Abstract
Ca2+ release from internal stores (sarcoplasmic reticulum or SR) in smooth muscles is initiated either via pharmaco-mechanical coupling due to the action of an agonist and involving IP3 receptors, or via excitation-contraction coupling, mostly involving L-type calcium channels in the plasmalemma (DHPRs), and ryanodine receptors (RyRs), or Ca2+ release channels of the SR. This work focuses attention on the structural basis for the coupling between DHPRs and RyRs in phasic smooth muscle cells of the guinea-pig urinary bladder. Immunolabeling shows that two proteins of the SR: calsequestrin and the RyR, and one protein the plasmalemma, the L-type channel or DHPR, are colocalized with each other within numerous, peripherally located sites located within the caveolar domains. Electron microscopy images from thin sections and freeze-fracture replicas identify feet in small peripherally located SR vesicles containing calsequestrin and distinctive large particles clustered within small membrane areas. Both feet and particle clusters are located within caveolar domains. Correspondence between the location of feet and particle clusters and of RyR- and DHPR-positive foci allows the conclusion that calsequestrin, RyRs, and L-type Ca2+ channels are associated with peripheral couplings, or Ca2+ release units, constituting the key machinery involved in excitation-contraction coupling. Structural analogies between smooth and cardiac muscle excitation-contraction coupling complexes suggest a common basic mechanism of action.
INTRODUCTION
Activation of muscle contraction requires a cytoplasmic calcium transient. In smooth muscle this can be initiated by two different mechanisms, both involving release of Ca2+ from an internal store, the sarcoplasmic reticulum (SR), that are appropriately termed pharmaco-mechanical coupling and excitation-contraction (e-c) coupling (Bozler, 1969; Somlyo and Somlyo, 1970, 1994; Somlyo et al., 1971; Bolton et al., 1999). Pharmaco-mechanical coupling does not require surface membrane depolarization. The IP3 receptor, which is relatively abundant in smooth muscle, is the final target of the cascade of events initiated by an agonist and leading to internal Ca2+ release (Somlyo et al., 1988). E-c coupling instead involves voltage-dependent L-type calcium channels (DHPRs) which mediate Ca2+ entry and whose activity results in Ca2+ release via ryanodine receptors (RyRs; Xu et al., 1994) or SR calcium release channels. Recently, depolarization-induced Ca2+ release from internal stores in the absence of extracellular calcium has been demonstrated in a smooth muscle (del Valle-Rodriguez et al., 2003). This latter phenomenon raises the further intriguing possibility that some component of voltage-dependent Ca2+ release in smooth muscle may involve the successive activity of the two types of release channels IP3s (via a G-protein) and RyRs (del Valle-Rodriguez et al., 2003) in a manner independent of Ca2+ permeation.
Smooth muscles occur in a variety of functional types and vary greatly in the relative proportions of IP3- and RyR-mediated stores, in the detailed mechanism of activation and in the amount and intracellular localization the SR (Nixon et al., 1994; Bolton et al., 1999). Nonetheless, it is to be expected that a certain commonality in the arrangement of Ca2+-signaling system components and in their mode of action should exist. This article focuses on the structures that are at the basis of the depolarization-dependent calcium release requiring activity of DHPRs and RyRs. In the guinea-pig urinary bladder, DHPRs are responsible for an inward current of ≈10 μA/cm2 (Klöckner and Isenberg, 1985a,b, 1991, 1994; Ganitkevich and Isenberg, 1990) and Ca2+ transients generated by Ca2+ currents are amplified by Ca2+ release through peripherally located RyRs (Ganitkevich and Isenberg, 1992). In this as in other smooth muscles, spontaneous and/or induced Ca2+ release events (sparks) are either directly observed in a peripheral location or determined to be peripheral by their immediate effect on the Ca2+-activated K+ channels (Fay, 1995; Nelson et al., 1995; Mironneau et al., 1996; Gordienko et al., 1998, 2001; ZhuGe et al., 1998, 2002; Bolton et al., 1999; Pérez et al., 1999; Jaggar et al., 2000; Herrera et al., 2001; Kirber et al., 2001; Ohi et al., 2003). Calcium release has also been directly detected in subplasmalemmal location (Bond et al., 1984). A relationship between spark and RyR sites has been demonstrated (Herrera et al., 2001; Ohi et al., 2003).
The preferred location of spark sites suggests aggregations of RyRs in clusters. By electron microscopy, components of the SR located in proximity to the plasmalemma have been detected in several smooth muscles (Gabella 1971, 1972; Somlyo et al., 1971). Small clusters of “feet” connect these peripheral SR vesicles to the plasmalemma (Somlyo et al., 1971; Devine et al., 1972; Somlyo and Franzini-Armstrong, 1985). By comparison with skeletal and cardiac muscle and on the basis of the spark location, it is likely that these feet represent RyRs. Smooth muscle also contains calsequestrin, in an isoform that is either the same or closely related to the cardiac type (Wuytack et al., 1987). Calsequestrin of smooth muscle has been identified by immunolabeling in small peripherally and internally located foci (Etter et al., 1993; Villa et al., 1993) and by electron microscopy in the small peripheral SR cisternae associated with the plasmalemma and bearing feet (Somlyo and Franzini-Armstrong, 1985).
We have analyzed the distribution of e-c coupling-related proteins in smooth muscle cells of the guinea pig bladder using 3D-immunofluorescence, freeze fracture and thin section electron microscopy. The results show that DHPRs, RyRs, and calsequestrin are located in close proximity to each other, forming a complex analogous to that found in calcium release units (CRUs) of striated muscles (see Sutko and Airey, 1996; Franzini-Armstrong and Protasi, 1997 for reviews). We propose that these complexes are not only the sites of spontaneous calcium sparks, but also provide the structural basis for an interaction between DHPRs and RyRs, and thus for e-c coupling-related Ca2+ release. As previously shown (Somlyo et al., 1971, see Bolton et al, 1999), the e-c coupling related complexes are located in caveolar domains of the smooth muscle cell membrane. We however note that in most cases caveolae and the SR-surface complexes are not directly associated.
