Abstract
The contribution of cytokines and chemokines to resistance and susceptibility to African trypanosomiasis remains controversial. In the present study, the levels of type I and type II cytokines and of the MCP-1 chemokine were compared during the early and late stages of Trypanosoma congolense infection in susceptible BALB/c and resistant C57BL/6 mice. Moreover, the status of macrophage activation was compared in these animals by analyzing the inducible nitric oxide synthase-arginase balance, tumor necrosis factor secretion, and expression of the FIZZ1 and YM genes. Data show that changing from a predominant type I cytokine environment in the early stage of infection to a predominant type II cytokine environment and an enhanced MCP-1 secretion in the late stage of infection correlates with resistance to T. congolense. Concomitantly, macrophage activation evolves from a classical to a predominant alternative phenotype. We further confirmed that the simultaneous occurrence of type I/type II cytokines in the early stage of infection in susceptible BALB/c mice, reflected by the presence of macrophages exhibiting a mixed classical/alternative activation phenotype, is associated with uncontrolled parasite growth and early death. Interleukin-4 (IL-4) and IL-13 signaling did not influence the susceptibility of BALB/c mice to T. congolense infection and interestingly were not the main trigger to alternative macrophage activation. In T. congolense-resistant C57BL/6 mice, our results corroborated the induction of FIZZ1 and YM gene expressions with the alternative pathway of macrophage activation. In susceptible BALB/c mice, however, YM but not FIZZ1 induction reflected the emergence of alternatively activated macrophages. Hence, the FIZZ1 and YM genes may be useful markers to discriminate between distinct populations of alternatively activated macrophages.
In sub-Saharan Africa, parasites such as Trypanosoma brucei and Trypanosoma congolense cause sleeping sickness in humans and Nagana disease in cattle. During infection with these extracellular parasites, complex interactions between the host immune system and parasite survival strategies occur. The control of African trypanosomiasis requires among others the contribution of variant specific glycoprotein-specific B- and T-cell responses (10, 35, 37) as well as the macrophage/monocyte phagocyte system (5, 18, 40).
As regulators of the immune response, cytokines orchestrate a type I and/or a type II immune response, thereby influencing the outcome of the disease. In this regard, the precise role of individual cytokines during African trypanosomiasis remains controversial. There is little doubt that a type I cytokine-based immune response contributes to the control of infection, although the relative importance of distinct cytokines may vary. For instance, gamma interferon (IFN-γ) was shown to be essential in Trypanosoma brucei rhodesiense and T. brucei brucei murine models through activation of macrophages (10, 30). On the other hand, the role of this cytokine in resistance to T. congolense has not been investigated, although susceptible mice produce higher amounts of IFN-γ than resistant mice (43).
Resistance to T. congolense infection in mice was suggested to rely upon the interleukin-12 (IL-12)-dependent synthesis of immunoglobulin G2a antibodies against parasite antigens and increased secretions of tumor necrosis factor and nitric oxide (18). The role of type II cytokines in resistance to African trypanosomes is even more speculative because they were reported to exert deleterious (44), protective (1, 14, 30), or no effect (38) on the outcome of the disease. The discrepancy in the relative importance of cytokines in resistance to African trypanosomiasis may result from the use of different parasite and/or mouse strains. In addition, most of the studies focused on the immune response elicited in the early stage of infection, omitting the late/chronic stage of the disease.
Chemokines may also influence the type I versus type II balance during an immune response (16, 17). In this respect, increased levels of RANTES, MCP-1, MIP1α, and MIP2 chemokine mRNAs were observed in the spleen and brain of T. brucei-infected animals (19, 39). However, the possible role of these chemotactic proteins in resistance or susceptibility to African trypanosomiasis has not been investigated.
In order to further analyze the importance of type I versus type II cytokines and the MCP-1 chemokine in resistance to African trypanosomes, their levels were quantified in the spleen and blood compartments during the early and late stages of T. congolense infection in a susceptible (BALB/c) and a resistant (C57BL/6) mouse model (42). In addition, the status of macrophage activation, which is influenced by the cytokine environment (8), was compared in the peritoneal compartment of T. congolense-infected susceptible and resistant mouse strains. In particular, the secretion of nitric oxide and tumor necrosis factor, reflecting the occurrence of classically activated macrophages in a type I cytokine environment, and arginase activity, reflecting the development of alternatively activated macrophages in a type II cytokine environment, were determined in the course of infection. In addition, the mRNA levels of the FIZZ1 (found in inflammatory zone) gene and the YM gene family, whose enhanced expression was recently correlated with alternative macrophage activation (6, 34), were quantified.
MATERIALS AND METHODS
Parasites and animals.
Female 8- to 12-week-old BALB/c and C57BL/6 mice (Harlan, Zeist, The Netherlands), and IL-4−/− (31) and IL-4 receptor alpha (IL-4Rα)−/− (26) mice were intraperitoneally inoculated with 5 × 103 T. congolense variant antigen type 13 (Tc13) organisms (32, 33) (kindly provided by Henry Tabel, University of Saskatchewan, Saskatchewan, Canada). Parasitemia was monitored by tail blood puncture every 2 to 4 days with a hemacytometer. Animal experimentation guidelines of the Ethics Commission for Laboratory Animals of the Free University of Brussels were followed.
Experimental design.
Mice were infected so that, at the time of the experiment, age-matched animals from both the early and late stages of infection were available. Levels of cytokines, nitric oxide, and arginase activity were quantified in serum and spleen or resident peritoneal exudate cell cultures of three infected mice at each stage of infection. For each parameter, results were expressed as the mean ± standard error of three infected animals tested individually and compared to the same parameters assessed in three noninfected mice. Results are representative of at least five independent experiments performed. Statistical analyses were assessed by Student's t test. A P value of <0.05 was considered significant.
Cell culture.
At the time of the experiments, spleen and resident peritoneal exudate cells from noninfected and infected mice were prepared and resuspended in RPMI 1640 (Gibco) supplemented with fetal calf serum (10%), 100 U of penicillin per ml, 100 μg of streptomycin per ml, 2 mM l-glutamine, and 5 × 10−5 M 2-mercaptoethanol as described previously (28). Cytokine and NO2 levels were determined in culture supernatants of cells (2 × 106/ml) stimulated or not with concanavalin A (2.5 μg/ml).
