Abstract
The biological activities of ceramides show a large variation with small changes in molecular structure. To help understand how the structure regulates the activity of this important lipid second messenger, we investigated the interfacial features of a series of synthetic ceramide analogs in monomolecular films at the argon-buffer interface. To minimize differences arising from the N-acyl moiety, each analog had either a N-hexadecanoyl or a N-cis-4-hexadecenoyl moiety amide linked to the nitrogen of the sphingosine backbone. We found that the trans 4,5-unsaturation in the sphingosine backbone promoted closer packing and lower compressibilities of ceramide analogs in interfaces relative to comparable saturated species. Moreover, structures with this feature exhibited dipole potentials as much as 150–250 mV higher than comparable compounds lacking 4,5-unsaturation. The results support the hypothesis by M.C. Yappert and co-workers that trans unsaturation in the vicinity of C4 of the sphingoid backbone augments intramolecular hydration/hydrogen bonding in the polar region. This intramolecular hydration may allow the close packing of the ceramide molecules and engender their high dipole potentials. These properties of ceramides and their analogs may be important determinants of biological function.
INTRODUCTION
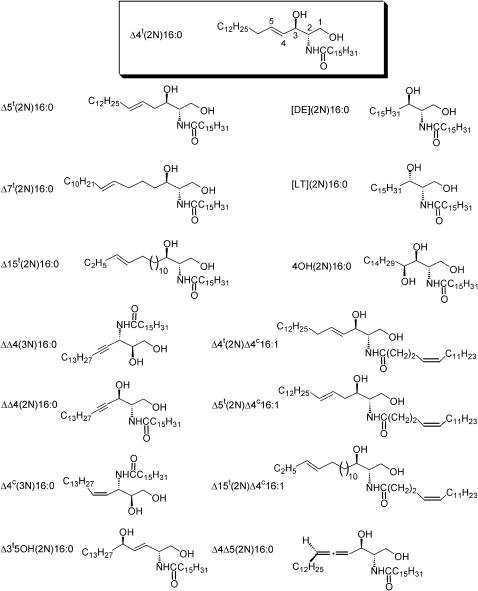
Sphingolipids are important structural and functional components of biological membranes. They differ from glycerol ester based lipids by being built not on a 1,2-diacyl-sn-glycerol backbone but on ceramide (Merrill and Sandhoff, 2002). In ceramide, a long-chain N-acyl group, which is sometimes hydroxylated, is attached through an amide linkage to the 2-amino group of sphingosine (1,3-dihydroxy-2-amino-4-trans-octadecene in the d-erythro (2S,3R) configuration; see Fig. 1). Complex sphingolipids have a ceramide backbone coupled to a polar headgroup. For example, sphingomyelin contains a phosphocholine moiety esterified to the C-1 hydroxy group of ceramide, whereas glycosphingolipids have various carbohydrates linked to the C1 position of ceramide. Both sphingomyelin and glycosphingolipids are significant components of the exofacial leaflet of plasma membranes. Among sphingolipid classes, much variation exists, not only in the headgroup components attached to ceramide but also in the structural features of the long-chain N-acyl group and sphingoid base that combine to form ceramide itself. As examples, in plants (soybean and wheat), the sphingoid base often contains two double bonds (at C4–C5 and C8–C9) and branching methyl groups. In the lens of the human eye, a high proportion of sphingomyelin has a 4,5-dihydro (sphinganine) core, in which the 4,5-double bond has been reduced. In the brain, some of the sphingoid bases are modified in having an additional hydroxy group at C6 (Chun et al., 2003b) or in having the double bond and the hydroxy group located at carbons 3 and 5 (Chun et al., 2003a), respectively. Prevalent among fungi and plants is phytosphingosine (4d-hydroxysphinganine), in which the 4,5-double bond is reduced and an additional hydroxy group is present at C4.
FIGURE 1.
The structures and codes of d-erythro-ceramide and its analogs used in this study.
Ceramides are both precursors and degradation products of sphingomyelin and glycosphingolipids (Merrill and Sandhoff, 2002). Ceramide generation is an important signaling event that can have different consequences depending on cell type (Kolesnick, 1991; Kolesnick et al., 2000). Because of ceramide's important roles in many physiological events, both natural ceramide and synthetic analogs have been used to study mechanisms of action and have been tested as potential therapeutic agents in an array of diseases (Kester and Kolesnick, 2003; Chalfant et al., 2004; Struckhoff et al., 2004). Many studies have shown that relatively small alterations of ceramide structure can have profound consequences on biological activity. As one example, d-erythro-ceramide induces apoptosis in cells, but the equivalent species lacking the C4-trans double bond were inactive (Bielawska et al., 1993) or very weakly active (Karasavvas et al., 1996). Another study showed that the C3-hydroxy group and C4-trans double bond were both required for the fusion of Semliki Forest virus with target membranes (Corver et al., 1995), whereas other studies in the same system showed that the d-erythro diastereomer was also mandatory (Moesby et al., 1995), as was the C4-trans double bond (He et al., 1999).
A large body of literature addresses the mechanisms by which ceramide exerts its effects on cellular homeostasis and pathogenesis (e.g., Menaldino et al., 2003). Although specific liganding interactions, e.g., with kinase suppressor of ras, ceramide-activated protein phosphatases, and protein kinase ζ (Ruvolo, 2003) are important, the physical properties of ceramides as membrane constituents play a significant functional role (van Blitterswijk et al., 2003; Cremesti et al., 2002). Like diacylglycerol, ceramide is a cone-shaped molecule with a relatively compact headgroup (Huang et al., 1999) that binds little water (Jendrasiak and Smith, 2001). These properties contribute to curvature stress that promotes hexagonal phase formation and membrane trafficking through vesiculation and fusion (van Blitterswijk et al., 2003). The nitrogen-containing ceramide molecule differs from glycerol ester lipids in that it forms a cyclic, planar resonance structure and can engage in both inter- and intramolecular hydrogen bonding in which the C(O)NH group can serve as both a hydrogen bond donor and acceptor (Pascher, 1976). These characteristics of ceramides, coupled with their generally saturated and often hydroxylated N-acyl chain, cause ceramides to undergo lateral phase separation in model membranes at relatively low mole fractions (Holopainen et al., 2001). More so than even sphingomyelin or glycosphingolipids, ceramides can promote the formation of gel or liquid-ordered domains in membranes (Wang and Silvius, 2003) and may displace cholesterol from such phases (Megha and London, 2004). These properties of ceramide can have a marked effect on signaling processes in cells mediated through so-called lipid rafts (Simons and Ikonen, 1997). Ceramides also appear to be unique among naturally occurring lipids in their ability to form channels in both natural and model membranes through which proteins up to 60 kD may diffuse (Siskind and Colombini, 2000; Siskind et al., 2002).
