Abstract
Purified M2 protein from the Udorn strain of influenza virus was reconstituted into planar lipid bilayers from liposomes. In 1 mM HCl, the single-channel conductance was measured as 6 pS with open probability of ≤0.03. The current voltage curve is linear over the achievable voltage range. The current amplitude is amantadine sensitive. In HCl solutions, the single-channel current was essentially invariant with changes in [Cl−], [Na+], and [tetraethylammonium] ([TEA+]), but dependent on [H+]. The reversal potential, determined with asymmetrical hydrogen chloride solution, is very close to the equilibrium potential of hydrogen. This appears to be the first report of single-channel proton currents with the full-length M2 protein.
INTRODUCTION
The influenza A virus M2 protein is a small (97-amino acid) transmembrane helix protein that tetramerizes (Sugrue and Hay, 1991; Sakaguchi et al., 1997) to pass protons into virus particles. Upon endocytosis of the virus, the low pH environment of the endosome activates the channel, allowing acidification of the virus, destabilization of matrix protein, and release of viral RNA (Hay, 1992; Grambas and Hay, 1992). In some species, the virus also protects nascent hemagglutinin in the Golgi apparatus from acid degradation by forming proton channels in Golgi membranes (Grambas and Hay, 1992; Hay, 1992).
Wild-type M2 protein was reported to form erratic single channels in planar bilayers when reconstituted from dissolved protein (Tosteson et al., 1994). Since then, considerable progress has been made with electrophysiological, neutron diffraction, infrared absorption, and circular dichroism studies of the protein function in liposomes and cellular expression systems. In CV-1 cells, Xenopus oocytes, and mouse erythroleukemia cells, amantadine-sensitive currents have been shown to be acid- (but not voltage-) gated and highly selective for H+ over Na+ (Chizhmakov et al., 1996; Mould et al., 2000a,b; Chizhmakov et al., 2003; Shimbo et al., 1996; Wang et al., 1993). Acid activation appears to be associated with protonation of His-37 (Wang et al., 1995), which projects into the channel and appears to form the selectivity filter for the channel (Wang et al., 1995). From infrared spectroscopic measurements, mutational, structural, and molecular dynamics simulations, there have been two major hypotheses suggested for the proton transport mechanism (Pinto et al., 1997; Mould et al., 2000a.; Okada et al., 2001; Tang et al., 2002), a His-shutter and a His-shuttle mechanism. A simple variant mechanism combines both notions: the His tetrad forms a gate that opens slightly as a proton approaches, just enough to pass a proton but not other ions (Smondyrev and Voth, 2002). Amantadine and rimantidine block the M2 channel (e.g., Wang et al., 1993; Chizhmakov et al., 1996) but it is not yet clear whether the mechanism is intrachannel block (Duff et al., 1994; Astrahan et al., 2004) or allosteric block (Pinto and Lamb, 1995). Trp-41 appears to be involved in the acid-gating mechanism (Tang et al., 2002). However, no reports of single-channel current measurements with the intact protein have appeared since the initial report of Tosteson et al. (1994).
The pore is formed by a four-helix bundle, as determined from the pattern of disulfide linked dimers formed with Cys mutants in the transmembrane region (Bauer et al., 1999). The transmembrane portion of the protein, residues 22–46 (TMP), also forms channels at pH 2.3 (Duff and Ashley, 1992) and has recently been studied by solid-state NMR, where it has a helical tilt of 32–38° with respect to the lipid bilayer normal (Kovacs and Cross, 1997; Song et al., 2000; Wang et al., 2000, 2001; Nishimura et al., 2002). This tilt angle is somewhat larger than was used in structural models during earlier molecular modeling studies (Kukol et al., 1999; Pinto et al., 1997; Zhong et al., 1998; Forrest et al., 2000; and Schweighofer and Pohorille, 2000). However, the lipid bilayer thickness dependence of the intact-protein tetramerization constant (Cristian et al., 2003) and solid-state NMR studies (Tian et al., 2002, 2003) suggest that the tilt angle might be smaller for the intact protein.
Determination of the single-channel proton conductance of M2 protein has been a difficult task because single-channel proton conductance can only be observed at significant H+ concentration, i.e., at low pH. Thus the reconstitution system must utilize lipid conditions that can withstand low pH without disruption of the pH-sensitive (i.e., gated) protein structure. Conditions have now been identified that appear to fulfill these requirements.
Our ultimate goal is to identify reconstitution conditions that yield a homogeneous structural population, to assay the functional activity, and to characterize the channel behaviors at the single-channel level to allow correlations of channel function with structure. Here we report experiments with a modified Udorn construct, which was purified and reconstituted in a mixture of dimyristoyl phosphatidylcholine (DMPC) and dimyristoyl phosphatidylglycerol (DMPG) liposomes. This protein produces single-channel activity after spontaneous fusion of lipid vesicles into phospholipid bilayers, at low pH. The unitary currents and proton selectivity in HCl solution are consistent with observations of the physiological protein conductance mentioned above. Preliminary reports of these results have appeared (Vijayvergiya et al., 2003, 2004).
