Abstract
The protective effect of therapeutic oral immunization with homologous and heterologous formalin-inactivated Helicobacter pylori cells given together with cholera toxin as an adjuvant was evaluated with C57BL/6 mice infected with H. pylori Sydney strain 1 (SS1). The bacteria used for immunization were strains that were either homologous or heterologous with regard to the O antigen (i.e., the Lewis antigen [Le antigen]) expressed by the lipopolysaccharide of the infecting H. pylori SS1 strain. We found that repeated oral immunization with inactivated H. pylori SS1 cells can significantly inhibit an existing infection (P < 0.001) and that the protection induced by such therapeutic immunization extends to protection against reinfection (P < 0.001). A similar level of protection was also achieved by immunization with another inactivated H. pylori strain having the same O antigen (Le antigen) as the infecting H. pylori SS1 strain. In contrast, immunization with inactivated strains expressing a heterologous O antigen, Lex, provided less protection or no protection. Immunization with H. pylori lysate preparations, on the other hand, resulted in significant comparable protection whether the lysates were prepared from an Lex strain or an Ley strain. Postimmunization gastritis was seen in mice that were protected after vaccination but not in unimmunized or unprotected mice. In conclusion, therapeutic immunization with inactivated H. pylori whole-cell vaccines may provide strong protection both against experimental H. pylori infection and against later reinfection.
Helicobacter pylori is the cause of chronic active gastritis, duodenal ulcers, and gastric ulcer disease and is a risk factor for gastric cancer (3, 18). H. pylori infections can be treated with antibiotics in combination with proton pump inhibitors, which results in the resolution of gastritis and a decrease in the rate of relapse of duodenal ulcers (B. J. Marshall, J. R. Warren, and C. S. Goodwin, Letter, Lancet i:836-837, 1989). However, problems such as high costs, poor patient compliance, and increasing occurrence of H. pylori resistant strains are major drawbacks in treatment. An alternative strategy to control the disease is to develop a safe and effective vaccine. The introduction of a mouse model of H. pylori infection using Sydney strain 1 (SS1), which causes persistent infection and chronic gastritis, has been an important advance in vaccine studies (15).
Although vaccines are usually given prophylactically to protect against infections, therapeutic vaccination, possibly in conjunction with antibiotic therapy, of an already infected individual is the most attractive strategy for immunologic intervention against H. pylori. To date, prophylactic and therapeutic vaccine studies in mice performed with various putative vaccine candidate antigens (e.g., bacterial lysate preparations, urease, CagA, catalase, Hsp60, lipoprotein, outer membrane vesicles, and neutrophil-activating protein together with a mucosal adjuvant) have revealed various degrees of protection (7, 8, 13, 26, 28). Studies with other gastrointestinal pathogens have shown that orally administered inactivated whole-cell vaccines can effectively protect against infection and disease (10, 23), and in a recent study in humans immunization with inactivated whole H. pylori cells gave rise to mucosal B-cell and T-cell responses in a majority of the volunteers (14).
The lipopolysaccharide (LPS) O antigen of H. pylori has been shown to contain different Lewis blood group antigens (Le antigens) that allow serotyping. Thus, in this study we chose to evaluate the protective effect of oral therapeutic immunization with formalin-killed H. pylori cells. Our analysis included a comparison of the protective efficacy of inactivated H. pylori whole-cell vaccines having homologous and heterologous LPS O antigens expressing different Le antigens to the protective efficacy of the H. pylori SS1 challenge strain, as well as an evaluation of the levels of vaccine-induced protection against a later reinfection challenge. We used this approach in order to evaluate the feasibility of a low-cost whole-cell vaccine against H. pylori infection in humans.
MATERIALS AND METHODS
Animals.
C57BL/6 male or female mice that were 6 to 8 weeks old were purchased from B&K Universal (Sollentuna, Stockholm, Sweden) and were housed in microisolators in the animal house at Göteborg University. All experiments were approved by the ethics committee of the National Board for Laboratory Animals (ethical permit 291/99).
H. pylori strains.
H. pylori mouse-adapted strain SS1, kindly provided by Lee et al. (15), was used for all infections and also for immunization after inactivation. In addition, three clinical isolates from our strain collection, designated Hel 305, Hel 309, and Hel 312, were used after inactivation; all strains were stored at −70°C in Luria broth with 10% glycerol until they were used. All strains were CagA+ and VacA+.
Determination of LPS and Lewis phenotype.
