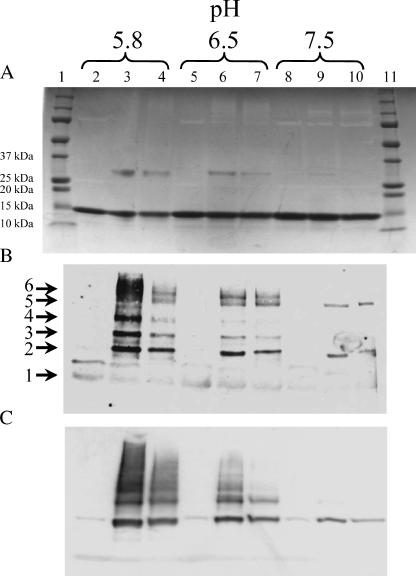
To assess the possibility of pH-dependent dimerization of Cx43CT, solutions of purified, recombinant Cx43CT at differing pH values were combined with the irreversible cross-linking agent EDC. EDC, a zero-length cross-linker, reacts with carboxylic acid and primary amine-containing molecules. Protein species were separated by SDS-PAGE (10 μg per lane) and stained with Coomassie blue (Fig. 1 A). Lanes 1 and 11 correspond to the molecular weight standards. The 14 kDa bands seen across the gel correspond to the monomeric form of Cx43CT. Lanes 2, 5, and 8 correspond to samples that were not exposed to the cross-linking agent. The pH of the solvent varied from 5.8 to 7.5. An ∼29 kDa band was apparent in lanes 3 and 4, 6 and 7, and—very faintly—lane 9. The cross-linked bands had the expected mobility for dimers of Cx43CT, and an immunoblot experiment confirmed that the product was indeed Cx43CT (Fig. 1, B and C).
FIGURE 1.
Cross-linking recombinant Cx43CT with EDC. (A) Cx43CT, at concentrations of 7.0 (lanes 2, 3, 5, 6, 8, and 9) and 1.0 (lanes 4, 7, and 10) μg/μL in PBS buffer (pH indicated above each panel). Equal amounts of protein (10 μg) were run on a 15% SDS-PAGE. The molecular mass standards for the 10, 15, 20, 25, and 37 kDa bands have been labeled. Proteins in the gel presented in panel A were transferred to nitrocellulose membrane and analyzed by immunoblot analysis using Zymed polyclonal anti-Cx43 antibody (B) or 181A polyclonal anti-Cx43 antibody (C).
In addition to the dimer at pH 5.8 and 6.5, immunoblot analysis revealed higher molecular weight aggregates. The molecular weights for these bands correlated to tri-, tetra-, penta-, and hexamers of Cx43CT polypeptides (numbers 3, 4, 5, and 6 in Fig. 1 B) cross-linked. Although dimeric and some trimeric species were seen for the Cx43CT polypeptide at pH 7.5, oligomerization was much more prevalent at the low pH, suggesting a pH dependence to the dimerization process. Interestingly, it can be seen in Fig. 1 A that equal amounts of protein were loaded for all lanes; yet, the two antibodies were not as reactive toward the monomer as they were toward the higher molecular weight aggregates. Though the conditions are far from those present when a connexin molecule oligomerizes into a functional gap junction in a mammalian cell, Cx43CT oligomerization did not exceed that which occurs in a connexon.
