Abstract
We examined the influence of cross-bridge cycling kinetics on the length dependence of steady-state force and the rate of force redevelopment (ktr) during Ca2+-activation at sarcomere lengths (SL) of 2.0 and 2.3 μm in skinned rat cardiac trabeculae. Cross-bridge kinetics were altered by either replacing ATP with 2-deoxy-ATP (dATP) or by reducing [ATP]. At each SL dATP increased maximal force (Fmax) and Ca2+-sensitivity of force (pCa50) and reduced the cooperativity (nH) of force-pCa relations, whereas reducing [ATP] to 0.5 mM (low ATP) increased pCa50 and nH without changing Fmax. The difference in pCa50 between SL 2.0 and 2.3 μm (ΔpCa50) was comparable between ATP and dATP, but reduced with low ATP. Maximal ktr was elevated by dATP and reduced by low ATP. Ca2+-sensivity of ktr increased with both dATP and low ATP and was unaffected by altered SL under all conditions. Significantly, at equivalent levels of submaximal force ktr was faster at short SL or increased lattice spacing. These data demonstrate that the SL dependence of force depends on cross-bridge kinetics and that the increase of force upon SL extension occurs without increasing the rate of transitions between nonforce and force-generating cross-bridge states, suggesting SL or lattice spacing may modulate preforce cross-bridge transitions.
INTRODUCTION
A fundamental property of cardiac muscle is its ability to increase contractile force when stretched, facilitating the matching of cardiac output with venous return (Frank-Starling's Law of the Heart). The strong length dependence of cardiac contraction manifests as an enhancement of force at all levels of intracellular [Ca2+] when sarcomere length (SL) is increased (Gordon et al., 2000; Fuchs, 2002; Konhilas et al., 2002a). It is generally accepted that force in both skeletal and cardiac muscle is a product of the availability of myosin binding sites on thin filament actin (the level of thin filament activation), which is dependent on the position of the dimeric regulatory protein tropomyosin (Tm) on the surface of the two helical actin strands that compose thin filaments. Tm overlays 7 actin monomers along each strand, with head-to-tail overlap between adjacent Tms. Each Tm binds in the overlap region with a troponin complex (Tn), which contains a Ca2+-binding subunit (TnC), an actin binding subunit (TnI) and a subunit (TnT) that anchors TnC/TnI to Tm (see Gordon et al., 2000 for review). In the absence of Ca2+ TnI binds to actin, holding Tm in a position that inhibits myosin head-actin (cross-bridge) interaction (“blocked” state), and when Ca2+ binds to TnC, TnI binding to actin is decreased, allowing Tm to assume a position that is permissive to cross-bridge binding (“closed” state). As cross-bridge binding becomes stronger Tm is further displaced on the thin filaments exposing more strong binding surface on actin, allowing force generation (“open” state) (Lehrer and Geeves, 1998; Geeves and Holmes, 1999; Gordon et al., 2000). Thus in striated muscle the availability of thin filament binding sites for cross-bridges is determined by a balance of the effects of Ca2+-binding to troponin and strong cross-bridge formation on the position of Tm. In cardiac muscle, and to a lesser extent in skeletal muscle, the ability of cross-bridges to activate thin filaments is further enhanced by a cross-bridge-dependent increase in the affinity of troponin for Ca2+ (Hofmann and Fuchs, 1987; Wang and Fuchs, 1994; Martyn et al., 2001).
It has been suggested that increasing force at longer SL results from the accompanying decrease in lateral myofilament lattice spacing (Fuchs and Wang, 1996; Fuchs, 2002) which increases the proximity of myosin heads to actin in skinned cardiac muscle (Konhilas et al., 2002b; Martyn et al., 2004). When lattice spacing is decreased by osmotic compression, independent of changes in SL, the Ca2+-sensitivity of force increases, as it does when SL is increased (McDonald and Moss, 1995; Fuchs and Wang, 1996). Additionally, reduced lattice spacing has been suggested to increase the probability of strong cross-bridge binding and force generation, as evidenced by an increased rate of isometric force redevelopment (ktr) when SL is stretched in maximally activated skinned skeletal muscle fibers (McDonald et al., 1997). This important hypothesis has not been systematically tested in cardiac muscle. If increasing SL increases force by enhancing the probability of cross-bridge transition from nonforce into force-generating states, then force and ktr would be likely to have a similar SL or lattice-spacing dependence.
Although there is evidence supporting a role for lattice spacing in force regulation, it has recently been suggested that SL alters force independent of changes in lattice spacing. Konhilas et al. (2002b) found a lack of correlation between the Ca2+-sensitivity of force and the lattice spacing of relaxed skinned cardiac muscle. Consistent with this idea, the Ca2+ sensitivity of force was found to decrease at longer SL when passive force was reduced by selective digestion of titin, suggesting a role for titin stretch in enhancing cardiac contractile activation (Cazorla et al., 2001; Fukuda et al., 2001). However, recent evidence indicates that titin strain may affect force by altering lattice spacing (Fukuda et al., 2003). Finally, our own observations of an increase in the attached, nonforce-bearing cross-bridge population as lattice spacing is compressed raises the possibility that the SL and lattice-spacing dependence of force may be mediated by altering the probability of the initial, preforce cross-bridge interactions (Martyn et al., 2004). Thus the molecular mechanisms by which changes in SL and lattice-spacing modulate cardiac contractile activation remains poorly understood (Konhilas et al., 2002b; Moss and Fitzsimons, 2002).
To date most evidence suggests that the steep SL dependence of force in cardiac muscle results from the sensitivity of cardiac thin filaments to activation by cross-bridges, coupled with changes in the probability of strong cross-bridge interactions as SL or lattice spacing is altered; decreasing SL or increasing lattice spacing would cause not only a decrease in strong cross-bridge binding but also a corresponding decrease of thin filament activation. A role for strong cross-bridge binding in defining the SL and lattice spacing dependence of contractile activation is emphasized by several observations. For example, inhibition of force during Ca2+-activation reduces the SL dependence of Ca2+-binding to cardiac thin filaments (Hofmann and Fuchs, 1987; Wang and Fuchs, 1994) and the corresponding conformational changes in cardiac troponin C (cTnC) (Martyn and Gordon, 2001), emphasizing the ability of cross-bridges to not only enhance thin filament activation but also its length dependence. On the other hand when cross-bridge binding was enhanced by elevated MgADP, the Ca2+-sensitivity of activation was increased whereas the effects of SL on force-pCa (pCa = −log[Ca2+]) relations were diminished (Fukuda et al., 2000). Similarly, the addition of exogenous strong binding, slowly cycling myosin heads eliminated the SL-dependence of force-pCa relations in cardiac myocytes (Fitzsimons and Moss, 1998). The latter two interventions have in common enhanced thin filament activation by slowly cycling cross-bridges, implying that interventions that either inhibit or enhance strong cross-bridge interactions and their contribution to thin filament activation diminish the SL dependence of activation in cardiac muscle. Finally, in addition to cross-bridge binding, the kinetics of cross-bridge cycling could influence the SL dependence of contraction, as implied by observations that the apparent kinetics of cross-bridge and thin filament activation may be coupled in striated muscle (Hancock et al., 1997; Regnier et al., 1998; Adhikari and Wang, 2004).
