Abstract
The Na+-Ca2+ exchanger is a plasma membrane protein expressed at high levels in cardiomyocytes. It extrudes 1 Ca2+ for 3 Na+ ions entering the cell, regulating intracellular Ca2+ levels and thereby contractility. Na+-Ca2+ exchanger activity is regulated by intracellular Ca2+, which binds to a region (amino acids 371–508) within the large cytoplasmic loop between transmembrane segments 5 and 6. Regulatory Ca2+ activates the exchanger and removes Na+-dependent inactivation. The physiological role of intracellular Ca2+ regulation of the exchanger is not yet established. Yellow (YFP) and cyan (CFP) fluorescent proteins were linked to the NH2- and CO2H-termini of the exchanger Ca2+ binding domain (CBD) to generate a construct (YFP-CBD-CFP) capable of responding to changes in intracellular Ca2+ concentrations by FRET efficiency measurements. The two fluorophores linked to the CBD are sufficiently close to generate FRET. FRET efficiency was reduced with increasing Ca2+ concentrations. Titrations of Ca2+ concentration versus FRET efficiency indicate a KD for Ca2+ of ∼140 nM, which increased to ∼400 nM in the presence of 1 mM Mg2+. Expression of YFP-CBD-CFP in myocytes, generated changes in FRET associated with contraction, suggesting that NCX is regulated by Ca2+ on a beat-to-beat basis during excitation-contraction coupling.
INTRODUCTION
Cytosolic Ca2+ is a ubiquitous regulator that controls cellular processes by setting the activity of many proteins. Among these proteins is the Na+-Ca2+ exchanger (NCX). NCX is a plasma membrane protein particularly abundant in myocardium that uses the energy of the transmembrane Na+ gradient to extrude Ca2+ from the cell after each contraction (Philipson and Nicoll, 2000). During each reaction cycle, NCX transports three Na+ ions into the cell to extrude one Ca2+ ion. By pumping Ca2+ out of the cell, the NCX protein plays an important role in cardiac Ca2+ homeostasis and contractility.
Structural studies show that NCX has nine transmembrane segments with a large intracellular loop located between transmembrane segments 5 and 6 (Nicoll et al., 1999). This large intracellular loop has multiple regulatory regions. In particular, residues 371–508 form a regulatory Ca2+ binding site (Levitsky et al., 1994). Ca2+ bound to this site is not transported but activates exchange activity and removes a Na+-dependent inactivation process (Hilgemann et al., 1992b; Levitsky et al., 1994). The exact Ca2+ concentration dependence of the Ca2+ regulatory effect is uncertain.
Electrophysiological approaches have determined apparent affinity values ranging from 22 nM (whole cell measurements) to 400 nM (giant excised patches) (Hilgemann et al., 1992a). These conflicting results affect interpretation of the physiological role of Ca2+ regulation in cardiac cells. The cytoplasmic Ca2+ concentration in a resting cardiac myocyte is ∼100 nM. Thus, with an apparent KD of 22 nM, the Ca2+ binding site of the exchanger would be saturated at resting conditions, thereby excluding the possibility of a dynamic tuning effect of Ca2+ on the exchanger. In contrast, the lower apparent affinity constant of 400 nM would enable increases in cytoplasmic Ca2+ to up-regulate the exchanger during the excitation-contraction coupling cycle.
To characterize the Ca2+ dependency of regulation of the exchanger, we isolated the Ca2+ binding site of the exchanger and determined Ca2+ sensitivity both in vivo and in vitro. To this end, we placed amino acid residues 371–508 (Ca2+ binding domain, CBD) between two fluorescent proteins (the yellow YFP and cyan CFP variants of green fluorescent protein) to produce the protein YFP-CBD-CFP. Upon excitation, YFP and CFP act in a concerted manner to give fluorescent resonance energy transfer or FRET. FRET is the nonradiative transfer of energy from a fluorescent donor to another excitable moiety; FRET is well established as a technique to determine protein-protein interactions and intramolecular conformational changes (Stryer, 1978). The efficiency of this reaction is strongly dependent on the distance between the two fluorophores: a small variation in distance can cause large changes in FRET efficiency (Stryer, 1978).
Since the exchanger Ca2+ binding domain has been shown to undergo major Ca2+-induced conformational changes (Levitsky et al., 1994), we predicted that upon binding Ca2+, YFP-CBD-CFP would alter conformation causing a change in the distance or angle between the two fluorophores and therefore a change in FRET efficiency.
We show that increasing intracellular Ca2+ reduces FRET efficiency suggesting a relaxation of the peptide. Finally, we also demonstrate that YFP-CBD-CFP can sense changes in intracellular Ca2+ within physiological concentration ranges found in actively contracting neonatal myocytes. These results strongly support a physiological role of secondary regulation of the exchanger by Ca2+.
Our results suggest that it is possible to determine a physiologically important function by examining a protein “module” in isolation from its parent: in this case, the core Ca2+ binding region of the Na+-Ca2+ exchanger.