MATERIALS AND METHODS
Preparation and expression of M2 protein
M2 was expressed and purified using previously published methods (Tian et al., 2002). Briefly, the M2 protein, Udorn variety with a six-His tag at the C-terminus and serine substitutions for C19 and C50 was expressed in BL21 (DE3) cells using the PET 39 plasmid and purified from exclusion bodies with a Ni affinity column. The resulting solution showed a single band in a sodium dodecylsulphate gel. Protein concentration was determined using the bicinchoninic acid method. The protein was reconstituted into DMPC and DMPG lipids (4:1 molar ratio; Avanti Polar Lipids, Alabaster, AL) at a 1:5 protein/lipid (w/w) ratio using 1% n-octyl β-D-glucopyranoside, and then dialyzed three times. The dialyzed sample was centrifuged and resuspended as liposomes in aqueous solution.
Preparation of lipids and solutions
Lipid bilayers were composed of a mixture of phosphatidylethanolamine, phosphatidylcholine, phosphatidylserine (all from brain), and cholesterol (molar ratio, 4:1:1:2, dispersed in n-decane) (Avanti Polar Lipids), which is referred to below as 4112. Chloroform solutions of the lipids were first mixed, the chloroform was evaporated with nitrogen gas to form a thin film, and then the lipid was suspended in n-decane by vortexing. For these lipids, the phosphate pK ≪ 3 and serine carboxylate pK is ∼3 (Tocanne and Teissié, 1990).
Aqueous solutions of hydrochloric acid (Aldrich Chemical, Milwaukee, WI), sodium chloride (Columbus Chemical Industries, Columbus, WI), and tetraethylammonium (TEA) chloride (Sigma, St. Louis, MO) were used without further purification. The solution pH was adjusted to the desired pH by adding HCl.
Electrophysiological recordings
Lipid bilayers were formed by painting the lipids dispersed in n-decane across the aperture (60–150 μm in diameter) of a polyethylene pipette inserted into a Teflon chamber (Busath and Szabo, 1988). Membrane currents were measured using a Warner BC-525C bilayer clamp amplifier. For each experiment, data were low-pass filtered with a cutoff frequency fc = 100 Hz and collected continuously, at 300 samples/s, usually for 30–60 min after bilayer formation. Data were collected on a Macintosh computer with a NI-DAQ data acquisition board (National Instruments, Austin, TX) and IGOR Pro (Version 3.01; Wave Metrics, Lake Oswego, OR). All experiments were performed at temperatures between 22° and 24°C.
A membrane potential was applied by way of Ag-AgCl electrodes. In a typical experiment, a bilayer was first formed in symmetrical solutions without protein added and checked for 5–15 min for stability and lack of channel activity. Then the M2 protein was incorporated by addition of a small aliquot (10 μl) of a concentrated solution to the cis chamber under continuous stirring to give a final concentration of ∼3 μg protein/ml. Channel activity was typically observed to develop after 5–10 min of stirring, at which point the stirring was ceased.
To determine the effect of amantadine, experiments were performed taking care not to break the bilayer. First, the presence of channels in the membrane was confirmed by observing channel activity in the absence of amantadine. Then aliquots of amantadine solution were added to both chambers to a concentration of 300 μM. The need to mix the solution by stirring was balanced with care to avoid breaking the membrane. For determination of the reversibility of the amantadine effect, fresh solutions, without amantadine, were perfused into the cis chamber and currents recorded afterwards.
The reversal potential, Erev, was obtained from single-channel current measurements at various voltage levels with asymmetrical hydrochloric acid solutions, 1 mM HCl in the trans and 5 mM HCl in the cis chamber. In a typical experiment, 1 mM HCl was placed in both the chambers, M2 was added to the cis chamber with continuous stirring, and then a calculated amount of additional HCl was added in the cis chamber to make the concentration 5 mM. The channels were observed at several voltages and the voltage at which the current-voltage relationship passes through the zero-current axis was evaluated. The Nernst potential for H+, taking activity coefficients for HCl (assumed equal for the two ions) into account, is −40.6 mV. The experiments were done with electrodes immersed directly in the bath, and therefore results had to be adjusted for the differential chloride potential, which is also expected to be −40.6 mV to yield an expected uncorrected reversal potential of −81.2 mV. The accuracy of the measurements and the assumptions about the chloride potentials were confirmed using control experiments with gramicidin channels. The use of high-salt bridges was avoided for convenience in the experiment and to avoid assumptions about contamination and diffusion potentials.