In this study we tested two hypotheses. First, we tested the hypothesis that increasing SL (and decreasing lattice spacing) enhances force generation by increasing the rate of cross-bridge transitions into strong force producing states. This was done by comparing the steady-state force-pCa and ktr-pCa relations at 2.0 and 2.3 μm SL over the full range of Ca2+-activation. Second, we tested whether the contribution of cross-bridges to the SL or lattice-spacing dependence of force is dependent on actin-myosin cycle kinetics. Cross-bridge kinetics were accelerated by replacement of ATP with 2-deoxy-ATP (dATP) as the contractile substrate (Regnier et al., 2000) or slowed by lowering [ATP] from 5 mM to 0.5 mM. The effects of these interventions on the SL dependence of force-pCa and ktr-pCa relations were determined. Whereas the Ca2+-sensitivity of force and maximal force were increased at both long and short SL by dATP, the SL dependence of force-pCa relations were unaffected. In contrast, lowering [ATP], which slowed cross-bridge cycling, also increased the Ca2+-sensitivity of force yet the SL-dependence of force-pCa relations was significantly decreased. Surprisingly, under all nucleotide conditions tested, decreasing SL significantly decreased the maximum Ca2+-activated force without a corresponding decrease in maximum ktr, whereas at equivalent intermediate submaximal forces ktr was actually higher at the shorter SL. Preliminary reports have been previously published in abstract form (Martyn and Gordon, 2001; Kreutziger et al., 2003; Adhikari et al., 2004).
METHODS
Cardiac trabeculae preparation
Single cardiac trabeculae were prepared from male Sprague-Dawley rats (200–250 gm) as described previously (Martyn and Gordon, 2001). Briefly, rats were anesthetized by an intraperitonial injection of pentobarbital (50 mg/kg) and their hearts were removed rapidly and placed in oxygenated, physiological salt solution (in mM): 100 NaCl, 24 NaHCO3, 2.5 KCl, 1 MgS04.7H2O, 1 NaHPO4, and 1 CaCl2. Small trabeculae (100–200 μm diameter, 1–2 mm length) were dissected from the right ventricle and their surface membranes were rendered permeable to bathing solution constituents by exposing them for 12 h at 4°C to a skinning solution containing (in mM): 100 KCl, 9.0 MgCl2, 4.0 MgATP, 5.0 K2EGTA (ethylene glycol-bis-(b-aminoethylether)-N,N,N,N-tetraacetic acid), 10 MOPS (3-(n-morpholino) propane sulfonic acid), 1% (v/v) nonionic detergent Triton X-100, pH 7.0, and 50% (v/v) glycerol and 1% (v/v) protease inhibitor cocktail (P-8340, Sigma). The skinned trabeculae were stored in the same solution without Triton X-100 at −20°C and used for experiments within 1 week. On average, trabeculae dimensions were 1500 ± 50 × 156 ± 10 μm (length × diameter; mean ± SE; n = 20).
Mechanics measurements
The ends of single trabeculae were wrapped in aluminum foil T-clips for attachment to a force transducer (Model 400A, 2.2 kHz resonant frequency, Cambridge Technology, Watertown, MA) and a servomotor (model 300, Cambridge Technology, Watertown, MA) tuned for a 350-μs step response. SL was measured and adjusted under relaxing conditions using laser diffraction techniques as described previously (Chase et al., 1994a). Force, length and SL signals were low pass filtered (fc = 40% of the sampling rate) with a CyberAmp 380 (Axon Instruments, Foster City, CA) and recorded digitally with a 12-bit resolution. All experiments were done at 15°C.
The rate of force redevelopment after a release-restretch protocol (ktr), was measured by applying a shortening ramp (∼15% muscle length (L0) at 4 L0 s−1) that reduced force to zero, followed by a fast (350 μs) under-damped restretch to the original muscle length. ktr was calculated from the halftime of force redevelopment (Chase et al., 1994b). Unloaded shortening velocity (Vu) was determined using the slack test method (Edman, 1975).
Solutions
Solutions contained (in mM): 15 phosphocreatine, 15 EGTA, 80 MOPS, 1 Mg2+, 135 Na+ + K+, 1 dithiothreitol (DTT), 250 units/ml−1 creatine kinase (CK, Sigma), and 5 mM ATP, or 0.5 mM ATP, or 5 mM 2 deoxy-ATP (dATP; Sigma) at pH 7.0 and ionic strength 0.17 M. Mg2+ affinities of dATP and ATP were assumed to be the same. For activation solutions, [Ca2+] (expressed as pCa = −log[Ca2+]) was varied by adding Ca2+-propionate. In a subset of experiments dextran T500 (4% w/v) was added to all solutions.
Data analysis
Force-pCa data were fit by the Hill equation,
 |
where Fmax is the maximally activated force, nH is the Hill coefficient or slope and pCa50 is the pCa at which force is half-maximal. The reported pCa50 and nH values represent the means of the values from the individual fits, ± standard error of the means. Means are compared with Student's t-test with significance at the 95% confidence level (P < 0.05). Modeling of ktr with a six-state cross-bridge cycle was carried out as described previously (Regnier and Homsher, 1998).
RESULTS
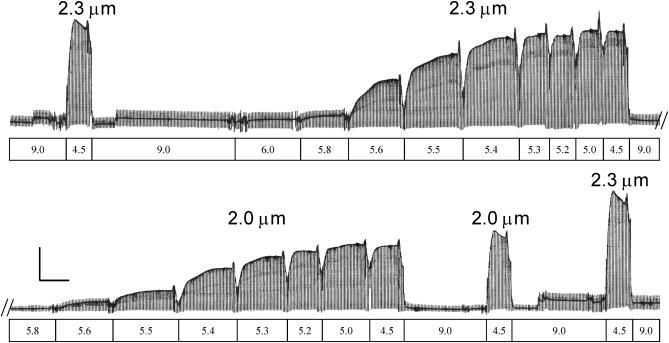
The Ca2+-dependence of isometric steady-state force and force redevelopment rate (ktr) at SL 2.0 and 2.3 μm were determined for single-skinned trabeculae at 15°C using the protocol illustrated in Fig. 1. In this example shown for 5 mM ATP, after an initial maximal activation at SL 2.3 μm the trabecula was activated at SL 2.3 μm step-wise by bathing it in solutions of decreasing pCa. Steady-state force and ktr were measured at each pCa. Initial SL was then adjusted to 2.0 μm and the protocol was repeated, followed by a final set of maximal activations at SL 2.0 and 2.3 μm (Fig. 1). The order of initial SL was alternated and experimental results were rejected if force at pCa 4.5 (Fmax) declined by more than 15% at 2.3 μm SL. Additionally, comparison between dATP or low ATP and corresponding ATP controls were carried out between different sets of trabeculae obtained from the same hearts to avoid excessive Fmax decline.
FIGURE 1.
Experimental protocol and example trace of trabecula activation at SL 2.0 and 2.3 μm with 5 mM ATP. SL and solution pCa are indicated above and below the trace. A single trabecula (length 1.4 mm, diameter 198 μm) at SL 2.3 μm was maximally activated (pCa 4.5) and relaxed (pCa 9.0), followed by SL adjustment (2.3 μm) and a series of step-wise activations from submaximal to maximal activations (pCa 6.0–4.5). The trabecula was then relaxed, adjusted to SL 2.0 μm and subjected to another series of step-wise activation and relaxation. Finally, to assess reproducibility, the trabecula was maximally activated (pCa 4.5) at SL 2.0 and 2.3 μm as shown. The periodic transients in the trace are due to a release-restretch protocol. The vertical calibration bar is 10 mN/mm2 and the horizontal bar is 100 s.