An underappreciated physical difference between sphingolipids and glycerol ester lipids is their electrostatic behavior in membranes. Even for uncharged lipid species there exists a dipole potential between the bulk water and the membrane interior. Its value can be hundreds of millivolts. Because of the small distances involved, this results in a field strength at the lipid-water interface of the order of 106 V/cm (Brockman, 1994). This contribution to the total field strength is 1–2 orders of magnitude greater than the effects of a typical transmembrane potential of 10–100 mV. Not only do such fields regulate protein conformation (Porschke, 1997) and interfacial enzyme activities (Maggio, 1999), but also lateral potential differences, which may arise as a consequence of the heterogeneous distribution of lipid species (above), are major determinants of lipid domain size and shape (McConnell and de Koker, 1996). It was recognized many years ago that the dipole potential of sphingomyelin is greater than that of diacylphosphatidylcholine at a comparable area, and it was postulated that this difference arises from an induced dipole in the C4-trans double bond of sphingosine (Shah and Schulman, 1967). More recently, Kuikka et al. (2001) used chirally pure d-erythro-N-palmitoylsphingomyelin and the comparable dihydrosphingomyelin and showed that removal of the C4-trans double bond does, indeed, lower the potential by 50–100 mV when the potentials are compared at comparable areas. Ceramides also exhibit high potentials (Maggio et al., 1978; Holopainen et al., 2001) that may exceed those of sphingomyelin (Fanani and Maggio, 1997), but a direct comparison with a dihydroceramide has not, to our knowledge, been reported.
The molecular basis of the unique biological activities of ceramides that modulate membrane structure and function is as yet unknown. Monolayer membranes are an excellent model system in which to measure the packing and electrostatic properties of lipids and seek to correlate structure-activity relationships (Brockman, 1999). Indeed, an early study provides a clue as to the all-or-none functional consequences of removing the ceramide double bond noted above. A comparison of the surface pressure-molecular area isotherms of N-stearoylceramide and N-stearoyl-4,5-dihydroceramide showed that the latter is markedly expanded at low surface pressure values, although both species collapse at similar areas (Löfgren and Pascher, 1977). Thus, despite their structural similarity, removal of the double bond induces changes in interfacial conformation and compressibility that regulate the packing behavior of dihydroceramide in membranes. In our measurements of interfacial packing and potential, we have characterized a series of 15 defined compounds related to the fundamental structure of dihydroceramide possessing a 16-carbon acyl moiety linked to the nitrogen of ceramide. Emphasis was on the role of unsaturation in the polar region of the sphingoid base, but compounds with other rearrangements/variations in this region were also evaluated. The results show that the presence of the C4-trans double bond or a similar structure imparts unique packing and potential properties to the ceramide molecule. It is striking that almost all variations of the natural ceramide structure lacking this unsaturation result in lower packing densities and interfacial potentials.
MATERIALS AND METHODS
Materials
Hexane (nonspectral grade 99.9+%) and 2-propanol (ultrapure) for monolayer studies were B&J Brand High Purity solvents from Burdick and Jackson (Muskegon, MI). Water was purified by reverse osmosis, charcoal filtration, deionization, ion exchange, and ultraviolet irradiation in a system (Millipore, Bedford, MA) designed to lower total organics to <5 ppb. Subphase buffer (pH 6.6) was 10 mM potassium phosphate (Sigma, St. Louis, MO), 0.1 M NaCl (ACS grade, Mallinckrodt, Paris, KY), and 1.5 mM sodium azide (Sigma).
The abbreviations for the ceramide analogs studied (see Fig. 1) are as follows: Δ4t(2N)16:0, d-erytho-Δ4-ceramide or (2S,3R)-N-palmitoylsphingosine; Δ4t(2N)Δ4c16:1, (2S,3R)-2-N-(cis-4-hexadecenoyl)sphingosine; ΔΔ4(2N)16:0, (2S,3R)-2-palmitoylamido-4-octadecyne-1,3-diol; Δ5t(2N)16:0, (2S,3R)-2-palmitoylamido-(5E)-octadecene-1,3-diol; Δ5t(2N) Δ4c16:1, (2S,3R)-2-N-(cis-4-hexadecenoylamido)-(5E)-octadecene-1,3-diol; Δ3t5OH(2N)16:0, (2R,5R)-2-palmitoylamido-(3E)-octadecene-1,5-diol; Δ7t(2N)16:0, (2S,3R)-2-palmitoylamido-(7E)-octadecene-1,3-diol; Δ15t (2N)16:0, (2S,3R)-2-palmitoylamido-(15E)-octadecene-1,3-diol; [DE](2N) 16:0, (2S,3R)-N-palmitoylsphinganine; [LT](2N)16:0, (2S,3S)-N-palmitoylsphinganine; Δ15t(2N) Δ4c16:1, (2S,3R)-2-N-(cis-4-hexadecenoyl)-(15E)-octadecene-1,3-diol; 4OH(2N)16:0, l-lyxo-phytoceramide or (2S,3S,4S)-N-palmitoylphytosphingosine; Δ4Δ5(2N)16:0, (2S,3R,5R)-2-palmitoylamidooctadeca-4,5-diene-1,3-diol; ΔΔ4(3N)16:0, (2S,3R)-3-palmitoylamido-4-octadecyne-1,2-diol; and Δ4c(3N)16:0, (2S,3R)-3-palmitoylamido-(4Z)-octadecene-1,2-diol. Δ4t(2N)16:0 was purchased from BIOMOL Research Laboratories (Plymouth Meeting, PA). The syntheses of most of the other analogs have been reported previously: Δ5t(2N)16:0 and Δ5t(2N)Δ4c16:1 (He et al., 1999); Δ3t5OH(2N)16:0 (Chun et al., 2003a); and 4OH(2N)16:0, [DE](2N)16:0, Δ4t(2N)Δ4c16:1, Δ7t(2N)16:0, Δ15t(2N)16:0, Δ15t(2N) Δ4c16:1, ΔΔ4(2N)16:0, ΔΔ4(3N)16:0, and Δ4c(3N)16:0 (He et al., 2000). [LT](2N)16:0 was synthesized by a modification of the procedure described by He et al. (2000), and the stereoselective synthesis of Δ4Δ5(2N)16:0 from a chiral propargylic alcohol will be reported elsewhere.
Methods
All lipid samples were dissolved in hexane/2-propanol/water (70:30:2.5). The concentrations were determined by weight using a Cahn Electrobalance (Cahn Instruments, Cerritos, CA).
Surface pressure-potential-molecular area isotherms were measured using a computer controlled, Langmuir-type film balance (Li et al., 2001). The trough was maintained in an atmosphere of humidified argon that had been passed through a series of seven filters selected to remove possible organic and particulate contaminants. Samples (51.67 μl) were applied from 250-μl vials onto the buffer surface using an autosampler as described (Brockman et al., 1984), and, after a waiting period of 4 min to allow for solvent evaporation, the monolayer was compressed at ≤4 Å2/min molecule.