This work does not address the question of pharmaco-mechanical coupling, even though this alternative Ca2+ release mechanism is well represented in the guinea pig detrusor muscle.
MATERIALS AND METHODS
Guinea pigs of either sex (∼300g) were killed by cervical dislocation followed by bleeding.
Western blotting
A crude membrane preparation was obtained from a homogenate of whole guinea pig bladders by pelleting at 30,000 × g the supernatant from an initial 14,000 × g centrifugation. Membranes from a high KCL extraction of rabbit skeletal muscle and from rabbit neonate urinary bladder and stomach preparations were used as controls. 50 μg of the skeletal muscle membranes and 150 μg of various bladder fractions were separated on SDSPAGE and stained with Amido-black stain, after transfer to PVDF solid support. The blot was probed with Sheep6 Anti-DHPR 1:500 (Pragnell et al., 1991; Arikkath et al., 2003) and a rabbit anti-sheep IgG peroxidase at 1:5000 dilution as secondary antibody.
3-D immunofluorescence
The urinary bladder was removed and cut into chunks of ∼1 mm3 and the cells were enzymatic isolated according to Klöckner and Isenberg (1985a). The isolated cells were fixed in 2% paraformaldehyde, permeabilized by 0.1% tritonX 100, blocked, and incubated with primary and secondary antibodies essentially as described (Scriven et al., 2000). Primary antibodies were: antivinculin, a mouse monoclonal directed against chicken gizzard vinculin, clone Vin 11-5 from Sigma; C3-C33, a mouse monoclonal directed against canine cardiac (type 2) ryanodine receptors, which also recognizes amphibian RyR homologous to mammalian RyR3, a gift of Dr. Gerhard Meissner, University of North Carolina (Lai et al., 1992); anticalsequestrin, an affinity purified rabbit polyclonal directed against canine cardiac calsequestrin, gift of Dr. Larry Jones, Indiana University (Mahony and Jones, 1986); Sheep 6, an affinity-purified sheep polyclonal antibody, which reacted with α1 and β-subunits of the mammalian skeletal DHPR, gift of Dr. Kevin P. Campbell, University of Iowa (Pragnell et al., 1991); and π-9, a polyclonal antibody that recognizes the Na+/Ca2+ exchanger of toad stomach smooth muscle (Moore et al., 1993), a gift of Dr. Kenneth D. Philipson. Fluorescently labeled secondary antibodies (either FITC or Texas Red) were obtained from Jackson Immunobiologicals. The cover slips were mounted onto glass slides in a medium composed of 90% glycerol, 10% 10X PBS, 2.5% w/v triethylendiamine, and 0.02% NaN3 and containing small beads (180 nm diameter, Molecular Probes, Eugene, OR) that have broad excitation and emission spectra and acted as fiduciary markers to align the three-dimensional image sets.
Images were acquired, deconvolved, processed, and analyzed as described in great detail in Moore et al. (1993). Briefly, the voxels are 122 nm in x and y; z-planes were acquired at 250 nm intervals; 30–40 planes were acquired for each cell. For statistical analysis, the null hypothesis is that the proteins are distributed randomly and independently of each other. The mean of a binomial distribution can then be defined as (Np), and its variance as Np(1−p), where N is the number of voxels on the membrane and p is the probability that a voxel contains both proteins. The null hypothesis is accepted if the observed overlap falls within the mean ± 1 SD. If the observed overlap is significantly less, then the proteins are distributed in different regions of the membrane. If the observed overlap is significantly greater, then the proteins are codistributed. As a final step, and only for visualization, the z axis is interpolated to produce cubic voxels.
Electron microscopy
The urinary bladders were fixed in situ, while expanded by an injection of 4% glutaraldehyde in 0.1M Na-Cacodylate buffer into the lumen. For thin sectioning, strips from the fixed bladder walls were postfixed in 2% OsO4 for 2 h at room temperature and then contrasted in saturated uranyl acetate for 4 h at 60°C. Some muscles were treated for 36 h in 0.1% tannic acid at room temperature before the osmium fixation. The samples were embedded in Epon 812, and the sections stained in uranyl acetate and lead (Sato, 1968) for ∼8 min each. For freeze fracture, thin strips from the fixed bladder walls were infiltrated with 30% glycerol, frozen in liquid nitrogen-cooled propane, and fractured. The fractured surfaces were shadowed with platinum either at 45° unidirectionally or at 25° while rotating and then replicated with carbon in a freeze fracture apparatus (Balzers, model BFA 400; Balzers S.p.A., Milan, Italy). Sections and replicas were photographed in an EM 410 (Philips Electron Optics, Mahwah, NJ). The dimensions of intramembranous particles were measured in freeze-fracture from one bladder. The width was measured in a direction perpendicular to the direction of shadowing and the height was determined from the length of the platinum free “shadow,” which is (approximately) equal to the height, given an ∼45° angle of platinum deposition.
SR volumes were obtained using a point-counting morphometric approach (Weibel, 1979) from eight guinea-pig urinary bladders, three tissue-blocks per animal, with three sections per block and seven photographs per section. Low magnifications were used for cell, extracellular space and mitochondria and higher magnifications (25,000) for the contribution of mitochondria, caveolae, and SR. The volume fractions of the cell organelles were calculated from the ratios of caveolae/mitochondria, central SR/mitochondria and peripheral SR/mitochondria as well as mitochondria /cell, after subtraction of the extracellular space. SR was considered peripheral if its membranes were located within a distance <80 nm from the sarcolemma. In tangential sections of the cell membrane, where clusters of caveolae were recognizable, SR was considered peripheral when located within the clusters of caveolae. Data represent mean ± SE of the 8 bladders. The area of plasmalemma occupied by clusters of large particles was measured using the National Institutes of Health Image program.