The LPS diversity of the different clinical isolates and H. pylori SS1 was determined by a dot blot test by using specific monoclonal antibodies (MAbs) raised against purified LPS as described previously (31). In this assay, MAbs against H. pylori SS1 LPS (LPS SS1 3:6) and strain Hel 73 LPS (LPS Hel 73 9:10) were used. These MAbs were screened by using a panel of 58 clinical isolates collected from our clinical studies over a period of 5 years, and their specificities were compared with those of commercial MAbs against Lex and Ley. These analyses showed that MAb LPS Hel 73 9:10 reacts specifically with Lex and MAb LPS SS1 3:6 reacts specifically with Ley and that 90% of the clinical isolates tested expressed either Lex, Ley, or Lexy. The MAbs reacted equally well with live bacteria and corresponding formalin-inactivated bacteria or lysates which were used for the immunization studies. Thus, according to the reactivities with different MAbs, H. pylori strains SS1 and Hel 309 expressed Ley and not Lex and Hel 305 and Hel 312 expressed Lex and not Ley.
Preparation of inactivated H. pylori cells and antigens for immunization. (i) Inactivated bacteria.
Bacteria from −70°C stock cultures were grown on Columbia iso agar plates. Each culture was then transferred to 200 ml of brucella broth (Difco Laboratories, Detroit, Mich.) supplemented with antibiotics (10 μg of vancomycin per ml, 10 U of polymyxin B per ml, 10 μg of trimethoprim per ml) and 5% newborn calf serum (Biochrome AG, Berlin, Germany). The flask was then flushed with a microaerophilic gas mixture containing 10% CO2, 6% O2, and 84% N2 for approximately 5 min and incubated in a shaking incubator at 37°C for 24 h. After centrifugation at 13,000 × g and two washes with phosphate-buffered saline (PBS), the bacteria were suspended in PBS to an optical density of 1.5, corresponding to 7.5 × 109 cells/ml, and formaldehyde was added to a final concentration of 0.01 M. After incubation at 37°C on a slow shaker for 2 h followed by overnight shaking at room temperature, the bacteria were washed three times in PBS and then resuspended in PBS to an optical density of 1.5 and stored at 4°C until they were used. The inactivated bacterial culture was plated on horse blood agar plates, and no colonies were detected, indicating that there was complete inactivation.
(ii) Lysates.
Lysates of H. pylori SS1 and Hel 305 were prepared as previously described (8). The protein contents were determined by measuring the absorbance at 280 nm and calculating the amounts of protein in the samples. Each lysate was snap frozen in liquid nitrogen and was stored in aliquots at −70°C until it was used.
(iii) MP.
Whole-membrane proteins (MP) were prepared from strains Hel 305 and SS1 as previously described (1, 16).
Infection, immunization, and reinfection of mice.
H. pylori strain SS1 was cultured as described above. After centrifugation, the bacteria were resuspended in brucella broth without antibiotics to a final optical density of 1.5, corresponding to 7.5 × 109 cells/ml. The cultures were then serially diluted and plated on horse blood agar plates to determine the infectious dose. C57BL/6 mice were each orally infected with approximately 3 × 108 CFU in brucella broth under anesthesia (Metofane Shering Plough Inc., Madison, N.J.). For studies on the in vivo kinetics of SS1 infection, female mice (five or more mice per time point) were infected and sacrificed 3 days and 1, 2, 3, 8, 10, and 24 weeks after infection. The number of bacteria in the mouse stomachs at each time point was quantified as described below.
(i) Therapeutic immunization.
The efficacy of therapeutic immunization was determined by administering four weekly oral doses consisting of either 500 μl of inactivated bacteria (3 × 109 inactivated cells/mouse) plus 10 μg of cholera toxin (List Biologicals, Campbell, Calif.) or 100 μl of a lysate preparation corresponding to 400 μg of protein plus 10 μg of cholera toxin 2 weeks after the initial infection.
(ii) Reinfection model.
For the reinfection experiments the immunized mice were treated with a combination of omeprazole (Losec; 0.4 mg/dose in physiological saline; Astra, Mölndal, Sweden), metronidazole (1.35 mg/dose; Dumex, Copenhagen, Denmark), and amoxicillin (5 mg/dose; Scand Pharm, Dublin, Ireland) daily for 5 days starting 1 week after the last immunization dose. One week after the antibiotic treatment was completed, the mice were each reinfected with 3 × 108 CFU of H. pylori SS1 as described above.
(iii) Quantitation of bacteria in the mouse stomach.
Groups of mice were killed 1 week after the last booster immunization to assess the protection induced by therapeutic immunization or 2 weeks after reinfection to assess the protection after reinfection. One-half of each stomach was excised, washed in PBS, and homogenized in 2 ml of brucella broth, and serial 10-fold dilutions were plated on Skirrow blood agar plates. The plates were incubated under microaerophilic conditions for 6 days before colonies were counted. Protection factors were calculated by determining the inverse of the ratio of the number of bacteria in individual mice in the vaccinated groups to the geometric mean number of bacteria in the infected control mice in the same experiment.
Serum antibody titers.
Blood was collected from the axillary plexus immediately before the mice were killed. Serum antibody titers were determined by an enzyme-linked immunosorbent assay (12) by using MP of H. pylori SS1 for coating at room temperature overnight. Sera from individual mice in each group were tested at an initial dilution of 1/10, followed by serial threefold dilution, and the antibody titers were expressed as the reciprocals of the sample dilutions that gave an absorbance that was 0.4 U above the background value.