RESULTS
Importance of C-4 unsaturation
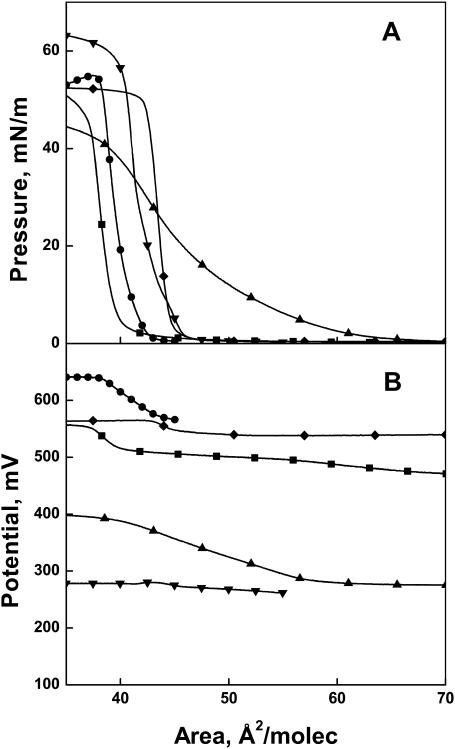
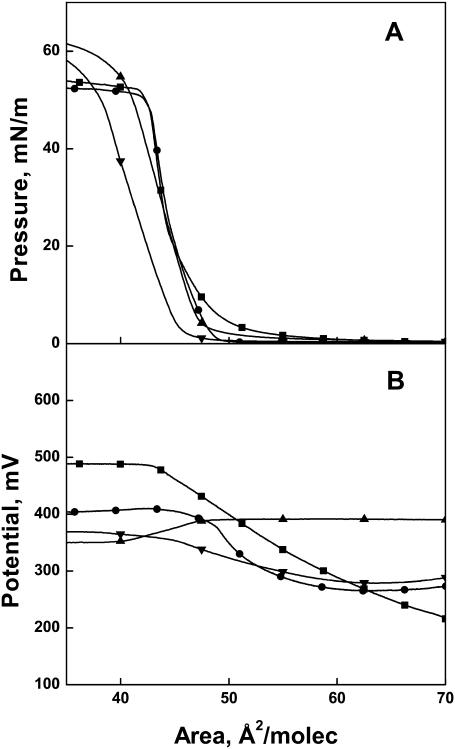
Ceramide has chiral centers at C2 and C3 of the backbone that give rise to diastereomers. The d-erythro (2S,3R) and l-threo (2S,3S) stereoisomers, which differ by inversion of the chiral center at C3, are compared in Fig. 2 A. The surface pressure-molecular area isotherms show a marked effect of configuration on surface packing of the molecules. Whereas the naturally occurring d-erythro-dihydroceramide ([DE](2N)16:0) lifts off at ∼65 Å2/molecule and rises gradually until collapsing at 41–40 Å2/molecule, the l-threo diastereomer ([LT](2N)16:0) lifts off at ∼46 Å2/molecule and exhibits a condensed monolayer behavior until it collapses at ∼40 Å2. The relatively expanded behavior of the d-erythro diastereomer at low surface pressures confirms an earlier observation (Löfgren and Pascher, 1977), but to our knowledge the interfacial behavior of the l-threo diastereomer has not been previously reported. The moduli of compression of the curves, evaluated at 35 mN/m, differ by almost sevenfold (Table 1). Although this surface pressure is, fortuitously, that at which the isotherms intersect, it was chosen for comparisons throughout this study because 35 mN/m or higher in monomolecular films corresponds to the lipid packing densities found in cellular bilayer membranes (MacDonald and Simon, 1987; Marsh, 1996).
FIGURE 2.
Role of C4 unsaturation in regulating the surface behavior of ceramide analogs. (A) Surface pressure-molecular isotherms and (B) dipole potential-molecular area isotherms for Δ4t(2N)16:0 (▪), ΔΔ4(2N)16:0 (•), [DE](2N)16:0 (▴), [LT](2N)16:0 (▾), and Δ4Δ5(2N)16:0 (♦).
TABLE 1.
Physical properties of ceramide analogs at 35 mN/m
| Compound* | Area | Modulus of compression | Dipole potential |
|---|---|---|---|
| Å2/molecule | mN/m | mV | |
| Δ4t(2N)16:0 | 37.8 | 850 | 546 |
| ΔΔ4(2N)16:0 | 39.1 | 871 | 628 |
| Δ5t(2N)16:0 | 39.6 | 362 | 441 |
| Δ3t5OH(2N)16:0 | 40.3 | 286 | 364 |
| Δ7t(2N)16:0 | 40.3 | 338 | 390 |
| Δ15t(2N)16:0 | 40.9 | 361 | 417 |
| [DE](2N)16:0 | 40.9 | 128 | 384 |
| [LT](2N)16:0 | 41.2 | 880 | 277 |
| Δ4t(2N) Δ4c16:1 | 41.8 | 679 | 547 |
| Δ15t(2N) Δ4c16:1 | 42.8 | 266 | 371 |
| 4OH(2N)16:0 | 43.1 | 358 | 366 |
| Δ4Δ5(2N)16:0 | 43.1 | 1070 | 561 |
| ΔΔ4(3N)16:0 | 43.5 | 825 | 481 |
| Δ4c(3N)16:0 | 43.7 | 643 | 408 |
| Δ5t(2N) Δ4c16:1 | 44.7 | 222 | 365 |
Except for [LT](2N)16:0, 4OH(2N)16:0, and Δ3t5OH(2N)16:0, all analogs had substituents at C2 and C3 with stereochemistry equivalent to naturally occurring d-erythro-ceramide.
The introduction of a 4,5-trans double bond into d-erythro-dihydroceramide to give the naturally occurring d-erythro-ceramide (Δ4t(2N)16:0) makes the isotherm highly condensed at all surface pressures; the molecular area decreases to ∼38 Å2, as observed earlier (Löfgren and Pascher, 1977). Additional unsaturation in the form of a 4,5-triple bond (ΔΔ4(2N)16:0) expands the isotherm area by only ∼1 Å2 and increases the modulus of compression at 35 mN/m slightly to afford a value comparable to that of l-threo-dihydroceramide ([LT](2N)16:0).
The introduction of a cumulated double bond system (C=C=C), as in the 4,5/5,6-allenyl structure (Δ4Δ5(2N)16:0), into the d-erythro-dihydroceramide ([DE](2N)16:0) backbone maintains the condensed isotherm shape, but at 35 mN/m the molecular area is increased to ∼43 Å2, and the compressibility modulus rises to 1070 mN/m. The latter is the highest modulus among all of the compounds studied (Table 1).
Like the surface pressure-molecular area isotherms, the interfacial potentials accompanying them show a range of behavior (Fig. 2 B). Over the range of molecular areas from before liftoff to collapse shown in Fig. 2 A, l-threo-dihydroceramide ([LT](2N)16:0) has a nearly invariant interfacial potential (Fig. 2 B) with a value of 277 mV at 35 mN/m (Table 1). d-erythro-Dihydroceramide ([DE](2N)16:0) shows a rising potential as the monolayer surface pressure increases, reaching 384 mV at 35 mN/m. This is >100 mV higher than that of l-threo-dihydroceramide ([LT](2N)16:0) and far lower than the value of 546 mV for d-erythro-ceramide (Δ4t(2N)16:0) at 35 mN/m (Table 1). Relative to d-erythro-ceramide (Δ4t(2N)16:0), with its C4-trans unsaturation, the alkynyl (ΔΔ4(2N)16:0) and allenyl (Δ4Δ5(2N)16:0) moieties in the ceramide analogs increase the potential by an additional 82 and 15 mV, respectively. Thus, the introduction of unsaturation into the dihydroceramide backbone near C4 generates ceramide species that are both highly incompressible and exhibit relatively high dipole potentials.