METHODS
Molecular biology
The CBD of NCX was placed between the donor moiety, cyan fluorescent protein, and the acceptor moiety, yellow fluorescent protein. pEYFP C-1 and pECFP N-1 (Clontech, Palo Alto, CA) were ligated together; an NheI/EcoRI fragment (768 bp) from pEYFP C-1 was inserted into NheI/EcoR1 digested pECFP N-1 to generate pYFP-CFP. The Ca2+ binding domain of the exchanger, amino acid residues 371–508, was amplified with two primers such that the 5′ primer incorporated an XhoI restriction site and five codons encoding glycine residues before residue 371 (underlined) (AGATCTCGAGGCGGCGGCGGCGGCAGTAAGATCTT CTTTGAACAA) and the 3′ primer incorporated five codons encoding glycine residues and an EcoR1 restriction site after residue 508 (underlined) (GCTGGCATCTTTACTTTTGAGGAGGGAGGAGGAGGAGGAATTCTGCA). The PCR product was subcloned into pYFP-CFP using XhoI and EcoRI restriction enzymes. The final construct YFP-CBD-CFP was confirmed by sequencing.
Cell culture
HEK293, Hela cells, and rat neonatal cardiac myocytes were grown in 35 mm glass bottom Petri dishes in standard culture medium. Rat neonatal myocytes were prepared as described previously (Mitra and Morad, 1985); 24 h after isolation cardiac myocytes were deprived of serum. Petri dishes were pretreated with either fibronectin (Sigma Chemical, St. Louis, MO) or Cell-Tak (Becton Dickinson Labware, Franklin Lakes, NJ). Transfection of cDNAs was accomplished using GenePORTER (GFTinc., San Diego, CA).
FRET measurements
Data were acquired using a Nikon Eclipse TE300 microscope equipped with a mercury lamp (Nikon, A-G Heinze, Lake Forrest, CA), a 40× oil immersion lens (N.A. 1.2) and the following filter cubes (nm): 1. CFP cube (CFPF): excitation (EX) 436 ± 20, emission (EM) 480 ± 40, dichroic 455 (longpass, DCLP); 2. YFP cube (YFPF): EX 500 ± 20, EM 535 ± 30, DCLP 515; FRET cube (FRETF): EX 436 ± 20, EM 535 ± 30, DCLP 455 (Chroma Technologies, Rockingham, VT). Alternatively, LEDs (Light Emitting Diodes, Lumileds; San Jose, CA) were used as light sources, two emitting at 455 ± 20 nm (royal blue) and one emitting at 505 ± 15 nm (cyan). The royal blue lights were placed in front of the CFP and FRET filter, whereas the cyan light was located in front of the YFP filter; LEDs were controlled by the camera driven shutter. This setup eliminated the fluctuations typically associated with a mercury lamp. Experiments were performed at room temperature.
Steady-state FRET
Sequential images were captured with a SPOTII digital camera (Diagnostic, Sterling Heights, MI). Control experiments confirm that correction factors values for donor (RD) and acceptor (RA) (measured as described in the results) were independent of level of expression. RA and RD were found to be 0.54 ± 0.01 (n = 12) and 0.73 ± 0.01 (n = 15), respectively. These correction factors were utilized to determine the true FRET signal indicated as FRETC and measured as follows: FRETC = FRETF − RD × CFPF − RA × YFPF, where FRETF, CFPF, and YFPF indicate the type of filter with which the light was acquired from a cell expressing both fluorophores. Cells were illuminated using the mercury lamp. Images were analyzed with the National Institutes of Health Scion Image Program and background was subtracted before FRET calculations. Before the experiment, external medium was replaced with PBS.
FRET efficiency was quantified using the acceptor photobleaching approach (Stryer, 1978; Llopis et al., 2000). For these measurements, wide-field illumination was obtained using the LED lights. YFP photobleaching was completed after ∼15 min of constant illumination (YFPF). CFP emission images were acquired before and after YFP photobleaching using the CFPF. Images were analyzed by quantifying the amount of fluorescence inside the cell. These values were used to determined FRET efficiency (E), calculated as follow: E = 1−ICFPb/ICFPa, where I is the intensity of CFP emission before (CFPb) and after (CFPa) YFP photobleaching.
Control cells were maintained in the presence of PBS, whereas cells in 0 Ca2+ were incubated in 160 mM MOPS, 1 mM EGTA, 5 μM ionomycin pH 7.4 (adjusted with KOH) for ∼10 min prior experiment.
Real time FRET
Measurements were performed using the MultiSpec Micro-Imager system from Optical Insight (Santa Fe, NM) that allows monitoring of CFP and YFP fluorescence emissions simultaneously. The ratio between YFP and CFP emission is then calculated as an indicator of FRET efficiency. The MultiSpec Micro-Imager is directly attached to the microscope and is equipped with a 505 nm long-pass dichroic filter used to separate the CFP signal from the YFP signal, a CFP emission filter (480 ± 30 nm) and an YFP emission filter (535 ± 40 nm). The excitation filter was 436 ± 20 nm. Images were captured with a fast high-resolution digital camera (CoolSNAP-fx 12bit Cooled Monochrome Digital Camera, Roper Scientific, Tucson, AZ) and acquired in a PC using the MetaFluor6.1 Fluorescence ratio imaging system (Universal Imaging Corporation, Downingtown, PA). For all experiments only cells with nonsaturating levels of fluorescence are used. Cells were maintained in Tyrode's solution supplemented with 1.8 mM CaCl2 (pH = 7.4).
