Abstract
Mouse and human neuroglobins, as well as the hemoglobins from Drosophila melanogaster and Arabidopsis thaliana, were recombinantly expressed in Escherichia coli, and their ligand-binding properties were studied versus temperature. These globins have a common feature of being hexacoordinated (via the distal histidine) under deoxy conditions, as evidenced by a large amplitude for the alpha absorption band at 560 nm and the Soret band at 426 nm. The transition from the hexacoordinated form to the CO bound species is slow, as expected for a replacement reaction Fe-His → Fe → FeCO. The intrinsic binding rates would indicate a high oxygen affinity for the pentacoordinated form, due to rapid association and slow (100 ms–1 s) dissociation. However, the competing protein ligand results in a much lower affinity, on the order of magnitude of 1 torr. In addition to decreasing the affinity for external ligand, the competitive internal ligand leads to a weaker observed temperature dependence of the ligand affinity, since the difference in equilibrium energy for the two ligands is much lower than that of ligand binding to pentacoordinated hemoglobin. This effect could be of biological relevance for certain organisms, since it could provide a globin with an oxygen affinity that is nearly independent of temperature.
INTRODUCTION
The recently discovered neuroglobin (Ngb) (Burmester et al., 2000) and cytoglobin (Burmester et al., 2002; Trent and Hargrove, 2002) share a common feature with the hemoglobins (Hbs) from Drosophila melanogaster and Arabidopsis thaliana. Under deoxy conditions, they display absorption spectra characteristics of a hexacoordinated heme, in which the protein forms two bonds with the iron atom, in addition to the four Fe-N bonds within the heme group. The proximal ligand is a histidine which is conserved for all globins. Based on sequence comparison and the crystallographic structure (Pesce et al., 2003), the distal protein ligand of Ngb is also a histidine, which is highly conserved in vertebrates; replacement of the E7 histidine by a leucine changes the optical properties of the mutant (Dewilde et al., 2001). The distal histidine in Ngb can be displaced by the usual ligands of ferrous hemoglobin such as O2, CO, or NO.
Although Hbs and myoglobins (Mbs) are certainly among the best studied proteins (e.g., Dickerson and Geis, 1983; Wittenberg and Wittenberg, 1990), the possible functions of hexacoordinated globins are less well-defined. Most hexacoordinated globins are present at comparatively low concentrations, and it is uncertain if they provide a distinct function for oxygen diffusion and delivery to the aerobic cell metabolism (Burmester et al., 2000, 2002; Trent and Hargrove, 2002). However, hexacoordinated globins show an oxygen affinity similar to that of Mb or Hb, suggesting a conserved function over the long period since the genetic divergence.
This reversible binding of a protein residue to the heme is a characteristic intermediate to certain cytochromes, which always remain hexacoordinated, and the usual Hbs, which are pentacoordinated in the deoxy state. Note that the dioxygenase cytochrome P450 is also pentacoordinated in the deoxy form, and that human Hb is capable of forming the His-Fe-His complex, referred to as a hemichrome (Winterbourn and Carell, 1974). Thus a small change in protein structure may be sufficient to change the iron from 5- to 6-coordinated.
In addition human Ngb and cytoglobin possess cysteine residues, capable of forming internal disulphide bonds which may influence the overall oxygen affinity (Hamdane et al., 2003). This leads to a sample heterogeneity which complicates the analysis of the ligand-binding kinetics, since two sets of parameters must be considered, in addition to possible relaxation phenomena (Trent et al., 2001a) and dimer formation (Dewilde et al., 2001; Hamdane et al., 2003). To obtain the rate parameters for the pure conformations, we have reanalyzed the data by numerical integration. In this study we consider the temperature dependence of the ligand affinities, and show that the competition with the internal protein ligand could be a mechanism for obtaining a weak temperature dependence for the binding of external ligands.
MATERIALS AND METHODS
The recombinant globins from D. melanogaster and A. thaliana (sequence U94999 in GenBank database; Trevaskis et al., 1997) and human and mouse Ngbs were expressed in E. coli and further purified as previously described (Dewilde et al., 2001; Hankeln et al., 2002). Due to sample heterogeneity as detected by HPLC or in the ligand-binding kinetics, a further purification was made with an FPLC Akta Purifier (Amersham Pharmacia Biotech, Uppsala, Sweden) using a column Hitrap DEAE sepharose Fast Flow.
Spectra and ligand-binding kinetics
Spectral measurements were made with an SLM DW2000 spectrophotometer or a HP 8453 diode array spectrophotometer. Under air at 37°C, the samples (10 μM on a heme basis in 4 × 10 mm quartz cuvettes) oxidize within an hour; this form was taken to be the ferric state. The deoxy sample was obtained by equilibration under nitrogen, and adding an excess of sodium dithionite. The oxidized species were reduced under air to record the oxy ferrous spectrum using the enzymatic system NADPH/NADP-ferredoxin as described by Hayashi et al. (1973). All ligand-binding experiments were performed in 100 mM potassium phosphate at pH 7.0. Samples were varied in temperature between 10 and 50°C.
Heme ligation model spectra
Hemin from bovine was dissolved in 0.02 N NaOH at a concentration for the stock solution of 1 g/l. Hemin was then diluted in 100 mM phosphate buffer at pH 8.0 containing 2% CTAB (hexadecyltrimethylammonium bromide) with 100 mM of axial base ligand either imidazole or 2methyl-imidazole. CTAB induces the formation of micelles which help hemin to solubilize by hydrophobic interaction and to dissociate, since in aqueous solution hemin tends to form dimers or higher aggregates. After a thorough deoxygenation under nitrogen gas of the heme compound solutions in an optical cuvette sealed with a rubber cap, sodium dithionite was added (0.2 mM final concentration) to reduce the hemin.