Positional specificity of aliphatic unsaturation
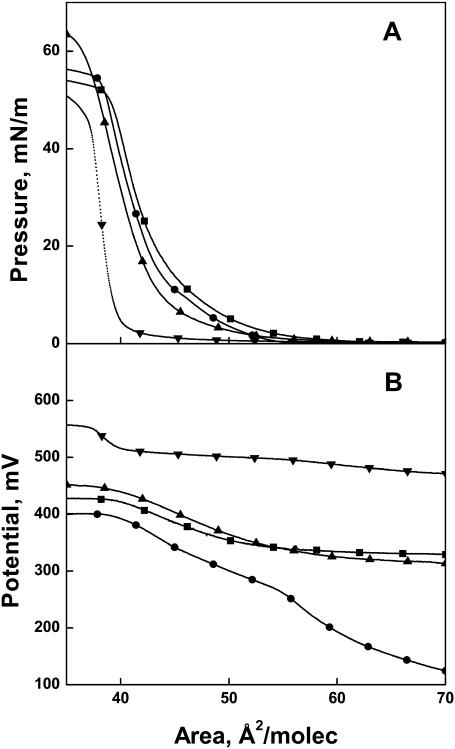
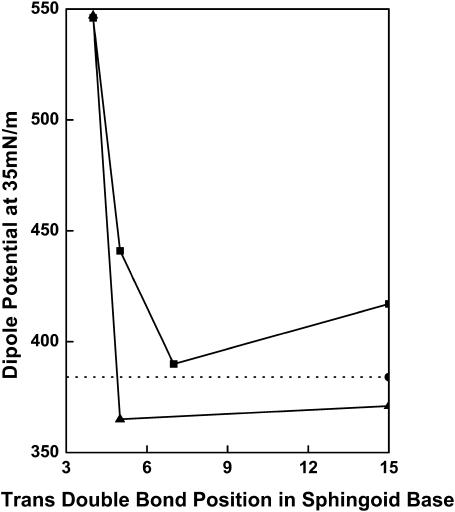
The importance of the position of the trans double bond in the aliphatic chain was tested by comparing d-erythro species in which the double bond is moved progressively farther from the polar region of the sphingoid base. As shown in Fig. 3 A, moving the unsaturation from C4 to C5 (Δ5t(2N)16:0) expands the isotherm at lower pressures to an area greater than that of d-erythro-ceramide (Δ4t(2N)16:0), whose isotherm from Fig. 2 A is shown as a dotted line for comparison. Accordingly, this change lowers the modulus of compression to an intermediate value, but it remains more than twice that of d-erythro-dihydroceramide ([DE](2N)16:0) (Table 1). Moving the trans double bond farther toward the methyl terminus of the aliphatic chain (Δ7t(2N)16:0 and Δ15t(2N)16:0) had little additional effect on the areas and compressional moduli of the surface pressure-area isotherms at 35 mN/m (Table 1). The dipole potential-area isotherms for the dihydroceramide species having a trans double bond at C5, C7, or C15 (Δ5t(2N)16:0, Δ7t(2N)16:0, and Δ15t(2N)16:0) are comparable to each other (Fig. 3 B) and to that of d-erythro-dihydroceramide ([DE](2N)16:0) (Fig. 2 B). The potential values are, however, more than 100 mV lower than that for d-erythro-ceramide (Δ4t(2N)16:0) at almost any molecular area we examined. Thus, the presence of a trans double bond beyond C4 on the aliphatic chain, or its complete absence, not only prevents the close packing behavior observed with ceramide but also lowers the interfacial potential.
FIGURE 3.
Role of trans double bond position in the sphingoid base in regulating surface behavior of ceramide analogs. (A) Surface pressure-molecular area isotherms and (B) surface potential-molecular area isotherms for Δ15t(2N)16:0 (▪), Δ7t(2N)16:0 (•), Δ5t(2N)16:0 (▴), and Δ4t(2N)16:0 (▾).
Role of N-acyl unsaturation
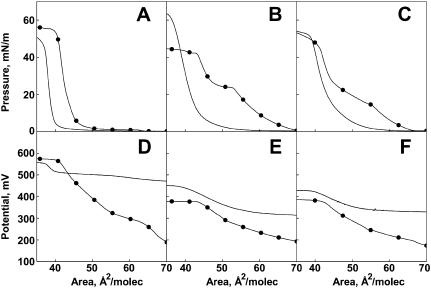
Variants of three of the compounds described above, i.e., the C4-, C5-, and C15-trans dihydroceramide (Δ4t(2N)16:0, Δ5t(2N)16:0, and Δ15t(2N)16:0), were synthesized in which the N-hexadecanoyl moiety was replaced with an N-cis-4-hexadecenoyl moiety (Δ4t(2N) Δ4c16:1, Δ5t(2N) Δ4c16:1, and Δ15t(2N) Δ4c16:1). The effects of this change are shown by pairwise comparison of the isotherms for the saturated (no symbols) and 4-cis-unsaturated analogs in Fig. 4, A–C. Fig. 4 A shows that the installation of the 4-cis double bond to the N-acyl group of ceramide (Δ4t(2N) Δ4c16:1) increases the molecular area at 35 mN/m by ∼4 Å2, whereas the isotherm maintains its condensed character at all but the very lowest surface pressures, where a more expanded phase can be discerned. The introduction of the 4-cis double bond to the N-acyl chain also reduces the modulus of compression at 35 mN/m by 20% (Table 1). With more distal placement of the trans double bond of the sphingoid base (Δ5t(2N) Δ4c16:1 and Δ15t(2N) Δ4c16:1), 4-cis-unsaturation in the N-acyl moiety stabilizes a more expanded surface state up to intermediate surface pressures with condensed behavior returning at high pressures. The onset of condensed phase formation occurs at a higher surface pressure for the C5-trans analog (Δ5t(2N) Δ4c16:1) than for the corresponding C15-trans compound (Δ15t(2N) Δ4c16:1), indicating less efficient packing of the C5-trans analog when the 4-cis double bond is present in the N-acyl chain. Taken together, the isotherms for the three 4-cis unsaturated species again emphasize the unique effect of the C4-trans double bond of the sphingoid base on molecular packing.
FIGURE 4.
Comparison of the surface behavior of N-4-cis-hexadecenoyl- and N-hexadecanoylceramide analogs. (A–C) Surface pressure-molecular area isotherms and (D–F) surface potential-molecular area isotherms for (A and D) Δ4t(2N)16:1 and Δ4t(2N)16:0; (B and E) Δ5t(2N)16:0 and Δ5t(2N)16:1; and (C and F) Δ15t (2N)16:0 and Δ15t(2N)16:1. Lines without symbols denote the N-hexadecanoyl species.
The dipole potential-molecular area isotherms for the compounds shown in Fig. 4, D–F, reflect the presence of the more expanded state that is seen in all of the 4-cis N-acyl species (Fig. 4, A–C) and again emphasize the unique properties of the 4-trans sphingoid base. Whereas the potentials for the C5- and C15-trans compounds having the 4-cis-unsaturated N-acyl moiety (Δ5t(2N) Δ4c16:1 and Δ15t(2N) Δ4c16:1) remain lower than their saturated chain counterparts at all areas, the C4-trans species potential exceeds that of the corresponding N-hexadecanoyl species at lower areas. Moreover, if the two isotherms in Fig. 4 D are compared at 35 mN/m, their potentials are identical (Table 1).