Molecular mass determination
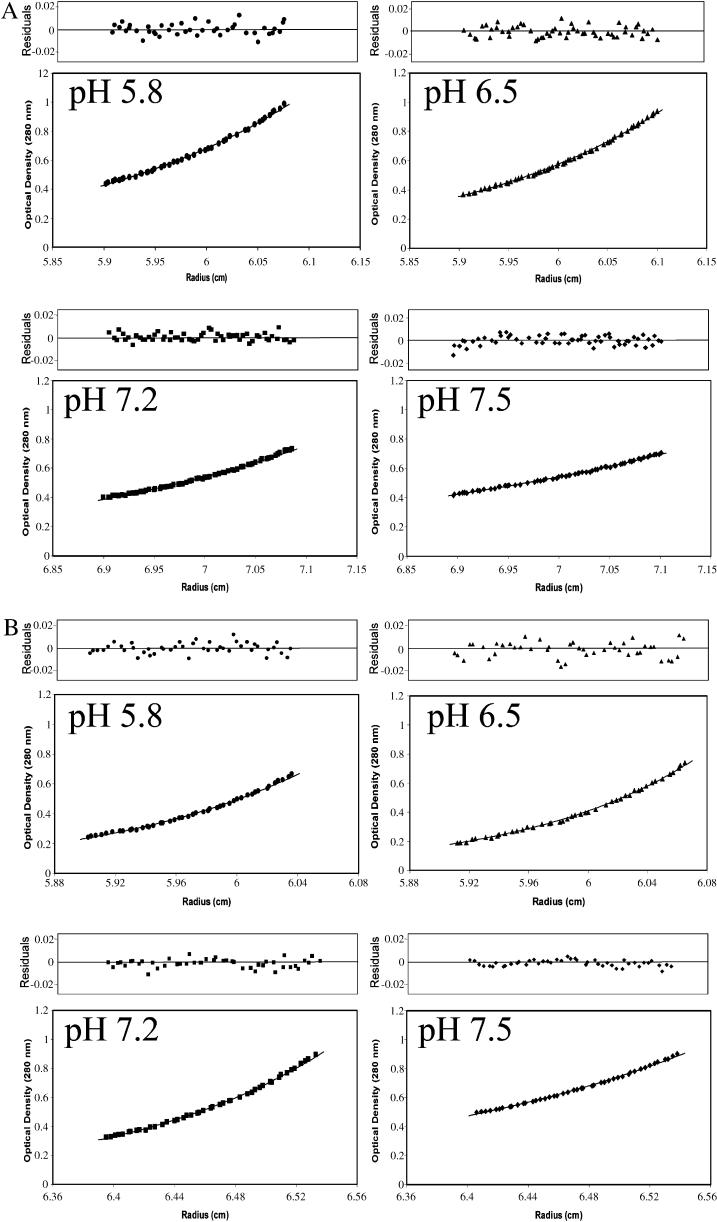
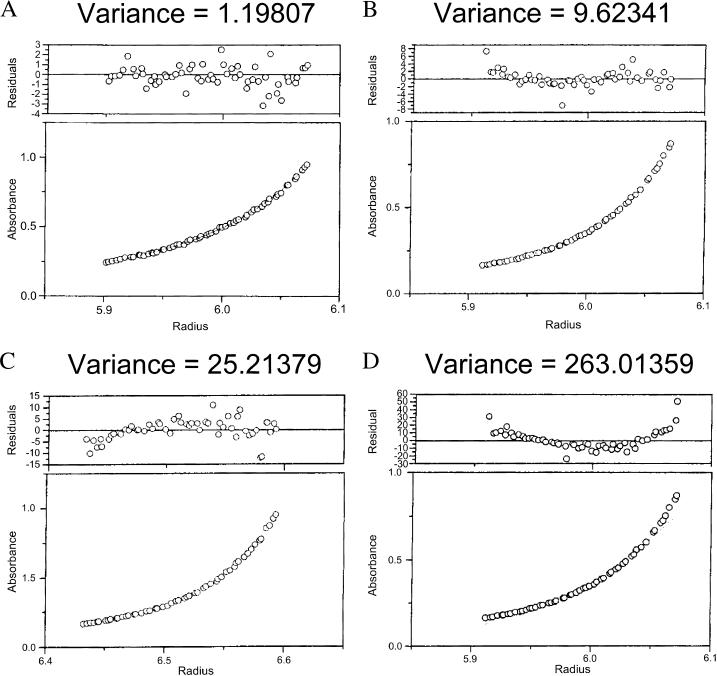
The observation of pH-dependent oligomerization of Cx43CT was confirmed by sedimentation equilibrium analysis. This method also allowed us to more accurately quantify the stoichiometry of oligomerization at different pH values. Plots of optical density at equilibrium as a function of radius for different pH values at two rotor speeds are shown in Fig. 2. Each plot was best fit by a function derived from a self-association model to determine the fraction of protein in specific oligomeric states (Beckman XL-A/XL-I software package). A monomer-dimer-trimer model showed excellent convergence, as demonstrated by the minimum deviation seen in the residuals (top plot on each panel) and a weighted variance approaching unity (Fig. 3). In contrast, fits of data to single component (i.e., nonoligomeric), monomer-dimer, and monomer-dimer-tetramer models had weighted variance values significantly greater than one. The results obtained from the monomer-dimer-trimer model were used to calculate the fraction of total protein that existed in the dimer form at each pH value. At a pH of 7.5, only 12% of the total protein content was in a dimer conformation. A slight reduction in the pH of the solvent to 7.2 increased the fraction of dimers to 40%. When the pH of the solvent was reduced to 6.5 and 5.8, more than 84% and 86%, respectively, of the Cx43CT molecules were dimerized (percent based on subunit concentration; extinction coefficient ∼12,450 cm−1M−1). Other than a small amount of trimer (<1%), the nondimerized form of Cx43CT was found as a monomer at all pH values tested. These results strongly support the notion of a pH-dependent dimerization of Cx43CT. Additional experiments were conducted to identify the specific residues involved in this process of self-association.
FIGURE 2.
Distribution of recombinant Cx43CT at sedimentation equilibrium. The concentrations of Cx43CT in PBS buffer (pH indicated in each box) at equilibrium (A, 18,000 rpm; B, 26,000 rpm) are shown as a function of radius. The solid lines are the theoretical curves. The calculated subunit molecular mass for Cx43CT at all pH values (5.8, 6.5, 7.2, 7.5) was 14.4 kDa, which is in excellent agreement with that deduced from the sequence of Cx43CT (14.2 kDa).
FIGURE 3.
Validation of the monomer-dimer-trimer model to describe the self-association of Cx43CT. Cx43CT in PBS buffer (pH 5.8) at equilibrium was used to identify the correct self-association model to fit the sedimentation equilibrium data. The same data were fit to (A) monomer-dimer-trimer, (B) monomer-dimer-tetramer, (C) monomer-dimer, and (D) monomer self-association models. The best fit, as indicated by the lowest variance value, is the monomer-dimer-trimer model.