RESULTS
Western blotting for DHPR in the urinary bladder
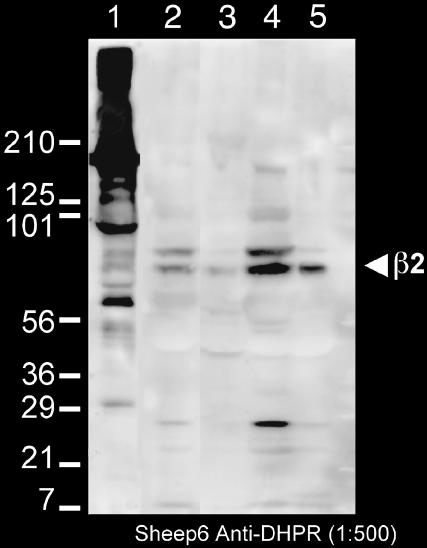
The β-subunit is an integral component of the DHPR (Leung et al., 1988). In skeletal muscle, the β-subunit is targeted to CRUs together with the channel forming α1 subunit (Neuhuber et al., 1998; Gerster et al., 1999) and is important in excitation-contraction coupling (Gregg et al., 1996). Either the β2 or the β3 isoforms are usually present in smooth muscle (Hohaus et al., 2000; Reimer et al., 2000). The Sheep 6 polyclonal antibody used for detecting the L-type Ca2+ channel reacted with both the α1 and β-subunits of rabbit skeletal muscle (Fig. 1, lane 1; Pragnell et al., 1991; Arikkath et al., 2003). Reactivity of the antibody in the urinary bladder smooth muscle was tested by Western blotting of whole homogenates and soluble and crude membrane fractions from two guinea pig urinary bladders. The antibody detected a prominent 70-kDa band in the crude membrane fraction (Fig. 1, lanes 2–5) and this is the only band that is detected in the purified membrane fraction (lane 5). This molecular weight is appropriate for the β2 subunit, although slightly higher than the skeletal ∼60 kDa band. The antibody does not recognize the α1 subunit of smooth muscle L-type channels. Given the fact that by far the predominant Ca2+ channels in the guinea pig urinary bladder are L-type (Schneider et al., 1991), labeling by the anti β-subunit antibody is expected to reliably detect the position of these channels.
FIGURE 1.
Western blot of a membrane preparation from rabbit skeletal muscle (1) and of several fractions from a crude preparation of membranes from the guinea pig urinary bladder (2–5). The sheep6 anti-DHPR (L-type Ca channel) serum that was used in the immunolabeling recognizes two bands at ∼100 and 60 kDa in the rabbit SR (Pragnell et al., 1991; Arikkath et al., 2003), corresponding to α1 and β-subunits and a band at ∼70 kDa in the smooth muscle crude membranes, which is about the size of the β2 subunit. The crude sheep antisera made against a rabbit skeletal muscle preparation combined with a rabbit anti-sheep secondary antibody results in a nonspecific reaction against high molecular weight components the rabbit membranes (lane 1). Other nonspecific bands (e.g., the ∼27 kDa band in lane 4, which may be the light chain of the antibody being picked up by the secondary are absent from the purified membrane fraction, lane 5). (Lane 1) Membrane fraction from rabbit skeletal muscle; (Lane 2) guinea pig urinary bladder homogenate; (Lane 3) 14,000 × g pellet; (Lane 4) 30,000 × g pellet; and (Lane 5) final membrane fraction.
The nonspecific reaction at high molecular weights in lane 1 for the rabbit fraction is due to the use of crude sheep antisera made against a rabbit skeletal muscle preparation enriched in DHPR, in combination with a rabbit anti-sheep secondary antibody, on rabbit skeletal muscle membranes. This nonspecific reaction is not observed with the guinea pig preparation. Other nonspecific bands are described in the figure legend.
Immunofluorescence
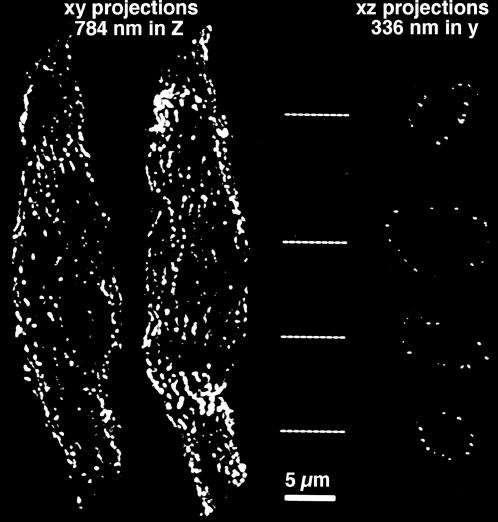
3-D image restoration was used to locate the position of fluorescently labeled secondary antibodies in the isolated smooth muscle cells. Fig. 2 illustrates the position of vinculin, a marker for the sites of dense plaque adhesion, where the contractile material transmits tension to the sarcolemma (North et al., 1993). The reconstructed images (left) show fluorescent pixels that are distributed like strings of pearls along stripes running at a shallow angle relative to the long axis of the cell, separated by nonfluorescent intervals. The XZ section (right), acquired from the positions indicated by the dashed lines, shows the pixels of vinculin fluorescence only at the cell periphery, forming a ring that encloses the cytoplasm of the cell. The Na+/Ca2+ exchanger; calsequestrin; the ryanodine receptor; and the β-subunit of the L-type Ca2+ channel are all located in small discrete spots either at the plasmalemma or very close to it (not shown). Thus, despite the fact that the SR is distributed in the interior of the cell as well as at its periphery, both calsequestrin and the ryanodine receptor are mostly distributed at discrete sites along the periphery of the cell in these smooth muscle cells.
FIGURE 2.