To quantify FIZZ1 and YM gene expression, adherent peritoneal exudate cells were prepared by dispensing 107 cells in a 10-cm tissue culture dish. After incubation at 37°C for 3 h in a humidified incubator containing 5% CO2, plates were washed with supplemented RPMI 1640 warmed at 37°C until no nonadherent cells or parasites were visible by microscopic examination. The adherent populations contained 80 to 90% macrophages as determined by cytofluorimetric analysis based on coexpression of CD11b and F4/80 (not shown).
Quantification of cytokines.
Cytokines were quantified in cell culture supernatants and in blood serum (collected on heparin) with specific sandwich enzyme-linked immunosorbent assays (ELISAs) for IFN-γ, IL-4, IL-10, MCP-1 (PharMingen, Erembodegem-Aalst, Belgium), or tumor necrosis factor and IL-13 (R&D Systems, Abingdon, United Kingdom). ELISA was performed in accordance with the manufacturers' protocols. Reported cytokine levels were the maximal levels observed after 1 day of culture for IL-4 and IL-13 and 3 days of culture for IFN-γ, IL-10, MCP-1, and tumor necrosis factor.
Quantification of nitric oxide and arginase activity.
Levels of nitric oxide in cell culture supernatants (collected after 3 days of culture) were determined by quantifying NO2 with the Greiss reagent as described previously (29). Arginase activity was measured in peritoneal exudate cell lysates as previously described (29). Briefly, 106 cells were lysed with 100 μl of 0.1% Triton X-100. After 30 min on a shaker, arginase was activated in the presence of 100 μl of 25 mM Tris-HCl (pH 7.5) and 35 μl of 10 mM MnCl2 (10 min, 56°C). l-Arginine hydrolysis was conducted by incubating the cell lysates with 100 μl of 0.5 M l-arginine (pH 9.7) at 37°C for 1 h. The reaction was stopped with 800 μl of H2SO4 (96%)-H3PO4 (85%)-H2O (1:3:7, vol/vol/vol). The urea produced was quantified at 540 nm after addition of 40 μl of α-isonitrosopropiophenone (dissolved in 100% ethanol), followed by heating at 100°C for 20 min. One unit of enzyme was defined as the amount that catalyzed the formation of 1 μmol of urea per min.
Semiquantitative RT-PCR.
Total RNA from adherent peritoneal exudate cells (3 × 106 cells) was prepared with the Trizol reagent (Gibco-BRL) as recommended by the suppliers. One microgram of DNase I-treated RNA was reverse-transcribed with oligo(dT) and Superscript II reverse transcriptase (Gibco-BRL). Each PCR cycle consisted of 1 min of denaturation at 94°C, 45 s of annealing at 55°C, and 1 min of extension at 72°C. The PCR primers were FIZZ1 sense (5′-TCCCAGTGAATACTGATGAGA-3′), FIZZ1 antisense (5′-CCACTCTGGATCTCCCAAGA-3′), YM sense (5′-GGGCATACCTTTATCCTGAG-3′), YM antisense (5′-CCACTGAAGTCATCCATGTC-3′), β-actin sense (5′-ACACTGTGCCCATCTACGAG-3′), and β-actin antisense (5′-TCAACGTCACACTTCATGATG-3′). The amplicon sizes were 213, 304, and 381 bp for FIZZ1, YM, and β-actin, respectively. The primers used did not allow discrimination of the different YM isotypes (15).
The amount of template cDNA and the number of PCR cycles were optimized so that the analysis of PCR products could be carried out within the linear range of amplification. β-Actin was used as a control to ensure that the observed differences in the expression levels of each gene in different cell samples were not due to differences in the amount of template cDNA. The results of the PCR analyses were confirmed in at least three independent experiments.
RESULTS
Parasitemia and survival of BALB/c and C57BL/6 mice infected with T. congolense.
The course of infection was compared in BALB/c and C57BL/6 mice infected with T. congolense parasites. BALB/c mice infected with T. congolense showed a drastically reduced survival time (8 ± 2 days) compared to C57BL/6 mice (160 ± 25 days). In BALB/c mice, T. congolense parasites grew exponentially, leading to parasitemia levels higher than 109/ml when the animals died. In C57BL/6 mice, on the other hand, the first peak of parasitemia was controlled at 3 × 108 parasites/ml. Thereafter, minor waves of parasitemia were observed until the animals died. In agreement with previous reports (42), these data indicate that BALB/c mice are extremely susceptible to T. congolense parasites (higher parasitemia, shorter survival), while C57BL/6 mice are relatively resistant (lower parasitemia, longer survival).
Cytokine productions by spleen cells from BALB/c and C57BL/6 mice infected with T. congolense.
The levels of type I (IFN-γ) and type II (IL-10, IL-4, and IL-13) cytokines as well as the chemotactic cytokine MCP-1 were quantified in culture supernatants of spleen cells from BALB/c and C57BL/6 mice infected with T. congolense in the early (6 days) and late (8 weeks) stages of infection.
In the early stage of infection, spleen cells from infected BALB/c mice produced, both spontaneously and after concanavalin A stimulation, increased levels of type I and type II cytokines and of the MCP-1 chemokine compared to spleen cells from noninfected mice (P < 0.02) (Table 1). At this stage of infection, spleen cells from T. congolense-infected C57BL/6 mice, activated or not with concanavalin A, produced increased amounts of IFN-γ, IL-10, IL-4, and MCP-1 (P < 0.02) but not IL-13 compared to spleen cells from noninfected mice (Table 1). The spontaneous or concanavalin A-induced IL-4, IL-13, and MCP-1 secretions upon infection were higher in BALB/c than in C57BL/6 mice.
TABLE 1.