LIGAND-BINDING KINETICS
O2 and CO bimolecular recombination rates (kon) were measured after flash photolysis with 10 ns YAG laser pulses of 160 mJ at 532 nm (Quantel, Les Ulis, France). The standard detection wavelength was 436 nm, but other detection wavelengths were used to separate the signals for the binding of CO, O2, and the protein ligand.
Samples were equilibrated under oxygen (air or 1 atm O2) or CO (0.01, 0.1, or 1 atm) in 1 or 4 mm optical cuvettes. Additional CO levels were obtained by mixing one of the standards with nitrogen. A typical kinetic curve is obtained from the average of at least ten measurements, with at least 4 s between photolysis pulses to allow sample recovery.
The rate of transition from the penta- to hexacoordinated protein ligand was extracted from the bimolecular kinetics after CO flash photolysis at various CO concentrations. This family of curves was fitted together with the same microscopic rate constants. Nonlinear fittings were performed using Scientist program (MicroMath Scientist, Salt Lake City, UT). The procedure for least squares fitting uses a modified Powell algorithm to find minima for each parameter. We also used our own program giving a numerical treatment of the competitive binding kinetics, taking into account the time-dependent ligand concentration. The first phase after CO photodissociation involves competition of the internal and external ligand association, and is followed by a slow phase of replacement of the internal ligand by CO. The phase of replacement allows the estimation of the protein ligand dissociation rate.
The concentrations of the three species versus time were simulated using the following differential equations:
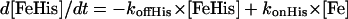 |
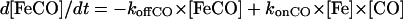 |
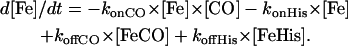 |
By numerical integration of the above equations, the observed absorption change after the flash photolysis was simulated considering the different species concentration and their relative extinction coefficients. For the flash photolysis experiments there are generally 3-rate coefficients to be varied (on-rates for CO, on- and off-rate for histidine), since the CO dissociation occurs on a longer timescale.
Note that the solvent CO concentration [CO] is usually taken as a constant equilibrium value [CO]eq, which depends on the gas partial pressure and solubility coefficient; however, at low CO levels one must take into account the CO photodissociated from the protein; the total solvent CO then varies in time, depending on the fraction (f) of protein bound with CO:
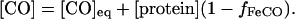 |
A simple competitive reaction between CO and His for heme rebinding predicts a biphasic kinetic pattern. The overall rates λ of two phases are a function of the microscopic rate coefficients, and can be approximated by the following equations; note that these approximations are not required for the numerical integration.
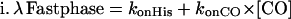 |
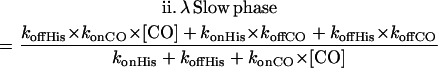 |
In the case where CO dissociation is very slow, the form reduces to
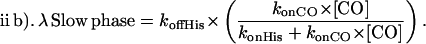 |
Finally, when CO binds more rapidly than histidine (konCO × [CO] ≫ konHis) the slow phase rate simplifies to λ ≈ koffHis.
Similarly the O2 dissociation rate can be measured by CO replacement after flash photolysis. By mixing pure O2 and CO gases in near equal amounts, the sample will be essentially CO bound at equilibrium; the fraction with histidine bound, as well as the histidine rebinding rate can be considered as negligible. After photolysis of CO, one observes essentially oxygen binding in the rapid phase, and the rate of the slow replacement phase depends on the bimolecular association rates for both external ligands and the O2 dissociation rate. By contrast the CO dissociation occurs on a timescale slow compared to the O2 or the internal histidine dissociation, so CO rebinding can be treated as an irreversible process.
The O2 and histidine dissociation rates were also measured by a replacement reaction after rapid mixing using a Biologic stopped-flow apparatus. The samples were mixed with buffer containing a high concentration of a competing ligand; the buffered solution contained 20 mM sodium dithionite and was equilibrated under 1 atm CO. After mixing, the final CO concentration was typically 0.5–0.7 mM. The kinetics at different wavelengths were followed from 2 ms to 10 s.
CO dissociation rates for the Ngb samples were measured by displacing CO with an excess of nitric oxide (0.1 atm); in this case the reaction is slow enough that it could be followed using the diode-array spectrophotometer with detection between 500 and 700 nm. If the partition coefficient between the O2 and CO affinities is low, which is the case for Ngb, the CO dissociation rate can be measured by replacement with O2. The same rate can be determined by mixing a carboxylated sample with a deoxygenated buffer containing an excess of potassium ferricyanide. In all globin species the CO dissociation is much slower than that of oxygen or the internal protein ligand.
Relative amplitudes
In addition to the rate coefficients, the relative proportion of the two phases is simulated; the fraction of slow phase depends on both the rates and the extinction coefficients of the three spectral forms: the pentacoordinated, the hexacoordinated (with internal ligand) species and the stable HbCO form that determines the baseline absorbance before photolysis. An accurate estimation of the extinction coefficients for each species at each wavelength of detection is a critical parameter since there is a compensation between the spectral parameters and the rate coefficients.