Effects of headgroup stereoisomerism
The structural variations discussed above all involved the introduction of unsaturation into the N-acyl dihydroceramide molecule. Other alterations that have been made include rearrangement of the headgroup substituents and introduction of a third hydroxy group. One is a compound analogous to that with a triple bond at C4 (Fig. 2) but with the reversed positioning of the hydroxy and amino groups at C2 and C3 (ΔΔ4(3N)16:0). Although the presence of a triple bond at C4 had little effect on the packing relative to a 4-trans double bond in the natural sphingoid base (Fig. 2), moving the nitrogen-containing group to C3 and the hydroxy group to C2 caused the molecular area to increase by ∼4.5 Å2. The condensed shape of the isotherm was retained, as shown by the decrease of only 5% in the modulus of compression at 35 mN/m (Table 1). The dipole potential of the C2-OH/C3-N-palmitoyl analog (ΔΔ4(3N)16:0) was only slightly lower than that of the natural C2-N-palmitoyl/C3-OH ceramide (Δ4t(2N)16:0) (Table 1). A similar C2-OH/C3-N-palmitoyl regioisomer having a 4-cis, rather than the natural 4-trans, double bond in the sphingoid base (Δ4c(3N)16:0) exhibited a surface pressure-area isotherm remarkably similar to that of the C2-OH/C3-N-palmitoyl/C4-alkynyl compound (Δ4t(2N)16:0), except for being more expanded at low pressures. However, this compound had both a significantly lower modulus and potential at 35 mN/m compared with the C2-OH/C3-N-palmitoyl/4-alkynyl regioisomer (Δ4t(2N)16:0).
Other headgroup variations with the nitrogen at the natural C2 position also increased the molecular area relative to the natural ceramide with its C4-trans double bond (Fig. 5 A). The removal of the double bond and the addition of a hydroxy group at C4 gave a phytoceramide (4OH(2N)16:0) whose packing properties are very similar to the two C2-OH/C3-N-palmitoyl compounds (ΔΔ4(3N)16:0 and Δ4c(3N)16:0) up to ∼35 mN/m but with rapidly decreasing modulus at higher pressures. Its dipole potential at 35 mN/m was 366 mV, similar to those of several of the other compounds studied (Table 1). However, its potential decreased in the region over which surface pressure increased with decreasing area (Fig. 5). This makes it unique among the set of compounds studied in that its dipole moment is negative. A naturally occurring variant of ceramide with a 3-trans double bond and a hydroxy group at C5 (Δ3t5OH(2N)16:0) was more compact than the other regioisomers studied but still significantly more expanded than the natural ceramide with its 3-hydroxy-4-trans structure (Δ4t(2N)16:0). The molecular area and potential of this species at 35 mN/m were very close to those of d-erythro-dihydroceramide ([DE](2N)16:0), but its modulus of compression was twice as great (Table 1).
FIGURE 5.
Role of polar headgroup structure in regulating surface behavior of ceramide analogs. (A) Surface pressure-molecular isotherms and (B) surface potential-molecular area isotherms for ΔΔ4(3N)16:0 (▪), Δ4c(3N)16:0 (•), 4OH(2N)16:0 (▴), and Δ3t5OH(2N)16:0 (▾).
DISCUSSION
Unsaturation and the packing of ceramides
The presence or absence of the 4-trans double bond in the sphingoid backbone of d-erythro-ceramide has an all-or-none effect on the activity of the molecule in inducing apoptosis (Bielawska et al., 1993), an effect that appears to be independent of the N-acyl chain length (reviewed in Kolesnick et al., 2000). More recently, it has also been shown that both short and long N-acyl chain ceramides, but not dihydroceramides, form channels in mitochondrial (Siskind et al., 2002) and in model (Siskind and Colombini, 2000) membranes. This difference is perplexing because the energy-minimized structures of the d-erythro-ceramides and dihydroceramides studied here are nearly identical (not shown). The previous observation of (Löfgren and Pascher, 1977), confirmed in Fig. 2, is that ceramide (Δ4t(2N)16:0) packs far better with itself at low surface pressures than does dihydroceramide. It has been argued on the basis of NMR solution data acquired at differing states of hydration that ceramide exhibits a high degree of intramolecular hydrogen bonding between the amide proton and the hydroxy groups at C1 and C3. This conformation, which occurs even at full hydration, involves two tightly associated water molecules and occurs with both short and long acyl chain homologs (Li et al., 2002). Additional support is provided by simulations that show hydrogen bonding between a water molecule and the 4-trans double bond of compounds analogous to the headgroup region of the sphingosine base (Vorobyov et al., 2002). In dihydroceramide, this tight intramolecular interaction with water is precluded and the intramolecular hydrogen-bond network is weakened (Li et al., 2002).
If the ceramide headgroup and its associated water molecules comprise an internally satisfied hydrogen-bond network, there would conceivably be a reduced propensity for ceramide to form hydrogen bonds with the surrounding lipid and water molecules. Therefore, there would be minimal steric repulsion as these hydrogen-bonded ceramide-water complexes approach. At close proximity, van der Waals attractive forces between the saturated aliphatic and N-acyl moieties will result in the tight packing and high compressional modulus observed (Fig. 2 and Table 1). Indeed, the low molecular area of ceramide (Δ4t(2N)16:0), on a per-chain basis, is not only the smallest of all of the 15 compounds studied but is equivalent to that observed for close packing of linear hydrocarbon chains (Abrahamsson et al., 1978). This feature of ceramide may contribute to its striking propensity to promote and stabilize the formation of sphingolipid/cholesterol-rich rafts (Xu et al., 2001; Wang and Silvius, 2003).
In contrast to ceramide with its internally satisfied hydrogen-bond network, d-erythro-dihydroceramide ([DE](2N)16:0) shows a greater tendency toward intermolecular interactions between molecules that involves bridging through interfacial water (Li et al., 2002). This tendency may explain its liftoff at much larger molecular areas, atypical isotherm shape, and low compressional modulus. These packing properties may be a result not of repulsive interactions between the lipid molecules, per se, but of strongly hydrogen-bonded water molecules being progressively excluded from between the lipid headgroups as the molecular area is reduced. For this reason the limiting molecular area, i.e., at high surface pressures, should, and does, approach that of ceramide. The l-threo-dihydroceramide diastereomer ([LT](2N)16:0) exhibits a higher modulus of compression than the d-erythro stereoisomer ([DE](2N)16:0) (Fig. 2), and does not pack as tightly as the 4-trans unsaturated ceramide (Δ4t(2N)16:0) at high surface pressures. The reasons for this are not clear but presumably involve a different pattern of hydration.