Filtered NOE
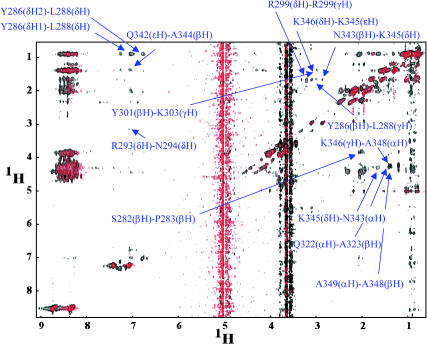
We have recently used NMR to determine the resonance assignments for Cx43CT (Sorgen et al., 2002). Here, we conducted a 3D 13C F1-edited, F3-13C/15N-filtered NOE experiment (Lee et al., 1994) to confirm intermolecular self-association and identify the resonance peaks that were affected by dimerization (Fig. 4). This approach further allowed us to determine whether the interaction between individual molecules was parallel; that is, whether energy was transferred from the amino acid in one polypeptide to the amino acid in the equivalent position in the homologous molecule. The two thick vertical lines across the map correspond to resonances originating from the water molecules, as well as a small trace of glycerol in the solvent. A control map, shown by the red contours, was first acquired using only a double-labeled species of the protein (13C15N-labeled Cx43CT). Since resonance peaks originating from the labeled protein were filtered, red contours represent “background signals” originating from the small fraction of the protein that did not incorporate the labeled amino acids and/or from nonoptimized pulse-field gradients (Lee et al., 1994). A second map, shown by the black contours, was obtained when the unlabeled species of Cx43CT was mixed with the 13C15N-labeled species at a 1:1 molar ratio. Signals that overlapped (black overlapping on red) were identified as background signals and not actual energy transfers between the species. However, the black contours that did not overlap corresponded to resonance peaks resulting from the transfer of energy between a labeled molecule and one that was unlabeled. Given that the resonance peaks had been already assigned for each amino acid in the Cx43CT sequence (Sorgen et al., 2002), this experiment allowed us to identify the specific amino acids involved in the intermolecular interaction. These results confirmed the presence of self-association. Moreover, it permitted the assignment of the specific areas of dimerization. These were: M281–N295, R299–Q304, S314–I327, and Q342–A348. A sampling of the resonance peaks (14 from a total of 30) from each region are identified and labeled in Fig. 4 (see arrows). Interestingly, region M281–N295 includes amino acids thought to be part of the SH3 binding domain of Cx43 (Kanemitsu et al., 1997; Zhou et al., 1999), whereas regions S314–I327 and Q342–A348 have been identified as containing a secondary, α-helical structure (Sorgen et al., 2002).