SL dependence of steady-state force
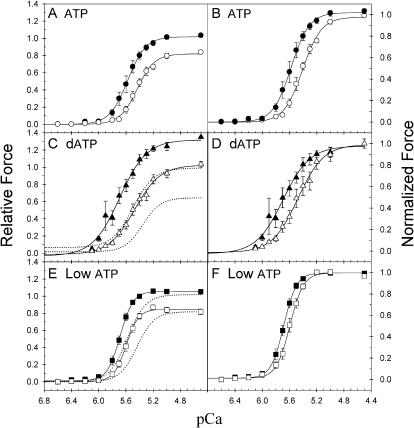
Force-pCa relations obtained with different nucleotide conditions are summarized in Fig. 2. Steady-state isometric force is plotted against pCa at SL 2.0 and 2.3 μm for 5 mM ATP (Fig. 2, A and B), 5 mM dATP (Fig. 2, C and D) and 0.5 mM ATP (Fig. 2, E and F). For comparison the data are normalized either to the Fmax in 5 mM ATP at 2.3 μm SL (Fig. 2, A, C, and E) or to the Fmax at each SL under each condition (Fig. 2, B, D, and F). The force-pCa data from individual trabeculae were fit with the Hill equation and the mean (± SE) pCa50 and nH values are given in Table 1. Decreasing SL-reduced steady-state force at all Ca2+ concentrations in all three conditions (Fig. 2). In ATP when SL was decreased from 2.3 to 2.0 μm, the Fmax decreased by 23 ± 4% (n = 15), the pCa50 decreased by 0.16 (ΔpCa50) and the nH decreased by 1.0 (Fig. 2, A and B; Table 1).
FIGURE 2.
SL dependence of Ca2+-activation of force under differing nucleotide conditions. Force-pCa relations are compared at SL 2.3 (solid symbols) and 2.0 (open symbols) μm for 5.0 mM ATP (A, B; •, ○), 5.0 mM dATP (C, D; ▴, ▵) and 0.5 mM ATP (E, F; ▪, □). Force is scaled to either the Fmax in ATP at 2.3 μm SL (left panels) or to the respective Fmax for each condition and SL (right panels). The error bars represent mean ± SE from 8, 10, and 13 trabecula for ATP, dATP, and low ATP, respectively. The data was fit with the Hill equation and the corresponding values of pCa50 and nH are included in Table 1. The dotted curves in C and E are the Hill fit curves for the corresponding sets of control (5.0 mM ATP) experiments.
TABLE 1.
Summary of fitted parameters for force-pCa and ktr-pCa relations for 5 mM ATP (ATP), 5 mM dATP (dATP), and 0.5 mM ATP (low ATP)
| ATP
|
dATP
|
Low ATP
|
||||
|---|---|---|---|---|---|---|
| 2.3 μm | 2.0 μm | 2.3 μm | 2.0 μm | 2.3 μm | 2.0 μm | |
| Fmax | 1.0 | 0.84 ± 0.02 | *1.35 | 1.04 ± 0.03 | *1.06 | 0.82 ± 0.03 |
| pCa50 | 5.55 ± 0.04 | 5.39 ± 0.03 | 5.66 ± 0.04 | 5.46 ± 0.05 | 5.70 ± 0.02 | 5.61 ± 0.02 |
| nH | 5.1 ± 0.4 | 4.1 ± 0.2 | 3.8 ± 0.5 | 3.3 ± 0.3 | 5.5 ± 0.4 | 5.3 ± 0.5 |
| ktr(max), s−1 | 10.3 ± 1.5 | 11.8 ± 1.2 | 16.7 ± 1.7 | 18.2 ± 2.0 | 7.9 ± 0.9 | 9.8 ± 1.3 |
| pCa50(ktr) | 5.26 ± 0.04 | 5.16 ± 0.05 | 5.41 ± 0.06 | 5.35 ± 0.08 | 5.56 ± 0.03 | 5.51 ± 0.04 |
Values shown are averages of 15 (ATP), 10 (dATP), and 13 (low ATP) experiments with errors shown as mean ± SE.
The relative values of Fmax for dATP and low ATP were determined in separate experiments.
When dATP was substituted for ATP steady-state force increased at all activating pCa at both SL compared to the corresponding controls (Fig. 2 C, dotted lines). We previously reported an increased rate of cross-bridge cycling with dATP, as evidenced by increased ktr and unloaded shortening velocity (Vu) in skinned cardiac muscle (Regnier et al., 2000). The increase in Fmax and pCa50 by dATP was similar at both lengths, so that the difference in pCa50 (ΔpCa50) between the two SLs was comparable to ATP (Fig. 2, C and D; Table 1). In contrast nH at each SL decreased relative to ATP control (Table 1). The increase in Fmax and pCa50 and the decrease in nH observed with dATP is similar to elevated [ADP] (Fukuda et al., 2000). However, unlike dATP, elevated [ADP] causes a reduction of ΔpCa50 (Fukuda et al., 2000). Thus although the faster cross-bridge cycling with dATP increased the apparent level of thin filament activation (increased Fmax and increased pCa50 with respect to ATP), this did not lead to a reduction in the SL-dependence of activation.
When cross-bridge cycling rate was reduced with 0.5 mM ATP (low ATP), there was no significant change in Fmax at either SL relative to ATP (Fig. 2 E). Slowed cross-bridge cycling at low ATP was evidenced by a decrease of Vu at maximal activation (pCa 4.5) at SL 2.3 μm from 3.5 ± 0.4 L0s−1 (n = 10) with ATP to 2.2 ± 0.5 (n = 6) with low ATP (P < 0.05). At SL 2.3 μm pCa50 increased by 0.13 (Table 1) with low ATP, which is similar to that of dATP. However, at SL 2.0 μm low ATP increased pCa50 by 0.2, approximately twice that found for dATP (0.09), leading to a reduction of ΔpCa50 (Fig. 2, E and F; Table 1). At both SL nH in low ATP was higher than with dATP and either similar to (2.3 μm SL) or greater (2.0 μm) than with ATP (Table 1). A similar increase in the Ca2+-sensitivity of force and reduction in ΔpCa50 has been reported with elevated MgADP in skinned cardiac muscle (Fukuda et al., 2000). These results suggest that conditions that slow cross-bridge detachment from strongly bound states lead to a decrease in the SL dependence of the Ca2+-sensitivity of force in cardiac muscle.