In vitro FRET measurements
HEK293 cells were cultured in 100 × 20 mm regular culture dishes and transfected with 5 μg DNA. Two to 3 days after transfection, cells were rinsed once with a solution containing 160 mM MOPS, 1 mM EGTA, adjusted to pH 7.2 with KOH and then solubilized using 0.5% Triton X-100, dissolved in 2 ml of the same buffer. After centrifugation, the supernatant was collected and placed in a quartz cuvette mounted in a spectrofluorometer (Photon Technology International, Model C-44/2000, Lawrenceville, NJ). CFP and YFP emission spectra were obtained by exciting at 434 nm and measuring emission between 460–600 nm. Both excitation and emission slits were set at 4 nm.
For ratio measurements, samples were excited at 434 nm and emission was measured at 472 and 524 nm. CaCl2 was added directly to the cuvette equipped with a magnetic stir bar. After addition of Ca2+, a shift of 0.01–0.02 pH units was measured at the end of the experiment. Control experiments using cytoplasmic CFP and YFP show that this change in pH did not affect the spectra of the fluorophores and did not significantly alter the affinity of EGTA for Ca2+, since the replacement of EGTA with 1 mM BAPTA, a less pH sensitive calcium buffer, did not modify the results (not shown).
Free [Ca2+] was calculated using the MAXC program.
RESULTS
Previous studies have demonstrated that the Na+-Ca2+ exchanger has a Ca2+ regulatory binding site, which activates the exchanger (DiPolo, 1979; Hilgemann, 1992a). Biochemical evidence localized this regulatory site to lie within the large cytoplasmic loop of the exchanger, specifically between residues 371 and 508 (Levitsky et al., 1994). This region was shown to undergo substantial change in conformation upon the binding of Ca2+, as indicated by a shift in electrophoretic mobility during SDS-PAGE in the presence or absence of Ca2+ (Levitsky et al., 1994). These results provided the rational for creating a system to noninvasively monitor these movements in vivo and to determine the range of Ca2+ concentrations required to produce conformational changes. To achieve this goal, we constructed a peptide consisting of the CBD of the exchanger (amino acid residues 371–508) with the NH2- and CO2H-termini linked to YFP and CFP (YFP-CBD-CFP) (Fig. 1 A), respectively. This construct is analogous to a well characterized Ca2+-indicator protein consisting of CFP-calmodulin-calmodulin binding peptide-YFP, known as Cameleon (Miyawaki et al., 1997). Binding of Ca2+ to the Cameleon produces an increase in FRET efficiency indicating a shift in the protein to a more compact state in the presence of Ca2+.
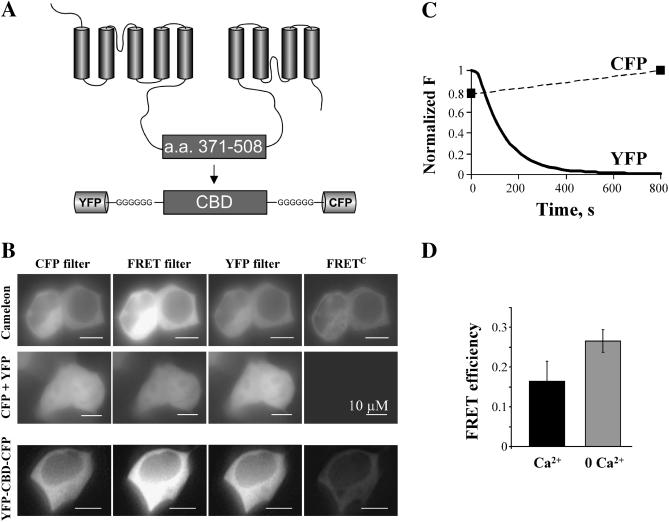
FIGURE 1.
FRET determination using the three cubes and the acceptor photobleaching techniques. (A) Schematic representation of the Na+-Ca2+ exchanger topology and the YFP-CBD-CFP construct. (B) An example of assessment of FRET using the three cubes method for the following cytoplasmic constructs: cameleon, a well-characterized construct generating Ca2+-dependent FRET (Miyawaki et al., 1997); CFP and YFP coexpressed; and the exchanger Ca2+ binding domain linked to YFP and CFP (YFP-CBD-CFP). The images were acquired with the CFP, YFP, and FRET filters and FRETC was calculated as described in the text. Notice the lack of FRET in cells expressing cytoplasmic CFP and YFP. (C) Example of increase in the CFP emission after YFP photobleaching in a cell expressing YFP-CBD-CFP. CFP images were acquired immediately before and after YFP photobleaching. After YFP photobleaching, achieved with maximal excitation at 500 nm for 800 s, CFP emission increased by 22%, demonstrating the presence of FRET. (D) FRET efficiency was determined using the increase in CFP emission (see Methods). Cells incubated with 5 μM ionomycin in the presence of 1 mM EGTA (0 Ca2+) show an increase in FRET efficiency, as compared to untreated cells (Ca2+ ∼100 nM). FRET efficiency values are 0.27 ± 0.03 (n = 7) and 0.16 ± 0.05 (n = 3) (p < 0.05), respectively. The result indicates that low Ca2+ favors FRET.