Three methodologies were used to determine the spectral differences between all species involved after CO dissociation: i), Photobleaching: since the histidine dissociation is slow (1 s), the sample can be photodissociated again before the replacement reaction. By using the maximum repetition rate of 10 Hz for the laser, the sample can be shifted toward the hexacoordinated state to better calibrate the spectral difference. To favor the histidine rebinding, the lowest (1%) CO atmosphere was used. Finally the amplitudes were normalized relative to the signal at elevated temperature, where there is little geminate recombination. ii), Increase in the temperature: since activation energies are higher for the internal ligand compared to those for CO, elevated temperatures will favor the histidine rebinding at low [CO] concentration. A good test is to check that the CO rebinding is negligible consists in the variation of the laser intensity. No change after normalization of the fast rate amplitude which obviously depends on both ligand binding rates (and the extinction coefficients for the different species) means that the histidine association is the dominant binding reaction. iii) variation of [CO]: the relative absorption for the hexacoordinated species compared to the preflash baseline absorption (CO species) and the 100 ns absorption that happens before the bimolecular ligand rebinding (pentacoordinated species) can also be estimated by plotting the fast phase amplitude of the CO binding kinetics versus [CO]. The shape of the curve follows a rectangular hyperbola for which the asymptote at low [CO] is related to the millisecond absorption after the internal ligand rebinding.
These different methods are valid as long as the experimental conditions allow the His binding to be competitive with CO rebinding. One cannot rule out that for some hexacoordinated globins the latter condition is difficult or even impossible to achieve. For instance if the ratio of CO to His rates is high, or if the His dissociation is too fast, it will be difficult to highly populate the His bound form. Nevertheless the flash photolysis method is the only method that allows the determination of the ligand binding rate constants for such globins. The stopped-flow rapid mixing device is useful for the dissociation rate measurements.
RESULTS
Optical spectra
The deoxy form of these Hbs shows a typical hemochrome absorption spectrum; large amplitudes for the alpha band (560 nm) and the Soret band (426) are characteristic of the six coordinated form (Dewilde et al., 2001). Based on the sequence and comparison to other hemoproteins, the most likely binding scheme would be His-Fe-His. The hexacoordinated spectra for the globin species present in this work were almost identical. In particular the 560 nm α-peak was two times higher than that of the 530 nm β-peak at 293 K with a minimum between at 545 nm. This result indicates a strong proportion of hexacoordinated species relative to pentacoordinated species.
The same features were observed by comparison between the visible spectra of deoxy heme bis-imidazole against deoxy heme bound to the hindered 2-methyl imidazole which gives rise to a classical deoxy Hb pentacoordinated heme spectrum (data not shown). The simulation of a mixed spectrum arising from an equal contribution of hexacoordinated and pentacoordinated spectra using a deoxy Hb spectrum or the deoxy spectrum for the HE7L mouse Ngb mutant predicts a 20–30% decrease of the α/β-ratio associated with a weaker β-band (data not shown). The deoxy spectra for human and mouse Ngbs (Dewilde et al., 2001) as well as Drosophila (Hankeln et al., 2002) and Arabidopsis globins were in agreement with our histidine binding constant measurement at 293 K which predicts at least 95% of hexacoordinated deoxy heme at equilibrium especially for the Ngb species.
Ligand-binding kinetic analysis
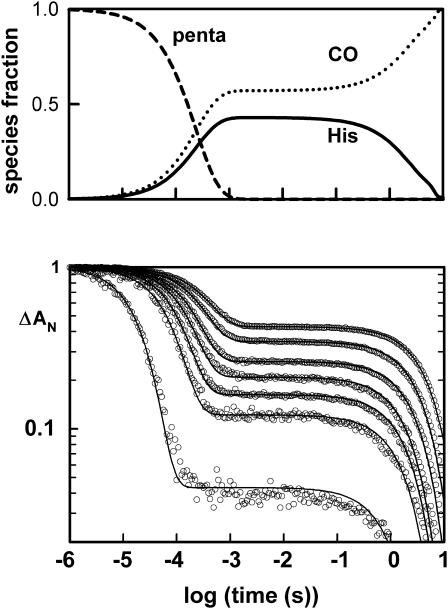
The kinetics after CO photodissociation exhibit a typical biphasic pattern (Fig. 1). The fast phase corresponds to a competitive binding of the internal and external ligands; the rate therefore involves the sum of the ligand association rates. The CO binding kinetics for the human Ngb were measured at different CO concentration (Fig. 1) to change the CO versus histidine binding rates; samples equilibrated under 1 atm (100%) CO show mainly the association of the external ligand (bottom curve in bottom panel in Fig. 1). The rate of histidine binding (1800/s, see Table 1) was best measured for samples under the lowest (1%) CO level. The rates of binding of CO and the distal histidine would be similar at a CO concentration of 50 μM (5% CO atmosphere). Note that the His rebinding is also the main reaction at 1% CO for Arabidopsis and Drosophila globins.
FIGURE 1.
Flash photolysis kinetics for human Ngb at 298 K, with detection at 436 nm. (Top panel) Species populations versus time. (Lower panel). Recombination kinetics at different CO concentrations. After photolysis of CO, there will be a competitive binding of CO and His ligands to the vacant sites. For the fraction that binds histidine, there is a slow replacement reaction to return to the more stable CO bound state. Consequently for lower CO concentrations, more histidine will bind and the amplitude of the slow phase increases. The biphasic kinetics at different CO concentrations will therefore provide information on the association rate of both ligands, as well as histidine dissociation. The Ngb is a mutant of human Ngb without cysteines to prevent the formation of disulfide bridges; the mutant and wild-type Ngb incubated with dithiotreitol (not shown) exhibit similar ligand-binding properties (Hamdane et al., 2003).
TABLE 1.