Fluorescent images of a single enzymatically dissociated smooth muscle cell from the guinea pig urinary bladder immunolabeled with an antibody against vinculin. These images have been deconvolved and thresholded; no other image processing steps were performed. The two images on the left are xy projections in which the cells were bisected (along the xy plane) to prevent superposition of the front and back surfaces. The images on the right are xz projections of the same cells, but after having been rotated 90° round the x axis; the images are 336 nm deep in y and were acquired from the locations indicated by the dashed lines. The images show that vinculin is clearly located at the cell surface, where it is distributed in longitudinal stripes. The scale bar is 5 μm.
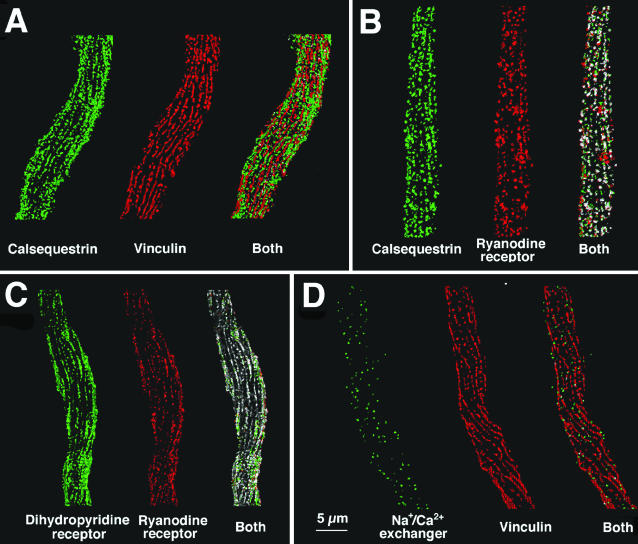
Double immunolabeling was used to define the spatial relationships between the peripherally located proteins at the cell surface. Since dense bodies and caveolae occupy distinct domains of the surface membrane (Gabella, 1971), vinculin was used as a positive marker for the dense bodies domains and a negative marker for the caveolar domains. Fig. 3 A shows double labeling for calsequestrin (green) and vinculin (red). Both proteins are disposed as strings of beads arranged along long pitch longitudinal helices, but the strings of beads for the two proteins are on separate stripes of membrane. Thus the great majority of the green calsequestrin pixels fall apart from the red vinculin pixels in the superimposed image (right). Statistical evaluation suggests that the probability of coincidence of the two proteins is significantly less than that expected from a random distribution (p < 0.001, see Table 1). That is, the distribution of the two proteins is not random and is mutually exclusive, indicating that the SR vesicles containing calsequestrin are located in correspondence of the plasmalemma's caveolar domains, which exclude vinculin-containing dense bodies.
FIGURE 3.
Double immunofluorescence images comparing the distribution of vinculin, calsequestrin, ryanodine receptor, L-type Ca channel (β-subunit) and the Na+/Ca2+ exchanger in single dissociated cells from the guinea-pig bladder. As in Fig. 2, one-half of the cell is displayed. For each of the image pairs, one image has been pseudocolored red and the other green. In the overlapped images, the voxels are colored white if they have identical three-dimensional coordinates for the two single images; otherwise, the voxel remain green or red. Since intensity information cannot be converted into number of protein copies in a given voxel, the intensity information has been removed from the data. Voxel that were above threshold are either green or red, and overlapped voxels are pure white. (A) Calsequestrin (green) and vinculin (red) are located at foci that occupy separate longitudinal stripes of membrane. (B and C) Foci of calsequestrin (green) and ryanodine receptor (red) and foci of Ca channels (green, labeled by the β-subunit) and ryanodine receptor (red) occupy the same membrane stripes and show considerable overlap. (D) Differently from the jSR components, the Na+/Ca2+ exchanger (green) is distributed over the entire cell surface and is not excluded from the vinculin (red) containing stripes, although it does not overlap with vinculin. See Table 1 for quantitative details. Bar = 5 μm.
TABLE 1.
Analysis of immunolabel overlap for various proteins
| Labeled pair | Observed overlap | Overlap expected due to chance* | Probability observed overlap is due to chance |
|---|---|---|---|
| DHPR with RyR | 61.3 ± 11.7 (4) | 18.4 | p < 0.001 |
| RyR with DHPR | 59.3 ± 9.4 | 17.8 | |
| Calsequestrin with RyR | 49.4 ± 9.2 (4) | 18.4 | p < 0.001 |
| RyR with calsequestrin | 43.0 ± 8.6 | 16.0 | |
| Vinculin with calsequestrin | 9.5 (1) | 33.6 | p < 0.001 |
| Calsequestrin with vinculin | 7.5 | 26.6 | |
| Vinculin with Na+/Ca2+ exchanger | 0.5 (1)† | 1.2 | p < 0.001 |
| Na+/Ca2+ exchanger with vinculin | 15.6 | 32.4 |
Overlap was calculated in accordance with binomial distribution prediction. The observed overlap is the percentage of voxels (mean ± SE) containing the first listed molecule that also contained the second. The number in parenthesis is the number of cells examined; each cell provides a large number of data (one for each acquired voxel, several thousands total).
The percentage of coincident voxels stained for these molecules are different from each other because the membrane contained a greater number of voxels labeled for vinculin than for the Na+/Ca2+ exchanger.
The positions of calsequestrin, RyRs, and DHPRs relative to each other are shown in Fig. 3, B and C. Both calsequestrin (Fig. 3 B, green) and Ca2+ channels (Fig. 3 C, green) are colocalized with RyRs (Fig. 3, B and C, red). The number of coincident voxels (shown in white) is highly significant, and much greater than predicted by a random distribution (see Table 1). This indicates that peripherally located SR vesicles contain both calsequestrin and ryanodine receptors and that they are in close proximity to patches of plasmalemma containing DHPRs. A rough count indicates that the number of discrete sites positive for the three proteins is between 100 and 200 on each cell surface.