Histology.
Strips of the entire greater curvature of the stomach were cut, fixed in 4% phosphate-buffered formalin (Histolab AB, Göteborg, Sweden), and embedded in paraffin. Sections that were 5 μm thick were cut and stained with hematoxylin and eosin. The slides were then graded according to the Sydney system of scoring gastritis (5).
Statistical analysis.
A nonparametric Mann-Whitney test was used for comparisons between groups performed with Graphpad Pris software (GraphPad Software Inc., San Diego, Calif.).
RESULTS
H. pylori SS1 infection in C57BL/6 mice.
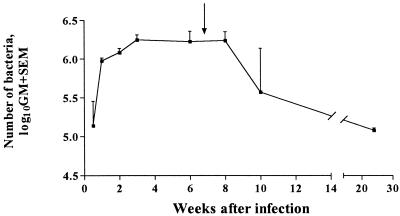
The kinetics of H. pylori colonization was studied by using groups of infected mice and was monitored for up to 6 months after infection. A rapid increase in the mean number of bacteria (CFU) per mouse stomach was seen between days 3 and 7 after colonization (Fig. 1). Thereafter, the mean number of bacteria continued to increase more slowly for 4 weeks and was then constant until at least 8 weeks after the initial infection. During the following 4 months, the mean number of bacteria in the stomachs decreased 20-fold and the variation between individual mice was considerably greater than earlier during infection. Significantly higher (P < 0.05) levels of colonization (mean, 2.5-fold) were observed in male mice than in female mice in repeat experiments (data not shown).
FIG. 1.
Kinetics of H. pylori infection. Female C57BL/6 mice were infected with H. pylori SS1 and sacrificed at various times (3 days to 24 weeks) after infection (five or more mice per time point). The data are the geometric mean (GM) + standard error of the mean number of bacteria (CFU) at each time point. The arrow indicates the time when mice were sacrificed following infection and therapeutic immunization.
Therapeutic protection by vaccination with inactivated H. pylori strains with homologous and heterologous Le antigens.
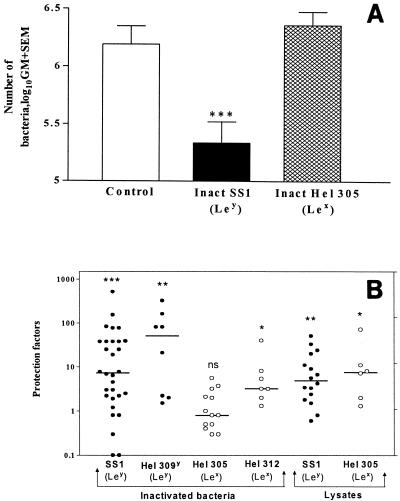
The protective effect of therapeutic immunization with inactivated whole-cell H. pylori was evaluated by using H. pylori SS1-infected mice. Repeated administration of inactivated homologous-cell H. pylori SS1 (Ley) resulted in a significant (P < 0.001) reduction (mean, 10-fold) in the bacterial load (Fig. 2A). However, no reduction in the bacterial load was observed when mice were immunized with inactivated whole-cell H. pylori Hel 305 having a different Le antigen (Lex) than the infecting H. pylori SS1 strain (Fig. 2A). To clarify the observed serotype (Le antigen) specificity in host protection against H. pylori infection, we analyzed the protective effects (protection factors) induced by immunization with additional strains of inactivated bacteria, as well as lysate preparations having O antigen with homologous or heterologous Le antigen. A strong protective effect (>10-fold; P < 0.001) was induced by immunization with inactivated H. pylori strain Hel 309 (Ley), which is homologous to the infecting strain with regard to Le antigen expression (Fig. 2B). Immunization with inactivated Hel 312, which has a heterologous Le antigen (Lex), resulted in only a threefold reduction (P < 0.05) (Fig. 2B). However, the protective effects of immunization with lysate preparations from both H. pylori SS1 (homologous Le antigen) and strain Hel 305 (heterologous Le antigen) (Fig. 2B) were comparable.
FIG. 2.
(A) Effect of therapeutic immunization with formalin-inactivated homologous H. pylori cells. Infected nonimmunized mice (Control), mice immunized with inactivated homologous strain H. pylori SS1 [Inact SS1 (Ley)], and mice immunized with inactivated heterologous strain Hel 305 [Inact Hel 305 (Lex)] were used. Only female mice were used in this experiment. Statistical significance was evaluated by the Mann-Whitney test. Three asterisks indicate that the P value was <0.001. (B) Protection induced by inactivated bacteria and lysates. Therapeutic immunization with inactivated SS1 (Ley), inactivated Hel 309 (Ley), Hel 305 (Lex), or Hel 312 (Lex) cells or with lysates of strain SS1 or Hel 305 was performed. The results were compared with the results for corresponding infected gender controls as both males and females were used in this study. Symbols: •, immunization with homologous LPS preparations (Ley); ○, immunization with heterologous preparation (Lex). The horizontal lines indicate the median value for each group. Three asterisks indicate that the P value was <0.001; two asterisks indicate that the P value was <0.01; and one asterisk indicates that the P value was <0.05. ns, not significant.