Detection of FRET from YFP-CBD-CFP
To determine if the fluorophores linked to the CBD were sufficiently close to generate FRET, we expressed YFP-CBD-CFP in mammalian cells and assessed FRET by using both the three cubes method and the acceptor photobleaching technique (Stryer, 1978; Sorkin et al., 2000; Erickson et al., 2001). Fig. 1 B shows HEK293 cells expressing YFP-CBD-CFP and the corresponding corrected FRET (FRETC) examined with the three cubes method. Briefly, FRETC was obtained by using three filters indicated as CFP, YFP, and FRET. The CFP and YFP filters allow excitation and detection of light from the CFP and YFP fluorophores, respectively. The FRET filter is a hybrid filter, which permits excitation of the donor (CFP) and detection of light from the acceptor (YFP). Ideally, any signal detected through the FRET filter would originate from transfer of energy from CFP to YFP and therefore represent FRET. However, the emission spectra of CFP and YFP partially overlap, and the emitted light measured through the FRET filter is contaminated by the fluorescence due to the direct excitation of both fluorophores. To dissect these components (Sorkin et al., 2000; Erickson et al., 2001) CFP and YFP were expressed separately and for each fluorophore, we divided the emission from the FRET filter by the emission measured with either the CFP (for CFP) or YFP (for YFP) filters. This correction factor (R) is independent of the amount of fluorescent protein and was utilized to determine the true FRET signal (FRETC; see Methods). As is shown in Fig. 1 B, we successfully detected FRET generated by both YFP-CBD-CFP and Cameleon. To corroborate our findings, we coexpressed cytoplasmic CFP and YFP in the same cells and, as expected, no FRET was detected.
FRET was also determined by acceptor photo bleaching (YFP). This method relies on the concept that when FRET occurs part of the energy of excited CFP is transferred to YFP resulting in decreased in CFP emission (Stryer, 1978). This energy transfer is eliminated by bleaching of YFP and leads to an increase in CFP emission. Fig. 1 C illustrates the time course of YFP photobleaching, obtained with a constant illumination of the cells at 500 ± 20 nm for ∼15 min, and the corresponding increase in CFP emission of YFP-CBD-CFP expressed in HEK293 cells. CFP and YFP values were normalized to their own maximum. After complete YFP bleaching, the CFP emission increased by 22%, indicative of FRET occurring between the two fluorophores. Photobleaching of YFP in cells coexpressing cytoplasmic CFP and YFP did not modify CFP emission (not shown).
The increase in CFP emission was used to determine the efficiency with which energy was transferred from CFP to YFP (FRET efficiency; see Methods), which is dependent on fluorophore proximity (Stryer, 1978). The average value of FRET efficiency obtained from cells expressing YFP-CBD-CFP and incubated in PBS was lower than that in cells treated with 5 μM ionomycin in the presence of 1 mM EGTA to chelate cytoplasmic Ca2+ (see lower panel, Fig. 1 D). The result indicates that cytoplasmic Ca2+ drives the exchanger Ca2+ binding domain into a conformation that decreases FRET.
The Ca2+ binding region of the exchanger senses physiological Ca2+ concentrations
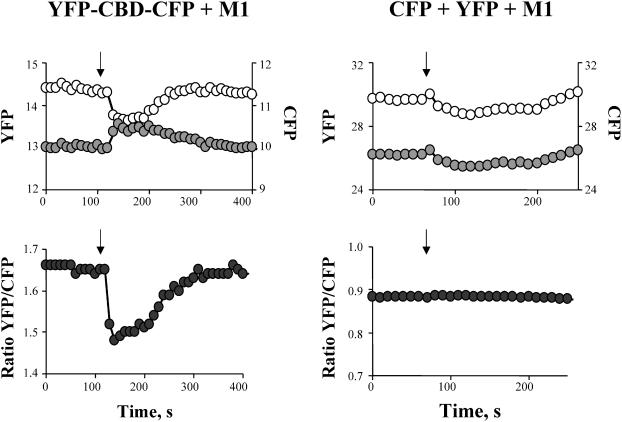
Since the three cubes and the acceptor photobleaching methods are only useful to determine FRET under steady-state conditions, we utilized a ratiometric approach to monitor changes in FRET during interventions. When FRET occurs, excitation of CFP causes YFP emission at the expense of CFP emission. Hence, the YFP/CFP fluorescence ratio is a measure of FRET efficiency. Fig. 2 shows how the CFP and YFP emission and the corresponding YFP/CFP ratio value changed upon addition of 10 μM carbachol to the external solution of HeLa cells expressing YFP-CBD-CFP and the muscarinic receptor M1. Activation of M1 by carbachol creates a transient increase in cytoplasmic Ca2+ (Felder, 1995), which led to a decrease in FRET. As shown in the right panel of Fig. 2, HeLa cells coexpressing cytoplasmic CFP, YFP, and M1 receptor do not show any change in the FRET ratio value, corroborating the specificity of the effect.
FIGURE 2.
Ca2+ decreases FRET: effect of changes in intracellular Ca2+ on CFP (open circles) and YFP (shaded circles) emissions (top) and corresponding YFP/CFP ratio (bottom), from HeLa cells expressing YFP-CBD-CFP (right) or CFP + YFP (left) in the presence of the muscarinic receptor M1. Cytoplasmic Ca2+ was increased by activation of the muscarinic receptor M1 with the agonist carbachol. The YFP/CFP ratio of cells expressing YFP-CBD-CFP significantly decreased with the induced rise in intracellular Ca2+ concentration, whereas the ratio from cells expressing cytoplasmic CFP and YFP remained constant. Arrows indicate the addition of 10 μM carbachol to the external Tyrode's solution.