Ca2+ and SL dependence of force redevelopment rate (ktr)
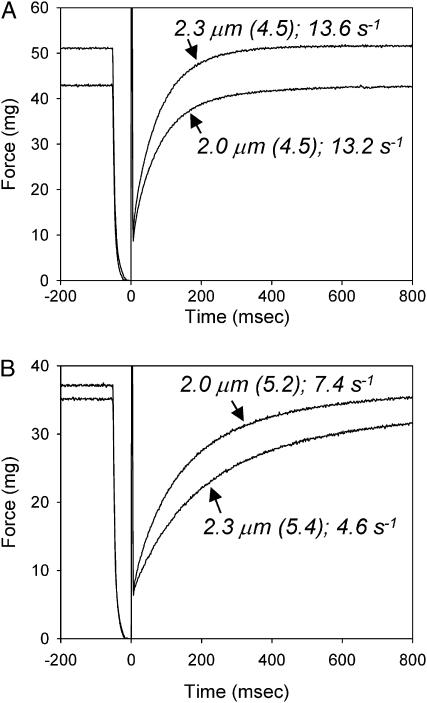
The steady-state data clearly indicate that cross-bridge kinetics can influence the SL-dependence of force-pCa relations in skinned cardiac muscle (Fig. 2; Table 1). To test the idea that elevated force at longer SL results from an increased probability of cross-bridge transition into force-generating states, we measured the Ca2+, SL, and nucleotide dependence of ktr. Although the SL dependence of ktr has been measured in skeletal fibers, a similar analysis has not yet been reported in cardiac muscle until now. Representative traces of force redevelopment (with ATP) obtained during ktr measurements at 2.0 and 2.3 μm SL are compared in Fig. 3 at maximal Ca2+-activation (Fig. 3 A) and at similar levels of submaximal Ca2+-activated force (∼70% Fmax at 2.3 μm SL; Fig. 3 B). The ktr-pCa and ktr-force data for all nucleotide conditions and both SLs are summarized in Figs. 4 and 5. Maximum values of ktr at 2.0 μm SL with ATP, dATP, and low ATP were 11.8 ± 1.2 s−1 (n = 15), 18.2 ± 2.1 s−1 (n = 10) and 9.8 ± 1.3 s−1 (n = 13), respectively. Corresponding values at 2.3 μm SL were (ATP) 10.3 ± 1.5 s−1, (dATP) 16.7 ± 1.7 s−1, and (low ATP) 7.9 ± 0.9 s−1. The data demonstrate that during maximal Ca2+-activation lowering [ATP] reduces ktr whereas substitution of dATP for ATP increases ktr, indicating slower and faster cross-bridge cycling kinetics, respectively, at both SLs (Table 1).
FIGURE 3.
Comparison of example force traces at SL 2.3 and 2.0 μm obtained during ktr measurements from a single skinned trabecula (length 1.1 mm, diameter 140 μm) during similar levels of submaximal force activation (pCa 5.2 for SL 2.0 μm and pCa 5.4 for SL 2.3 μm) (A) and during maximal activation (pCa 4.5) (B). The SL, pCa (in brackets), and value of ktr are indicated next to each trace.
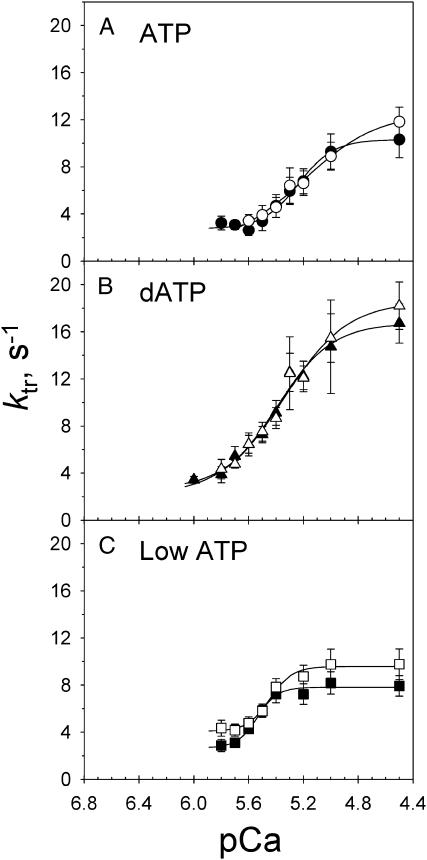
FIGURE 4.
ktr-pCa relations during Ca2+-activation at SL 2.3 (solid symbols) and 2.0 (open symbols) μm in 5 mM ATP (A), 5 mM dATP (B) and 0.5 mM or low ATP (C). Symbols are as in Fig. 2. Data, with error bars representing mean ± SE, were obtained from 8, 10, and 13 trabecula with ATP, dATP, and low ATP, respectively. The solid lines represent fitted curves through the mean data points for each condition.
FIGURE 5.
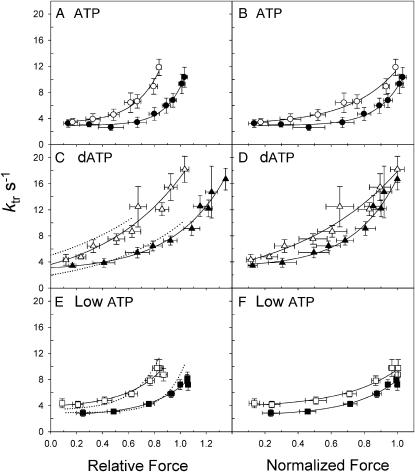
SL dependence of ktr-force relations for data obtained from fibers shown in Fig. 4. Symbols are as in Figs. 2 and 4. Data are shown with force expressed relative to the Fmax in ATP at SL 2.3 μm (left panels) or normalized to the Fmax for each condition and SL (right panels). The dotted curves in C and E are from the corresponding sets of control (5.0 mM ATP) experiments.
The Ca2+ dependence of ktr is illustrated in Fig. 4 for control ATP (A), dATP (B), and low ATP (C). The data were fit by the Hill equation to obtain the pCa50 values under the different nucleotide conditions (Table 1). Both dATP and low ATP caused an apparent shift of ktr-pCa relations to higher pCa relative to ATP, as found for force (Fig. 2). However, in contrast to the force-pCa relations (Fig. 2), the Ca2+-dependence of ktr was unaffected by changes in SL between 2.0 and 2.3 μm with ATP, dATP, or low ATP (Fig. 4). This comparison is important because in cardiac muscle increasing SL increases both force and Ca2+-binding to thin filaments (Wang and Fuchs, 1994). The results in Fig. 4 suggest that increased thin filament Ca2+-binding at longer SL may not correlate with changes in the kinetics of isometric force redevelopment (ktr).
To more directly compare the activation dependence of ktr for each nucleotide condition, ktr is replotted against the relative force for each pCa, with force expressed either relative to Fmax at SL 2.3 μm (Fig. 5, A, C, and E) or normalized to Fmax for each SL (Fig. 5, B, D, and F). For each nucleotide condition, a higher [Ca2+] was required to obtain the same level of force at SL 2.0 μm compared to SL 2.3 μm (Fig. 2). At the lowest forces ktr was the same under all three nucleotide conditions and both SLs (∼4 s−1). With increasing force ktr increased nonlinearly at each SL to an apparent maximum at Fmax. The range of ktr changes during activation at each SL was approximately fourfold for ATP (Fig. 5, A and B), approximately sixfold for dATP (Fig. 5, C and D), and two- to threefold for low ATP (Fig. 5, E and F). As noted above, maximum ktr was similar at SL 2.0 and 2.3 μm for each condition, whereas at intermediate matched-force levels ktr was greater at SL 2.0 μm for all three nucleotide conditions, independent of whether force was expressed as relative or normalized values. This and the lack of a significant SL-dependence of maximal ktr contrasts with a previous report in skinned skeletal fibers, which showed that ktr was slower at shorter SL than at longer SL during maximal Ca2+ activation (McDonald et al., 1997).
When ktr-force curves are compared between dATP and ATP (Fig. 5 C; solid versus dotted lines, respectively) ktr is similar over the range of forces common to both conditions at each SL, whereas force and ktr are both elevated by dATP at the highest Ca2+ concentrations. The data in Fig. 5 extend our observations (Regnier et al., 2004) to include shorter SL and supports the idea that dATP may enhance maximal force by increased thin filament activation, even at pCa 4.5. In Fig. 5, B, D, and F, force is normalized to the maximum value at each SL which assumes that activation is independent of SL and is maximal for each nucleotide condition. However, this assumption may not be correct as it has been shown that decreasing SL reduces the amount of Ca2+-bound to thin filaments (Wang and Fuchs, 1994). Even so, independent of the method of force normalization, ktr is faster for equivalent force levels at shorter SL when cross-bridge cycling rate is varied by each nucleotide condition. This idea is discussed in more detail below (see Discussion). Interestingly, at SL 2.0 μm ktr values at submaximal activation levels are slightly higher in low ATP than the control ATP (Fig. 5 E), reminiscent of our observations in skeletal fibers (Regnier et al., 1998) where we suggested that thin filament activation was elevated at low Ca2+ concentrations by slowed cross-bridge cycling.