Protection against reinfection by vaccination with inactivated H. pylori strains with homologous and heterologous Le antigens.
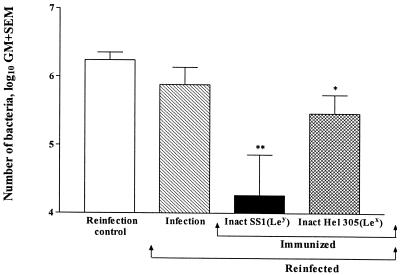
The effect of therapeutic immunization with inactivated whole bacteria on protection against reinfection was also studied. This was done by immunizing mice, which were initially infected with SS1 bacteria, with inactivated SS1 (homologous Le antigen) or inactivated Hel 305 (heterologous Le antigen). After 1 week the mice were treated with antibiotics to eradicate any residual H. pylori SS1, and they were reinfected with H. pylori SS1 1 week after antibiotic treatment was completed. Naive mice that were infected at the reinfection time point were used as controls, and all mice were sacrificed 2 weeks after reinfection. Quantitative culture of gastric samples revealed a significant, >10-fold decrease (P < 0.001) in the number of bacteria in the reinfected mice that had been immunized with homologous inactivated H. pylori SS1 compared to the number of bacteria in the reinfection controls (Fig. 3). In the mice immunized with inactivated strain Hel 305 with heterologous Le antigen and reinfected with strain SS1, a fourfold decrease (P < 0.05) in the mean number of bacteria recovered from the stomach was also seen (Fig. 3). Infection in the absence of active immunization failed to protect against a later reinfection challenge (Fig. 3).
FIG. 3.
Protection after reinfection. H. pylori SS1-infected mice were immunized four times weekly with either inactivated H. pylori SS1 [Inact SS1(Ley)] or inactivated Hel 305 [Inact Hel 305(Lex)] or were not immunized (Infection). The bars indicate the geometric mean (GM) numbers of bacteria 2 weeks after reinfection, and the number of bacteria in the reinfection control is included for comparison. Two asterisks indicate that the P value was <0.01, and one asterisk indicates that the P value was <0.05.
Specific serum antibody response in infected and vaccinated mice.
The serum immunoglobulin G (IgG) and IgM antibody responses to a membrane preparation (MP) from H. pylori SS1 were monitored before and after therapeutic immunization in the various immunization groups. The preexisting serum antibody titers against H. pylori antigens at the onset of the experiment were approximately 100 in all groups of mice. Infection in the absence of immunization resulted in enzyme-linked immunosorbent assay titers of 800 to 6,000 in individual mice (data not shown). Immunization with SS1 cells or lysate resulted in mean titers that were three- to fourfold higher than the titers in the infected and unimmunized mice. Immunization with heterologous inactivated bacteria did not induce higher mean titers to MP of H. pylori SS1 than infection alone. There was no correlation between antibody titers and the numbers of CFU in the mouse stomach as analyzed for individual mice.
Protection in vaccinated mice and its relationship to postimmunization gastritis.
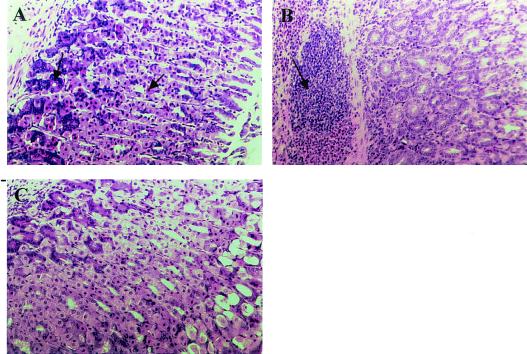
To evaluate a possible effect of immunization on the extent of gastritis, sections of the mouse stomach were stained with hematoxylin and eosin and graded in a blind fashion according to the Sydney system for scoring gastritis. In infected control mice slight infiltration of mononuclear cells into the lamina propria was seen. However, all mice had normal histology in the corpus region of the stomach, with intact zymogen-secreting chief cells and acid-secreting parietal cells (Fig. 4A and Table 1). In contrast, in mice immunized with inactivated SS1 cells or lysate and in six of eight mice immunized with Hel 309 (Ley), infiltration of leukocytes into the mucosa and submucosa and a loss of chief cells and parietal cells were seen, indicating that there was ongoing inflammation in the gastric mucosa (Fig. 4B and Table 1). Mice that had been immunized with inactivated cells with heterologous LPS (strains Hel 305 [Lex] and Hel 312 [Lex]) showed little or no inflammation, and their stomach sections were comparable to those of infected control mice (Fig. 4C and Table 1). Inflammation was associated with protection and with reduction in the number of bacteria after reinfection of mice that were initially immunized either with inactivated SS1 or with inactivated Hel 305 (Table 1). Slight infiltration was seen after reinfection in the absence of active immunization, but the histological appearance of the corpus mucosa was normal.