Rates of CO rebinding to hexacoordinated Hb after flash photolysis and stopped-flow
| konCO (/M/s) (×107) | konO2 (/M/s) (×107) | koffO2 (/s) | KO2 (nM) | konHis(/s)* | koffHis (/s)† | KHis | P50 (torr) | |
|---|---|---|---|---|---|---|---|---|
| Mouse NGb | 5.5 | 20 | 0.4/0.5† | 2.2 | 1000 | 0.5/0.6† | 2000 | 2.2 |
| Human NGb | 5.0 | 17 | 0.7 | 4.1 | 1800 | 0.6 | 3000 | 6.8 |
| Drosophila Hb† | 1.0 | 6.4 | 1/1.1† | 16 | 550 | 30/40† | 18 | 0.1 |
| Arabidopsis Hb | 5.0 | 15 | 2.1/3.2† | 14 | 1600 | 34/43† | 50 | 0.3 |
From Hankeln et al. (2002).
Measured by stopped-flow.
‡Calculated using the formula P50 = (1/KO2)/(1 + KHis).
Experimental conditions: 100 mM potassium phosphate, 2 mM dithiotreitol, dtt pH 7.0, 25°C.
Flash photolysis experiments show a high rate of recombination for O2 or CO to the pentacoordinated form. However, once the distal histidine binds, binding of these ligands is quite slow, as the rate-limiting step involves dissociation of the protein ligand. One can estimate the rate for histidine dissociation form the rate of the slow phase, which corresponds to the replacement reaction from FeHis to FeCO.
Once bound, both oxygen and CO show a slow dissociation; this would indicate a very high intrinsic affinity of these ligands for the pentacoordinated form. However, the observed affinity for human Ngb (1 torr for oxygen at 37°C, Dewilde et al., 2001) is average due to competition with the protein ligand. For the species studied in this work, the histidine affinity (KHis) lies between 10 and 4000 (Table 1), which decreases the observed O2 affinity (KO2, obs) by the same factor:
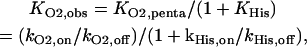 |
where the intrinsic affinity for the pentacoordinated form is the ratio of the on to off rates.
[CO] dependence
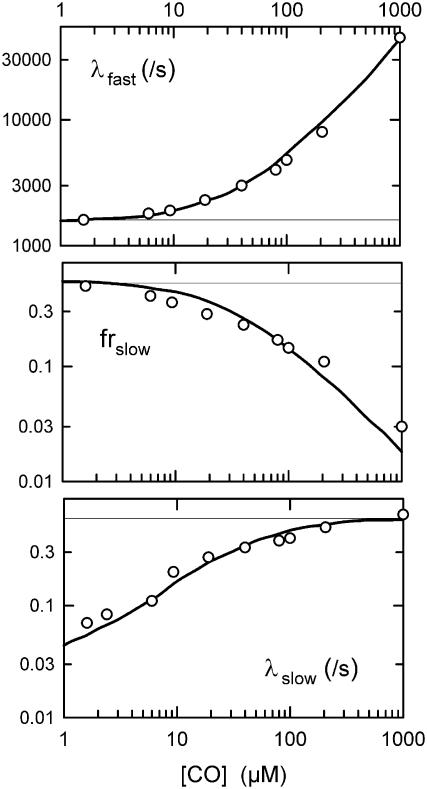
In Fig. 2, top panel, the rate of the fast phase for the CO-binding kinetics versus [CO] for human Ngb is shown. As expected the observed rate depends linearly on the CO concentration; however at low [CO] values, the histidine binding becomes competitive, and eventually a plateau is achieved which corresponds to the rate of association for the internal ligand.
FIGURE 2.
Global simulation used to describe the rebinding kinetics versus CO concentration. The observed biphasic kinetics were decomposed into the two rate coefficients and the fraction occurring as the slow phase; the points are the experimentally observed values. The solid lines are based on simulation using a hexacoordination model to take into account the ligand competition.
The fraction of slow phase versus [CO] is shown in Fig. 2, middle panel. At low [CO], where the rate of histidine binding exceeds that of CO, the fraction of slow phase is related to the relative absorption of the hexacoordinated species, the microseciond transient species, the microsecond transient pentacoordinated species, and CO bound species at equilibrium.
The rate of slow phase versus [CO] is shown in Fig. 2, bottom panel. As expected this curve reaches a plateau at high [CO] for which the rate value approaches the His dissociation rate. As a control, the transient difference spectrum, at 100 ms after photolysis (A(t = 100 ms) − A(preflash)), was well simulated, as for the other species, by the difference between ground spectra for the CO and the deoxy hexacoordinated forms (data not shown).
We obtained the same values by stopped-flow device by mixing a deoxygenated sample of Ngb into a CO-saturated buffer. This suggests that no relaxation process occurs for the Ngb or the Drosophila globin, after a long incubation in the absence of external ligands. The same conclusions can be drawn from the comparison between kinetic and equilibrium measurements (data not shown). This latter comparison is generally important to make sure that the model of ligand competition we are using is correct. For Ngb the isotherm of CO binding is easy to measure by tonometry, whereas for a myoglobin the much higher CO affinity prevents an accurate evaluation at equilibrium. Once the CO affinity is known, the O2 affinity can be estimated by the measurement of the partition coefficient for binding both ligands using an accurate mixture of CO and O2 gas. Based on the absorption spectra one can obtain a good approximation of the CO and O2 affinities measured at equilibrium provided that an adequate reducing system is present. Note that a rapid autoxidation involving consumption of the O2 molecules will lead to a decreasing oxygen concentration; this can be avoided by continuous stirring.