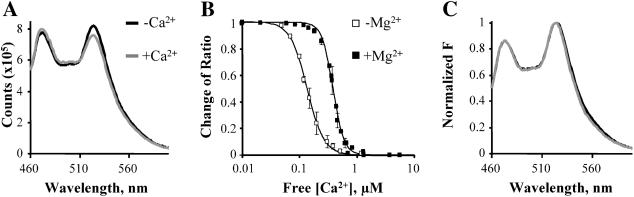
To determine the range of Ca2+ concentrations to which YFP-CBD-CFP responded, the construct was expressed in HEK293 cells and then used for in vitro studies. After detergent lysis of the cells in an EGTA-buffered, low Ca2+ solution, the FRET ratio of YFP-CBD-CFP was monitored in a spectrofluorimeter after additions of Ca2+. Fig. 3 A shows the emission spectra of solubilized YFP-CDB-CFP before and after addition of 1 μM Ca2+. Ca2+ induced a decrease in the FRET ratio value. The changes in FRET were plotted versus free Ca2+ concentration and the values were fit to the Hill function to determine the apparent dissociation constant (KD). As shown in Fig. 3 B, the fit of the averages from 8 different experiments gave a KD for Ca2+ of 140 nM and a Hill coefficient of 2.9. Since Mg2+ competes for Ca2+ at the regulatory site (Levitsky et al., 1994; Wei et al., 2002), we also measured the KD for Ca2+ in the presence of 1 mM Mg2+ (n = 4). The apparent Ca2+ affinity for FRET was shifted to 383 nM. However, Mg2+ by itself did not evoke any changes in FRET up to a concentration of 3 mM (not shown), perhaps suggesting that Mg2+ can interact with the Ca2+-binding site of the exchanger but is incapable of generating a detectable conformation change.
FIGURE 3.
Fluorescence properties of YFP-CBD-CFP in vitro. (A) Emission spectra of solubilized YFP-CBD-CFP (excited at 434 nm) in the absence or presence of 1 μM free Ca2+. Ca2+ addition induced a decrease in the YFP/CFP ratio without altering the spectral properties of YFP-CBD-CFP. (B) Changes in the emission ratio versus Ca2+ concentration. Each point represents the average of eight experiments. Emission ratios were normalized to the value obtained in the absence of Ca2+ and fit to the Hill function (1/1 + ([Ca2+]/KD)n), where KD is the dissociation constant and n the Hill coefficient. The Ca2+ titration curve has a KD of 140 nM and a Hill coefficient of 2.9. Addition of 1 mM Mg2+ (▪) induced a shift in the KD value to 383 nM. (C) Emission spectra of YFP-CBD-YFP (excited at 434 nm) before and after an eightfold dilution. Both spectra were normalized to emission at 514 nm.
GFP molecules can potentially form dimers (Tsien, 1998), and we investigated the possibility of intermolecular FRET induced by the dimerization of YFP-CBD-CFP. The efficiency of dimerization of adjacent molecules is dependent on protein concentration. Fig. 3 C shows that this is not the case, since the ratio between YFP/CFP was not affected by a eightfold dilution of YFP-CBD-CFP. The result indicates that FRET occurs exclusively between CFP and YFP of the same molecule and measures the intrinsic movements of the connecting moiety; i.e., it gives an indication of the movements of the Ca2+ binding domain of the Na+-Ca2+ exchanger.
FRET is altered by mutations known to reduce the Ca2+ affinity of the exchanger
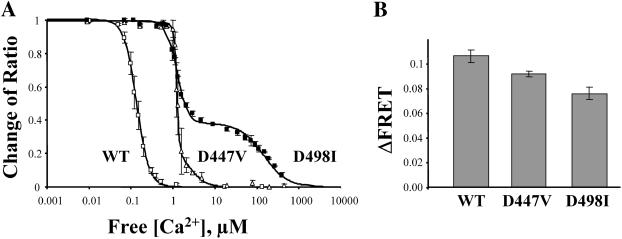
Previous studies have shown that point mutations (D447V and D498I) within the Ca2+ binding domain of the exchanger decreased Ca2+ affinity (Matsuoka et al., 1995). Using FRET, we measured the Ca2+ dependency of YFP-CBD-CFP with either D447 or D498 mutated. As shown in Fig. 4 A, mutation of the aspartate at position 447 to valine shifted the Ca2+ affinity. The response to Ca2+ was best fit with a linear combination of two Hill functions with apparent Ca2+ affinities of 1.2 and 2.5 μM and relative contributions of 76% and 24%, respectively. The mutation also decreased the total change in FRET (ΔFRET) by 13% compared to WT (Fig. 4 B). Replacement of aspartate 498 with an isoleucine also exposed two Ca2+ binding sites with apparent affinities of 1.2 and 130 μM (relative contributions were 59% and 41%, respectively (Fig. 4 A) and reduced the total change in FRET by 29% (Fig. 4 B).
FIGURE 4.