Effects of lattice compression on force and ktr at SL 2.0 μm
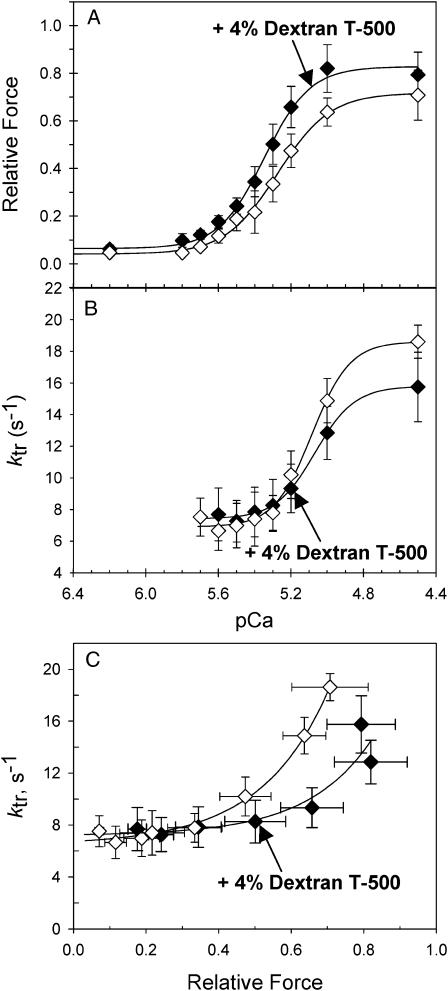
To determine if SL effects on force and ktr (Figs. 2, 4, and 5) are correlated with accompanying changes in myosin-actin proximity, the myofilament lattice spacing was compressed osmotically using 4% dextran T500 (w/v) at SL 2.0 μm. This level of compression was chosen to produce a small increase in force (12%) to mimic force changes that occur when SL is stretched. The decrease in lattice spacing by this dextran concentration in skinned trabeculae is greater than what occurs by increasing SL from 2.0 to 2.3 μm under relaxing conditions (Konhilas et al., 2002b; Martyn et al., 2004), but is comparable to that occurring in intact trabeculae over the same length range (Irving et al., 2000). Unfortunately, similar comparisons of lattice spacing during active contractions are unavailable. As seen in Fig. 6 A osmotic compression with 4% dextran increased pCa50 from 5.27 ± 0.01 (n = 5) to 5.35 ± 0.02 (n = 5) and Fmax by ∼12%. In contrast, Fig. 6 B illustrates that osmotic compression with 4% dextran had no effect on the pCa50 of the ktr -pCa relationship (5.10 ± 0.04, n = 5 versus 5.07 ± 0.01, n = 5 for 4% dextran), which is similar to the data in Fig. 4 where SL is altered. The corresponding values of ktr are plotted against the relative level of force (normalized to Fmax at SL 2.3 μm) in Fig. 6 C. As with increasing SL (Fig. 5, A and B), ktr was lower at intermediate and maximally activated matched force levels in the presence of 4% dextran at 2.0 μm SL (Fig. 6 C). These data demonstrate that compression of the lattice spacing at SL 2.0 μm produces similar changes in both force and ktr as that of increasing SL from 2.0 to 2.3 μm. These observations support the idea (Fuchs and Wang, 1996; Fuchs, 2002; Fukuda et al., 2003) that increased contractile activation at longer SL in cardiac muscle could be due, at least in part, to the accompanying decrease in the distance between thick and thin filaments.
FIGURE 6.
Force-pCa relations (A), ktr-pCa relations (B), and ktr-force relations (C) are illustrated for data obtained at SL 2.0 μm with (♦) and without (⋄) the osmotic compression with 4% dextran T500. Data (mean ± SE) were obtained from 5 trabecula. The solid lines are fitted curves through the mean data points.
DISCUSSION
In this study we tested the hypothesis that the kinetics of actin-myosin cross-bridge interactions are important determinants of the steep SL sensitivity of cardiac contractile activation (Frank-Starling effect). In addition we tested whether the rate of isometric force redevelopment (ktr) covaries with force when SL is changed, as might be expected if SL-dependent changes in the probability of strong cross-bridge interaction were the basis of the SL-dependence of force generation (the Frank-Starling relationship). In the presence of dATP the magnitudes of both force and ktr at maximal activation increased compared to ATP, as did the Ca2+-sensitivity of force and ktr, whereas the effect of SL on pCa50 (ΔpCa50) was not significantly different from the ATP controls (Table 1). When cross-bridge kinetics were slowed by lowering [ATP] from 5.0 to 0.5 mM, the Ca2+-sensitivity of force-pCa and ktr-pCa relations increased (as for dATP), whereas Fmax was unchanged and maximum ktr decreased. In contrast to both ATP and dATP ΔpCa50 was diminished and nH was increased with 0.5 mM ATP (Table 1). Importantly we found that at the same level of force, ktr was faster at the shorter SL over the entire range of submaximal [Ca2+] under all tested conditions (Fig. 5). Finally, the compression of myofilament lattice spacing at SL 2.0 μm by 4% dextran led to similar changes in the force and ktr as that of increasing SL alone (Fig. 6). As discussed below, these results provide important insights into the relationship between the cross-bridge binding and kinetics and the SL dependence of force generation in cardiac muscle.
Effects of cross-bridge cycling kinetics on the SL dependence of Ca2+- activation
The hypothesis that the SL dependence of force in cardiac muscle is influenced by the kinetics of actin-myosin interactions is suggested by our observations that submaximal ktr in skinned skeletal fibers is dependent on the kinetics of Ca2+-binding to thin filaments. It also suggests kinetic coupling occurs between thin filament regulatory transitions and cross-bridge cycling (Chase et al., 1994b; Regnier et al., 1996, 1998, 1999). Our current data supports this hypothesis for cardiac muscle because the effect of SL change on force was influenced by cross-bridge kinetics. With dATP cross-bridge binding and cycling kinetics were enhanced, as evidenced by increased Fmax (Fig. 2; Table 1) and ktr (Fig. 5; Table 1), respectively. Both Fmax and pCa50 of steady-state force were increased by dATP (relative to ATP) to the same extent at both SL, whereas ΔpCa50 was comparable to ATP. Thus when cross-bridge binding is enhanced with faster kinetics, the SL-dependence of force is unaltered even though Ca2+-sensitivity of force increased at each SL. This contrasts with the effects of slower cross-bridge kinetics on the SL and Ca2+-dependence of force with low [ATP] (Fig. 2, E and F; Table 1) where ΔpCa50 decreased compared to ATP. Fukuda et al. (2000) reported a similar reduction of ΔpCa50 when cross-bridge cycling was reduced with 0.1 mM MgADP in skinned cardiac muscle. However, although elevated [MgADP] increased Fmax, there was also an elevated force in the absence of Ca2+ (Fukuda et al., 1998), implying Ca2+-independent activation of thin filaments. In our experiments there was no Ca2+-independent activation with either dATP or low [ATP] because resting force was not elevated at either SL (data not shown). Interestingly, with low [ATP] pCa50 increased more at the shorter SL, relative to ATP (Fig. 2, E and F; Table 1) causing ΔpCa50 to decrease, as found for elevated [ADP] (Fukuda et al., 2000). Both elevated [ADP] and lowering [ATP] to 0.5 mM reduce the rate of cross-bridge detachment and could therefore affect the apparent cooperativity of the force-pCa relationship. Although Fmax did not increase when [ATP] was lowered, as it did with elevated [ADP] (Fukuda et al., 2000), the apparent cooperativity (nH) of force-pCa relations increased at the shorter SL, indicating enhanced cooperative activation by strong binding cross-bridges. This is consistent with the higher cooperative activation of cardiac versus skeletal muscle by cross-bridges in the rigor state (Metzger, 1995). The effect of low ATP on nH might be expected to be greatest under conditions where cross-bridge binding is reduced, such as at short SL and lower force, as we observed (Fig. 2, E and F; Table 1).