Spontaneous and induced cytokine secretion by spleen cells from BALB/c and C57BL/6 mice during the early (6 days) and late (8 weeks) stages of T. congolense infectiona
| Conditions | Mouse strain | Infected | Infection stage | Mean secretion (pg/ml) ± SE
|
||||
|---|---|---|---|---|---|---|---|---|
| IFN-γ | IL-10 | IL-4 | IL-13 | MCP-1 | ||||
| Spontaneous | BALB/c | No | 400 ± 48 | 10 ± 2 | 6 ± 3 | 6 ± 3 | 250 ± 56 | |
| Yes | Early | 2,500 ± 236a | 200 ± 19a | 50 ± 10a | 30 ± 10a | 1,800 ± 168a | ||
| C57BL/6 | No | 200 ± 33 | 15 ± 3 | 5 ± 3 | 5 ± 2 | 100 ± 29 | ||
| Yes | Early | 850 ± 90a | 250 ± 35a | 15 ± 3a | 5 ± 2 | 250 ± 50a | ||
| Yes | Late | 150 ± 21c | 50 ± 15ac | 25 ± 5ad | 10 ± 2ad | 2,400 ± 350ad | ||
| Concanavalin A induced | BALB/c | No | 7,500 ± 450e | 200 ± 16e | 50 ± 15e | 20 ± 5e | 300 ± 58 | |
| Yes | Early | 9,000 ± 854ae | 540 ± 47ae | 600 ± 115ae | 120 ± 20ae | 2,200 ± 363a | ||
| C57BL/6 | No | 6,000 ± 514e | 75 ± 12e | 24 ± 5 | 10 ± 2e | 50 ± 18 | ||
| Yes | Early | 10,000 ± 964ae | 320 ± 30ae | 50 ± 15ae | 10 ± 2e | 250 ± 74a | ||
| Yes | Late | 500 ± 54bce | 250 ± 50ae | 300 ± 25ade | 50 ± 5ade | 3,410 ± 500ad | ||
Letters following values indicate statistical significance. a, significantly higher compared to noninfected animals; b significantly lower compared to noninfected animals; c significantly lower compared to the early stage of infection; d significantly higher compared to the early stage of infection; e significantly higher compared to spontaneous release in the same mouse strain.
In the absence of concanavalin A stimulation, induction of IL-10 secretion upon infection was comparable in both strains, while the induction of IFN-γ secretion was higher in BALB/c than in C57BL/6 mice. On the contrary, after mitogenic activation, spleen cells from infected C57BL/6 mice showed a lower induction of IL-10 but enhanced induction of IFN-γ secretion compared to spleen cells from infected BALB/c mice, possibly indicating a lower level of T-cell activation but enhanced type I cytokine polarization in spleen cells from infected C57BL/6 than from infected BALB/c mice.
In the late stage of infection, spontaneous IFN-γ secretion by spleen cells from T. congolense-infected C57BL/6 mice returned to the level in spleen cells from noninfected mice, while concanavalin A-induced IFN-γ secretion was strongly inhibited compared to that in spleen cells from noninfected mice (P < 0.001) (Table 1). In parallel, spontaneous and concanavalin A-induced IL-10 secretion remained increased in the supernatants of spleen cells from infected C57BL/6 mice compared to spleen cells from noninfected mice (P < 0.01), but to lower levels than in the early stage of infection. Finally, the levels of IL-4, IL-13, and MCP-1 increased significantly from the early to the late stage of infection in spleen cells from infected C57BL/6 mice, both without and after concanavalin A stimulation (P < 0.05). Hence, in the late stage of T. congolense infection, spleen cells from infected resistant C57BL/6 mice showed reduced type I and enhanced type II cytokine and MCP-1 secretions compared to the early stage of infection.
Cytokine levels in serum from BALB/c and C57BL/6 mice infected with T. congolense.
The levels of type I and type II cytokines as well as MCP-1 were quantified in the serum from BALB/c and C57BL/6 mice infected with T. congolense in the early (6 days) and late (8 weeks) stages of infection. Throughout infection, IL-13 and MCP-1 serum levels were below detection levels in the two mouse strains.
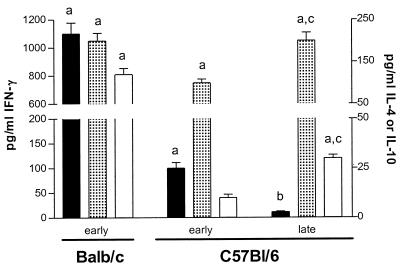
During the early stage of infection in BALB/c mice, an increase in the serum concentrations of IFN-γ, IL-10, and IL-4 was observed compared to noninfected animals (P < 0.01) (Fig. 1). Increased IFN-γ and IL-10 concentrations compared to noninfected animals were noted as well in serum from C57BL/6 mice at this stage of infection (P < 0.05), while the increase in IL-4 concentration was not significant.
FIG. 1.
Cytokine levels in serum from BALB/c and C57BL/6 mice infected with T. congolense. During the early (6 days) and late (8 weeks) stages of infection, IFN-γ (solid bars), IL-10 (dotted bars), and IL-4 (open bars) cytokines were quantified in the serum of BALB/c and C57BL/6 mice (mean ± standard error, n = 3). Cytokine levels in serum from noninfected animals were below the detection limit (<5 pg/ml). a, significantly higher compared to noninfected animals; b, significantly lower compared to the early stage of infection; c, significantly higher compared to the early stage of infection.
In the late stage of infection in C57BL/6 mice, serum levels of IFN-γ dropped to values observed in noninfected animals, while the levels of IL-4 and IL-10 increased compared to the early stage of infection (P < 0.05) (Fig. 1).
These data confirm that, as in the spleen, the presence of a mixed type I/type II cytokine environment in the serum of T. congolense-susceptible BALB/c mice. In resistant C57BL/6 mice, decreased type I and enhanced type II cytokine secretions are observed in the late compared to the early stage of infection.
Status of macrophage activation during T. congolense infection in BALB/c and C57BL/6 mice.
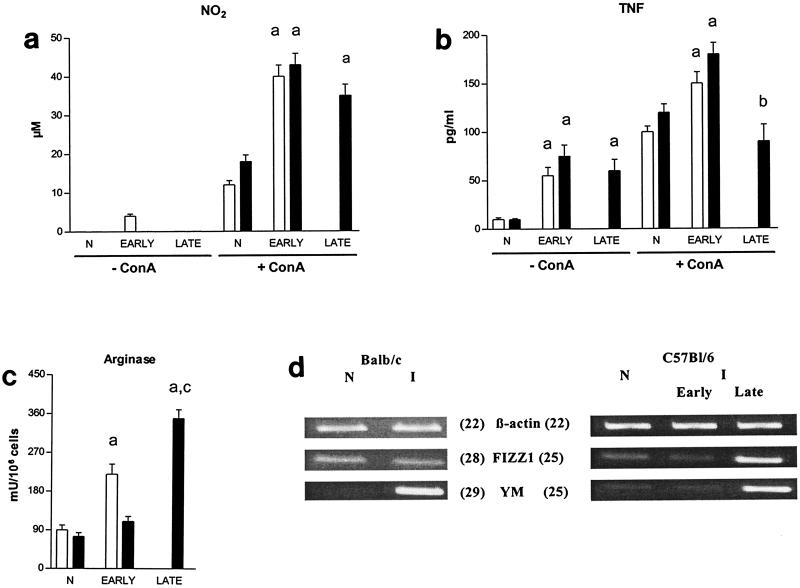
To evaluate the status of macrophage activation, spontaneous nitric oxide (assessed by NO2 accumulation) and tumor necrosis factor secretions were determined in the culture supernatants of peritoneal exudate cells from T. congolense-infected BALB/c and C57BL/6 mice. Since we were interested in macrophage-T-cell cytokine interactions during infection, concanavalin A-induced nitric oxide and tumor necrosis factor secretions were measured as well. In parallel, arginase activity was quantified in lysates of peritoneal exudate cells from infected mice. Finally, the expression of the genes coding for FIZZ1 and YM was compared in adherent peritoneal exudate cells from infected mouse populations by semiquantitative RT-PCR.