FIGURE 4.
Mapping inter-Cx43 carboxyl terminal interactions from a 3D 13C F1-edited, F3-13C/15N-filtered NOE experiment. 13C15N-Cx43CT was titrated with unlabeled Cx43CT to a 1:1 molar ratio at pH 5.8 (black). 13C15N-Cx43CT alone was used as the control for leakage through the filter (red). Labeled is a sample of the intermolecular interactions. α, β, δ, γ, ɛ, and H symbolize the alpha, beta, gamma, delta, and epsilon protons.
DISCUSSION
We have used NMR, cross-linking, and sedimentation equilibrium to characterize the process of pH-dependent self-association of the carboxyl terminal domain of Connexin43. Our data strongly suggest that acidification of the solvent leads to dimerization of the protein. We further show that the areas of dimerization include a putative SH3 binding domain, as well as two regions found to contain secondary structure (Sorgen et al., 2002). These results lead to the hypothesis that dimerization of the CT domain may be one of the structural changes involved in the chemical regulation of Cx43. However, some technical aspects need to be considered.
Technical considerations
All experiments presented in this article were obtained in vitro from isolated protein fragments in solution. The conditions are therefore substantially different from those likely to be present in the microenvironment of the Cx43CT domain when integrated in a gap junction plaque. Therefore, the results presented in this article need to be interpreted with caution. However, a number of previous studies indicate that the isolated Cx43CT domain retains at least some of its functional properties. For example, the Cx43CT fragment can be phosphorylated in vitro by kinases known to modify the behavior of Cx43 gap junctions (Cooper et al., 2000; Lampe and Lau, 2000). This same isolated domain can bind in vitro to known Cx43 molecular partners such as ZO-1 (Toyofuku et al., 2001), tubulin (Giepmans et al., 2001), and the SH3 domain of Src (Kanemitsu et al., 1997; Duffy et al., 2004). Finally, coexpression experiments in cells show that the CT fragment can act as an independent domain to rescue the pH sensitivity of truncated Cx43 channels (Morley et al., 1996). The same applies to the ability of Cx43 to be regulated by insulin and insulin-like growth factor (Homma et al., 1998), c-Src (Zhou et al., 1999) and transjunctional voltage (Moreno et al., 2002). These studies show that both in vitro and in vivo the isolated Cx43CT domain retains biochemical and functional properties consistent with those found in the full-length channels. Furthermore, our results show that dimerization occurs in vitro even at neutral pH (7.0). It is therefore likely that in those studies where the CT fragment has been used, at least a fraction of the protein species was present in a dimerized state. Whether the ability of Cx43CT to act as a kinase substrate, a molecular partner and/or a functional “gating particle”, is affected by the dimerization state remains to be determined.