Data analysis
Current transitions reflecting channel openings and closings were detected and analyzed with the computer programs TAC and TACfit (Version 2.5; Skalar Instruments, Seattle, WA). Digital filtering was applied, usually with a cutoff of 30 Hz. The upper limit on the open probability, p, was obtained assuming the smallest possible number of channels in the membrane. If each channel event were actually due to a separate molecule instead, the estimated p would be lower by up to a few orders of magnitude. From analysis of the apparent number of independent molecular channels in the membrane, N, assuming an open-state probability, p, closed-state probability, q = 1 − p, and binomially distributed conductance levels,
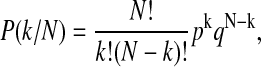 |
(1) |
the observed proportions of time spent with k = 0, 1, 2,… channels conducting failed to discriminate whether a few or several hundred channels were present due to the short channel lifetimes. This was equally true for histogram comparison, variance analysis, and maximum likelihood approaches. Therefore, we simply report the upper limit here.
The mean channel lifetime was calculated by the following equation:
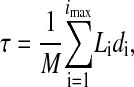 |
(2) |
where a continuous current trace extending from one baseline segment to another is divided completely into imax constant-current segments of duration, di. Li = 0, 1, 2,… is the number of channels conducting for each flat segment. M is the number of channel events, meaning the number of on (or off) transitions. Bursting behavior was not evident in the data and was not evaluated.
RESULTS
Proton conductance
Single-channel currents were measured with symmetrical HCl, pH 3, solution after reconstitution from liposomes to planar lipid bilayers. The 4112 bilayers were stable for the full range of pHs tested, 2.6–8.0. For pH ≤ 4 the channel currents can be easily observed within a few minutes after stirring the cis chamber with liposomes containing M2 protein.
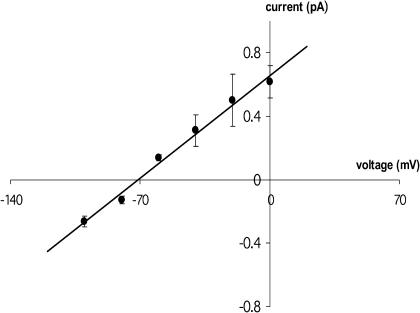
The current recordings at various voltages (Fig.1 A) show that the square channels have one principal conductance state. The open-channel current is proportional to voltage (Fig.1 B) in the accessible range for this lipid. The channel opens at positive as well as negative voltages and is stable for a few tenths of a second. The single-channel current transitions fall within a narrow peak (Fig. 2) and are reproducible from experiment to experiment. The mean channel lifetime and maximum value of the open-state probability, pmax from four experiments at 50 mV were 0.23 sec and ≤0.03, respectively, where pmax is estimated under the assumption that there are ≥Nmax channels in the membrane, Nmax being the largest number of channels seen to be open simultaneously in a given experiment, which was always between 1 and 3. Because of the small amount of simultaneity, it is not possible to be sure that there are not more channels in the membrane in these experiments, including up to 100 or more, with proportionately lower p. We therefore give only the upper limit on p. Control experiments without addition of M2 protein routinely show no channel activity.
FIGURE 1.
M2 single-channel currents. (A) M2 current traces at different voltages. A representative sample of single channels observed in a symmetrical 1-mM HCl solution. A 4112 lipid bilayer was used (see Methods); M2 delivered in DMPC/DMPG 4:1. (B) Single-channel current versus voltage plot of M2 in a 4112 lipid bilayer at pH 3. Error bars represent ±1 SD of the best-fit normal distribution for the experiment shown in A.
FIGURE 2.
Distribution of single-channel M2 current transitions from a typical experiment.
Amantadine sensitivity
The M2 channel amplitude is reduced by ∼50% with addition of 300 μM amantadine at pH 3 as shown in Fig. 3. This reduction in amplitude of the current has been observed in more than 10 experiments. The block can be readily observed at low membrane potentials, i.e., around +50 mV. Using perfusion experiments, there was no increase in amplitude after removal of amantadine for washouts of >5 min (data not shown). On some occasions a decrease in channel frequency was apparent, although this is difficult to validate statistically. With a different assay in our lab, i.e., hydrogen uptake by vesicles at pH 7 using the same sample of M2, a similar concentration of amantadine (1 mM) was also observed to produce ∼80% block (Moffat et al., 2004). Therefore, the M2 sample used here is sensitive to amantadine.
FIGURE 3.
Effect of amantadine on M2-induced currents. (A) Time course of the currents (1 mM HCl, pH 3) before and after the addition of amantadine to 300 μM. (B) Bar plot comparing mean single-channel current amplitude at +50 mV before and after amantadine addition. (Planar lipid bilayer mixture: 4112; M2 delivered in DMPC/DMPG 4:1).
Hydrogen selectivity of channel
To ascertain whether the channel is more permeable to protons than chloride ions in acidic conditions, reversal potentials have been observed in the presence of a concentration gradient for HCl (Fig. 4). The bilayer was formed in asymmetrical baths of 1 mM HCl (trans) and 5 mM HCl (cis) and the currents have been measured at different voltages (cis relative to trans). The uncorrected reversal potential is −70 mV. After correction for chloride potentials at the Ag-AgCl electrodes (−35 mV), the channel reversal potential (−35 mV) closely resembles the equilibrium potential for hydrogen, −40.8 mV (after correction for activity coefficients). This clearly indicates that hydrogen is the principal conducting ion.