By comparison, the distribution of Na+/Ca2+ exchanger molecules is distinctly different from that of the three components above. The foci positive for the Na+/Ca2+ exchanger are few, small, and randomly disposed over the whole cell (Fig. 3 D, exchanger, green; vinculin, red). There is no tendency toward alignment in longitudinally oriented lines, and no exclusion from the vinculin-labeled stripes. However, even though vinculin and the exchanger are present within the same general areas of membrane, visual inspection, and statistical analysis (Table 1) indicates that vinculin and the exchanger are not colocalized. The conclusion is that the exchanger is excluded from the sites of adhesion plaques (containing vinculin), but is distributed both within and between the caveolae-containing stripes and thus has a location different from that of the RyR-DHPR channel clusters.
Electron microscopy
Thin sections
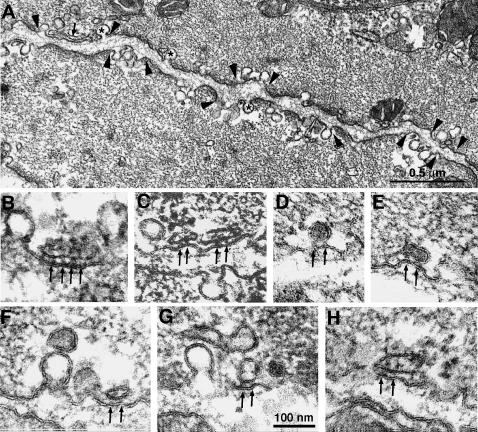
Cross sections of the smooth muscle cells show two structurally different regions that alternate with each other along the cell periphery (Fig. 4 A). In one region, the plasmalemma is associated with peripheral dense bodies, which appear continuous with the myofibrils. These correspond to the vinculin positive sites seen by immunolabeling and are characterized by a dense subplasmalemmal matrix. In the second region, the subplasmalemmal area is free of visible subsurface cytoskeletal components, but numerous caveolae invaginate from the surface membrane (Fig. 4 A, between arrowheads, some caveolae are marked by asterisks. See also Gabella, 1971, 1972; Somlyo et al., 1971; Devine et al., 1972). Vesicular and tubular SR vesicles are located at the cell periphery within the caveolar domains and are excluded from the dense bodies' domains (Fig. 4 A, small arrow). In general these peripheral SR profiles are less frequent than the caveolae, but being larger and more convoluted in shape, they occupy a percentage of the cell volume (2.8%) approximately equal to that of caveolae (2.7%). In addition to the peripheral SR, the bladder smooth muscle contains less frequent internal SR components, which occupy 1.3% of the cell volume, thus resembling, in this respect, other phasic smooth muscles (Nixon et al., 1994).
FIGURE 4.
Electron microscopy of cross-sectioned smooth muscle cells from the guinea pig urinary bladder showing two structurally different domains, which alternate with each other along the cell periphery. (A) Between arrowheads are membrane segments (caveolar domains) associated with caveolae (asterisks) and with peripheral SR cisternae (arrow). Between these segments are regions in which the plasmalemma is associated with peripheral dense bodies, which in turn are continuous with the myofibrils. (B–H) Details of peripheral SR profiles within caveolar domains. The peripheral SR vesicles shown have a dense content and are coupled to the surface membrane by large densities, the feet (arrows). The area of junction is small and it contains 2–3 feet. Close association of SR vesicles with a caveolae via feet are rare (F, arrow). Bar = 100 nm.
Some of the peripheral SR vesicles form junctions (peripheral couplings) with the surface membrane and are thus called junctional SR (jSR) (Fig. 4, B–H). jSR vesicles are identified on the basis of three structural features. The vesicles are apposed to the surface membrane across a narrow junctional gap of constant width; relatively large densities, the feet, are located within the junctional gap (Fig. 4, B–H, arrows); the vesicle lumen is occupied by an electron dense content. Note that even though these jSR profiles are located within the caveolar domains, they form junctions directly with the surface membrane, but not with the caveolae. In general, SR profiles that are immediately adjacent to caveolae (upper vesicle in Fig. 4 G) do not bear feet and do not make specific associations with caveolae via feet, and thus they are not junctional. One single, possible exception is shown in Fig. 4 F, where one SR vesicle associated with an individual caveola seems to bear a single foot.
The majority of jSR vesicles are very small, and the junctional gap contains only two feet (Fig. 4, C–H). Three or more feet (Fig. 4 B) are rarely seen. No feet are detected outside the junctional gap. The average number of feet is 2.3 ± 0.8/vesicle profile (mean ± 1 SD, n = 33 vesicles) and the average center to center distance between feet is 27.2 ± 3.9 nm. The average area of close proximity between small feet-bearing SR vesicles and the surface membrane can be estimated from the following consideration. On the average the junctions show a line of 2.3 feet. Assuming that feet are disposed in an orthogonal arrangement, as in skeletal muscle the total feet/junction number would (2.3)2. Small deviations from this arrangement would not appreciably change the number. Given the 27.2 nm average center to center distance between feet, the average area occupied by feet is 3913 nm2.