Peritoneal exudate cells from infected C57BL/6 and BALB/c mice spontaneously produced undetectable and marginal levels of nitric oxide, respectively (Fig. 2a). In the early stage of infection, concanavalin A-stimulated peritoneal exudate cells from T. congolense-infected BALB/c and C57BL/6 mice produced increased nitric oxide levels compared to peritoneal exudate cells from noninfected animals (P < 0.05). At this stage of infection, spontaneous and concanavalin A-induced tumor necrosis factor levels in supernatants of peritoneal exudate cells from infected mice increased in both BALB/c and C57BL/6 mice compared to peritoneal exudate cells from noninfected mice (P < 0.02) (Fig. 2b). Peritoneal exudate cells from BALB/c but not C57BL/6 mice in the early stage of infection exhibited increased arginase activity compared to peritoneal exudate cells from noninfected mice (P < 0.001) (Fig. 2c).
FIG. 2.
Status of macrophage activation during T. congolense infection in BALB/c and C57BL/6 mice. Spontaneous and concanavalin A-induced NO2 (a) and tumor necrosis factor (b) secretion was quantified in the supernatants of peritoneal exudate cells from BALB/c (open bars) and C57BL/6 (solid bars) mice (mean ± standard error, n = 3) in the early (6 days) and late (8 weeks) stages of infection and compared to that in noninfected (N) animals. In parallel, arginase activity was quantified in peritoneal exudate cell lysates (c), and expression levels of the β-actin, FIZZ1, and YM genes was analyzed in noninfected (N) and infected (I) adherent peritoneal exudate cells (d). The number of PCR cycles performed is indicated in parentheses. a, significantly higher compared to noninfected animals; b, significantly lower compared to the early stage of infection; c, significantly higher compared to the early stage of infection.
YM but not FIZZ1 gene expression was induced in peritoneal exudate cells from T. congolense-infected BALB/c mice compared to peritoneal exudate cells from noninfected mice. In peritoneal exudate cells from C57BL/6 animals, FIZZ1 and YM expression in the early stage of infection was slightly decreased and similar, respectively, compared to peritoneal exudate cells from noninfected mice (Fig. 2id). Thus, in the early stage of T. congolense infection, peritoneal exudate cells from infected susceptible BALB/c mice displayed characteristics of both classical and alternative macrophage activation, while in resistant C57BL/6 mice, classically activated macrophages were elicited.
In peritoneal exudate cells from late-stage infected C57BL/6 mice, concanavalin A-induced nitric oxide levels remained increased compared to peritoneal exudate cells from noninfected mice (P < 0.01), but were only slightly lower than in the early stage of infection (Fig. 2a). Similarly, spontaneous tumor necrosis factor secretion by peritoneal exudate cells from infected mice tended to be lower in the late than in the early stage of infection, while concanavalin A-induced tumor necrosis factor secretion by peritoneal exudate cells from infected mice returned to that in peritoneal exudate cells from noninfected mice (Fig. 2b). Finally, in the late stage, the levels of arginase activity (P < 0.001) as well as expression of FIZZI and YM were significantly increased in peritoneal exudate cells from infected mice compared to the levels in peritoneal exudate cells from uninfected and infected mice from the early stage of infection (Fig. 2c and d). Taken together, peritoneal macrophages from resistant C57BL/6 mice evolved from a polarized classical mode of activation in the early stage of infection to a more pronounced alternative mode of activation in the late stage of infection.
Influence of IL-4 and IL-13 on the course of infection in BALB/c mice infected with T. congolense.
To investigate the role of IL-4 and IL-13 in the susceptibility of BALB/c mice to T. congolense, the course of infection was compared in IL-4−/−, IL-4Rα−/−, and syngeneic wild-type BALB/c mice. Since both the IL-4 and IL-13 receptor share the IL-4Rα chain, IL-4Rα−/− mice lack both IL-4 and IL-13 signaling (2, 27).
IL-4−/− and IL-4Rα−/− mice were as susceptible to infection as their wild-type counterparts, showing similar exponential and uncontrolled parasite growth (>109/ml) and similar survival time (8 ± 2 days). The absence of IL-4 and IL-13 signaling did not significantly influence the secretion of IFN-γ, IL-4, or IL-10 by spleen cells from T. congolense-infected wild-type BALB/c mice, reduced MCP-1 secretion to a large extent, and abolished the production of IL-13. These effects were slightly less pronounced in the sole absence of IL-4 signaling (i.e., in IL-4−/− mice) (not shown).
Influence of IL-4 and IL-13 on macrophage activation status during T. congolense infection in BALB/c mice.
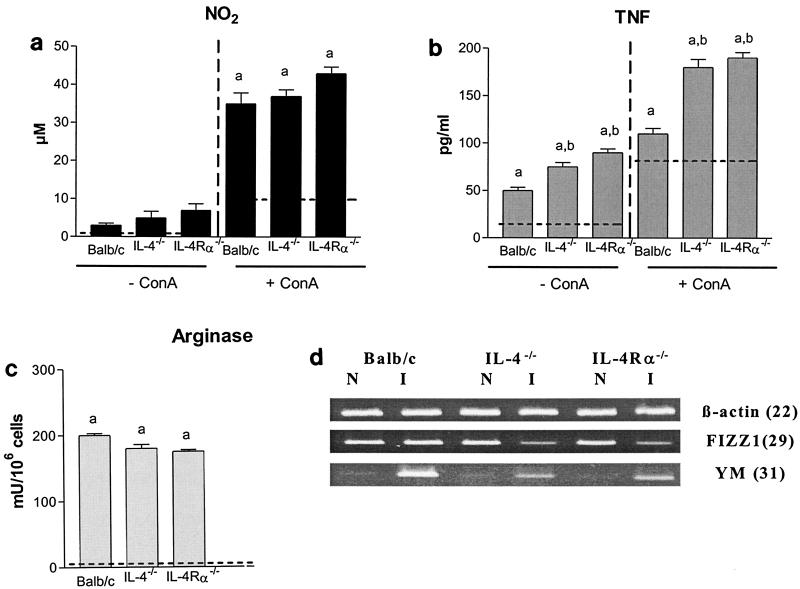
The spontaneous and concanavalin A-induced secretion of nitric oxide and tumor necrosis factor was determined in culture supernatants of peritoneal exudate cells from T. congolense-infected IL-4−/−, IL-4Rα−/−, and wild-type BALB/c mice. In parallel, arginase activity was quantified in lysates of peritoneal exudate cells from infected mice, and expression levels of the FIZZ1 and YM genes were analyzed in adherent peritoneal exudate cells from infected mouse populations.