Dimerization of other channel proteins
Our data show that the CT domain of Cx43 tends to dimerize in response to low pH. This is not the first study showing that intracellular domains of channel proteins undergo dimerization. Tetrameric channel proteins such as the HCN channels, the IP3 receptor, and the NMDA receptor have been dubbed “dimers of dimers” because of the self-association of specific subunit domains (Galvan and Mignery, 2002; Sun et al., 2002; Schorge and Colquhoun, 2003). In some cases, dimerization has been shown in vitro using isolated protein fragments and then corroborated in functional channels (Galvan and Mignery, 2002; Leach et al., 2003). Dimerization seems to be more than a simple biochemical phenomenon. Indeed, in the case of cyclic nucleotide gated channels, dimerization of a regulatory domain of the protein substantially modifies channel function (Matulef and Zagotta, 2002; Ulens and Siegelbaum, 2003). Analogous to the structure of tetrameric channels as “dimers of dimers”, we speculate that Cx43 hemichannels may exist as “trimers of dimers” and their oligomeric state may have a regulatory role on the function of Cx43. It is worth noting that connexin dimerization on Western blots has been observed ever since the initial gap junction isolation studies on liver (see Traub et al., 1982; Green et al., 1988). Although most apparent for Cx32 (VanSlyke and Musil, 2000), antibody-recognized bands of the approximate dimeric mobility have also been seen for Cx40 (Matesic et al., 2003) and for Cx43 (Hossain et al., 1994; VanSlyke and Musil, 2000; Roger et al., 2004), thus supporting the possibility that Cx43 dimerization may occur in the setting of an assembled channel.
Dimerization of Cx43CT and regulation of Cx43 gap junctions
Analysis of the specific residues involved in dimerization identified four specific areas: M281–N295, R299–Q304, S314–I327, and Q342–A348. Region M281–N295 is interesting from the point of view of Cx43 regulation. Serine 282 is a substrate for MAPK phosphorylation (Warn-Cramer et al., 1996,1998). An additional MAPK phosphorylation site, Serine 279 (Warn-Cramer et al., 1996,1998), is very close to the dimerization site and its structure may be affected by the oligomeric state of the protein. Moreover, region 271–287 is thought to act as an SH3 binding domain, critical for the interaction of Cx43 with v-Src (Kanemitsu et al., 1997). It is therefore possible that regulation by these two kinases (MAPK and v-Src) may either change the dimerization state of the protein or involve access to a binding site modified by dimerization. It is also worth noting that deletion of amino acids 281–300 renders Cx43 channels less sensitive to acidification-induced uncoupling (Ek-Vitorin et al., 1996). A similar result is obtained when a peptide corresponding to amino acids 271–287 of Cx43 is injected in the intracellular space of Cx43-expressing cells (Calero et al., 1998). We speculate that dimerization may be a part of the pH gating process and the 271–287 peptide competitively inhibits the pH-dependent dimerization of the native CT domains. Finally, regions 314–327 and 342–348 correspond to areas of the primary sequence where high-order structure has been found (Sorgen et al., 2002). We speculate that dimerization may involve formation of a coiled-coil structure between the two subunits. Further structural studies will aim at solving the structure of the Cx43CT dimer and characterizing the effects of dimerization on other intramolecular interactions, such as the pH-dependent binding of Cx43CT to the cytoplasmic loop domain (Duffy et al., 2002).
In summary, the work described here has enabled us to further understand pH-dependent changes in the structure of Cx43. We show in vitro dimerization of the Cx43CT domain, a region of the Cx43 protein often used for in vitro studies of biochemical modifications of the Cx43 gap junctions. We further show that the fraction of the total protein present in dimer form is a function of the pH of the solvent. Finally, we show that some of the regions of dimerization are also involved in regulation of Cx43 channels, thus opening the possibility that dimerization may be a structural component of the regulation of gap junctions.
Acknowledgments
We thank Mark Girvin and Sean Cahill for their teaching, insight, and helpful suggestions about the NMR experiments performed for this project. We would also like to thank Wanda Coombs for purification of the Cx43CT used in this study and Michael Brenowitz for assistance with the sedimentation equilibrium experiments.
This work was supported by United States Public Health Service grants F32 GM20504, HL39707, NS41282, MH65495, and GM5769.