Relation of ceramide and sphingomyelin packing
The proposal (Yappert and Borchman, 2004) that stronger intermolecular hydrogen bonds between water and dihydrosphingomyelin molecules, compared with sphingomyelin, are responsible for its higher gel-to-liquid crystal transition temperature is consistent with the surface pressure-molecular area properties of ceramide (Δ4t(2N)16:0) and dihydroceramide ([DE](2N)16:0) described above. Also consistent with dihydrosphingomyelin exhibiting stronger intermolecular bonds through water is the finding that N-hexadecanoyldihydrosphingomyelin has a lower onset pressure than N-hexadecanoylsphingomyelin for the transition from the liquid-expanded to a condensed monolayer state (Kuikka et al., 2001). Although this behavior appears to be the opposite of that observed with ceramide and dihydroceramide (Fig. 2 A), it must be noted that the phosphocholine headgroup of both dihydrosphingomyelin and sphingomyelin limits their close approach at low surface pressures. Specifically, extrapolation of the isotherms of the nearly identical condensed states for N-hexadecanoyldihydrosphingomyelin and sphingomyelin (Kuikka et al., 2001) to zero pressure gives a molecular area of ∼55 Å2, a value much greater than the liftoff area of ∼40 Å2 of d-erythro-N-hexadecanoylceramide (Δ4t(2N)16:0) (Fig. 2 A). Thus, the lower onset of the transition pressure for N-hexadecanoyldihydrosphingomyelin reflects the greater stability of its condensed state in a range of molecular areas over which it has formed a network of intermolecular hydrogen bonds with neighboring lipid and water molecules.
Unsaturation and the dipole properties of ceramides
The value of lipid dipole potential is influenced by the presence of unsaturation, particularly cis unsaturation, in the aliphatic moieties of the lipids. For phosphatidylcholines it has been argued, however, that this is an indirect effect resulting from the effects of unsaturation on the spacing of lipid headgroups (Clarke, 2001). With ceramide, which is tightly packed, this is clearly not the case. A trans double bond in a hydrocarbon chain in a medium of uniform dielectric constant should not make a direct contribution to the dipole potential. However, the C4 double bond of ceramide is near both other functional groups, i.e., the C2-amido and C3-hydroxy groups, and is near the lipid-water interface, where dielectric changes markedly. Therefore, the double bond is expected to contribute to the dipole potential. Within the context of the hydration properties of ceramide noted above, the high potential value could also arise in part as a consequence of the interaction of water with the double bond in the ceramide headgroup. Water is a major contributor to the dipole potential (Brockman, 1994). The area and potential isotherms for the other unsaturated ceramide analogs are consistent with this notion. Changing the 4-trans double bond to a triple bond (ΔΔ4(2N)16:0) causes the smallest increase in molecular area relative to ceramide, ∼1 Å2, but the increased unsaturation increases the dipole potential by another 82 mV. Likewise, the 4-allenyl moiety (Δ4Δ5(2N)16:0) increases the potential by 15 mV although its diene-type structure precludes a packing density as high as that of ceramide (Fig. 2). Finally, even moving the N-acyl amide function from C2 to C3 of the sphingosine base although maintaining unsaturation at C4 (ΔΔ4(3N)16:0) results in a potential of 481 mV, a value higher than any of the other species studied that did not have unsaturation at C4.
As the trans double bond is moved progressively farther from the polar region of the sphingoid base and its hydrogen-bonding functionalities, its ability to remain hydrated should diminish and the electronic environment of the double bond should become more symmetric. This was tested with ceramide analogs in which the trans double bond was increasingly distal from the sphingosine headgroup (Fig. 3, A and B). Moving the double bond one or more carbons distal from C4 precluded the close packing observed with ceramide, but the isotherms of the three isomers (Δ5t(2N)16:0, Δ7t(2N)16:0, and Δ15t(2N)16:0) were similar. They were also less expanded than that of d-erythro-dihydroceramide ([DE](2N)16:0). This suggests that the proposed π-hydrogen bond between the double bond and the intramolecularly hydrogen-bonded water (Li et al., 2002) that appears to promote the close packing of ceramide (Fig. 2 A) is diminished or eliminated. The dipole potential data are consistent with this interpretation. As shown in Fig. 6, moving the double bond to C5 (Δ5t(2N)16:0) reduces the potential by >100 mV with little additional change up to C15 (Δ7t(2N)16:0 and Δ15t(2N)16:0). Importantly, beyond C5 the potential value is similar to that of d-erythro-dihydroceramide ([DE](2N)16:0) (Fig. 6, dotted line), which lacks the double bond. This observation suggests that the more distal double bonds are in a symmetrical environment and are less hydrated than the double bond at C4. The addition of a cis double bond at C4 of the N-acyl chain (Δ4t(2N) Δ4c16:1) has the expected expanding effect and induces liquid-expanded monolayer states at larger areas (Fig. 4 A). Despite its increased area, N-4-cis-hexadecenoylceramide (Δ4t(2N) Δ4c16:1) has the same potential at 35 mN/m as N-hexadecanoylceramide (Δ4t(2N)16:0) (Table 1). Moreover, the 4-cis-hexadecenoyl derivatives of the 5- or 15-trans unsaturated ceramides (Δ5t(2N) Δ4c16:1 and Δ15t(2N) Δ4c16:1) show the same drop in potential to a value similar to that of d-erythro-dihydroceramide ([DE](2N)16:0) as was observed with the N-hexadecanoyl analogs (Fig. 6). Thus, the behavior of the area and potentials of the sphingosine analogs supports the notion that the unique hydration and asymmetric electronic environment of the 4-trans compound give rise to high dipole potentials. This may partially explain why biological activity depends on the double bond being between C4 and C5 rather than between C5 and C6 (He et al., 1999).
FIGURE 6.
Dominance of the sphingoid base double bond position in determining the dipole potential at 35 mN/m N-acyl saturated and unsaturated ceramide analogs; N-hexadecanoylceramides (▪), N-4-cis-hexadecenoylceramides (▴), and dihydroceramide (·······).
Comparison of ceramide and sphingomyelin dipole potentials
A notable property of ceramide (Δ4t(2N)16:0) is its high dipole potential of 546 mV at 35 mN/m, a value 162 mV higher than that of d-erythro-dihydroceramide ([DE](2N)16:0) and 269 mV higher than that of the l-threo diastereomer ([LT](2N)16:0) (Table 1). Not only does the difference of 162 mV far exceed that observed between N-palmitoylsphingomyelin and dihydrosphingomyelin (Kuikka et al., 2001), the absolute potential of ceramide at 35 mN/m is greater than that of sphingomyelin by 240 mV. Thus, the generation of ceramide from sphingomyelin in a membrane at 35 mN/m will cause not only an area contraction of ∼30%, estimated from Fig. 2 and data in Kuikka et al. (2001), but will also result in an approximate doubling of the local electrostatic field. Obviously, such a change should markedly affect those membrane phenomena, as noted in the Introduction, that are regulated by dipole potential.