Both spontaneously and after concanavalin A stimulation, peritoneal exudate cells from infected IL-4−/− and IL-4Rα−/− mice tended to secrete higher levels of nitric oxide, secreted higher levels of tumor necrosis factor (P < 0.01), and showed slightly lower arginase activity than peritoneal exudate cells from infected wild-type BALB/c animals (Fig. 3a, b, and c).
FIG. 3.
Role of IL-4 and IL-13 in macrophage activation status during T. congolense infection in BALB/c mice. During the early stage (6 days) of infection, spontaneous and concanavalin A (ConA)-induced NO2 (a) and tumor necrosis factor (b) secretion was quantified in the supernatants of peritoneal exudate cells from IL-4−/−, IL-4Rα−/−, and wild-type BALB/c mice (mean ± standard error, n = 3). In parallel, arginase activity was quantified in lysates from peritoneal exudate cells (c). Dotted lines represent levels in peritoneal exudate cells from naïve animals. Finally, expression levels of the β-actin, FIZZ1, and YM genes were analyzed in adherent peritoneal exudate cells from the noninfected (N) and infected (I) animals (d). The number of PCR cycles performed is indicated in parentheses. a, significantly higher than in noninfected animals; b, significantly higher than in BALB/c mice.
YM expression in peritoneal exudate cells from infected mice was induced in IL-4−/− and IL-4Rα−/− mice, although to lower extent than in wild-type BALB/c mice. Finally, compared to peritoneal exudate cells from noninfected mice, the expression of FIZZ1 in peritoneal exudate cells from infected mice was not triggered in wild-type BALB/c mice and was even impaired in IL-4−/− and IL-4Rα−/− mice (Fig. 3d).
Thus, despite the absence of IL-4 and IL-13, macrophages from the peritoneal compartment of T. congolense-infected BALB/c mice displayed the characteristics of both classical and alternative activation.
DISCUSSION
In the present study, we compared the course of African trypanosome infection in BALB/c and C57BL/6 mice, which are susceptible and resistant, respectively, to T. congolense (42). Cytokine levels in the spleen and the serum, as well as the status of macrophage activation in the peritoneal compartment, were analyzed at both the early and late stages of infection. It is worthwhile mentioning that modulations of cytokine and MCP-1 releases in the lymph nodes of infected BALB/c and C57BL/6 mice were similar to those observed in the spleen (not shown).
In the early stage of infection with T. congolense, a mixed type I/type II cytokine environment was reported to occur in the spleen and the plasma of susceptible BALB/c mice, while a type I cytokine environment developed in resistant C57BL/6 mice. Based on this time-limited information, it was suggested that a type I cytokine environment may be protective during T. congolense infection (42). Importantly, we show that, from the late stage of infection, type II cytokine production occurs in T. congolense-resistant C57BL/6 mice (Table 1). Changes in cytokine patterns in these animals were paralleled by changes in macrophage activation in the course of infection, from a classical to a predominant alternative phenotype (Fig. 2).
The modulations of cytokine environment and macrophage activation status during T. congolense infection in resistant animals resemble those occurring during infection with the phospholipase C (PLC)−/− T. brucei brucei variant which, like T. congolense, induces a chronic infection in C57BL/6 mice (survival, 160 ± 10 days) (28). Moreover, the inability to switch from a type I to a type II cytokine pattern and from classically to alternatively activated macrophages in the course of infection was correlated with the susceptibility of C57BL/6 mice to wild-type T. brucei brucei (survival, 35 ± 3 days) (28, 29). Finally, enhanced IL-4 and IL-10 mRNA levels and decreased levels of nitric oxide were shown to correlate with increased tolerance in T. congolense-infected cattle (24, 41).
Together, these data indicate that a switch from a dominant type I to a predominant type II immune response may be a general mechanism for resistance to African trypanosomiasis, both in murine models and in natural infections. By mounting a type I immune response and classically activated macrophages, infected animals may overcome the first and most aggressive wave of parasitemia, possibly through the secretion of nitric oxide and tumor necrosis factor, which are trypanostatic and trypanolytic, respectively (23, 40, 45). The emergence of a type II cytokine environment in the late stage of infection may favor the development of alternatively activated macrophages during African trypanosomiasis. These alternatively activated macrophages could reduce the inflammatory responses induced by classically activated macrophages, increasing the resistance of infected animals (8, 29). However, we do not exclude that alternatively activated macrophages impair effective parasite control and favor the progression of African trypanosomiasis to a chronic phase. Indeed, these cells were shown to potently inhibit T-cell responses during Brugia malayi nematode and T. brucei brucei infection (20, 22, 29, 36).
Mechanisms behind the switch from a type I to a type II cytokine environment during African trypanosome infection are currently unknown. In this regard, MCP-1 was reported to favor the polarization towards a type II cytokine response during Leishmania infection and Schistosoma egg granuloma formation (9, 21). Therefore, high MCP-1 levels occurring during the late stage of infection in mice resistant to T. congolense (Table 1) or to PLC−/− T. brucei brucei (our unpublished observations) may play a role in type II cytokine polarization. Concomitantly, MCP-1 may contribute to the anti-inflammatory processes of alternatively activated macrophages (4, 47).
As mentioned above, our data further confirm that the simultaneous occurrence of type I and type II cytokines in the early stage of infection is detrimental to the host. Indeed, it correlates with uncontrolled parasite growth and early death of T. congolense-susceptible BALB/c mice and is reflected by the emergence of macrophages displaying the characteristics of both classical and alternative activation. It is unclear whether this mixed phenotype originates from distinct macrophage populations. Analyses of infection in IL-4−/− and IL-4Rα−/− mice, lacking IL-4 production and IL-4/IL-13 signaling, respectively (2, 27), revealed that the type II cytokines IL-4 and IL-13 do not contribute to the susceptibility of BALB/c mice to T. congolense. The susceptibility to T. congolense may essentially be governed by the IFN-γ/IL-10 balance during the early stage of infection, as described previously for T. brucei brucei (30) and other infections (7, 12, 13). Accordingly, neutralization of IL-10 or IFN-γ prolongs, though moderately, the life span of T. congolense-infected BALB/c mice (43, 44).