FIGURE 4.
Ion selectivity of the M2 protein channel. Current-voltage relationship plotted near the reversal voltage in asymmetrical bath conditions (HCl, pH 2.3 cis, and pH 3 trans). The lipid mixture was 4112. Erev observed at −70 mV which is close to EH under these conditions. Error bars are as in Fig. 1 B. Two other experiments showed nearly identical results.
In experiments with 150 mM NaCl or 150 mM TEACl at pH 3, the current magnitude is essentially the same as was observed with HCl pH 3, suggesting that M2 is selective for H+ over these other ions (Na+, Cl−, and TEA+) under these acidic conditions (Fig. 5).
FIGURE 5.
M2 ionic selectivity: Dependence of ion current on ion species. M2 current traces as observed with different solutions: (a) 1 mM HCl; (b) 150 mM NaCl, pH3; and (c) 150 mM TEACl, pH 3. The results show that hydrogen is the only permeating ion. (Planar lipid bilayer mixture: 4112; M2 delivered in DMPC/DMPG 4:1).
M2 experiments at pH 5, 6, and 7 (with 5 mM TEACl to stabilize the electrodes) do not show any channel activity (data not shown), as expected for hydrogen currents at such a low H+ concentration. At pH 4, the channel activity can be observed, and at lower pH the magnitude of current increases moderately (Fig. 6), also as expected for a proton-selective channel. The rise of log(current) with respect to log[H+] has a slope of <1 throughout the observed range, suggesting a saturable permeation mechanism
FIGURE 6.
Current versus concentration. isc versus [H+] plotted logarithmically (on both axes) for M2 in symmetrical HCl solutions at Vm = +50 mV. Lipid bilayer mixture: 4112. Each point corresponds to the mean ± SD from three experiments.
DISCUSSION
Relationship of M2 to other channels
Considerable work has been done with proton conductance pathways, which are important in bioenergetics and pH regulation (see review by DeCoursey, 2003). Transport could proceed via hydrodynamic hydronium flow, Grotthus transport, or relay through titratable sites. Hydrodynamic hydronium flow is found in nonhydroxylic solvents and is associated with modest ion mobility, similar to that of other cations. Grotthus transport is characterized by hydrogen ions hopping from one water molecule to the next and, hence, the mobility of hydrogen in water is higher than that of other cations. Grotthus transport in bulk solutions is suggested by the increasing proton mobility sequence observed in methanol, water, D2O, and ice, an increasing viscosity sequence. Relay through titratable sites is characterized by protons binding to titratable sites, which may lead to saturable pH-dependent conductance. Gramicidin channels provide a particularly simple and elegant example of water-wire Grotthus conductance, and have been evaluated both experimentally (Levitt et al., 1978; Akeson and Deamer, 1991; Cukierman et al., 1997; Chernyshev et al., 2003; Phillips et al., 1999; Gowen et al., 2002) and theoretically (Sagnella and Voth, 1996; Pomès and Roux, 1996, 2002; Zhu and Schulten, 2003). Mechanisms in more complex pathways are coming under scrutiny (e.g., Roux et al., 1996) although structural information is sometimes sparse.
The M2 channel is of special interest both because of its medicinal relevance and its small size, which makes it amenable to structural characterization. Given its high selectivity for protons over Na+, M2 appears to function as a highly selective channel. The mechanism of transport in this protein should be relevant to other proton channels in which tight regulation of leakage is crucial to protein function.
This represents the first report of hydrogen currents with the reconstituted intact protein. Although Tosteson et al. (1994) performed reconstitution experiments with the intact M2 protein, they did not make measurements at low pH and did not observe proton-selective channels. Rather, the poorly selective, erratic channels they observed appear essentially identical to phenomena we observed in preliminary experiments in which the protein was delivered to the planar bilayers by dipalmitoyl phosphatidylcholine vesicles. We take this to be a poorly formed conformation of the channel protein. In contrast, like the channels reported here, channels formed in bilayers with M2 TMP were more proton selective (Duff and Ashley, 1992). Interestingly, the M2 TMP channels were reported to be completely blocked by amantadine in a reversible fashion (Duff and Ashley, 1992), unlike the observed partial irreversible block reported here. Perhaps the differences are ascribable to differences in the N-terminal vestibule of the channel as suggested by Astrahan et al. (2004).