Temperature effect
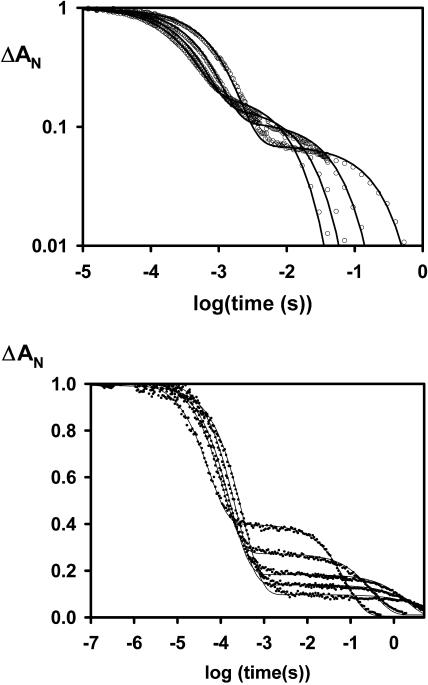
The kinetics at different temperatures, after photodissociation of CO from the Hb of Drosophila and the human Ngb, are shown in Fig. 3, a similar pattern was observed for mouse Ngb and Arabidopsis globin (data not shown). The relative amplitude of the slow phase depends on the fraction of the hemes that bind the internal ligand rather than CO. A change in the slow fraction indicates a change in the ratio of the association rates. As the temperature increases, a larger fraction of slow phase is observed, indicating a higher activation energy of binding for the internal (His) ligand relative to that for the binding of a diatomic ligand. The spectral form is distinct for each phase, as they correspond to different mixture of liganded states. The slow return to the CO form for the hexacoordinated His-Fe-His form occurs via a replacement reaction: Fe-His ↔ Fe → Fe-CO. As expected the rate of this phase increases with higher temperatures; the rate also increases with higher CO concentration, saturating when the rate approaches the histidine off rate.
FIGURE 3.
(Top panel) Ligand binding kinetics Hb D. melanogaster. The temperature was varied from 283 K to 323 K (bottom to top, at t = 10 ms), for samples equilibrated under 0.1 atm CO. Since the activation energies are different for CO and histidine, the kinetics versus temperature show a different fraction of slow phase. (Bottom panel) Rebinding kinetics for Ngb versus T.
Dissociation rates
Histidine
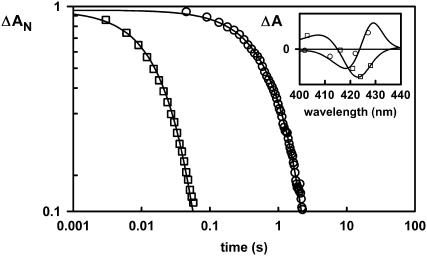
The replacement reaction in the flash photolysis measurements can also be observed by the stopped-flow technique. Mixing the hexacoordinated Drosophila Hb with CO saturated buffer requires ∼1 s to obtain the CO form, consistent with the slow rate of dissociation for the distal histidine (Fig. 4). These stopped-flow kinetics were analyzed at several wavelengths in the Soret region to assess whether the observed signal corresponds to that expected based on the static spectra (inset, Fig. 4). The measurement of the full amplitude for the reaction is another strong support that at equilibrium the pentacoordinated fraction is very low. The same kinetic features were observed for the other hexacoordinated globin species.
FIGURE 4.
Stopped-flow kinetics of the histidine (□) and oxygen (○) replacement by CO for Drosophila Hb. At high CO concentrations, the observed kinetics gives a direct measurement of the ligand dissociation rates. The inset shows the amplitude of the signal in the Soret region in comparison with the static differential spectra.
Oxygen dissociation
Based on stopped-flow (Fig. 4) or flash photolysis data, the O2 dissociation from Ngb is slow, requiring over a second at 298 K. This suggests a stabilization of the O2 ligand via hydrogen bonding, most likely with the distal histidine and/or the residue at position B10, as for Ascaris Hb (Carver et al., 1992; De Baere et al., 1994) even though other structural determinants may play a role.
The O2 affinity can be estimated from the binding rates for O2 and His; however, direct measurements provide a control that the binding scheme assumed is correct. Equilibrium experiments were made in selected cases to confirm the validity of the overall scheme of competitive ligand binding.
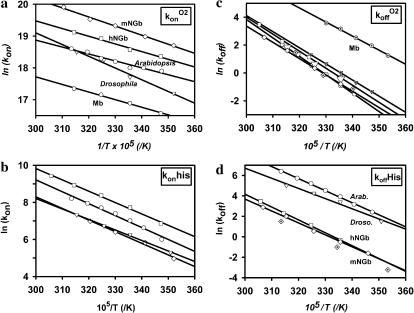
The temperature dependence of the association and dissociation rates for O2 and the internal ligand are shown in Fig. 5. The Arrhenius representation for each parameter allows an estimation of the activation energies (E); the difference between association and dissociation activation energies being equivalent to the equilibrium energy of binding (ΔE, Table 2). For each hexacoordinated species, the activation energies for O2 or His dissociation are similar. A higher energy barrier is observed for the association of His compared to the diatomic ligands O2 (Fig. 5) or CO (data not shown). Note that the entropy values are generally positive underlying that the transition state for the ligand dissociation reaction less ordered than the ground state.
FIGURE 5.