Macrophages from T. congolense-infected mice lacking IL-4 and/or IL-13 signaling produced levels of nitric oxide and arginase activity similar to those in BALB/c mice but secreted increased levels of tumor necrosis factor (Fig. 3). Moreover, expression of the YM gene was still induced in the absence of IL-4/IL-13 signaling in infected BALB/c mice. Hence, it seems that in T. congolense-infected susceptible mice, macrophages still display a mixed classical/alternative phenotype in the absence of IL-4 and/or IL-13 signaling. Importantly, these results indicate that alternatively activated macrophages can occur independently of IL-4/IL-13 signaling in BALB/c mice, corroborating the natural propensity of these animals to develop alternatively activated macrophages (25). To gain more insight on the role of IL-4 and IL-13 in resistance to T. congolense, the course of infection and modulation of immune responses in IL-4−/− and IL-4Rα−/− C57BL/6 mice should be investigated.
Discrimination between murine classical and alternatively activated macrophages is so far based mainly on differential arginine metabolism via inducible nitric oxide synthase and arginase (8). Molecular markers allowing the identification of different macrophage populations remain scarce. However, recent reports have shown that the FIZZ1 and YM genes are induced in alternatively activated macrophages during Brugia malayi, Trichinella spiralis, and PLC−/− T. brucei brucei infections, as well as in the context of allergic pulmonary inflammation (3, 6, 11, 34, 46). The function of these genes in the modulation of the immune responses is unknown, although they were suggested to downregulate inflammatory processes (11).
Our results further corroborate expression of the FIZZ1 and YM genes in alternatively activated macrophages elicited from the late stage of T. congolense infection in resistant C57BL/6 mice. However, despite evidence for the presence of alternative macrophage activity in the early stage of T. congolense infection in susceptible BALB/c mice, only the expression of the YM gene was induced. Hence, distinct populations of alternatively activated macrophages expressing different gene repertoires are elicited in T. congolense-resistant and -susceptible mice. Interestingly, the expression of FIZZ1 in alternatively activated macrophages correlates better with the resistance of C57BL/6 mice to T. congolense than the induction of arginase activity or the enhanced expression of YM genes, since the last two parameters were also detected in alternatively activated macrophages from susceptible BALB/c mice.
IFN-γ was found to block the induction of both FIZZ1 and YM expression in in vitro IL-4-treated macrophages (34). Accordingly, the expression of these genes is observed in the late but not the early stage of T. congolense infection in C57BL/6 mice, i.e., when these animals are sensitized to produce IL-4 but not IFN-γ. On the other hand, we cannot exclude that in T. congolense-infected BALB/c mice, IFN-γ antagonizes the expression of the FIZZ1 gene more efficiently than of YM genes in in vivo-elicited alternatively activated macrophages. The induction of YM expression in alternatively activated macrophages is at least partially IL-4/IL-13 independent in T. congolense-infected BALB/c mice. Similarly, the induction of YM expression in B. malayi-infected C57BL/6 mice was found to be IL-4 independent (6). In contrast, YM expression in alternatively activated macrophages elicited during PLC−/− T. brucei brucei infection in (C57BL/6 × BALB/c)F1 mice or during allergic pulmonary disease in BALB/c mice was IL-4/IL-13 dependent (34, 46). Hence, it may well be possible that use of the FIZZ1 and YM genes as molecular markers for distinct populations of alternatively activated macrophages and their type II cytokine dependence for expression varies from mouse to mouse strain and/or depends on the pathological situation.
In conclusion, we have correlated the presence of type I/type II cytokine environments with resistance versus susceptibility to African trypanosomes. Resistance to T. congolense, like that to T. brucei brucei (28), correlates with the predominant secretion of type I cytokines in the early stage of infection to a more pronounced secretion of type II cytokines and MCP-1 in the late stage of infection. A change in the status of macrophage activation in the course of infection, from classical to predominant alternative, reflects the changed cytokine pattern. We have also found that the type II cytokines IL-4 and IL-13 do not modulate the susceptibility of BALB/c mice to T. congolense infection and are not the main stimuli inducing alternatively activated macrophages in these mice. Finally, our data suggest that the FIZZ1 and YM genes may be suitable markers to discriminate among populations of alternatively activated macrophages.
Acknowledgments
We thank Henry Tabel for helpful discussion. Furthermore, we are grateful to Ella Omasta and Martine Gobert for excellent technical assistance and administration.
Fund for Scientific Research Flanders (FWO) performed in frame of an Interuniversity Attraction Pole Program. G.R. is a postdoctoral fellow of the Institute for Promotion of Innovation by Science and Technology in Flanders (IWT-Vlaanderen, Brussels). F.B. is the holder of a Wellcome Trust Research Senior Fellowship for Medical Science in South Africa (grant no. 056708/Z/99).