Mutation of aspartates 447 and 498 alters Ca2+ dependency of FRET signals. (A) Changes in FRET value versus free Ca2+ concentrations for WT and mutants D447V and D498I. Emission ratios were normalized to the value obtained in the absence of Ca2+ and fitted to a single Hill function for wild-type and to the sum of two Hill functions for mutants D447V and D498I. KD values are WT, 140 nM; D447V, 1.2 and 2.5 μM; and D498I, 1.2 and 130 μM. Each point is the mean of eight experiments for WT, five experiments for D497V, and six experiments for D498I. (B) The graph shows the total change in FRET signal (ΔFRET) for the WT and mutants D447V and D498I. Values are WT = 0.109 ± 0.005 (n = 8), D447V = 0.091 ± 0.003 (n = 5), and D498I = 0.075 ± 0.005 (n = 5). ΔF is the difference between the YFP/CFP value in 0 Ca2+ and YFP/CFP value in presence of high [Ca2+].
These data suggest that mutation of these residues not only decreases Ca2+ affinity but also partially hinders the rearrangement of the protein after the binding of Ca2+. The double mutant D447V-D498I showed a Ca2+ insensitive YFP/CFP ratio, although a full-length exchanger with this double mutation retains weak Ca2+ regulation (Levitsky et al., 1994; Matsuoka et al., 1995).
The exchanger Ca2+ binding domain undergoes conformational changes during excitation-contraction coupling
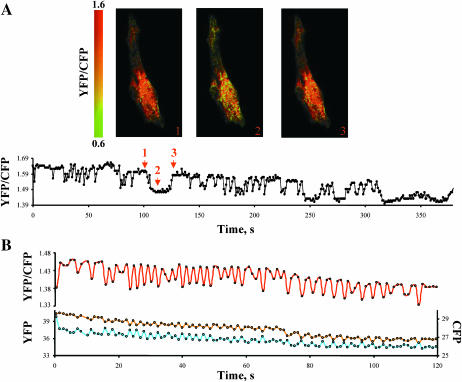
One objective of our study was to determine if the Ca2+ binding site of the Na+-Ca2+ exchanger could sense changes in cytoplasmic Ca2+ during each contraction cycle. To address this question, we expressed YFP-CBD-CFP in rat neonatal cardiac myocytes and monitored changes in FRET during spontaneous contractions. Three to four days after DNA transfection, media was replaced with regular Tyrodes solution and the spontaneous contractions of myocytes expressing YFP-CBD-CFP were monitored at room temperature. Fast acquisition of simultaneous images of CFP and YFP revealed a change in the YFP/CFP fluorescence ratio, which correlated with myocyte contraction. Consistent with our previous observations, the YFP/CFP fluorescence ratio was maximal during relaxation and decreased significantly during contractions when cytoplasmic Ca2+ was elevated (Fig. 5). Prolonged decreases in FRET correlated with protracted states of contractions (Fig. 5 A). Another example of a contracting cardiac myocyte is shown in Fig. 5 B. In this case the myocyte displayed fast twitches, which were associated with rapid changes in the FRET signal. The corresponding changes in both CFP and YFP emission are also shown for this myocyte and, as expected, opposite changes in YFP and CFP emission were associated with FRET oscillations. Similar results were observed in an additional 22 myocytes. The data indicate that the regulatory Ca2+ binding site of the exchanger responds to changes in Ca2+ that occur during excitation-contraction coupling. Control experiments monitoring the fluorescence of myocytes expressing cytoplasmic CFP and YFP or mutants D498I and D447V demonstrated no changes in FRET with contraction (data not shown).
FIGURE 5.
The Ca2+ binding site of the exchanger can sense changes in Ca2+ during excitation-contraction coupling. (A) An example of rat neonatal cardiomyocytes expressing YFP-CBD-CFP and the corresponding changes in YFP/CFP ratio during spontaneous activity of the myocytes. Arrows indicate the time when the images were acquired. Myocytes were kept in Tyrode's solution at room temperature and fluorescence was monitored each second. The vertical bar indicates the YFP/CFP ratio value in pseudocolor. The decrease in the ratio of YFP/CFP correlates with myocyte contraction (not shown). (B) Another example of changes in YFP/CFP ratio during spontaneous contractions of a different neonatal cardiac myocyte. In this case, the contractions were faster. The lower panel shows how the CFP and YFP emissions changed during time. Changes in YFP/CFP values were due to simultaneous and opposite oscillations in CFP and YFP emissions. Images were acquired every second.
DISCUSSION
The regulatory CBD of the Na+-Ca2+ exchanger consists of amino acid residues 371 to 508 (Levitsky et al., 1994). The affinity of the CBD for Ca2+ has been extensively studied using techniques such as 45Ca2+ overlay, radioisotopic flux, and electrophysiology. These techniques have characterized the general properties of Ca2+-induced regulation of the Na+-Ca2+ exchanger. Nevertheless, Ca2+ regulation is difficult to study in intact myocytes, and an accurate in vivo value for Ca2+ affinity has proven to be elusive with values ranging from 20–400 nM (Miura and Kimura, 1989; Hilgemann et al., 1992a; Reeves and Condrescu, 2003). Problems have included experimental limitations such as difficulties in controlling intracellular Ca2+ and measuring exchanger activity, lack of specific inhibitors of Na+-Ca2+ exchange, and partial overlap of the affinity curves for transported and regulatory Ca2+. In our study, this issue was addressed using a noninvasive optical approach to determine the range of cytoplasmic Ca2+ needed to activate the Na+-Ca2+ exchanger in living cells. By linking YFP and CFP fluorophores to the NH2- and CO2H-termini of the CBD of the exchanger, conformational changes of the protein were translated into changes in FRET, enabling the selective study of CBD in vitro and in vivo in contracting myocytes.