Proton conductance
Three key channel properties have been achieved with the reconstituted protein: 1), a stable conductance state representing a stable channel configuration; 2), amantadine sensitivity indicating the correct channel configuration; and 3), proton selectivity, also indicative of the correct channel configuration. Single-channel measurements are very valuable indicators of item 1, but to measure them, it is necessary to carry out the studies at pH <4. This is more acidic than can be typically used in cellular expression systems, but is not very much lower than the endosomal pH in which the channel is functional. However, both proton selectivity and amantadine block might be affected by such low pH, especially given the expected dominance of the tetrapronated state for the His tetrad below pH 4 (T. A. Cross et al., unpublished data). In particular, evidence from analytical ultracentrifugation indicates that amantadine affinity may be reduced at low pH (Salom et al., 2000).
In our experience, the presence of negatively charged lipid, DMPG, with DMPC in the delivery liposomes and the use of a mixture of lipids in planar bilayers are both crucial to maintain the channel conformation and enhance the channel incorporation. With these lipids, clean, square ion channel currents can be observed routinely at low pH. The single-channel conductance is 6 pS. The current-voltage curve is linear at low membrane potential (Fig. 1 B) as observed with other proton conducting channels such as gramicidin (Akeson and Deamer, 1991). The open-state probability and the mean channel lifetime are ≤0.03 and 0.2 s, respectively, which is comparable to other proton conducting channels (DeCoursey, 2003). The channels are very selective against Cl−, which is especially remarkable when one considers that the selectivity filter may have a net charge of +4 below pH 4.0. The permeability ratio, PH/PCl, is 19.7 according to the Goldman-Hodgkin-Katz equation, representing a very substantial preference for H+.
Amantadine sensitivity
The results with amantadine show that the channel is sensitive to 300 μM amantadine at pH 3. After addition of amantadine the channel amplitude is reduced by about half (Fig. 3). At higher pH, 80% block is observed in cellular systems with 50–100 μM amantadine (Wang et al., 1993). The single-channel currents are reduced uniformly, without any obvious increase in single-channel noise. This is suggestive of an allosteric block, consistent with the voltage independence and lack of excess channel noise observed with amantadine block in mouse erythroleukemia cells (Chizhmakov et al., 1996), with interpretations of mutations that confer amantadine resistivity (Pinto and Lamb, 1995), and with the observed slow blocking and unblocking rate constants (Wang et al., 1993). On the other hand, neutron diffraction and surface plasmon resonance studies with M2 TMP (Duff et al., 1994; Astrahan et al., 2004) suggest the presence of an intrachannel amantadine binding site. It is possible that at the low pH required to observe single-channel currents, the affinity of the intrachannel blocking site is reduced, perhaps by protonation of His imidazole groups in the channel, such that signs of intrachannel block are not apparent. This would be consistent with the observation that amantadine binding is reduced at low pH (Salom et al., 2000) in studies of the transmembrane helix of M2 in micelles, and that the amantadine IC50 in whole cell preparations is increased under acidic conditions (Wang et al., 1993). On the other hand, the fact that, in mouse erythroleukemia cells with very low-level currents, the amantadine block is low-noise and voltage-independent, even in weakly acidic conditions, suggests that even under neutral pH conditions, signs of intrachannel block may be elusive.
The amantadine block with M2 protein was found here to be irreversible on the tens of minutes timescale using a washout experiment. This is consistent with the irreversible block observed with whole-cell systems (Wang et al., 1993), and indicates very tight binding of amantadine with or near the protein.
Open-state probability
The probability of the open state can easily be determined when it is certain that there is only one channel in the membrane. However, with the possibility of many channels present, the apparent p must be divided by N, the number of channels present, to get the single-channel p. Binomial distribution statistics can often be applied to evaluate N and p under conditions where there is a significant probability of more than one channel conducting simultaneously. However, in our experiments, the channels are short-lived and rarely overlap, so this analysis yielded results consistent with any number of active channels. Therefore, we cannot say with certainty the values of p or N, and merely report here a maximum p (assuming low N, 1–3). Based on the protein density, calculated from the lipid area per headgroup, the approximate protein diameter, and the protein/lipid ratio to be ∼6000 tetramers/μm2, if the vesicles that fuse are ∼150 nm in diameter, we would expect each fusion to introduce ∼400 channels into the membrane. It is possible that all tetramers are active, but have a low p, or that only a fraction of the tetramers are active. From the current data, it is impossible to distinguish between these two possibilities. On the assumption that all the protein is in the tetrameric state, that all tetramers are active, and that our experiments typically represent a single vesicle-fusion event, p would be more accurately estimated to be 7.5 × 10−5.