FIG. 4.
Histopathology in infected and immunized mice. (A) Corpus mucosa of a mouse that was infected with H. pylori SS1 for 8 weeks, showing normal morphology. The arrows indicate chief cells and parietal cells. (B) Corpus mucosa of a mouse that was therapeutically vaccinated with inactivated H. pylori SS1 cells. Massive inflammation was seen, as indicated by the arrow. (C) Mucosa of a mouse that was therapeutically vaccinated with inactivated Hel 305. There are no signs of atrophy or inflammatory infiltrate.
TABLE 1.
Histopathology in the corpus region of the stomach in infected and vaccinated mice
| Group | Atrophya | Inflam- mationb | Protection factorc |
|---|---|---|---|
| Therapeutic immunization study groups | |||
| Controls | 0 | 0.5 | |
| Inactivated SS1 + CTd | 5 | 4 | 8 (4-12) |
| Inactivated Hel 305 + CT | 0 | 1 | 1 (0-2) |
| Inactivated Hel 309 + CT | 3 | 3 | 21 (1-46) |
| Inactivated Hel 312 + CT | 0.6 | 0.4 | 4.6 (2-7) |
| Lysate of SS1 + CT | 5 | 5 | 5 (3-7) |
| Lysate of Hel 305 + CT | 3 | 2 | 7 (2-12) |
| Reinfection study groups | |||
| Reinfection controls | 0 | 0.25 | |
| Infection | 0 | 1.4 | 2 (0-4) |
| Inactivated SS1 + CT | 5 | 3 | 93 (0.3-380) |
| Inactivated Hel 305 + CT | 5 | 4 | 6 (1-11) |
Atrophy was defined as the loss of chief cells and parietal cells and was graded on a scale of 0 to 6. Individual mice were scored, and the mean score for each group is shown.
Inflammation was graded on a scale of 0 to 6, individual mice were scored, and the mean score for each group is shown.
Protection factors were calculated by determining the inverse of the ratio of the number of bacteria in individual mice of the vaccinated group to the geometric mean number of bacteria in the infected control mice. The values in parentheses are ranges.
CT, cholera toxin.
DISCUSSION
In this study we used the established model of H. pylori SS1 infection in C57BL/6 mice (15) to examine the protective effect of therapeutic immunization with an inactivated H. pylori SS1 whole-cell vaccine. Inactivated whole-cell vaccines may be useful for mucosal immunization since they are easy to prepare on a large scale and present antigens on the bacterial surface to the mucosal immune system in an attractive form. Our results show that oral immunization with such a whole-cell vaccine prepared by formalin inactivation can induce protection with a efficacy comparable to that induced by immunization with whole-cell lysate but with a higher degree of serotype specificity, possibly reflecting differences in the protective immune mechanisms induced by the two types of vaccines.
In initial experiments we found that colonization of C57BL/6 mice with H. pylori SS1 is stable after the first week and during the following 6 to 8 weeks and that there is little variation between individual mice in the colonization pattern, which allows evaluation of protective immunogenicity during this period with relatively small groups of mice. In their original description of the mouse model, Lee et al. (15) did not describe the early kinetics of H. pylori SS1 infection in vivo. Here we found that the minimum time needed for optimal colonization by H. pylori SS1 in C57BL/6 mice is 2 weeks. In addition, we observed a marked difference in bacterial colonization between male and female mice, with the male mice exhibiting consistently greater colonization (mean, two- to threefold), suggesting that protection studies must include gender aspects. Aebischer et al. (2) recently reported a similar observation concerning a gender difference in H. pylori strain P76-infected BALB/c wild-type and IL-4Rα−/− knockout mice. These authors speculated that this difference may represent a novel factor which contributes eventually to the higher prevalence of gastric cancer in male patients (4).