Freeze-fracture
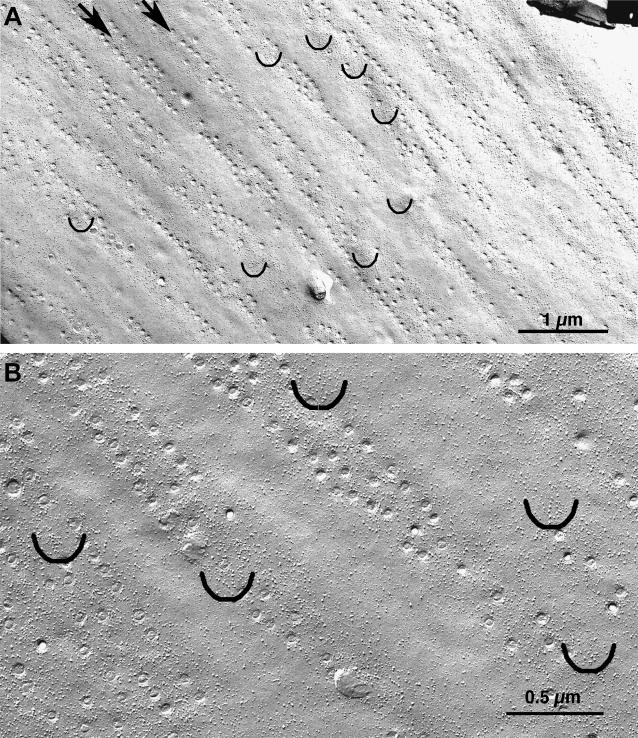
The two types of membrane domains (with and without caveolae) described above are clearly recognizable in freeze-fracture replicas, where the openings of caveolae are visible as small circles (Fig. 5 A). Caveolae are disposed in membrane domains having the shape of stripes that are oriented either longitudinally or at a slight angle to the longitudinal axis of the cell and occupy ∼50% of the total surface area. Caveolar domains are separated from each other by longitudinal stripes of smooth membrane with no invaginations, which correspond to the location of dense bodies.
FIGURE 5.
Freeze-fracture replicas of the smooth muscle cell plasmalemma, the longitudinal axis of the cell is at ∼45° in the image. (A) The opening of caveolae, visible as small circles, are confined to longitudinally oriented membrane stripes (two of them indicated by arrows), separated by membrane regions without invaginations which serve as sites of attachments of dense bodies. (B) Intramembrane particles of variable sizes are frequent in the caveolar domains and less frequent in the smooth membrane regions. Small groups of large particles are localized in defined patches of membrane within the caveolar domains. The patches are indicated by semicircles and are shown at higher magnification in Fig. 6. Bars = 1 μm and 0.5 μm.
Intramembrane particles of variable sizes are more frequent in proximity of caveolae and less frequent in the smooth membrane domains (Fig. 5 B). The caveolar domains, but not the smooth domains, also contain small clusters of large intramembrane particles residing within specialized patches of membrane. Semicircles in Fig. 5, A and B, mark the locations of such clusters and give an approximate indication of their frequency. The clusters are not well visible at the magnifications used in Fig. 5: details are shown in Fig. 6, which illustrates a selection of small clusters (between arrows). Each cluster is composed of closely spaced particles of large size. The membrane adjacent to the clusters is occupied by particles that are more variable in size and are located at more variable distances. We measured (see Methods) the apparent diameter and height of all particles within the small clusters and compared these two parameters with those of all particles contained in nearby areas of membrane of equal size, within the caveolae-containing stripes. Each set of measurements involved a pair of membrane patches (with and without clusters) in close proximity, thus ensuring equal platinum thickness deposition and angle of shadowing. The average width and height of all particles in clusters is 9.6 ± 0.2 nm and 9.3 ± 0.2 nm (mean ± 1 SE, 168 particles from 15 membrane patches), respectively, whereas the same parameters for particles in other areas of the surface are 7.3 ± 0.1 and 6.7 ± 0.2 nm (199 particles from 15 patches). The differences between the means of widths and heights for clustered and generic particles are extremely significant (Student's t-test, p < 0.0001).
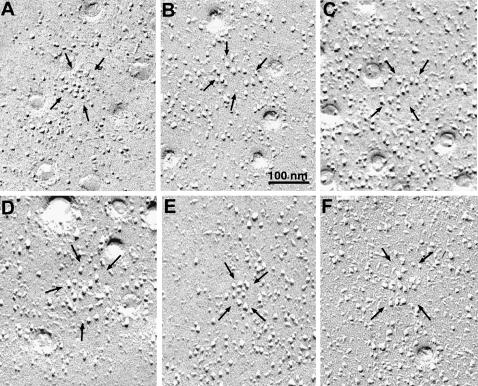
FIGURE 6.
Selected images of small membrane patches with large particles (between arrows). Within each patch there is a homogenous population of particles with the same characteristic large diameter and elongated shadow. Very few particles of smaller diameter are present in the same patches. By contrast, particles in the surrounding membrane are quite variable in size and height. Within the patches the particles have random arrangement and variable spacings.
The disposition of large particles within the specialized domains shows no evidence of order. The spacing between the particles is variable and no recognizable geometry is visible. In some domains the particles are fairly tightly clustered (Fig. 6, A and E), in others more disperse. The surface area of membrane patches occupied by clusters of large particles (measured as indicated in the method section) is 4310 ± 213 nm2. The average number of large particles within each patch is 6.9 ± 2.1 (mean ± SD, from 60 patches in freeze-fractures of four bladders). The membranes of caveolae do not have any particles in them, as in skeletal muscle (not shown; Dulhunty and Franzini-Armstrong, 1975).
DISCUSSION
Immunolabeling and electron microscopy of smooth muscle cells in the guinea pig urinary bladder provide complementary evidence for the presence of peripherally located complexes that have three important components of the Ca2+ cycle: the L-type Ca2+ channels, DHPRs, which initiate e-c coupling; the RyRs, responsible for Ca2+ release from the SR; and calsequestrin, which increases the total SR storing capacity for luminal Ca2+. Immunofluorescence suggests that both RyRs and Ca2+ channels colocalize with calsequestrin within voxels of 0.1 × 0.1 × 0.35 μm3, and that the sites with these components locate at or close to the cell periphery. Electron microscopy images confirm the existence of jSR vesicles containing feet and a dense content (presumably calsequestrin) that are indeed present right at the surface membrane and form peripheral couplings with it. Feet are identified as RyRs due to their size and to their location at the fiber periphery in correspondence of the RyR-positive foci identified by immunolabeling. The cytoplasmic domains of IP3 receptors are definitely smaller than those of RyRs, even though they have the same four leaf clover appearance (Katayama et al., 1996). On this basis, identification of the feet with IP3 receptors can be excluded.