The variation of current with pH
The results showing the variation of current amplitudes with varying pH at constant voltage is shown in Fig. 6. The logarithmic plot shows that the rise is sublinear (slope <1) throughout the observable range. This is consistent with the observation by Chizhmakov and colleagues (1996) that the M2 current at a membrane potential of +60 mV partially saturates below pH 4.5. This suggests the presence of a saturable site in the hydrogen permeation pathway. A simple three-site obligate relay model for such a permeation process is presented in Lear (2003). The model successfully explains the voltage and pH dependence of the whole MEL cell M2 currents reported by Chizhmakov et al. (2003). Our data do not conflict with this model's prediction of acid gating at high pH (because our studies are limited to the low pH range) nor with its prediction of maximal diffusion-limited influx into the channel based on the Smoluchowski equation with a capture radius of ∼1 Å. At pH 3, this equation predicts a maximum flux of 0.54 pA at room temperature, similar to that observed here. However, it is interesting to note that the high currents reported here would require consideration of an additional binding state with lower affinity than that deduced from the whole-cell data, pK = ∼5.6, because the dissociation rate constant, predicted for this case to be 8100 s−1, would be more than two orders of magnitude too low for the 0.6 pA-level (3.6 × 106 ions/s) currents we measure. It will be interesting to search for indications of alternative proton permeation mechanisms in future studies. Recent work has focused on the low conductance of H+ channels formed by M2 (Mould et al., 2001a; Lin and Schroeder, 2001). However, as Lin and Schroeder (2001) point out, the M2 permeability to H+ is similar to that of potassium channels and other channels. Part of the reason that vesicles take up protons slowly, leading to estimates of conductance in the aS range, is that low [H+] solution was used (pH 7.4 in most experiments, 5.7 in a few). Even pH 5.7 represents too low a [H+] to expect to see single channels. Also, p is lower than anticipated. The estimate of aS is for a time averaged conductance at pH 7, which is lower than the single-channel conductance at pH 3 by both the factor, p, and by 3–5 orders of magnitude in relation to the bath [H+]. Furthermore, we note that conductance is an ambiguous number when measurements are made in asymmetric solutions, even if voltages are in the linear I/V range for symmetric solutions.
In summary, when reconstituted in negatively charged lipid membranes, with acidic bathing solution, M2 forms a highly selective proton channel with a low probability of being in the open state and partial block by amantadine.
Acknowledgments
We are grateful to Larry Pinto for many helpful discussions. We are also grateful to Matthew Swenson for technical help.
This work was supported by National Institutes of Health grant AI23007.
References
- Akeson, M., and D. W. Deamer. 1991. Proton conductance by the gramicidin water wire. Model for proton conductance in the F1F0 ATPases? Biophys. J. 60:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrahan, P., I. Kass, M. A. Cooper, and I. T. Arkin. 2004. A novel method of resistance for influenza against a channel-blocking antiviral drug. Proteins. 55:251–257. [DOI] [PubMed] [Google Scholar]
- Bauer, C. M., L. H. Pinto, T. A. Cross, and R. A. Lamb. 1999. The influenza virus M2 ion channel protein. Probing the structure of the transmembrane domain in intact cells by using engineered disulfide cross-linking. Virology. 254:196–209. [DOI] [PubMed] [Google Scholar]
- Busath, D., and G. Szabo. 1988. Low conductance gramicidin A channels are head-to-head dimers of β6.3-helices. Biophys. J. 53:689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyshev, A., R. Pomès, and S. Cukierman. 2003. Kinetic isotope effects of proton transfer in aqueous and methanol containing solutions, and in gramicidin A channels. Biophys. Chem. 103:179–190. [DOI] [PubMed] [Google Scholar]
- Chizhmakov, I. V., F. M. Geraghty, D. C. Ogden, A. Hayhurst, M. Antoniou, and A. J. Hay. 1996. Selective proton permeability and pH regulation of the influenza virus M2 channel expressed in mouse erythroleukaemia cells. J. Physiol. 494:329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizhmakov, I. V., D. C. Ogden, F. M. Geraghty, A. Hayhurst, A. Skinner, T. Betakova, and A. J. Hay. 2003. Differences in conductance of M2 proton channels of two influenza viruses at low and high pH. J. Physiol. 546:427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristian, L., J. D. Lear, and W. F. DeGrado. 2003. Use of thiol-disulfide equilibria to measure the energetics of assembly of transmembrane helices in phospholipid bilayers. Proc. Natl. Acad. Sci. USA. 100:14772–14777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman, S., E. P. Quigley, and D. S. Crumrine. 1997. Proton conductance in gramicidin A and its dioxolane-linked dimer in different bilayers. Biophys. J. 73:2489–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey, T. E. 2003. Voltage-gated proton channels and other proton transfer pathways. Physiol. Rev. 83:475–579. [DOI] [PubMed] [Google Scholar]