References
- Calero, G., M. Kanemitsu, S. M. Taffet, A. F. Lau, and M. Delmar. 1998. A 17mer peptide interferes with acidification-induced uncoupling of connexin43. Circ. Res. 82:929–935. [DOI] [PubMed] [Google Scholar]
- Cascio, W. E., T. A. Johnson, and L. S. Gettes. 1995. Electrophysiologic changes in ischemic ventricular myocardium: I. Influence of ionic, metabolic, and energetic changes. J. Cardiovasc. Electrophysiol. 6:1039–1062. [DOI] [PubMed] [Google Scholar]
- Cooper, C. D., J. L. Solan, M. K. Dolejsi, and P. D. Lampe. 2000. Analysis of connexin phosphorylation sites. Methods. 20:196–204. [DOI] [PubMed] [Google Scholar]
- Delaglio, F., S. Grzesiek, G. W. Vuister, G. Zhu, J. Pfeifer, and A. Bax. 1995. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR. 6:277–293. [DOI] [PubMed] [Google Scholar]
- Duffy, H. S., A. W. Ashton, P. O'Donnell, W. Coombs, S. M. Taffet, M. Delmar, and D. C. Spray. 2004. Regulation of connexin43 protein complexes by intracellular acidification. Circ. Res. 94:215–222. [DOI] [PubMed] [Google Scholar]
- Duffy, H. S., P. L. Sorgen, M. E. Girvin, P. O'Donnell, W. Coombs, S. M. Taffet, M. Delmar, and D. C. Spray. 2002. pH-dependent intramolecular binding and structure involving Cx43 cytoplasmic domains. J. Biol. Chem. 277:36706–36714. [DOI] [PubMed] [Google Scholar]
- Ek-Vitorin, J. F., G. Calero, G. E. Morley, W. Coombs, S. M. Taffet, and M. Delmar. 1996. PH regulation of connexin43: molecular analysis of the gating particle. Biophys. J. 71:1273–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis, D., K. Stergiopoulos, J. F. Ek-Vitorin, F. L. Cao, S. M. Taffet, and M. Delmar. 1999. Connexin diversity and gap junction regulation by pHi. Dev. Genet. 24:123–136. [DOI] [PubMed] [Google Scholar]
- Galvan, D. L., and G. A. Mignery. 2002. Carboxyl-terminal sequences critical for inositol 1,4,5-trisphosphate receptor subunit assembly. J. Biol. Chem. 277:48248–48260. [DOI] [PubMed] [Google Scholar]
- Giepmans, B. N., and W. H. Moolenaar. 1998. The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr. Biol. 8:931–934. [DOI] [PubMed] [Google Scholar]
- Giepmans, B. N., I. Verlaan, T. Hengeveld, H. Janssen, J. Calafat, M. M. Falk, and W. H. Moolenaar. 2001. Gap junction protein connexin-43 interacts directly with microtubules. Curr. Biol. 11:1364–1368. [DOI] [PubMed] [Google Scholar]
- Goldberg, G. S., and A. F. Lau. 1993. Dynamics of connexin43 phosphorylation in pp60v-src-transformed cells. Biochem. J. 295:735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, C. R., E. Harfst, R. G. Gourdie, and N. J. Severs. 1988. Analysis of the rat liver gap junction protein: clarification of anomalies in its molecular size. Proc. R. Soc. Lond. B Biol. Sci. 233:165–174. [DOI] [PubMed] [Google Scholar]
- Homma, N., J. L. Alvarado, W. Coombs, K. Stergiopoulos, S. M. Taffet, A. F. Lau, and M. Delmar. 1998. A particle-receptor model for the insulin-induced closure of connexin43 channels. Circ. Res. 83:27–32. [DOI] [PubMed] [Google Scholar]
- Hossain, M. Z., L. J. Murphy, E. L. Hertzberg, and J. I. Nagy. 1994. Phosphorylated forms of connexin43 predominate in rat brain: demonstration by rapid inactivation of brain metabolism. J. Neurochem. 62:2394–2403. [DOI] [PubMed] [Google Scholar]