Arrhenius plots. Analysis of the flash photolysis data (open symbols), at different temperatures and ligand concentrations, yields the rates coefficients versus temperature for the globins of A. thaliana (circles), D. melanogaster (triangles), human Ngb (squares), mouse Ngb (diamonds), and horse heart myoglobin (hexagons). Note that we also measured the dissociation rate constants by the stopped-flow technique (same symbols are used as before, but cross-filled). A similar slope was observed for both methods; each data set was simulated independently. The Arrhenius plots indicate a higher activation energy for the dissociation rate coefficients compared to the association rates, and for the latter a higher activation energy for the internal histidine compared to the diatomic ligand.
TABLE 2.
Activation energies (Ea) and entropies (ΔS) for O2 and histidine binding to several globin species
| Activation energy (kcal mol−1) and entropy (cal deg−1 mol−1)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Histidine
|
Oxygen
|
O2, Observed
|
|||||||||
| Species | Eaoff | ΔSoff | Eaon | ΔSon | ΔEHIS | Eaoff | ΔSoff | Eaon* | ΔSon | ΔEO2 | ΔEO2–ΔEHIS |
| Arabidopsis | 23.5 | 26.7 | 11.5 | −2.9 | 12 | 25.5 | 26.9 | 8 | 0.1 | 17.5 | 5.5 |
| Drosophila | 19.5 | 13.0 | 11.5 | −9.5 | 8.5 | 23 | 17.6 | 10.5 | 9.6 | 12.5 | 4 |
| Mouse Ngb | 23 | 17.4 | 12.5 | −5.7 | 11 | 24.5 | 16.9 | 8.5 | 6.8 | 14.5 | 4 |
| Human Ngb | 25 | 23.7 | 12 | −3.5 | 13 | 24.5 | 21.3 | 8 | 2.8 | 16.5 | 3.5 |
| Horse Mb | - | - | - | - | - | 20 | 14.0 | 7.5 | −0.9 | 12.5 | 12.5 |
The energy for the oxygen on-rate included the correction for the heat of O2 solubility, taken as 3 kcal/mol. The rate coefficient is given by k = ν e −Ea/RT eΔS/R. Entropy values were calculated using a frequency factor of ν = 1013 s−1 for the unimolecular reactions and 1011 M−1 s−1 for the bimolecular reactions (Austin et al., 1975).
For all of the hexacoordinated species studied, the competition for O2 binding with the distal histidine leads to a weaker temperature dependence on the external ligand affinity, compared with a classical oxygen carrier such as the myoglobin. Although a variation of temperature from 0 to 40°C induces a decrease of the O2 affinity for the myoglobin by a factor of 19, the decrease is only a factor of 2.5 for the hexacoordinated globin species. In terms of equilibrium energy for oxygen binding, the usual pentacoordinated Mbs have an equilibrium energy nearly 3 times that of the reversibly hexacoordinated forms.
DISCUSSION
Ligand binding
The spectral and ligand-binding kinetics indicate a reversible hexacoordination of mouse and human Ngbs, as well as the Hbs from D. melanogaster and A. thaliana. In the absence of external ligands the protein will adapt the hexacoordinated His-Fe-His form. Moderate concentrations of oxygen or CO can replace the sixth ligand, indicating a favorable competition for these ligands. Based on the high on-rates, and low off-rates, the intrinsic affinities for oxygen and CO for the pentacoordinated form are quite high.
For the hexacoordinated Hbs, one must consider two types of ligand association: a rapid binding to the 5-coordinated form, and a slow binding to the hexacoordinated form, where the dissociation of the sixth ligand is usually the rate limiting step. The rapid recombination to the open binding site can be probed by flash photolysis; the rates for oxygen were very high, approaching the diffusion limit for ligand binding to Hb. If an external ligand is mixed with the deoxy hexacoordinated form, the observed ligand replacement requires ∼0.5–2 s at 25°C. At high external ligand concentration the observed rate approaches that for the dissociation of the protein ligand.
At low CO concentrations the association rate for the protein ligand may be faster than CO rebinding. One can then extract both the on and off rates for the histidine ligand. This method is also useful to probe the oxygen on/off rates, especially when the quantum yield for oxygen photodissociation is low.
The Hb species studied share common features: i), at least 95% of the deoxy form occurs as the hexacoordinated form, indicating a high affinity of the distal histidine for the heme. Note that our value for the histidine-binding coefficient of ∼1000 in Ngb is consistent with other studies, but not that of Trent et al. (2001b) who reported a value of 1; ii), a high intrinsic oxygen affinity for the pentacoordinated heme; iii), an overall oxygen affinity similar to Mb, resulting from the competition of both ligands for the heme; iv), a faster rate of autoxidation compared with a classical O2 carrier (Dewilde et al., 2001); and v), the objective of this study, a low temperature dependence for oxygen binding.
Temperature dependence
From the rates of ligand binding versus temperature, one can determine the ligand binding activation energy. The Arrhenius plots (Fig. 5) show characteristics typical of other Hbs; that is, a small energy barrier for oxygen association, but a higher activation energy for ligand dissociation reflecting the high intrinsic binding affinity.
The activation energy for histidine association was generally higher than that for oxygen or CO. This effect can be seen directly in the series of kinetic curves in Fig. 3: as the temperature is raised, the fraction of slow phase increases (more histidine binding), indicating a larger increase in rate for histidine relative to CO.