Editor: J. M. Mansfield
REFERENCES
- 1.Bakhiet, M., L. Jansson, P. Buscher, R. Holmdahl, K. Kristensson, and T. Olsson. 1996. Control of parasitemia and survival during Trypanosoma brucei brucei infection is related to strain-dependent ability to produce IL-4. J. Immunol. 157:3518-3526. [PubMed] [Google Scholar]
- 2.Brombacher, F. 2000. The role of interleukin-13 in infectious diseases and allergy. Bioessays 22:646-656. [DOI] [PubMed] [Google Scholar]
- 3.Chang, N. C., S. I. Hung, K. Y. Hwa, I. Kato, J. E. Chen, C. H. Liu, and A. C. Chang. 2001. A macrophage protein, Ym1, transiently expressed during inflammation is a novel mammalian lectin. J. Biol. Chem. 276:17497-17506. [DOI] [PubMed] [Google Scholar]
- 4.Chensue, S. W., K. S. Warmington, J. H. Ruth, P. S. Sanghi, P. Lincoln, and S. L. Kunkel. 1996. Role of monocyte chemoattractant protein-1 (MCP-1) in Th1 (mycobacterial) and Th2 (schistosomal) antigen-induced granuloma formation: relationship to local inflammation, Th cell expression, and IL-12 production. J. Immunol. 157:4602-4608. [PubMed] [Google Scholar]
- 5.Dempsey, W. L., and J. M. Mansfield. 1983. Lymphocyte function in experimental African trypanosomiasis. V. Role of antibody and the mononuclear phagocyte system in variant-specific immunity. J. Immunol. 130:405-411. [PubMed] [Google Scholar]
- 6.Falcone, F. H., P. Loke, X. Zang, A. S. MacDonald, R. M. Maizels, and J. E. Allen. 2001. A Brugia malayi homolog of macrophage migration inhibitory factor reveals an important link between macrophages and eosinophil recruitment during nematode infection. J. Immunol. 167:5348-5354. [DOI] [PubMed] [Google Scholar]
- 7.Gazzinelli, R. T., M. Wysocka, S. Hieny, T. Scharton-Kersten, A. Cheever, R. Kuhn, W. Muller, G. Trinchieri, and A. Sher. 1996. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T-cells and accompanied by overproduction of IL-12, IFN-γ and tumor necrosis factor-α. J. Immunol. 157:798-805. [PubMed] [Google Scholar]
- 8.Goerdt, S., and C. E. Orfanos. 1999. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity 10:137-142. [DOI] [PubMed] [Google Scholar]
- 9.Gu, L., S. Tseng, R. M. Horner, C. Tam, M. Loda, and B. J. Rollins. 2000. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature 404:407-411. [DOI] [PubMed] [Google Scholar]
- 10.Hertz, C. J., H. Filutowicz, and J. M. Mansfield. 1998. Resistance to the African trypanosomes is IFN-γ dependent. J. Immunol. 161:6775-6783. [PubMed] [Google Scholar]
- 11.Holcomb, I. N., R. C. Kabakoff, B. Chan, T. W. Baker, A. Gurney, W. Henzel, C. Nelson, H. B. Lowman, B. D. Wright, N. J. Skelton, G. D. Frantz, D. B. Tumas, F. V. Peale, Jr., D. L. Shelton, and C. C. Hebert. 2000. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J. 19:4046-4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holscher, C., M. Mohrs, W. J. Dai, G. Kohler, B. Ryffel, G. A. Schaub, H. Mossmann, and F. Brombacher. 2000. Tumor necrosis factor α-mediated toxic shock in Trypanosoma cruzi-infected interleukin 10-deficient mice. Infect. Immun. 68:4075-4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunter, C. A., L. A. Ellis-Neyes, T. Slifer, S. Kanaly, G. Grunig, M. Fort, D. Rennick, and F. G. Araujo. 1997. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. J. Immunol. 158:3311-3316. [PubMed] [Google Scholar]
- 14.Inoue, N., M. Inoue, K. Kuriki, H. Yamaguchi, H. Nagasawa, T. Mikami, K. Fujisaki, N. Suzuki, and H. Hirumi. 1999. Interleukin 4 is a crucial cytokine in controlling Trypanosoma brucei gambiense infection in mice. Vet. Parasitol. 86:173-184. [DOI] [PubMed] [Google Scholar]
- 15.Jin, H. M., N. G. Copeland, D. J. Gilbert, N. A. Jenkins, R. B. Kirkpatrick, and M. Rosenberg. 1998. Genetic characterization of the murine Ym1 gene and identification of a cluster of highly homologous genes. Genomics 54:316-322. [DOI] [PubMed] [Google Scholar]
- 16.Karpus, W. J., and K. J. Kennedy. 1997. MIP-1α and MCP-1 differentially regulate acute and relapsing autoimmune encephalomyelitis as well as Th1/Th2 lymphocyte differentiation. J. Leukoc. Biol. 62:681-687. [PubMed] [Google Scholar]
- 17.Karpus, W. J., N. W. Lukacs, K. J. Kennedy, W. S. Smith, S. D. Hurst, and T. A. Barrett. 1997. Differential CC chemokine-induced enhancement of T helper cell cytokine production. J. Immunol. 158:4129-4136. [PubMed] [Google Scholar]
- 18.Kaushik, R. S., J. E. Uzonna, J. R. Gordon, and H. Tabel. 1999. Innate resistance to Trypanosoma congolense infections: differential production of nitric oxide by macrophages from susceptible BALB/c and resistant C57BL/6 mice. Exp. Parasitol. 92:131-143. [DOI] [PubMed] [Google Scholar]
- 19.Liu, Y., Z. Li, and M. Bakhiet. 1999. Upregulation of the chemokines Rantes, MCP-1, MIP-1α and MIP-2 in early infection with Trypanosoma brucei brucei and inhibition by sympathetic denervation of the spleen. Trop. Med. Int. Health 4:85-92. [DOI] [PubMed] [Google Scholar]
- 20.Loke, P., A. S. MacDonald, A. Robb, R. M. Maizels, and J. E. Allen. 2000. Alternatively activated macrophages induced by nematode infection inhibit proliferation via cell-to-cell contact. Eur. J. Immunol. 30:2669-2678. [DOI] [PubMed] [Google Scholar]
- 21.Lu, B., B. J. Rutledge, L. Gu, J. Fiorillo, N. W. Lukacs, S. L. Kunkel, R. North, C. Gerard, and B. J. Rollins. 1998. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J. Exp. Med. 187:601-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacDonald, A. S., R. M. Maizels, R. A. Lawrence, I. Dransfield, and J. E. Allen. 1998. Requirement for in vivo production of IL-4, but not IL-10, in the induction of proliferative suppression by filarial parasites. J. Immunol. 160:1304-1312. [PubMed] [Google Scholar]
- 23.Magez, S., M. Geuskens, A. Beschin, H. del Favero, H. Verschueren, R. Lucas, E. Pays, and P. De Baetselier. 1997. Specific uptake of tumor necrosis factor-α is involved in growth control of Trypanosoma brucei. J. Cell Biol. 137:715-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mertens, B., K. Taylor, C. Muriuki, and M. Rocchi. 1999. Cytokine mRNA profiles in trypanotolerant and trypanosusceptible cattle infected with the protozoan parasite Trypanosoma congolense: protective role for interleukin-4? J. Interferon Cytokine Res. 19:59-65. [DOI] [PubMed] [Google Scholar]