First, we determined the presence of FRET between the two fluorophores linked to the CBD by using two well established independent techniques: the three cubes method (Sorkin et al., 2000; Erickson et al., 2001) and acceptor photobleaching (Llopis et al., 2000). Both techniques demonstrated that YFP-CBD-CFP expressed in mammalian cells generates FRET. To determine if FRET changed with Ca2+, we coexpressed YFP-CBD-CFP with the muscarinic receptor M1. Activation of M1 leads to release of Ca2+ from internal stores. Addition of carbachol, a M1 agonist, caused a rapid decrease in FRET; this reduction was transient, as Ca2+ is resequestered into the endoplasmic reticulum. These data indicate that the Ca2+ binding site of the Na+-Ca2+ exchanger can sense variations in cytoplasmic Ca2+ and that binding of Ca2+ induces a change in the conformation of the CBD. The Ca2+-dependent decrease in FRET could potentially be due to a conformational change of the CBD moving the CFP and YFP further apart or changing the orientation of the two fluorophores with respect to one another. A combination of these two mechanisms may also be occurring. Our results cannot discriminate between these possibilities. However, we favor the first interpretation since both CFP and YFP were linked to the Ca2+ binding domain by a series of glycines that likely confers flexibility to the orientation of the fluorophores. Interestingly, YFP-CBD-CFP shows a reduction in FRET upon Ca2+ binding, a behavior opposite to that observed with Cameleon, a fluorescent Ca2+ indicator that increases FRET efficiency upon binding Ca2+.
To determine the sensitivity of the regulatory Ca2+ binding site for Ca2+, we performed in vitro measurements. The FRET efficiency of expressed YFP-CBD-CFP from solubilized cells was assessed as a function of Ca2+ concentration. The Ca2+ needed to cause a 50% reduction in FRET was ∼140 nM in the absence of Mg2+ and ∼400 nM in the presence of 1 mM Mg2+. The rightward shift of the FRET Ca2+ affinity in the presence of Mg2+ corroborates previous studies (Levitsky et al., 1994; Wei et al., 2002) showing competition between the two ions for the Ca2+ regulatory site.
In sum, these results imply that a majority of Na+-Ca2+ exchangers are in an inactivated state at resting Ca2+ concentrations (∼100 nM) and that small increases in cytoplasmic Ca2+ content could have a large effect on Na+-Ca2+ exchanger activity, assuming that the Ca2+ affinity of the CBD reflects that of the intact exchanger. Activation of the exchanger would be maximal by ∼1 μM internal Ca2+.
Two clusters of aspartate residues located within the exchanger Ca2+ binding domain have previously been shown to be important for binding. Point mutations at either of these sites alter Ca2+ affinity as measured biochemically by 45Ca2+ overlay (Levitsky et al., 1994) and functionally by the giant excised patch technique (Matsuoka et al., 1995). Mutation of residues D447 or D498 lowered the apparent affinity for Ca2+ from 0.4 to 1.8 and 1.4 μM, respectively (Matsuoka et al., 1995). These same mutations also cause a rightward shift of the Ca2+ dependency when studied with the 45Ca2+ overlay technique (Levitsky et al., 1994). We investigated the changes in FRET associated with Ca2+ binding for the mutants D447V, D498I, and the double mutant D447V/D498I. Our data show that replacement of aspartate 447 with a valine altered the Ca2+ binding curve and revealed the presence of low and high affinity sites with KD values of 1.2 and 2.4 μM, respectively. The biphasic form of the Ca2+ titration was more accentuated by the replacement of aspartate 498 with valine since this mutation produced a drastic rightward shift of the low affinity site (KD >100 μM). The presence of a biphasic response of FRET to Ca2+ could represent the equilibrium of the protein between multiple states with different FRET efficiencies. In addition to alter Ca2+ affinity, significant reductions (10–30%) in ΔFRET was also observed with both mutants. Finally, a complete lack of change in FRET was observed when the two mutations were combined. The findings corroborate the importance of these residues in Ca2+ regulation of the exchanger.
To determine if activation of the Na+-Ca2+ exchanger by the binding of regulatory Ca2+ could occur during excitation-contraction coupling, we expressed YFP-CBD-CFP in neonatal cardiac myocytes and measured the change in FRET upon contraction. Our data clearly show oscillations in the YFP/CFP fluorescence ratio associated with myocyte contraction. FRET was maximal during relaxation and decreased during contraction. The changes in fluorescence were fast enough to track each contraction of the myocyte. The changes in FRET were not motion artifacts, as FRET changes were not detected when mutants with reduced Ca2+ affinity (D447V and D498I) were expressed in the myocytes.