Our data suggest that increased thin filament activation, as evidenced by increased Ca2+-sensitivity of force, does not by itself determine the SL-dependence of force-pCa relations. This is demonstrated by the observation that ΔpCa50 is unaffected by dATP and reduced by low [ATP], even though Ca2+-sensitivity of force is increased by both (Fig. 2; Table 1). Thus the question is, “why would the SL-dependence of activation be apparently unaffected by enhanced strong cross-bridge binding (more force and stiffness) with dATP?” The answer may lie in the faster kinetics of cross-bridge cycling with dATP (Regnier et al., 1998, 2000). Kinetic-coupling between thin filament activation processes and cross-bridge cycling has been proposed to explain the lower activation dependence of force redevelopment kinetics in cardiac (Hancock et al., 1997) and skeletal muscle when cross-bridge cycling (Regnier et al., 1998), TnC isoform or structure (Chase et al., 1994b; Regnier et al., 1999) or Ca2+-binding properties (Regnier et al., 1996) are altered. Increasing cross-bridge cycling rate with dATP (when thin filament activation kinetics are not altered) could increase coupling between cross-bridge binding and thin filament state (Regnier et al., 2004) thereby reducing or eliminating the effects of SL on thin filament activation mediated through altered cross-bridge binding. The opposite may occur with low ATP, resulting in a reduction of the SL dependence of force.
Does ΔpCa50 accurately describe changes in the Frank-Starling mechanism?
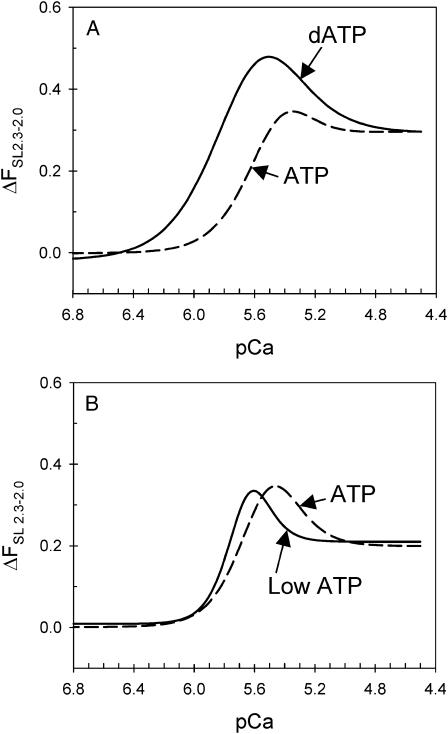
At the cellular level, the Frank-Starling effect in cardiac muscle can be described by the increase in force upon SL extension over the entire range of intracellular Ca2+ concentrations experienced under physiological conditions. The ΔpCa50 is therefore a limited indicator of the Frank-Starling effect, because the force difference between SL 2.0 and 2.3 μm depends not only on the respective pCa50 values but also on the respective changes in Fmax and slope (nH) of the individual force-pCa relations. This is illustrated in Fig. 7. Although ΔpCa50 varies little between dATP and ATP (Table 1) the difference in force (ΔFSL2.3–2.0) between 2.3 versus 2.0 μm is greater with dATP over the entire activation range (pCa 6.4–4.8, Fig. 7 A). Likewise, although low ATP diminished ΔpCa50, ΔFSL2.3–2.0 illustrates that the Frank-Starling effect is actually enhanced above pCa 5.6 and diminished below pCa 5.6 (Fig. 7 B). This analysis also hints that the cardiac Ca2+ regulatory mechanism may be “tuned,” such that a maximal effect of SL on force occurs at or near the peak Ca2+ concentration achieved during twitch contractions of intact myocytes (Fabiato, 1981).
FIGURE 7.
The “magnitude” of the Frank-Starling effect (the force difference between SL 2.3 and 2.0 μm) during Ca2+-activation for ATP, dATP, and low ATP. The force difference as a function of pCa were obtained by subtracting the corresponding Hill fit curves at SL 2.0 μm from SL 2.3 μm from Fig. 2, and are compared between dATP (A) and low ATP (B) and the corresponding ATP control curves.
The effects of SL and myofilament lattice spacing on ktr
Although the substitution of dATP for ATP and lowering [ATP] increased the Ca2+-sensitivity of ktr, ktr-pCa relations were unaffected by changing SL under all three conditions (Fig. 4; Table 1). The lack of an SL-dependence of ktr-pCa relations is explained by our observations that maximum ktr is relatively unaffected by changes in SL (Fig. 3 A), whereas at equivalent submaximal levels of force ktr was faster at SL 2.0 μm compared to SL 2.3 μm (Fig. 3 B). The increase in ktr at submaximal levels of force, when force was matched at both SL (Fig. 5), can be explained by a higher [Ca2+] needed to reach the same level of force at the shorter SL (Fig. 2). Put another way, it appears that [Ca2+] is the major determinant of ktr at both SL under all nucleotide conditions. However, increasing SL causes an enhanced binding of Ca2+ to cTnC in cardiac muscle (Wang and Fuchs, 1994; Martyn and Gordon, 2001), and the absence of SL effects on ktr–pCa relations implies that Ca2+-binding to cTnC does not directly modulate cross-bridge steps that are rate limiting to ktr. Thus the most likely effect of Ca2+ on ktr is via modulation of thin filament activation level.
Our observations that ktr values were either similar at maximum Ca2+-activated force or slightly faster at equivalent submaximal forces for 2.0 vs. 2.3 μm SL, independent of the nucleotide condition (Fig. 5) was surprising because McDonald et al. (1997) reported that maximal ktr was somewhat slower at shorter SL in skinned skeletal fibers. Furthermore, osmotic compression at SL 2.0 μm increased Fmax and pCa50 (Fig. 6 A) but had no effect on the ktr-pCa relations (Fig. 6 B), which is similar to that found for the SL increase from 2.0 μm to 2.3 μm without osmotic compression (Figs. 2, 4, and 5). These observations are significant because at SL 2.0 μm there was very little passive force and presumably no extension of titin, suggesting that changes in ktr and force cannot be attributed to a direct effect of titin on cross-bridge binding and that the effects of SL on force and ktr are, at least in part, a consequence of lattice spacing changes. Thus, at comparable levels of Ca2+-activation, increasing SL and decreasing lattice spacing (which increases the proximity of cross-bridge and thin filament binding sites) caused an apparent decrease, rather than increase, of the probability of cross-bridge transition into force generating states in cardiac muscle.