In a previous study of therapeutic immunization in the H. pylori SS1 mouse model, Ikewaki et al. (11) reported that as many as 60% of the animals were completely eradicated compared to nonimmunized mice, in contrast to both our results and the results of other workers who investigated therapeutic or prophylactic protection in a H. pylori SS1 mouse model (21, 30). The strong protection observed by Ikewaki et al. can be explained by the long-term experiments which they carried out (21 weeks) since we observed that at 10 weeks postinfection there was a decrease in the colonization level in infected nonimmunized mice. To our knowledge, our study is the first report of the therapeutic protective effect of a formalin-inactivated preparation of H. pylori SS1 in a mouse model. Recently, Kotloff et al. (14) described immunization with a formalin-inactivated H. pylori whole-cell vaccine together with a nontoxic heat-labile E. coli enterotoxin as an adjuvant in human volunteers. They observed increases in H. pylori-specific fecal IgA and serum IgG titers in the immunized volunteers, as well as T-cell responses in most of the immunized volunteers. However, in spite of the strong immune response generated, a urea breath test analysis 2 months after vaccination showed that the vaccinees remained positive for H. pylori; furthermore, there were no efforts to evaluate either a decrease in colonization after immunization or the serotype homology between the immunizing and infecting strains.
An interesting finding of our study, which has implications for the design of whole-cell vaccines against H. pylori infection in humans, was that inactivated Hel 305 cells did not provide therapeutic protection against H. pylori SS1 infection. We believe that this lack of protection was related to the differences in the Le antigen profiles of the immunizing and infecting strains, as we have found that the two strains have the same protein profile, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (data not shown). In contrast to the results obtained with whole Hel 305 cells with heterologous Le antigen, immunization with the lysate preparation from the same strain provided protection against an existing infection. The reason for this discrepancy may be the inherent antigenic differences between the two preparations. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis revealed an approximately 10-fold-larger amount of total protein in the immunization dose of lysate than in the dose of inactivated bacteria, and possibly this difference in protein content could contribute to the protection seen in the mice immunized with the lysate of strain Hel 305. However, the results of immunization with inactivated Lex-positive strain Hel 312 were unexpected, as there was a tendency for protection even though the strain was heterologous with regard to the Le antigen, which indicates that there are factors other than the Le antigens that may be involved in protection when formalin-inactivated strains of bacteria are used as whole-cell vaccines. Studies of the antibody response to the MP from H. pylori SS1 revealed slightly higher antibody responses in groups of mice that were given the SS1 cells than in groups of mice that were immunized with the heterologous bacterial preparation.
The mechanism of protection in the mice immunized with inactivated homologous cells or lysate could be speculated to be due to the ongoing inflammation in the stomach mucosa. Previous studies in our laboratory have shown that H. pylori-infected human volunteers mounted a considerably better immune response to an oral cholera vaccine in the stomach than uninfected volunteers (19). In continued studies we could show that an inflamed stomach was effective as an expression site for immune responses against H. pylori and may facilitate recruitment of immunocytes to the stomach (24).
In addition, we also studied whether therapeutic immunization with the various antigen preparations could protect against reinfection after eradication of the residual bacteria. In repeated experiments we clearly demonstrated that infection in the absence of active immunization was inefficient for providing protection against reinfection. This is in contrast to a recent study of Radcliff and Ferrero (25), who demonstrated that mice infected with a subinfectious dose of H. pylori SS1 (i.e., 15 CFU) had significantly lower bacterial loads after challenge with an infectious dose of 104 CFU. One reason for the discrepancy between these results and the results obtained in our study could be that Radcliff and Ferrero used a considerably lower (10,000-fold-lower) dose of bacteria for challenge and hence a weaker immune response could have been sufficient to inhibit bacterial colonization and multiplication. Thus, we believe that active immunization is required for protection against reinfection if a relatively high dose of the bacteria is used for challenge. These results have implications for the development of vaccines against H. pylori infection in areas where H. pylori infection is endemic, such as developing countries where reinfection with a presumably large inoculum may occur (9, 22, 32).
However, in agreement with Ermak et al. (6) we observed that in all immunization groups the decrease in bacterial load was associated with postimmunization gastritis, indicating that the effector mechanisms responsible for reducing the bacterial load also lead to gastritis. However, acute gastritis per se may not be harmful, as we have observed in our studies of human volunteers infected with H. pylori that asymptomatic individuals have acute chronic gastritis whose severity is comparable to that of the gastritis in patients with duodenal ulcers (17). In addition, we have recently observed that in mice gastritis caused by immunization with H. pylori does not lead to an autoimmune reaction since antisera from these mice do not react with stomach tissue; furthermore, postimmunization gastritis resolves after eradication of the bacteria with antibiotics, indicating that there is a lack of autoantibodies (27; S. Raghavan, M. Fredriksson, A.-M. Svennerholm, J. Holmgren, and E. Suri-Payer, unpublished observations). Studies by Mohammadi et al. (20), who used a Helicobacter felis infection and immunization model, have shown that enhanced gamma interferon (IFN-γ) production by mononuclear cells recruited to the gastric tissue could lead to infection-induced or immunization-induced gastritis in mice. Thus, in vivo neutralization of IFN-γ resulted in a reduction in the overall gastric inflammation score, and interestingly, neutralization of IFN-γ in the immunized mice resulted in detectable production of interleukin-4, which was absent in infected mice treated with anti-IFN-γ. Thus, in the immunized (protected) mice, but not in the infected mice, depletion of IFN-γ allowed local and systemic increases in Th2 cells that may have contributed to the protection observed in the immunized mice (20). Indeed, we have shown in a recent study that oral administration of H. pylori together with cholera toxin polarizes the immune response towards a Th2 response and induces protection against reinfection with the homologous challenge strain in the absence of any postimmunization gastritis (27). However, further studies need to be carried out to elucidate the role of postimmunization gastritis in protection and strategies to attain vaccine-induced protection in the absence of gastritis.