- Janse, M. J., and A. L. Wit. 1989. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol. Rev. 69:1049–1169. [DOI] [PubMed] [Google Scholar]
- Johnson, B. A., and R. A. Blevins. 1994. NMRView: a computer program for the visualization and analysis of NMR data. J. Biomol. NMR. 4:603–614. [DOI] [PubMed] [Google Scholar]
- Kanemitsu, M. Y., L. W. Loo, S. Simon, A. F. Lau, and W. Eckhart. 1997. Tyrosine phosphorylation of connexin 43 by v-Src is mediated by SH2 and SH3 domain interactions. J. Biol. Chem. 272:22824–22831. [DOI] [PubMed] [Google Scholar]
- Kleber, A. G., C. B. Riegger, and M. J. Janse. 1987. Electrical uncoupling and increase of extracellular resistance after induction of ischemia in isolated, arterially perfused rabbit papillary muscle. Circ. Res. 61:271–279. [DOI] [PubMed] [Google Scholar]
- Lampe, P. D., and A. F. Lau. 2000. Regulation of gap junctions by phosphorylation of connexins. Arch. Biochem. Biophys. 384:205–215. [DOI] [PubMed] [Google Scholar]
- Leach, R. N., M. R. Boyett, and J. B. Findlay. 2003. Expression, purification and spectroscopic studies of full-length Kir3.1 channel C-terminus. Biochim. Biophys. Acta. 1652:83–90. [DOI] [PubMed] [Google Scholar]
- Lee, W., M. J. Revington, C. Arrowsmith, and L. E. Kay. 1994. A pulsed field gradient isotope-filtered 3D 13C HMQC-NOESY experiment for extracting intermolecular NOE contacts in molecular complexes. FEBS Lett. 350:87–90. [DOI] [PubMed] [Google Scholar]
- Loo, L. W., J. M. Berestecky, M. Y. Kanemitsu, and A. F. Lau. 1995. pp60src-mediated phosphorylation of connexin 43, a gap junction protein. J. Biol. Chem. 270:12751–12761. [DOI] [PubMed] [Google Scholar]
- Matesic, D., T. Tillen, and A. Sitaramayya. 2003. Connexin 40 expression in bovine and rat retinas. Cell Biol. Int. 27:89–99. [DOI] [PubMed] [Google Scholar]
- Matulef, K., and W. Zagotta. 2002. Multimerization of the ligand binding domains of cyclic nucleotide-gated channels. Neuron. 36:93–103. [DOI] [PubMed] [Google Scholar]
- Moreno, A. P., M. Chanson, S. Elenes, J. Anumonwo, I. Scerri, H. Gu, S. M. Taffet, and M. Delmar. 2002. Role of the carboxyl terminal of connexin43 in transjunctional fast voltage gating. Circ. Res. 90:450–457. [DOI] [PubMed] [Google Scholar]
- Moreno, A. P., J. C. Saez, G. I. Fishman, and D. C. Spray. 1994. Human connexin43 gap junction channels. Regulation of unitary conductances by phosphorylation. Circ. Res. 74:1050–1057. [DOI] [PubMed] [Google Scholar]
- Morley, G. E., S. M. Taffet, and M. Delmar. 1996. Intramolecular interactions mediate pH regulation of connexin43 channels. Biophys. J. 70:1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, N. S., J. Coromilas, N. J. Severs, and A. L. Wit. 1997. Disturbed connexin43 gap junction distribution correlates with the location of reentrant circuits in the epicardial border zone of healing canine infarcts that cause ventricular tachycardia. Circulation. 95:988–996. [DOI] [PubMed] [Google Scholar]
- Roger, C., B. Mograbi, D. Chevallier, J. F. Michiels, H. Tanaka, D. Segretain, G. Pointis, and P. Fenichel. 2004. Disrupted traffic of connexin 43 in human testicular seminoma cells: overexpression of Cx43 induces membrane location and cell proliferation decrease. J. Pathol. 202:241–246. [DOI] [PubMed] [Google Scholar]