It has been proposed that ktr reflects the rate at which a new equilibrium is reached in cross-bridge distribution between nonforce and force producing states and is the sum of the apparent forward (fapp) and reverse (gapp) rate constants for these transitions in a two-state cross-bridge model (Brenner and Eisenberg, 1986). The steep dependence of ktr on [Ca2+] and force in skeletal fibers suggested that Ca2+ binding to thin filaments controls force redevelopment kinetics by increasing fapp (Brenner and Eisenberg, 1986). However, the regulation and activation dependence of ktr is likely to be more complicated. For example, thin filament activation kinetics limit ktr at submaximal forces in skeletal fibers (Chase et al., 1994b; Regnier et al., 1996), indicating that ktr represents the apparent kinetics of both thin filament activation and inherent cross-bridge cycle kinetics. If this is also true in cardiac muscle the lower activation dependence of ktr, compared to skeletal fibers, could be explained by a larger influence of thin filament kinetics even at high levels of Ca2+ activation (Hancock et al., 1997). It is also possible that cooperative activation of thin filaments by cross-bridges could affect the ktr-force relationship (Campbell, 1997), especially since the equilibrium activation level appears to be more dependent on strong cross-bridge binding in cardiac muscle (Hofmann and Fuchs, 1987; Wang and Fuchs, 1994; Martyn and Gordon, 2001; Regnier et al., 2004).
Possible explanations for a disproportionate effect of SL or lattice spacing on force and ktr when SL is extended from 2.0 to 2.3 μm (Fig. 5) or when lattice spacing is compressed at SL 2.0 μm (Fig. 6) are as follows. Cross-bridge binding to thin filaments depends not only on the equilibrium distribution of thin filament states but also on the proximity of thin filament binding sites to cross-bridges. The availability of thin filament sites should increase with decreasing lattice spacing because of increased proximity of the myofilaments, resulting in increased fapp. Our observation that weak cross-bridge binding in relaxed cardiac muscle is elevated when lattice spacing is compressed is consistent with this idea (Martyn et al., 2004). As a result force (proportional to fapp/(fapp+gapp); Brenner, 1988) should increase with increasing SL and reduced lattice spacing, as observed (Fig. 2). However, ktr (= fapp+gapp) should increase as well if gapp is unaffected by SL extension, as suggested by recent observations that the ratio of ATPase activity (proportional to gapp; Brenner, 1988) to isometric force is independent of SL between 2.0 and 2.2 μm (Wannenburg et al., 1997). This interpretation is contrary to our observations that maximum ktr is either unaffected by SL or slightly faster at short SL and significantly faster at intermediate levels of force (Fig. 5). The apparent contradiction is resolved if compressed lattice spacing and reduced radial cross-bridge strain at longer SL causes a reduction of gapp (Brenner and Yu, 1985; Goldman and Simmons, 1986) that compensates for the increase of fapp implied by elevated force. Conversely, at shorter SL and expanded lattice spacing the elevated radial cross-bridge strain may cause gapp to increase, compensating for the decrease of fapp (implied by lower force). As a result maximal ktr would vary little whereas force would be strongly SL dependent, as observed (Figs. 2 and 6). This idea is consistent with the observation that the ratio of ATPase activity to force during isometric contraction at maximal Ca2+-activation increases with decreasing SL in skinned cardiac muscle (Kentish and Stienen, 1994) and with the observation of decreased economy and efficiency of oxygen consumption relative to isometric force when muscle length is decreased in intact cardiac muscle (Holmes et al., 2002). We further tested this idea by modeling changes in force and ktr when the rate of cross-bridge detachment was increased twofold, as might occur at short SL, using a six-state cross-bridge model (Regnier and Homsher, 1998). Simulations predicted a 24% decrease in Fmax and an 18% increase in ktr. When SL was decreased from 2.3 to 2.0 μm, Fmax decreased 25% and average ktr was 14% greater at the short SL, close to the values predicted by the model. Slight decreases in the forward rate of force generation (fapp) coupled with smaller magnitude changes in cross-bridge detachment (gapp) yield quantitatively similar simulation results. Thus in cardiac muscle the steep decrease of force with decreasing SL may result from a simultaneous decrease in the probability of cross-bridge attachment coupled with an increased probability of cross-bridge detachment. These opposing changes in cross-bridge dynamics would result in little change of ktr, since the sum of fapp + gapp remains relatively constant. This result indicates that the steep decline of force at short SL in cardiac contraction is not accompanied by similar reductions in the rate of force development. Maintenance of the speed of force development with declining force at short length may help maintain rapid ventricular ejection during the later stages of systole in the heart, thus helping to maintain blood flow and ventricular emptying subsequent to the next period of diastole.
Acknowledgments
This work was supported by HL 61683 and AHA 040040N to M. Regnier, and HL67071 to D. A. Martyn. B. Adhikari was supported by Bioengineering Cardiovascular Training Grant HL 07403.
M. Regnier is an established investigator of the AHA.
References
- Adhikari, B., M. Regnier, and D. A. Martyn. 2004. At similar activation levels the rate of force redevelopment is faster at short sarcomere lengths and increased myfilament lattice spacing in skinned cardiac muscle. Biophys. J. 86:207a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari, B. B., and K. Wang. 2004. Interplay of troponin- and myosin-based pathways of calcium activation in skeletal and cardiac muscle: the use of W7 as an inhibitor of thin filament activation. Biophys. J. 86:359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner, B. 1988. Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proc. Natl. Acad. Sci. USA. 85:3265–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner, B., and E. Eisenberg. 1986. Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proc. Natl. Acad. Sci. USA. 83:3542–3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner, B., and L. C. Yu. 1985. Equatorial x-ray diffraction from single skinned rabbit psoas fibers at various degrees of activation. Changes in intensities and lattice spacing. Biophys. J. 48:829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, K. 1997. Rate constant of muscle force redevelopment reflects cooperative activation as well as cross-bridge kinetics. Biophys. J. 72:254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazorla, O., Y. Wu, T. C. Irving, and H. Granzier. 2001. Titin-based modulation of calcium sensitivity of active tension in mouse skinned cardiac myocytes. Circ. Res. 88:1028–1035. [DOI] [PubMed] [Google Scholar]
- Chase, P. B., D. A. Martyn, and J. D. Hannon. 1994a. Activation dependence and kinetics of force and stiffness inhibition by aluminiofluoride, a slowly dissociating analogue of inorganic phosphate, in chemically skinned fibres from rabbit psoas muscle. J. Muscle Res. Cell Motil. 15:119–129. [DOI] [PubMed] [Google Scholar]
- Chase, P. B., D. A. Martyn, and J. D. Hannon. 1994b. Isometric force redevelopment of skinned muscle fibers from rabbit with and without Ca2+. Biophys. J. 67:1994–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman, K. A. 1975. Mechanical deactivation induced by active shortening in isolated muscle fibres of the frog. J. Physiol. 246:255–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato, A. 1981. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J. Gen. Physiol. 78:457–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimons, D. P., and R. L. Moss. 1998. Strong binding of myosin modulates length-dependent Ca2+ activation of rat ventricular myocytes. Circ. Res. 83:602–607. [DOI] [PubMed] [Google Scholar]
- Fuchs, F. 2002. The Frank-Starling Relationship: Cellular and Molecular Mechanisms. In Advances in Muscle Research. R. J. Solaro and R. L. Moss, editors. Dordrecht, Kluwer, The Netherlands. 379–407.