In conclusion, we found that therapeutic immunization with inactivated H. pylori may be effective in protecting not only against an existing infection but also against reinfection. However, when a vaccine is designed by using inactivated bacteria, a mixture of strains expressing different Le antigens (at least Lex, Ley, and Lexy since they seem to be the most prevalent O antigens in H. pylori LPS [29]) should be used. Furthermore, studies to identify noninflammatory immune mechanisms that protect against H. pylori infection would be important in the development of a safe vaccine against H. pylori.
Acknowledgments
We thank the Swedish Medical Research Council (projects 16x-3382 and 16x-9084) and The Knut and Alice Wallenberg Foundation for providing financial support to GUVAX.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Achtman, M., G. Morelli, and S. Schwuchow. 1978. Cell-cell interactions in conjugating Escherichia coli: role of F pili and fate of mating aggregates. J. Bacteriol. 135:1053-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aebischer, T., S. Laforsch, R. Hurwitz, F. Brombacher, and T. F. Meyer. 2001. Immunity against Helicobacter pylori: significance of interleukin-4 receptor alpha chain status and gender of infected mice. Infect. Immun 69:556-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaser, M. J. 1990. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J. Infect. Dis. 161:626-633. [DOI] [PubMed] [Google Scholar]
- 4.Crespi, M., and F. Citarda. 1998. Helicobacter pylori and gastric cancer: what is the real risk? Gastroenterologist 6:16-20. [PubMed] [Google Scholar]
- 5.Dixon, M. F., R. M. Genta, J. H. Yardley, and P. Correa. 1996. Classification and grading of gastritis. The updated Sydney System International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 20:1161-1181. [DOI] [PubMed] [Google Scholar]
- 6.Ermak, T. H., R. Ding, B. Ekstein, J. Hill, G. A. Myers, C. K. Lee, J. Pappo, H. K. Kleanthous, and T. P. Monath. 1997. Gastritis in urease-immunized mice after Helicobacter felis challenge may be due to residual bacteria. Gastroenterology 113:1118-1128. [DOI] [PubMed] [Google Scholar]
- 7.Ferrero, R. L., J. M. Thiberge, I. Kansau, N. Wuscher, M. Huerre, and A. Labigne. 1995. The GroES homolog of Helicobacter pylori confers protective immunity against mucosal infection in mice. Proc. Natl. Acad. Sci. USA 92:6499-6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghiara, P., M. Rossi, M. Marchetti, A. Di Tommaso, C. Vindigni, F. Ciampolini, A. Covacci, J. L. Telford, M. T. De Magistris, M. Pizza, R. Rappuoli, and G. Del Giudice. 1997. Therapeutic intragastric vaccination against Helicobacter pylori in mice eradicates an otherwise chronic infection and confers protection against reinfection. Infect. Immun. 65:4996-5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gurel, S., F. Besisk, K. Demir, Z. Mungan, S. Kaymakoglu, G. Boztas, Y. Cakaloglu, O. Yeginsu, and A. Okten. 1999. After the eradication of Helicobacter pylori infection, relapse is a serious problem in Turkey. J. Clin. Gastroenterol. 28:241-244. [DOI] [PubMed] [Google Scholar]
- 10.Holmgren, J., A. M. Svennerholm, M. Jertborn, J. Clemens, D. A. Sack, R. Salenstedt, and H. Wigzell. 1992. An oral B subunit: whole cell vaccine against cholera. Vaccine 10:911-914. [DOI] [PubMed] [Google Scholar]
- 11.Ikewaki, J., A. Nishizono, T. Goto, T. Fujioka, and K. Mifune. 2000. Therapeutic oral vaccination induces mucosal immune response sufficient to eliminate long-term Helicobacter pylori infection. Microbiol. Immunol. 44:29-39. [DOI] [PubMed] [Google Scholar]
- 12.Johansson, E. L., C. Rask, M. Fredriksson, K. Eriksson, C. Czerkinsky, and J. Holmgren. 1998. Antibodies and antibody-secreting cells in the female genital tract after vaginal or intranasal immunization with cholera toxin B subunit or conjugates. Infect. Immun. 66:514-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keenan, J., J. Oliaro, N. Domigan, H. Potter, G. Aitken, R. Allardyce, and J. Roake. 2000. Immune response to an 18-kilodalton outer membrane antigen identifies lipoprotein 20 as a Helicobacter pylori vaccine candidate. Infect. Immun. 68:3337-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotloff, K. L., M. B. Sztein, S. S. Wasserman, G. A. Losonsky, S. C. DiLorenzo, and R. I. Walker. 2001. Safety and immunogenicity of oral inactivated whole-cell Helicobacter pylori vaccine with adjuvant among volunteers with or without subclinical infection. Infect. Immun. 69:3581-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee, A., J. O'Rourke, M. C. De Ungria, B. Robertson, G. Daskalopoulos, and M. F. Dixon. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112:1386-1397. [DOI] [PubMed] [Google Scholar]