- Duff, K. C., and R. H. Ashley. 1992. The transmembrane domain of influenza A M2 protein forms amantadine-sensitive proton channels in planar lipid bilayers. Virology. 190:485–489. [DOI] [PubMed] [Google Scholar]
- Duff, K. C., P. J. Gilchrist, A. M. Saxena, and J. P. Bradshaw. 1994. Neutron diffraction reveals the site of amantadine blockage in the influenza A M2 ion channel. Virology. 202:287–293. [DOI] [PubMed] [Google Scholar]
- Forrest, L. R., A. Kukol, I. T. Arkin, D. P. Tieleman, and M. S. P. Sansom. 2000. Exploring models of the influenza A M2 channel: MD simulations in a phospholipid bilayer. Biophys. J. 78:55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen, J. A., J. C. Markham, S. E. Morrison, D. D. Busath, T. A. Cross, E. J. Mapes, and M. F. Schumaker. 2002. The role of Trp side chains in tuning single proton conduction through gramicidin channels. Biophys. J. 83:880–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grambas, S., and A. J. Hay. 1992. Maturation of influenza A virus hemagglutinin—estimates of the pH encountered during transport and its regulation by the M2 protein. Virology. 190:11–18. [DOI] [PubMed] [Google Scholar]
- Hay, A. J. 1992. The action of adamantanamines against influenza A viruses: inhibition of the M2 ion channel protein. Semin. Virol. 3:21–30. [Google Scholar]
- Kovacs, F., and T. A. Cross. 1997. Transmembrane 4-helix bundle of influenza A M2 protein channel: structural implications from helix tilt and orientation. Biophys. J. 74:2511–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukol, A., P. D. Adams, L. M. Rice, A. T. Brunger, and I. T. Arkin. 1999. Experimentally based orientational refinement of membrane protein models: a structure for the influenza A M2 H+ channel. J. Mol. Biol. 286:951–962. [DOI] [PubMed] [Google Scholar]
- Lear, J. D. 2003. Proton conduction through the M2 protein of the influenza A virus; a quantitative, mechanistic analysis of experimental data. FEBS Lett. 552:17–22. [DOI] [PubMed] [Google Scholar]
- Levitt, D. G., S. R. Elias, and J. M. Hautman. 1978. Number of water molecules coupled to the transport of sodium, potassium, and hydrogen ions via gramicidin, nonactin or valinomycin. Biochim. Biophys. Acta. 512:436–451. [DOI] [PubMed] [Google Scholar]
- Lin, T., and C. Schroeder. 2001. Definitive assignment of proton selectivity and attoampere unitary current to the M2 ion channel protein of influenza A virus. J. Virol. 75:3647–3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat, C. J., M. D'Haenens, R. Davidson, V. Vijayvergiya, D. D. Busath, and D. J. Woodbury. 2004. Measurement of proton flux through influenza A viral protein M2. Biophys. J. 86:550a. (Abstr.) [Google Scholar]
- Mould, J. A., H. C. Li, C. S. Dudlak, J. Lear, A. Pekosz, R. A. Lamb, and L. H. Pinto. 2000a. Mechanism for proton conduction of the M2 ion channel of influenza A virus. J. Biol. Chem. 275:8592–8599. [DOI] [PubMed] [Google Scholar]
- Mould, J. A., J. E. Drury, S. M. Frings, U. B. Kaup, A. Pekosz, R. A. Lamb, and L. H. Pinto. 2000b. Permeation and activation of the M(2) ion channel of influenza A virus. J. Biol. Chem. 275:31038–31050. [DOI] [PubMed] [Google Scholar]
- Nishimura, K., S. Kim, L. Zhang, and T. A. Cross. 2002. The closed state of a H+ channel helical bundle combining precise orientational and distance restraints from solid state NMR. Biochemistry. 41:13170–13177. [DOI] [PubMed] [Google Scholar]
- Okada, A., T. Miura, and H. Takeuchi. 2001. Protonation of histidine and histidine-tryptophan interaction in the activation of the M2 ion channel from influenza A virus. Biochemistry. 40:6053–6060. [DOI] [PubMed] [Google Scholar]
- Phillips, L. R., C. D. Cole, R. J. Hendershot, M. Cotton, T. A. Cross, and D. D. Busath. 1999. Noncontact dipole effects on channel permeation. III. Anomalous proton conductance effects in gramicidin. Biophys. J. 77:2492–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto, L. H., G. R. Dieckmann, C. S. Gandhi, C. G. Papworth, J. Braman, M. A. Shaughnessy, J. D. Lear, R. A. Lamb, and W. F. DeGrado. 1997. Functionally defined model for the M2 proton channel of influenza A virus suggests a mechanism for its ion selectivity. Proc. Natl. Acad. Sci. USA. 94:11301–11306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto, L. H., and R. A. Lamb. 1995. Understanding the mechanism of action of the anti-influenza virus drug amantadine. Trends Microbiol. 3:271. [DOI] [PubMed] [Google Scholar]