A recent study measured ligand recombination of Ngb over a large temperature range (Kriegl et al., 2002). At very low temperatures, the solution is frozen and one observes only the internal geminate recombination, where the ligand is trapped within the protein and therefore rebinds to the same heme. Under liquid solvent conditions, the ligand may escape and then competes with other solvent ligands for available binding sites; the reaction is then bimolecular. The reported values for the activation energy for histidine dissociation are in agreement with our measurements. For the ligand association, we obtained larger values, but the difference in energies is about the same: histidine has an equilibrium binding energy ∼4 kcal/mol higher than the external ligand. For the CO association rate, these authors reported a surprising temperature dependence, considering three CO concentrations; however, they apparently did not include the histidine contribution to this phase. A higher energy at low CO levels is expected for the first phase, since the histidine contribution is larger. The multiple rates for the bimolecular phase were taken as evidence for several protein conformations. Trent et al. (2001a) also reported a model involving a protein transition between substrates. We were able to eliminate this heterogeneity in our samples to obtain a simpler two-phase form for the bimolecular kinetics. We did not observe a dynamic transition (on the millisecond to second timescale), but rather distinct conformations that can be isolated (Hamdane et al., 2003). At a fixed temperature, the flash and stopped-flow data at different CO levels could be simulated with a single set of rate coefficients; comparison to independent simulations at each CO level showed only a small change in rate and deviations in the rate coefficients were typically <20% and at most ∼50% in the case of human Ngb.
Implications for in vivo function?
It is not clear whether the “hexaglobins” studied in this work are involved in O2 diffusion and delivery. Alternative enzymatic functions could be proposed, since for instance oxyHb is efficient at removing NO·by formation of nitrate (Eich et al., 1996). But an NO·detoxification mechanism will require an enzymatic system to reduce the iron, a product of the reaction. One may also consider an electron transfer mechanism subsequent to the O2 binding.
It is interesting to note that the feature of hexacoordination does not imply a recent common ancestry of these types of Hbs. Rather these proteins have close relatives that are pentacoordinated and are involved in oxygen storage or delivery (Burmester et al., 2000, 2002; Hankeln et al., 2002). Thus hexacoordination appears a structural feature associated with Hbs that are expressed at comparatively low levels, though the functional relevance is not yet clear.
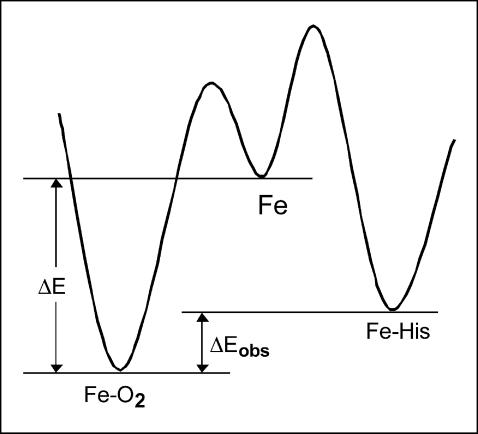
For the hexaglobins, the overall observed equilibrium energy for oxygen binding involves ligand replacement (as opposed to ligand binding to the pentacoordinated form) and corresponds to the difference in equilibrium energies for the two ligands. An energy diagram is presented in Fig. 6. As can be seen the activation energy for dissociation is similar for oxygen and histidine. The activation energy for the ligand association is larger for histidine than for oxygen. The overall observed energy of 4 kcal/mol for oxygen equilibrium binding is the difference in equilibrium energies for the two competing ligands; a value for oxygen binding to the pentacoordinated form is typically three times larger. A similar value (4.5 kcal/mol) was reported for Nostoc commune Hb which belongs to the hexaglobin family (Thorsteinsson et al., 1996), based on oxygen equilibrium binding measurements. Thus a general characteristic of the hexacoordinated forms is a low temperature dependence of binding of the external ligands.
FIGURE 6.
Energy diagram for human Ngb. For the usual pentacoordinated Hbs, the larger activation energy for ligand dissociation leads to a high temperature dependence of ligand binding. However, for the hexacoordinated Hbs, the energies for both internal (protein) and external ligands are involved. For the replacement reaction (His-Fe-His → His-Fe → His-Fe-O2), the overall difference in equilibrium energies results in a much weaker observed temperature dependence.
This effect could lead to novel properties of the hexaglobins. Since the oxygen is always in competition with the protein ligand, the observed affinity will depend on the partition coefficient of the two ligands. Thus any effector that changes either the oxygen or the histidine binding parameters will lead to a change in the observed oxygen affinity. For example, the dependence on temperature will depend on the equilibrium energies of both ligands, and a null or reverse dependence could be observed for one of the ligands. That is, the change in intrinsic affinity for the histidine could more than offset that of the external ligand (a higher equilibrium energy for histidine binding) resulting in a higher ligand affinity at higher temperatures; this reversed temperature dependence for ligand binding was observed for Ngb/CO complex (data not shown). The hexacoordination could be a way to obtain a temperature-independent oxygen affinity. We hypothesize that this particular functional property of hexacoordinated globins could be especially useful for poikilothermic organisms, unable to keep their body temperature stable. Since hexacoordinated globins are known to be phylogenetically very ancient (Burmester et al., 2000), their temperature independence might be regarded as an evolutionary relic of a time when organisms were exposed to vastly fluctuating temperature conditions in their environment.
Acknowledgments
This work was supported by INSERM, the Délegation Générale pour l'Armement (France), the Deutsche Forschungsgemeinschaft (Ha2103/3 and Bu956/5), the University of Antwerp, and a grant from the European Commission (contract No. QLG3-CT-2002-01548).
S.D. is a postdoctoral fellow of the Fund for Scientific Research, Flanders (FWO).