Structure and biological activity of ceramides
The two regioisomers of ceramide, having the N-hexadecanoyl moiety at C3 rather than at C2 (ΔΔ4(3N)16:0 and Δ4c(3N)16:0) (Fig. 3 A), were, together with other ceramide analogs, as effective as ceramide itself in promoting the membrane permeabilization by Vibrio cholerae cytolysin (Zitzer et al., 2001). The isotherms for these compounds are condensed and nearly identical above 20 mN/m but are expanded by ∼6 Å2 relative to ceramide. This shows that close packing of ceramide species are not required for this biological activity. One of these compounds has a C4-cis double bond (Δ4c(3N)16:0) and the other has a C4 triple bond (ΔΔ4(3N)16:0). Because the C4 triple bond analog of ceramide (ΔΔ4(2N)16:0) was nearly as compact as ceramide itself (Δ4t(2N)16:0), the expansion exhibited by the stereoisomer (ΔΔ4(3N)16:0) apparently arises from repositioning of the amido moiety rather than the unsaturation. Interestingly, although the C4 triple bond analog (ΔΔ4(3N)16:0) has a relatively high dipole potential, consistent with water binding as discussed above, the regioisomer with the 4-cis double bond (Δ4c(3N)16:0) has a potential more comparable to that of d-erythro-dihydroceramide ([DE](2N)16:0). The other regioisomer studied has the trans double bond at C3 and the hydroxy group at C5 (4OH(2N)16:0). This allows efficient packing at high surface pressures (Fig. 4 A) although the monolayer is much more compressible than that of ceramide or the other regioisomers. This naturally occurring compound has a low dipole potential, comparable to that of d-erythro-dihydroceramide ([DE](2N)16:0). Because it exhibits stronger antiproliferative activity than ceramide (Chun et al., 2003a), the antiproliferative activity of natural ceramides cannot arise solely as a consequence of their high dipole potentials.
CONCLUSION
Overall, this study shows that small variations in ceramide structure, known to have large affects on biological activity, also have large effects on the behavior of ceramides at interfaces. In particular, tightly packing and incompressible structures are associated with ceramides that bear a 2-N-acyl chain, as compared to 3-N-acyl chain, and with unsaturation at C4 of the sphingoid base. Such structures appear to promote tight water association through internal hydrogen bonding. Ceramide analogs with these properties also exhibit high dipole potentials that could contribute to their phase behavior in membranes and their regulation of biological activities. These high potentials appear to result from the both the hydration of the C4 unsaturation and its asymmetric electronic environment. The availability of derivatives such as these will allow the dependence of biological activities to be tested more rationally. For example, the 3-N-acyl regioisomer with a triple bond at C4 (ΔΔ4(3N)16:0) shares with ceramide (Δ4t(2N)16:0) a high dipole potential and a high modulus of compression. However, its area is ∼6 Å2 greater than that of ceramide. Likewise, there are a series of analogs that have almost identical areas at 35 mN/m but different dipole potentials and compressibilities. Lastly, the trends revealed in this study with respect to unsaturation may aid in the development of therapeutically useful ceramide analogs.
Acknowledgments
This work was supported by U.S. Public Health Service grants HL16660 (R.B.), HL49180 (H.L.B.), and HL GM45928 (R.E.B.) and by the Hormel Foundation (H.L.B. and R.E.B.).
References
- Abrahamsson, S., B. Dahlén, H. Löfgren, and I. Pascher. 1978. Lateral packing of hydrocarbon chains. Prog. Chem. Fats Other Lipids. 16:125–143. [DOI] [PubMed] [Google Scholar]
- Bielawska, A., H. M. Crane, D. Liotta, L. M. Obeid, and Y. A. Hannun. 1993. Selectivity of ceramide-mediated biology. Lack of activity of erythro-dihydroceramide. J. Biol. Chem. 268:26226–26232. [PubMed] [Google Scholar]
- Brockman, H. L. 1994. Dipole potential of lipid membranes. Chem. Phys. Lipids. 73:57–79. [DOI] [PubMed] [Google Scholar]
- Brockman, H. L. 1999. Lipid monolayers: why use half a membrane to characterize protein-membrane interactions? Curr. Opin. Struct. Biol. 9:438–443. [DOI] [PubMed] [Google Scholar]
- Brockman, H. L., J. M. Smaby, and D. E. Jarvis. 1984. Automation of surface cleaning and sample addition for surface balances. J. Phys. E: Sci. Instrum. 17:351–353. [Google Scholar]
- Chalfant, C. E., Z. Szulc, P. Roddy, A. Bielawska, and Y. A. Hannun. 2004. The structural requirements for ceramide activation of serine-threonine protein phosphatases. J. Lipid Res. 45:496–506. [DOI] [PubMed] [Google Scholar]
- Chun, J., H.-S. Byun, G. Arthur, and R. Bittman. 2003a. Synthesis and growth inhibitory activity of chiral 5-hydroxy-2-N-acyl-(3E)-sphingenines: ceramides with an unusual sphingoid backbone. J. Org. Chem. 68:355–359. [DOI] [PubMed] [Google Scholar]
- Chun, J., H.-S. Byun, and R. Bittman. 2003b. First asymmetric synthesis of 6-hydroxy-4-sphingenine-containing ceramides. Use of chiral propargylic alcohols to prepare a lipid found in human skin. J. Org. Chem. 68:348–354. [DOI] [PubMed] [Google Scholar]
- Clarke, R. J. 2001. The dipole potential of phospholipid membranes and methods for its detection. Adv. Colloid Interface Sci. 89:263–281. [DOI] [PubMed] [Google Scholar]
- Corver, J., L. Moesby, R. K. Erukulla, K. C. Reddy, R. Bittman, and J. Wilschut. 1995. Sphingolipid-dependent fusion of Semliki Forest virus with cholesterol-containing liposomes requires both the 3-hydroxyl group and the double bond of the sphingolipid backbone. J. Virol. 69:3220–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremesti, A. E., F. M. Goni, and R. Kolesnick. 2002. Role of sphingomyelinase and ceramide in modulating rafts: do biophysical properties determine biologic outcome? FEBS Lett. 531:47–53. [DOI] [PubMed] [Google Scholar]
- Fanani, M. L., and B. Maggio. 1997. Mutual modulation of sphingomyelinase and phospholipase A2 activities against mixed lipid monolayers by their lipid intermediates and glycosphingolipids. Mol. Membr. Biol. 14:25–29. [DOI] [PubMed] [Google Scholar]
- He, L., H.-S. Byun, and R. Bittman. 2000. A stereocontrolled, efficient synthetic route to bioactive sphingolipids: synthesis of phytosphingosine and phytoceramides from unsaturated ester precursors via cyclic sulfate intermediates. J. Org. Chem. 65:7618–7626. [DOI] [PubMed] [Google Scholar]
- He, L., H.-S. Byun, J. Smit, J. Wilschut, and R. Bittman. 1999. Enantioselective synthesis of a novel trans double bond ceramide analogue via catalytic asymmetric dihydroxylation of an enyne. The role of the trans double bond of ceramide in the fusion of Semliki Forest virus with target membranes. J. Am. Chem. Soc. 121:3897–3903. [Google Scholar]