- Pomès, R., and B. Roux. 1996. Structure and dynamics of a proton wire: A theoretical study of H+ translocation along the single-file water chain in the gramicidin A channel. Biophys. J. 71:19–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomès, R., and B. Roux. 2002. Molecular mechanism of H+ conduction in the single-file water chain of the gramicidin channel. Biophys. J. 82:2304–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux, B., M. Nina, R. Pomès, and J. C. Smith. 1996. Thermodynamic stability of water molecules in the bacteriorhodopsin proton channel: a molecular dynamics free energy perturbation study. Biophys. J. 71:670–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagnella, D. E., and G. A. Voth. 1996. Structure and dynamics of hydronium in the ion channel gramicidin A. Biophys. J. 70:2043–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi, T., Q. A. Tu, L. H. Pinto, and R. A. Lamb. 1997. The active oligomeric state of the minimalistic influenza virus M2 ion channel is a tetramer. Proc. Natl. Acad. Sci. USA. 94:5000–5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salom, D., B. R. Hill, J. D. Lear, and W. F. DeGrado. 2000. pH-dependent tetramerization and amantadine binding of the transmembrane helix of M2 from the influenza A virus. Biochemistry. 39:14160–14170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighofer, K. J., and A. Pohorille. 2000. Computer simulation of ion channel gating: The M2 channel on influenza A virus in a lipid bilayer. Biophys. J. 78:150–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimbo, K., D. L. Baddard, R. A. Lamb, and L. H. Pinto. 1996. Ion selectivity and activation of the M2 ion channel of influenza virus. Biophys. J. 70:1335–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smondyrev, A. M., and G. A. Voth. 2002. Molecular dynamics simulation of proton transport through the influenza A virus M2 channel. Biophys. J. 83:1987–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, Z., F. A. Kovacs, J. Wang, J. K. Denny, S. C. Shekar, J. R. Quite, and T. A. Cross. 2000. Transmembrane domain of M2 protein from influenza A virus studied by solid-state 15N polarization inversion spin exchange at magic angle NMR. Biophys. J. 79:767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugrue, R. J., and A. J. Hay. 1991. Structural characteristics of the M2 protein of influenza A viruses: Evidence that it forms a tetrameric channel. Virology. 180:617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, Y., F. Saitseva, R. A. Lamb, and L. H. Pinto. 2002. The gate of the influenza virus M2 proton channel is formed by a single tryptophan residue. J. Biol. Chem. 277:39880–39886. [DOI] [PubMed] [Google Scholar]
- Tian, C., P. F. Gao, L. H. Pinto, R. A. Lamb, and T. A. Cross. 2003. Initial structural and dynamic characterization of the M2 protein transmembrane and amphipathic helices in lipid bilayers. Protein Sci. 12:2597–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, C., K. Tobler, R. A. Lamb, L. H. Pinto, and T. A. Cross. 2002. Expression and initial structural insights from solid-state NMR of the M2 proton channel from influenza A virus. Biochemistry. 41:11294–11300. [DOI] [PubMed] [Google Scholar]
- Tocanne, J.-F., and J. Teissié. 1990. Ionization of phospholipids and phospholipid-supported interfacial lateral diffusion of protons in membrane model systems. Biochim. Biophys. Acta. 1031:111–142. [DOI] [PubMed] [Google Scholar]
- Tosteson, M. T., L. H. Pinto, L. J. Holsinger, and R. A. Lamb. 1994. Reconstitution of the influenza A virus in lipid bilayers. J. Membr. Biol. 142:117–126. [DOI] [PubMed] [Google Scholar]
- Vijayvergiya, V., T. A. Cross, and D. D. Busath. 2003. Ion channel studies of influenza virus M2 in planar lipid bilayers. Biophys. J. 84:488a (Abstr.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayvergiya, V., R. R. Wilson, A. Chorak, F. Gao, T. A. Cross, and D. D. Busath. 2004. Single channel conductance of influenza virus M2 protein in planar lipid bilayers. Biophys. J. 86:550a. (Abstr.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., J. Denny, C. Tian, S. Kim, Y. Mo, F. Kovacs, Z. Song, K. Nishimura, Z. Gan R. Fu, J. R. Quine, and T. A. Cross. Z. Gan R. Fu, J. R. Quine, and T. A. Cross. 2000. Imaging membrane protein helical wheels. J. Magn. Reson. 144:162–167. [DOI] [PubMed] [Google Scholar]
- Wang, F., S. Kim, F. Kovacs, and T. A. Cross. 2001. Structure of the transmembrane region of the M2 protein H+ channel. Protein Sci. 10:2241–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C., R. A. Lamb, and L. H. Pinto. 1995. Activation of the M2 ion channel of influenza virus: a role for the transmembrane domain histidine residue. Biophys. J. 69:1363–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C., K. Takeuchi, L. H. Pinto, and R. A. Lamb. 1993. Ion channel activity of influenza A virus M2 protein: characterization of the amantadine block. J. Virol. 67:5585–5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, Q., T. Husslein, P. B. Moore, D. M. Newns, P. Pattnaik, and M. L. Klein. 1998. The M2 channel of influenza A virus: a molecular dynamics study. FEBS Lett. 434:265–271. [DOI] [PubMed] [Google Scholar]
- Zhu, F., and K. Schulten. 2003. Water and proton conduction through carbon nanotubes as models for biological channels. Biophys. J. 85:236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]