We further propose that the large intramembranous particles clustered within small membrane patches located in the caveolar stripes are L-type channels, DHPRs, and that the channels are closely apposed to RyRs, based on the following considerations. First, the particle clusters are located in the caveolar stripes, as are the β-subunit-positive foci detected by immunolabeling. Comparison between immunolabeling for Ca2+ channels and for another plasmalemmal protein, the Na+/Ca2+ exchanger, confirms the ability of the technique to detect preferential clustering of the former but not of the latter in the caveolar domains. Secondly, the particles resemble those identified as L-type channels or DHPRs in skeletal and cardiac muscle (Block et al., 1988; Takekura et al., 1994; Sun et al., 1995; Protasi et al., 1998; Tijskens et al., 2003) on the basis of their size and of their tendency to cluster within membrane patches from which other proteins are at least partly excluded. Thirdly, the approximate size of the patches occupied by the particle clusters is very close to the size of the SR membrane surfaces that are covered by feet and that are closely associated with the surface membrane, thus forming the basis for the superimposition of RyR- and Ca2+-channel-positive voxels in the immunolabeled images. A recent publication (Ohi et al., 2003) differs from this report in that it finds a clustering of RyRs, but it fails to detect correspondence between RyR and DHPR locations in guinea pig smooth muscle of comparable origin. The reason for the difference is not clear. BKCa channels are activated by internal calcium release, indicating a proximity to RyRs (Benham and Bolton, 1986; Klöckner and Isenberg, 1992; Jaggar et al., 1998; Pérez et al., 1999; ZhuGe et al., 1998, 1999, 2002). It is possible that some of the particles within and/or in proximity of the clusters described above represent these channels.
The complexes formed by Ca2+ channels, RyR and calsequestrin in smooth muscle are structurally homologous to the those found in CRUs involved in excitation contraction coupling of striated (skeletal and cardiac) muscles (MacLennan and Wong, 1971; Meissner, 1975; Campbell et al., 1980; Cala and Jones, 1983; Jorgensen et al., 1983; Block et al., 1988; Takekura et al., 1994; Carl et al., 1995; Franzini-Armstrong and Kish, 1995; Sun et al., 1995; Scriven et al., 2000). If the particles in the surface membrane patches are L-type channels, then the Ca2+ channel/RyR ratio in smooth muscle CRUs is ∼2:1 and thus similar to that in CRUs of striated muscles (Block et al., 1988; Sun et al., 1995).
An immediate question raised by these findings is whether the structurally identified supramolecular complexes of smooth muscle are functionally active in e-c coupling, that is, are functional CRUs. This is supported by two findings. First, the peripheral location of the e-c coupling protein complexes in this smooth muscle is in agreement with the peripheral location of spark sites (see Introduction). Second, the relatively high frequency of such complexes (200–400/cell) is in agreement with the relatively large internal Ca2+ release during e-c coupling. CRUs of striated (cardiac and skeletal) muscles contain many more RyRs that those shown here for smooth muscle (compare with Franzini-Armstrong et al., 1999), but the size of sparks is comparable to that observed in striated muscles. This apparent discrepancy is explained by the fact that only a few of the available channels (1–5) are involved in spark production of striated muscles even if a larger number is available (Wang et al., 2004).
Functional studies of bladder smooth muscle cells indicate that Ca2+ current through L-type channels is the predominant pathway for Ca2+ influx (Schneider et al., 1991) and that this influx is followed by Ca2+ release from the SR, with a gain of ∼20-fold (Klöckner and Isenberg 1985b; Ganitkevich and Isenberg, 1991; Ganitkevich and Isenberg, 1992; Isenberg et al, 1992). Active release sites resulting in detectable sparks are very few in the resting muscle (Herrera et al., 2001), but up to 200–400/cell (these data) are available for activation during depolarization, probably due to spread from one initial release site to others (Bolton and Gordienko, 1998; Ohi et al., 2003).
Ca2+ channel-related particles in guinea pig bladder smooth muscle show no apparent order in their disposition within the small membrane patches. Comparison of their arrangement to that of dihydropyridine receptors (L-type channels) in CRUs of skeletal (Block et al., 1988; Takekura et al., 1994; Nakai et al., 1998) and cardiac (Carl et al., 1995; Sun et al., 1995; Protasi et al., 1998; Scriven et al., 2000) muscles indicates a closer structural similarity to the latter. In cardiac muscle, the location and disposition of DHPRs is ideal for an indirect interaction with RyR, perhaps via Ca2+-activated Ca2+ release (Herrmann-Frank et al., 1991). Indeed, the voltage-dependence of SR Ca2+ release in the smooth muscle is bell-shaped like the voltage-dependence of ICa through the DHPRs (Ganitkevich and Isenberg, 1991). In this it resembles that of cardiac myocytes and differs from the S-shaped dependence of skeletal muscle fibers (Morad and Cleemann, 1987). Both DHPRs and RyRs of smooth muscle are more closely related to the cardiac than to the skeletal type (Ertel et al., 2000). RyRs in smooth muscle are either type 2 (cardiac) or type 3 (Herrmann-Frank et al., 1991; Xu et al., 1994; Jiang et al., 2003).
The close spatial relationship between Ca2+ channels and RyR clusters described in these findings serves as the basis for the observed interaction and for Ca2+ release at the cell periphery, presumably in a manner analogous to that found in cardiac myocytes lacking T tubules (Junker et al., 1994; Kockskämper et al., 2001). Given the colocalization of calsequestrin and IP3 receptors in some smooth muscle (Villa et al., 1993), it is possible that the latter release channels may also be located close to the units described here and may provide an indirect link of the type suggested by del Valle-Rodriguez et al. (2003).