- 16.Lindholm, C., J. Osek, and A. M. Svennerholm. 1997. Quantification of conserved antigens in Helicobacter pylori during different culture conditions. Infect. Immun. 65:5376-5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindholm, C., M. Quiding-Jarbrink, H. Lonroth, A. Hamlet, and A. M. Svennerholm. 1998. Local cytokine response in Helicobacter pylori-infected subjects. Infect. Immun. 66:5964-5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall, B. J., and J. R. Warren. 1984. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet i:1311-1315. [DOI] [PubMed]
- 19.Mattsson, A., H. Lonroth, M. Quiding-Jarbrink, and A. M. Svennerholm. 1998. Induction of B cell responses in the stomach of Helicobacter pylori-infected subjects after oral cholera vaccination. J. Clin. Investig. 102:51-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohammadi, M., S. Czinn, R. Redline, and J. Nedrud. 1996. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J. Immunol. 156:4729-4738. [PubMed] [Google Scholar]
- 21.Pappo, J., D. Torrey, L. Castriotta, A. Savinainen, Z. Kabok, and A. Ibraghimov. 1999. Helicobacter pylori infection in immunized mice lacking major histocompatibility complex class I and class II functions. Infect. Immun. 67:337-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patchett, S., S. Beattie, E. Leen, C. Keane, and C. O'Morain. 1992. Helicobacter pylori and duodenal ulcer recurrence. Am. J. Gastroenterol. 87:24-27. [PubMed] [Google Scholar]
- 23.Qadri, F., C. Wenneras, F. Ahmed, M. Asaduzzaman, D. Saha, M. J. Albert, R. B. Sack, and A. Svennerholm. 2000. Safety and immunogenicity of an oral, inactivated enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine in Bangladeshi adults and children. Vaccine 18:2704-2712. [DOI] [PubMed] [Google Scholar]
- 24.Quiding-Jarbrink, M., H. Lonroth, I. Ahlstedt, J. Holmgren, and A. M. Svennerholm. 2001. Human gastric B cell responses can be induced by intestinal immunisation. Gut 49:512-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radcliff, F. J., and R. L. Ferrero. 2001. Effect of low-dose antigen exposure on development of immunity to Helicobacter pylori infection in mice. Infect. Immun. 69:5186-5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radcliff, F. J., S. L. Hazell, T. Kolesnikow, C. Doidge, and A. Lee. 1997. Catalase, a novel antigen for Helicobacter pylori vaccination. Infect. Immun. 65:4668-4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raghavan, S., A. Svennerholm, and J. Holmgren. 2002. Effects of oral vaccination and immunomodulation by cholera toxin on experimental Helicobacter pylori infection, reinfection, and gastritis. Infect. Immun. 70:4621-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satin, B., G. Del Giudice, V. Della Bianca, S. Dusi, C. Laudanna, F. Tonello, D. Kelleher, R. Rappuoli, C. Montecucco, and F. Rossi. 2000. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a protective antigen and a major virulence factor. J. Exp. Med. 191:1467-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simoons-Smit, I. M., B. J. Appelmelk, T. Verboom, R. Negrini, J. L. Penner, G. O. Aspinall, A. P. Moran, S. F. Fei, B. S. Shi, W. Rudnica, A. Savio, and J. de Graaff. 1996. Typing of Helicobacter pylori with monoclonal antibodies against Lewis antigens in lipopolysaccharide. J. Clin. Microbiol. 34:2196-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sutton, P., J. Wilson, and A. Lee. 2000. Further development of the Helicobacter pylori mouse vaccination model. Vaccine 18:2677-2685. [DOI] [PubMed] [Google Scholar]
- 31.Tinnert, A., A. Mattsson, I. Bolin, J. Dalenback, A. Hamlet, and A. M. Svennerholm. 1997. Local and systemic immune responses in humans against Helicobacter pylori antigens from homologous and heterologous strains. Microb. Pathog. 23:285-296. [DOI] [PubMed] [Google Scholar]
- 32.Veenendaal, R. A., A. S. Pena, J. L. Meijer, H. P. Endtz, M. M. van der Est, W. van Duijn, F. Eulderink, J. Kreuning, and C. B. Lamers. 1991. Long term serological surveillance after treatment of Helicobacter pylori infection. Gut 32:1291-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]