- Saez, J. C., V. M. Berthoud, A. P. Moreno, and D. C. Spray. 1993. Gap junctions. Multiplicity of controls in differentiated and undifferentiated cells and possible functional implications. Adv. Second Messenger Phosphoprotein Res. 27:163–198. [PubMed] [Google Scholar]
- Schorge, S., and D. Colquhoun. 2003. Studies of NMDA receptor function and stoichiometry with truncated and tandem subunits. J. Neurosci. 23:1151–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgen, P. L., H. S. Duffy, S. M. Cahill, W. Coombs, D. C. Spray, M. Delmar, and M. E. Girvin. 2002. Sequence-specific resonance assignment of the carboxyl terminal domain of Connexin43. J. Biomol. NMR. 23:245–246. [DOI] [PubMed] [Google Scholar]
- Spray, D. C., and M. V. Bennett. 1985. Physiology and pharmacology of gap junctions. Annu. Rev. Physiol. 47:281–303. [DOI] [PubMed] [Google Scholar]
- Stergiopoulos, K., J. L. Alvarado, M. Mastroianni, J. F. Ek-Vitorin, S. M. Taffet, and M. Delmar. 1999. Hetero-domain interactions as a mechanism for the regulation of connexin channels. Circ. Res. 84:1144–1155. [DOI] [PubMed] [Google Scholar]
- Sun, Y., R. Olson, M. Horning, N. Armstrong, M. Mayer, and E. Gouaux. 2002. Mechanism of glutamate receptor desensitization. Nature. 417:245–253. [DOI] [PubMed] [Google Scholar]
- Swenson, K. I., H. Piwnica-Worms, H. McNamee, and D. L. Paul. 1990. Tyrosine phosphorylation of the gap junction protein connexin43 is required for the pp60v-src-induced inhibition of communication. Cell Regul. 1:989–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thi, M. M., T. Kojima, S. C. Cowin, S. Weinbaum, and D. C. Spray. 2003. Fluid shear stress remodels expression and function of junctional proteins in cultured bone cells. Am. J. Physiol. Cell Physiol. 284:C389–C403. [DOI] [PubMed] [Google Scholar]
- Toyofuku, T., Y. Akamatsu, H. Zhang, T. Kuzuya, M. Tada, and M. Hori. 2001. c-Src regulates the interaction between connexin-43 and ZO-1 in cardiac myocytes. J. Biol. Chem. 276:1780–1788. [DOI] [PubMed] [Google Scholar]
- Traub, O., U. Janssen-Timmen, P. M. Druge, R. Dermietzel, and K. Willecke. 1982. Immunological properties of gap junction protein from mouse liver. J. Cell. Biochem. 19:27–44. [DOI] [PubMed] [Google Scholar]
- Ulens, C., and S. A. Siegelbaum. 2003. Regulation of hyperpolarization-activated HCN channels by cAMP through a gating switch in binding domain symmetry. Neuron. 40:959–970. [DOI] [PubMed] [Google Scholar]
- VanSlyke, J. K., and L. S. Musil. 2000. Analysis of connexin intracellular transport and assembly. Methods. 20:156–164. [DOI] [PubMed] [Google Scholar]
- Warn-Cramer, B. J., G. T. Cottrell, J. M. Burt, and A. F. Lau. 1998. Regulation of connexin-43 gap junctional intercellular communication by mitogen-activated protein kinase. J. Biol. Chem. 273:9188–9196. [DOI] [PubMed] [Google Scholar]
- Warn-Cramer, B. J., P. D. Lampe, W. E. Kurata, M. Y. Kanemitsu, L. W. Loo, W. Eckhart, and A. F. Lau. 1996. Characterization of the mitogen-activated protein kinase phosphorylation sites on the connexin-43 gap junction protein. J. Biol. Chem. 271:3779–3786. [DOI] [PubMed] [Google Scholar]
- Zhou, L., E. M. Kasperek, and B. J. Nicholson. 1999. Dissection of the molecular basis of pp60(v-src) induced gating of connexin 43 gap junction channels. J. Cell Biol. 144:1033–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]