References
- Austin, R. H., K. W. Beeson, L. Eisenstein, H. Frauenfelder, and I. C. Gunsalus. 1975. Dynamics of ligand binding to myoglobin. Biochemistry. 14:5355–5373. [DOI] [PubMed] [Google Scholar]
- Burmester, T., B. Ebner, B. Weich, and T. Hankeln. 2002. Cytoglobin: a novel globin type ubiquitously expressed in vertebrate tissues. Mol. Biol. Evol. 19:416–421. [DOI] [PubMed] [Google Scholar]
- Burmester, T., B. Weich, S. Reinhardt, and T. Hankeln. 2000. A vertebrate globin expressed in the brain. Nature. 407:520–523. [DOI] [PubMed] [Google Scholar]
- Carver, T. E., R. E. Brantley Jr., E. W. Singleton, R. M. Arduini, M. L. Quillin, G. N. Jr Phillips, and J. S. Olson. 1992. A novel site-directed mutant of myoglobin with an unusually high O2 affinity and low autooxidation rate. J. Biol. Chem. 267:14443–14450. [PubMed] [Google Scholar]
- Dewilde, S., L. Kiger, T. Burmester, T. Hankeln, V. Baudin-Creuza, T. Aerts, M. C. Marden, R. Caubergs, and L. Moens. 2001. Biochemical characterization and ligand binding properties of neuroglobin, a novel member of the globin family. J. Biol. Chem. 276:38949–38955. [DOI] [PubMed] [Google Scholar]
- De Baere, I., M. F. Perutz, L. Kiger, M. C. Marden, and C. Poyart. 1994. Formation of two hydrogen bonds from the globin to the heme-linked oxygen molecule in Ascaris hemoglobin. Proc. Natl. Acad. Sci. USA. 91:1594–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson, R. E., and I. Geis. 1983. Hemoglobin: Structure, Function, Evolution, and Pathology. Benjamin/Cummings, Menlo Park, CA.
- Eich, R. F., T. Li, D. D. Lemon, D. H. Doherty, S. R. Curry, J. F. Aitken, A. J. Mathews, K. A. Johnson, R. D. Smith, G. N. Phillips Jr., and J. S. Olson. 1996. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry. 35:6976–6983. [DOI] [PubMed] [Google Scholar]
- Hamdane, D., L. Kiger, S. Dewilde, B. N. Green, A. Pesce, J. Uzan, T. Burmester, T. Hankeln, M. Bolognesi, L. Moens, and M. C. Marden. 2003. The redox state of the cell regulates the ligand binding affinity of human neuroglobin and cytoglobin. J. Biol. Chem. 278:51713–51721. [DOI] [PubMed] [Google Scholar]
- Hankeln, T., V. Jaenicke, L. Kiger, S. Dewilde, G. Ungerechts, M. Schmidt, J. Urban, M. C. Marden, L. Moens, and T. Burmester. 2002. Characterization of Drosophila hemoglobin. Evidence for hemoglobin-mediated respiration in insects. J. Biol. Chem. 277:29012–29017. [DOI] [PubMed] [Google Scholar]
- Hayashi, A., T. Suzuki, and M. Shin. 1973. An enzymic reduction system for metmyoglobin and methemoglobin, and its application to functional studies of oxygen carriers. Biochim. Biophys. Acta. 310:309–316. [DOI] [PubMed] [Google Scholar]
- Kriegl, J. M., A. J. Bhattacharyya, K. Nienhaus, P. O. Deng, O. Minkow, and G. U. Nienhaus. 2002. Ligand binding and protein dynamics in neuroglobin. Proc. Natl. Acad. Sci. USA. 99:7992–7997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesce, A., S. Dewilde, M. Nardini, L. Moens, P. Ascenzi, T. Hankeln, T. Burmester, and M. Bolognesi. 2003. Human brain neuroglobin structure reveals a distinct mode of controlling oxygen affinity. Structure. 11:1087–1095. [DOI] [PubMed] [Google Scholar]
- Thorsteinsson, M. V., D. R. Bevan, R. E. Ebel, R. E. Weber, and M. Potts. 1996. Spectroscopical and functional characterization of the hemoglobin of Nostoc commune (UTEX 584 (Cyanobacterial)). Biochim. Biophys. Acta. 1292:133–139. [DOI] [PubMed] [Google Scholar]
- Trent, J. T., 3rd, A. N. Hvitved, and M. S. Hargrove. 2001a. A model for ligand binding to hexacoordinate hemoglobins. Biochemistry. 40:6155–6163. [DOI] [PubMed] [Google Scholar]
- Trent, J. T., 3rd, R. A. Watts, and M. S. Hargrove. 2001b. Human neuroglobin, a hexacoordinate hemoglobin that reversibly binds oxygen. J. Biol. Chem. 276:30106–30110. [DOI] [PubMed] [Google Scholar]
- Trent, J. T.,3rd, and M. S. Hargrove. 2002. A ubiquitously expressed human hexacoordinate hemoglobin. J. Biol. Chem. 277:19538–19545. [DOI] [PubMed] [Google Scholar]
- Trevaskis, B., R. A. Watts, C. R. Andersson, D. Llewellyn, M. S. Hargrove, J. S. Olson, E. S. Dennis, and W. J. Peacock. 1997. Two hemoglobin genes in Arabidopsis thaliana: the evolutionary origins of leghemoglobins. Proc. Natl. Acad. Sci. USA. 94:12230–12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn, C.C., and R. W. Carrell. 1974. Studies of hemoglobin denaturation and Heinz body formation in the unstable hemoglobins. J. Clin. Invest. 54:678–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg, B. A., and J. B. Wittenberg. 1990. Mechanisms of cytoplasmic hemoglobin and myoglobin function. Annu. Rev. Physiol. 19:217–241. [DOI] [PubMed] [Google Scholar]