In summary, the results demonstrate that the Ca2+ regulatory site of the Na+-Ca2+ exchanger can sense changes in Ca2+ within the physiological range and that the rearrangement of the protein upon the binding of Ca2+ is fast enough to follow the rapid variations in cytoplasmic Ca2+ that occur during excitation-contraction coupling. The same Ca2+-induced conformational changes that cause changes in FRET presumably also lead to activation of exchange activity. A caveat to our conclusions results from the fact that the data were obtained using a soluble form of the CBD that may not have identical characteristics to the Ca2+ binding site on the full length, intact exchanger protein. Also, Ca2+ levels in the subsarcolemmal environment where the CBD would normally be located may be different from those in the bulk cytoplasm. Further work to investigate these issues is underway.
Acknowledgments
This work was supported by Beginning Grant-In-Aid (0365104Y) from the American Heart Association Western States Affiliate (M.O.), by National Institutes of Health grant HL49101 (K.D.P.), and by the Laubish Fund (S.J.).
References
- DiPolo, R. 1979. Calcium influx in internally dialyzed squid giant axons. J. Gen. Physiol. 73:91–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson, M. G., B. A. Alseikhan, B. Z. Peterson, and D. T. Yue. 2001. Preassociation of calmodulin with voltage-gated Ca2+ channels revealed by FRET in single living cells. Neuron. 31:973–985. [DOI] [PubMed] [Google Scholar]
- Felder, C. C. 1995. Muscarinic acetylcholine receptors: signal transduction through multiple effectors. FASEB J. 9:619–625. [PubMed] [Google Scholar]
- Hilgemann, D. W., A. Collins, and S. Matsuoka. 1992a. Steady-state and dynamic properties of cardiac sodium-calcium exchange. Secondary modulation by cytoplasmic calcium and ATP. J. Gen. Physiol. 100:933–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann, D. W., S. Matsuoka, G. A. Nagel, and A. Collins. 1992b. Steady-state and dynamic properties of cardiac sodium-calcium exchange. Sodium-dependent inactivation. J. Gen. Physiol. 100:905–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitsky, D. O., D. A. Nicoll, and K. D. Philipson. 1994. Identification of the high affinity Ca2+-binding domain of the cardiac Na+-Ca2+ exchanger. J. Biol. Chem. 269:22847–22852. [PubMed] [Google Scholar]
- Llopis, J., S. Westin, M. Ricote, Z. Wang, C. Y. Cho, R. Kurokawa, T. M. Mullen, D. W. Rose, M. G. Rosenfeld, R. Y. Tsien, C. K. Glass, and J. Wang. 2000. Ligand-dependent interactions of coactivators steroid receptor coactivator-1 and peroxisome proliferator-activated receptor binding protein with nuclear hormone receptors can be imaged in live cells and are required for transcription. Proc. Natl. Acad. Sci. USA. 97:4363–4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka, S., D. A. Nicoll, L. V. Hryshko, D. O. Levitsky, J. N. Weiss, and K. D. Philipson. 1995. Regulation of the cardiac Na+-Ca2+ exchanger by Ca2+. Mutational analysis of the Ca2+-binding domain. J. Gen. Physiol. 105:403–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra, R., and M. Morad. 1985. A uniform enzymatic method for dissociation of myocytes from hearts and stomachs of vertebrates. Am. J. Physiol. 249:H1056–H1060. [DOI] [PubMed] [Google Scholar]
- Miura, Y., and J. Kimura. 1989. Sodium-calcium exchange current. Dependence on internal Ca and Na and competitive binding of external Na and Ca. J. Gen. Physiol. 93:1129–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki, A., J. Llopis, R. Heim, J. M. McCaffery, J. A. Adams, M. Ikura, and R. Y. Tsien. 1997. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 388:882–887. [DOI] [PubMed] [Google Scholar]
- Nicoll, D. A., M. Ottolia, L. Lu, Y. Lu, and K. D. Philipson. 1999. A new topological model of the cardiac sarcolemmal Na+-Ca2+ exchanger. J. Biol. Chem. 274:910–917. [DOI] [PubMed] [Google Scholar]
- Philipson, K. D., and D. A. Nicoll. 2000. Sodium-calcium exchange: a molecular perspective. Annu. Rev. Physiol. 62:111–133. [DOI] [PubMed] [Google Scholar]
- Reeves, J. P., and M. Condrescu. 2003. Allosteric activation of sodium-calcium exchange activity by calcium: persistence at low calcium concentrations. J. Gen. Physiol. 122:621–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin, A., M. McClure, F. Huang, and R. Carter. 2000. Interaction of EGF receptor and grb2 in living cells visualized by fluorescence resonance energy transfer (FRET) microscopy. Curr. Biol. 10:1395–1398. [DOI] [PubMed] [Google Scholar]
- Stryer, L. 1978. Fluorescence energy transfer as a spectroscopic ruler. Annu. Rev. Biochem. 47:819–846. [DOI] [PubMed] [Google Scholar]
- Tsien, R. Y. 1998. The green fluorescent protein. Annu. Rev. Biochem. 67:509–544. [DOI] [PubMed] [Google Scholar]
- Wei, S. K., J. F. Quigley, S. U. Hanlon, B. O'Rourke, and M. C. Haigney. 2002. Cytosolic free magnesium modulates Na/Ca exchange currents in pig myocytes. Cardiovasc. Res. 53:334–340. [DOI] [PubMed] [Google Scholar]