- Fuchs, F., and Y. P. Wang. 1996. Sarcomere length versus interfilament spacing as determinants of cardiac myofilament Ca2+ sensitivity and Ca2+ binding. J. Mol. Cell. Cardiol. 28:1375–1383. [DOI] [PubMed] [Google Scholar]
- Fukuda, N., H. Fujita, T. Fujita, and S. Ishiwata. 1998. Regulatory roles of MgADP and calcium in tension development of skinned cardiac muscle. J. Muscle Res. Cell Motil. 19:909–921. [DOI] [PubMed] [Google Scholar]
- Fukuda, N., H. Kajiwara, S. Ishiwata, and S. Kurihara. 2000. Effects of MgADP on length dependence of tension generation in skinned rat cardiac muscle. Circ. Res. 86:E1–E6. [DOI] [PubMed] [Google Scholar]
- Fukuda, N., D. Sasaki, S. Ishiwata, and S. Kurihara. 2001. Length dependence of tension generation in rat skinned cardiac muscle: role of titin in the Frank-Starling mechanism of the heart. Circ. Res. 104:1639–1645. [DOI] [PubMed] [Google Scholar]
- Fukuda, N., Y. Wu, T. C. Irving, and H. Granzier. 2003. Titin isoform variance and length dependence of activation in skinned bovine cardiac muscle. J. Physiol. 553.1:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeves, M. A., and K. C. Holmes. 1999. Structural mechanism of muscle contraction. Annu. Rev. Biochem. 68:687–728. [DOI] [PubMed] [Google Scholar]
- Goldman, Y. E., and R. M. Simmons. 1986. The stiffness of frog skinned muscle fibres at altered lateral filament spacing. J. Physiol. 378:175–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, A. M., E. Homsher, and M. Regnier. 2000. Regulation of contraction in striated muscle. Physiol. Rev. 80:853–924. [DOI] [PubMed] [Google Scholar]
- Hancock, W. O., L. L. Huntsman, and A. M. Gordon. 1997. Models of calcium activation account for differences between skeletal and cardiac force redevelopment kinetics. J. Muscle Res. Cell Motil. 18:671–681. [DOI] [PubMed] [Google Scholar]
- Hofmann, P. A., and F. Fuchs. 1987. Effect of length and cross-bridge attachment on Ca2+ binding to cardiac troponin C. Am. J. Physiol. 253:C90–C96. [DOI] [PubMed] [Google Scholar]
- Holmes, J. W., M. Hunlich, and G. Hasenfuss. 2002. Energetics of the Frank-Starling effect in rabbit myocardium: economy and efficiency depend on muscle length. Am. J. Physiol. Heart Circ. Physiol. 283:H324–H330. [DOI] [PubMed] [Google Scholar]
- Irving, T. C., J. Konhilas, D. Perry, R. Fischetti, and P. P. de Tombe. 2000. Myofilament lattice spacing as a function of sarcomere length in isolated rat myocardium. Am. J. Physiol. Heart Circ. Physiol. 279:H2568–H2573. [DOI] [PubMed] [Google Scholar]
- Kentish, J. C., and G. J. Stienen. 1994. Differential effects of length on maximum force production and myofibrillar ATPase activity in rat skinned cardiac muscle. J. Physiol. 475:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konhilas, J. P., T. C. Irving, and P. P. de Tombe. 2002a. Frank-Starling law of the heart and the cellular mechanisms of length-dependent activation. Pflugers Arch. 445:305–310. [DOI] [PubMed] [Google Scholar]
- Konhilas, J. P., T. C. Irving, and P. P. de Tombe. 2002b. Myofilament calcium sensitivity in skinned rat cardiac trabeculae: role of interfilament spacing. Circ. Res. 90:59–65. [DOI] [PubMed] [Google Scholar]
- Kreutziger, K. L., A. J. Rivera, D. A. Martyn, and M. Regnier. 2003. Effect of cross-bridge (CB) kinetics on sarcomere length (SL) dependence of force development in cardiac muscle. Biophys. J. 84:449a. [Google Scholar]
- Lehrer, S. S., and M. A. Geeves. 1998. The muscle thin filament as a classical cooperative/allosteric regulatory system. J. Mol. Biol. 277:1081–1089. [DOI] [PubMed] [Google Scholar]
- Martyn, D. A., B. B. Adhikari, M. Regnier, J. Gu, S. Xu, and L. C. Yu. 2004. Response of equatorial x-ray reflections and stiffness to altered sarcomere length and myofilament lattice spacing in relaxed skinned cardiac muscle. Biophys. J. 86:1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyn, D. A., and A. M. Gordon. 2001. Influence of length on force and activation-dependent changes in troponin c structure in skinned cardiac and fast skeletal muscle. Biophys. J. 80:2798–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyn, D. A., M. Regnier, D. Xu, and A. M. Gordon. 2001. Ca2+- and cross-bridge-dependent changes in N- and C-terminal structure of troponin C in rat cardiac muscle. Biophys. J. 80:360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, K. S., and R. L. Moss. 1995. Osmotic compression of single cardiac myocytes eliminates the reduction in Ca2+ sensitivity of tension at short sarcomere length. Circ. Res. 77:199–205. [DOI] [PubMed] [Google Scholar]
- McDonald, K. S., M. R. Wolff, and R. L. Moss. 1997. Sarcomere length dependence of the rate of tension redevelopment and submaximal tension in rat and rabbit skinned skeletal muscle fibres. J. Physiol. 501:607–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger, J. M. 1995. Myosin binding-induced cooperative activation of the thin filament in cardiac myocytes and skeletal muscle fibers. Biophys. J. 68:1430–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss, R. L., and D. P. Fitzsimons. 2002. Frank-Starling relationship: long on importance, short on mechanism. Circ. Res. 90:11–13. [PubMed] [Google Scholar]
- Regnier, M., and E. Homsher. 1998. The effect of ATP analogs on posthydrolytic and force development steps in skinned skeletal muscle fibers. Biophys. J. 74:3059–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnier, M., H. Martin, R. J. Barsotti, A. J. Rivera, D. A. Martyn, and E. Clemmens. 2004. Cross-bridge versus thin filament contributions to the level and rate of force development in cardiac muscle. Biophys. J. 87:1815–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnier, M., D. A. Martyn, and P. B. Chase. 1996. Calmidazolium alters Ca2+ regulation of tension redevelopment rate in skinned skeletal muscle. Biophys. J. 71:2786–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnier, M., D. A. Martyn, and P. B. Chase. 1998. Calcium regulation of tension redevelopment kinetics with 2-deoxy-ATP or low [ATP] in rabbit skeletal muscle. Biophys. J. 74:2005–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnier, M., A. J. Rivera, P. B. Chase, L. B. Smillie, and M. M. Sorenson. 1999. Regulation of skeletal muscle tension redevelopment by troponin C constructs with different Ca2+ affinities. Biophys. J. 76:2664–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnier, M., A. J. Rivera, Y. Chen, and P. B. Chase. 2000. 2-deoxy-ATP enhances contractility of rat cardiac muscle. Circ. Res. 86:1211–1217. [DOI] [PubMed] [Google Scholar]
- Wang, Y. P., and F. Fuchs. 1994. Length, force, and Ca2+-troponin C affinity in cardiac and slow skeletal muscle. Am. J. Physiol. 266:C1077–C1082. [DOI] [PubMed] [Google Scholar]
- Wannenburg, T., P. M. Janssen, D. Fan, and P. P. de Tombe. 1997. The Frank-Starling mechanism is not mediated by changes in rate of cross-bridge detachment. Am. J. Physiol. 273:H2428–H2435. [DOI] [PubMed] [Google Scholar]