- 25.Mills, C. D., K. Kincaid, J. M. Alt, M. J. Heilman, and A. M. Hill. 2000. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 164:6166-6173.10843666 [Google Scholar]
- 26.Mohrs, M., B. Ledermann, G. Kohler, A. Dorfmuller, A. Gessner, and F. Brombacher. 1999. Differences between IL-4- and IL-4 receptor α-deficient mice in chronic leishmaniasis reveal a protective role for IL-13 receptor signaling. J. Immunol. 162:7302-7308. [PubMed] [Google Scholar]
- 27.Murata, T., J. Taguchi, R. K. Puri, and H. Mohri. 1999. Sharing of receptor subunits and signal transduction pathway between the IL-4 and IL-13 receptor system. Int. J. Hematol. 69:13-20. [PubMed] [Google Scholar]
- 28.Namangala, B., P. De Baetselier, L. Brijs, B. Stijlemans, W. Noël, E. Pays, M. Carrington, and A. Beschin. 2000. Attenuation of Trypanosoma brucei is associated with reduced immunosuppression and concomitant production of Th2 lymphokines. J. Infect. Dis. 181:1110-1120. [DOI] [PubMed] [Google Scholar]
- 29.Namangala, B., P. De Baetselier, W. Noël, L. Brys, and A. Beschin. 2001. Alternative versus classical macrophage activation during experimental African trypanosomiasis. J. Leukoc. Biol. 69:387-396. [PubMed] [Google Scholar]
- 30.Namangala, B., W. Noël, P. De Baetselier, L. Brys, and A. Beschin. 2001. Relative contribution of interferon-gamma and interleukin-10 to resistance to murine African trypanosomiasis. J. Infect. Dis. 183:1794-1800. [DOI] [PubMed] [Google Scholar]
- 31.Noben-Trauth, N., G. Kohler, K. Burki, and B. Ledermann. 1996. Efficient targeting of the IL-4 gene in a BALB/c embryonic stem cell line. Transgenic Res. 5:487-491. [DOI] [PubMed] [Google Scholar]
- 32.Otesile, E. B., M. Lee, and H. Tabel. 1991. Plasma levels of proteins of the alternative complement pathway in inbred mice that differ in resistance to Trypanosoma congolense infections. J. Parasitol. 77:958-964. [PubMed] [Google Scholar]
- 33.Otesile, E. B., and H. Tabel. 1987. Enhanced resistance of highly susceptible BALB/c mice to infection with Trypanosoma congolense after infection and cure. J. Parasitol. 73:947-953. [PubMed] [Google Scholar]
- 34.Raes, G., P. De Baetselier, W. Noël, A. Beschin, F. Brombacher, and G. Hassanzadeh Gh. 2002. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J. Leukoc. Biol. 71:597-602. [PubMed] [Google Scholar]
- 35.Reinitz, D. M., and J. M. Mansfield. 1990. T-cell-independent and T-cell-dependent B-cell responses to exposed variant surface glycoprotein epitopes in trypanosome-infected mice. Infect. Immun. 58:2337-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schebesch, C., V. Kodelja, C. Muller, N. Hakij, S. Bisson, C. E. Orfanos, and S. Goerdt. 1997. Alternatively activated macrophages actively inhibit proliferation of peripheral blood lymphocytes and CD4+ T-cells in vitro. Immunology 92:478-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schleifer, K. W., H. Filutowicz, L. R. Schopf, and J. M. Mansfield. 1993. Characterization of T helper cell responses to the trypanosome variant surface glycoprotein. J. Immunol. 150:2910-2919. [PubMed] [Google Scholar]
- 38.Schopf, L. R., H. Filutowicz, X. J. Bi, and J. M. Mansfield. 1998. Interleukin-4-dependent immunoglobulin G1 isotype switch in the presence of a polarized antigen-specific Th1-cell response to the trypanosome variant surface glycoprotein. Infect. Immun. 66:451-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharafeldin, A., R. Eltayeb, M. Pashenkov, and M. Bakhiet. 2000. Chemokines are produced in the brain early during the course of experimental African trypanosomiasis. J. Neuroimmunol. 103:165-170. [DOI] [PubMed] [Google Scholar]
- 40.Tabel, H., R. S. Kaushik, and J. Uzonna. 1999. Experimental African trypanosomiasis: differences in cytokine and nitric oxide production by macrophages from resistant and susceptible mice. Pathobiology 67:273-276. [DOI] [PubMed] [Google Scholar]
- 41.Taylor, K., B. Mertens, V. Lutje, and R. Saya. 1998. Trypanosoma congolense infection of trypanotolerant N′Dama (Bos taurus) cattle is associated with decreased secretion of nitric oxide by interferon-γ-activated monocytes and increased transcription of interleukin-10. Parasite Immunol. 20:421-429. [DOI] [PubMed] [Google Scholar]
- 42.Uzonna, J. E., R. S. Kaushik, J. R. Gordon, and H. Tabel. 1999. Cytokines and antibody responses during Trypanosoma congolense infections in two inbred mouse strains that differ in resistance. Parasite Immunol. 21:57-71. [DOI] [PubMed] [Google Scholar]
- 43.Uzonna, J. E., R. S. Kaushik, J. R. Gordon, and H. Tabel. 1998. Experimental murine Trypanosoma congolense infections. I. Administration of anti-IFN-γ antibodies alters trypanosome-susceptible mice to a resistant-like phenotype. J. Immunol. 161:5507-5515. [PubMed] [Google Scholar]
- 44.Uzonna, J. E., R. S. Kaushik, J. R. Gordon, and H. Tabel. 1998. Immunoregulation in experimental murine Trypanosoma congolense infection: anti-IL-10 antibodies reverse trypanosome-mediated suppression of lymphocyte proliferation in vitro and moderately prolong the lifespan of genetically susceptible BALB/c mice. Parasite Immunol. 20:293-302. [DOI] [PubMed] [Google Scholar]
- 45.Vincendeau, P., S. Daulouede, B. Veyret, M. L. Darde, B. Bouteille, and J. L. Lemesre. 1992. Nitric oxide-mediated cytostatic activity on Trypanosoma brucei gambiense and Trypanosoma brucei brucei. Exp. Parasitol. 75:353-360. [DOI] [PubMed] [Google Scholar]
- 46.Webb, D. C., A. N. McKenzie, and P. S. Foster. 2001. Expression of the Ym2 lectin-binding protein is dependent on interleukin (IL)-4 and IL-13 signal transduction: identification of a novel allergy-associated protein. J. Biol. Chem. 276:41969-41976. [DOI] [PubMed] [Google Scholar]
- 47.Zisman, D. A., S. L. Kunkel, R. M. Strieter, W. C. Tsai, K. Bucknell, J. Wilkowski, and T. J. Standiford. 1997. MCP-1 protects mice in lethal endotoxemia. J. Clin. Investig. 99:2832-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]