- Holopainen, J. M., H. L. Brockman, R. E. Brown, and P. K. J. Kinnunen. 2001. Interfacial interactions of ceramide with dimyristoylphosphatidylcholine: impact of the N-acyl chain. Biophys. J. 80:765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H.-W., E. M. Goldberg, and R. Zidovetzki. 1999. Ceramides modulate protein kinase C activity and perturb the structure of phosphatidylcholine/phosphatidylserine bilayers. Biophys. J. 77:1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jendrasiak, G. L., and R. L. Smith. 2001. The effect of the choline head group on phospholipid hydration. Chem. Phys. Lipids. 113:55–66. [DOI] [PubMed] [Google Scholar]
- Karasavvas, N., R. K. Erukulla, R. Bittman, R. Lockshin, and Z. Zakeri. 1996. Stereospecific induction of apoptosis in U937 cells by N-octanoyl-sphingosine stereoisomers and N-octyl-sphingosine. The ceramide amide group is not required for apoptosis. Eur. J. Biochem. 236:729–737. [DOI] [PubMed] [Google Scholar]
- Kester, M., and R. Kolesnick. 2003. Sphingolipids as therapeutics. Pharmacol. Res. 47:365–371. [DOI] [PubMed] [Google Scholar]
- Kolesnick, R. N. 1991. Sphingomyelin and derivatives as cellular signals. Prog. Lipid Res. 30:1–38. [DOI] [PubMed] [Google Scholar]
- Kolesnick, R. N., F. M. Goñi, and A. Alonso. 2000. Compartmentalization of ceramide signaling: physical foundations and biological effects. J. Cell. Physiol. 184:285–300. [DOI] [PubMed] [Google Scholar]
- Kuikka, M., B. Ramstedt, H. Ohvo-Rekilä, J. Tuuf, and J. P. Slotte. 2001. Membrane properties of D-erythro-N-acyl sphingomyelins and their corresponding dihydro species. Biophys. J. 80:2327–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X.-M., M. M. Momsen, J. M. Smaby, H. L. Brockman, and R. E. Brown. 2001. Cholesterol decreases the interfacial elasticity and detergent solubility of sphingomyelins. Biochemistry. 40:5954–5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L., X. Tang, K. G. Taylor, D. B. DuPré, and M. C. Yappert. 2002. Conformational characterization of ceramides by nuclear magnetic resonance spectroscopy. Biophys. J. 82:2067–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfgren, H., and I. Pascher. 1977. Molecular arrangements of sphingolipids. The monolayer behaviour of ceramides. Chem. Phys. Lipids. 20:273–284. [DOI] [PubMed] [Google Scholar]
- MacDonald, R. C., and S. A. Simon. 1987. Lipid monolayer states and their relationships to bilayers. Proc. Natl. Acad. Sci. USA. 84:4089–4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio, B. 1999. Modulation of phospholipase A2 by electrostatic fields and dipole potential of glycosphingolipids in monolayers. J. Lipid Res. 40:930–939. [PubMed] [Google Scholar]
- Maggio, B., F. A. Cumar, and R. Caputto. 1978. Surface behaviour of gangliosides and related glycosphingolipids. Biochem. J. 171:559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh, D. 1996. Lateral pressure in membranes. Biochim. Biophys. Acta. 1286:183–223. [DOI] [PubMed] [Google Scholar]
- McConnell, H. M., and R. de Koker. 1996. Equilibrium thermodynamics of lipid monolayer domains. Langmuir. 12:4897–4904. [Google Scholar]
- Megha, and E. London. 2004. Ceramide selectively displaces cholesterol from ordered lipid domains (rafts). Implications for lipid raft structure and function. J. Biol. Chem. 279:9997–10004. [DOI] [PubMed] [Google Scholar]
- Menaldino, D. S., A. Bushnev, A. M. Sun, D. C. Liotta, H. Symolon, K. Desai, D. L. Dillehay, Q. Peng, E. Wang, J. Allegood, S. Trotman-Pruett, M. C. Sullards, and A. H. Merrill, Jr. 2003. Sphingoid bases and de novo ceramide synthesis: enzymes involved, pharmacology and mechanisms of action. Pharmacol. Res. 47:373–381. [DOI] [PubMed] [Google Scholar]
- Merrill, A. H., and K. Sandhoff. 2002. Sphingolipids: metabolism and cell signaling. In Biochemistry of Lipids, Lipoproteins and Membranes. D. E. Vance and J. E. Vance, editors. Elsevier, Amsterdam, The Netherlands. 373–407.
- Moesby, L., J. Corver, R. K. Erukulla, R. Bittman, and J. Wilschut. 1995. Sphingolipids activate membrane fusion of Semliki Forest virus in a stereospecific manner. Biochemistry. 34:10319–10324. [DOI] [PubMed] [Google Scholar]
- Pascher, I. 1976. Molecular arrangements in sphingolipids. Conformation and hydrogen bonding of ceramide and their implication on membrane stability and permeability. Biochim. Biophys. Acta. 455:433–451. [DOI] [PubMed] [Google Scholar]
- Porschke, D. 1997. Macrodipoles. Unusual electric properties of biological macromolecules. Biophys. Chem. 66:241–257. [DOI] [PubMed] [Google Scholar]
- Ruvolo, P. P. 2003. Intracellular signal transduction pathways activated by ceramide and its metabolites. Pharmacol. Res. 47:383–392. [DOI] [PubMed] [Google Scholar]
- Shah, D. O., and J. H. Schulman. 1967. Interaction of calcium ions with lecithin and sphingomyelin monolayers. Lipids. 2:21–27. [DOI] [PubMed] [Google Scholar]
- Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature. 387:569–572. [DOI] [PubMed] [Google Scholar]
- Siskind, L. J., and M. Colombini. 2000. The lipids C2- and C16-ceramide form large stable channels. Implications for apoptosis. J. Biol. Chem. 275:38640–38644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind, L. J., R. N. Kolesnick, and M. Colombini. 2002. Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. J. Biol. Chem. 277:26796–26803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struckhoff, A. P., R. Bittman, M. E. Burow, S. Clejan, S. Elliot, T. Hammond, Y. Tang, and B. S. Beckman. 2004. Novel ceramide analogs as potential chemotherapeutic agents in breast cancer. J. Pharmacol. Exp. Ther. 309:523–532. [DOI] [PubMed] [Google Scholar]
- van Blitterswijk, W. J., A. H. van der Luit, R. J. Veldman, M. Verheij, and J. Borst. 2003. Ceramide: second messenger or modulator of membrane structure and dynamics? Biochem. J. 369:199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobyov, I., M. C. Yappert, and D. B. DuPré. 2002. Energetic and topological analyses of cooperative σH- and πH-bonding interactions. J. Phys. Chem. 106:10691–10699. [Google Scholar]
- Wang, T.-Y., and J. R. Silvius. 2003. Sphingolipid partitioning into ordered domains in cholesterol-free and cholesterol-containing lipid bilayers. Biophys. J. 84:367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, X., R. Bittman, G. Duportail, D. Heissler, C. Vilcheze, and E. London. 2001. Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts). Comparison of cholesterol to plant, fungal, and disease-associated sterols and comparison of sphingomyelin, cerebrosides, and ceramide. J. Biol. Chem. 276:33540–33546. [DOI] [PubMed] [Google Scholar]
- Yappert, M. C., and D. Borchman. 2004. Sphingolipids in human lens membranes: an update on their composition and possible biological implications. Chem. Phys. Lipids. 129:1–20. [DOI] [PubMed] [Google Scholar]
- Zitzer, A., R. Bittman, C. A. Verbicky, R. K. Erukulla, S. Bhakdi, S. Weis, A. Valeva, and M. Palmer. 2001. Coupling of cholesterol and cone-shaped lipids in bilayers augments membrane permeabilization by the cholesterol-specific toxins streptolysin O and Vibrio cholerae cytolysin. J. Biol. Chem. 276:14628–14633. [DOI] [PubMed] [Google Scholar]