The close proximity of RyRs to the surface membrane has an important functional effect: their activity acts synergistically to that of the L-type Ca2+ channels in producing a substantial subplasmalemmal increase in [Ca2+], which in turn results in spontaneous transient outward currents (STOCs) via BKCa channels (Benham and Bolton, 1986; Klöckner and Isenberg, 1992; Jaggar et al., 1998; ZhuGe et al., 1998, 1999, 2002; Pérez et al., 1999) and in the apparent paradox of calcium induced relaxation (Fay, 1995; Jaggar et al., 1998). The relationship between small individual calcium “sparks” and STOC, and the nearby location of RyRs and BKCa channels indicate that most, but perhaps not all, RyR clusters are in sufficient proximity of BKCa channels to activate them by Ca release (ZhuGe et al., 1999, 2002; Kirber et al., 2001, Ohi et al., 2003).
In this study, location of RyRs in sites other than the peripherally located clusters were not detected either by electron microscopy or by immunolabeling, but this cannot be totally excluded. A small concentration of dispersed channels (see Lesh et al., 1998) would not be observed by electron microscopy. The antibody used recognizes mammalian RyR2, but also amphibian β-RyR, which is equivalent to RyR3. It is not clear whether it recognizes RyR2, RyR3, or both in smooth muscle, and whether it may miss some internally located RyRs of a different variety. In addition, a small number of channels dispersed in the membrane might not generate a sufficient signal even if recognized by the antibody. However, functional evidence both from sparks (see Introduction) and from activation of BKCa channels (see below) would favor a predominant subplasmalemmal position for active RyRs in this muscle.
Acknowledgments
This work is supported by Deutsche Forschungsgemeinschaft grant We/879 5-1 to M.F.G.; Deutsche Forschungsgemeinschaft grant SFB 598 to G.I.; and National Institutes of Health grant RO1 HL-48093 to C. F.-A.
References
- Arikkath, J., C. C. Chen, C. Ahern, V. Allamand, J. D. Flanagan, R. Coronado, R. G. Gregg, and K. P. Campbell. 2003. Gamma 1 subunit interactions within the skeletal muscle L-type voltage-gated calcium channels. J. Biol. Chem. 278:1212–1219. [DOI] [PubMed] [Google Scholar]
- Benham, C. D., and T. B. Bolton. 1986. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J. Physiol. 381:385–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block, B. A., T. Imagawa, K. P. Campbell, and C. Franzini-Armstrong. 1988. Structural evidence for direct interaction between the molecular components of the transverse tubules/sarcoplasmic reticulum junction in skeletal muscle. J. Cell Biol. 107:2587–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton, T. B., and D. V. Gordienko. 1998. Confocal imaging of calcium release events in single smooth muscle cells. Acta Physiol. Scand. 164:567–575. [DOI] [PubMed] [Google Scholar]
- Bolton, T. B., S. A. Prestwic, A. V. Zholos, and D. V. Gordienko. 1999. Excitation-contraction coupling in gastrointestinal and other smooth muscles. Annu. Rev. Physiol. 61:85–115. [DOI] [PubMed] [Google Scholar]
- Bond, M., T. Kitazawa, A. P. Somlyo, and A. V. Somlyo. 1984. Release and recycling of calcium by the sarcoplasmic reticulum in guinea-pig portal vein smooth muscle. J. Physiol. 355:677–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozler, E. 1969. Role of calcium in initiation of activity of smooth muscle. Am. J. Physiol. 216:671–674. [DOI] [PubMed] [Google Scholar]
- Cala, S. E., and L. R. Jones. 1983. Rapid purification of calsequestrin from cardiac and skeletal muscle sarcoplasmic reticulum vesicles by Ca2+-dependent elution from phenyl-sepharose. J. Biol. Chem. 258:11932–11936. [PubMed] [Google Scholar]
- Campbell, K. R., C. Franzini-Armstrong, and A. E. Shamoo. 1980. Further characterization of light and heavy sarcoplasmic reticulum vesicles. Identification of the ‘sarcoplasmic reticulum feet’ associated with heavy sarcoplasmic reticulum vesicles. Biochim. Biophys. Acta. 602:97–116. [DOI] [PubMed] [Google Scholar]
- Carl, S. L., K. Felix, A. H. Caswell, N. R. Brandt, J. P. Brunschwig, G. Meissner, and D. G. Ferguson. 1995. Immunolocalization of triadin, DHP receptors, and ryanodine receptors in adult and developing skeletal muscle of rats. Muscle Nerve. 18:1232–1243. [DOI] [PubMed] [Google Scholar]
- Del Valle-Rodriguez, A., J. Lopez-Barneo, and J. Urena. 2003. Ca2+ channel-sarcoplasmic reticulum coupling: a mechanism of arterial myocyte contraction without Ca2+ influx. EMBO J. 22:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine, C. E., A. V. Somlyo, and A. P. Somlyo. 1972. Sarcoplasmic reticulum and excitation contraction coupling in mammalian smooth muscle. J. Cell Biol. 52:690–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulhunty, A. F., and C. Franzini-Armstrong. 1975. The relative contributions of folds and caveolae to the surface membrane of frog skeletal muscle fibres at different sarcomere lengths. J. Physiol. 250:513–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertel, E. A., K. P. Campbell, M. M. Harold, F. Hofmann, Y. Mori, E. Perez-Reyes, A. Schwartz, T. P. Snutch, T. Tanabe, L. Birnbaumer, R. W. Tsien, and W. A. Catterall. 2000. Nomenclature of voltage-gated calcium channels. Neuron. 25:533–535. [DOI] [PubMed] [Google Scholar]
- Etter, E. F., K. D. Philipson, W. A. Carrington, K. E. Fogarty, L. M. Lifshitz, and F. S. Fay. 1993. Coupling of the Na+/Ca2+ exchanger, Na+/K+ pump and sarcoplasmic reticulum in smooth muscle. Nature. 365:657–660. [DOI] [PubMed] [Google Scholar]
- Fay, F. S. 1995. Calcium sparks in vascular smooth muscle: relaxation regulators. Science. 270:588–589. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong, C., and C. W. Kish. 1995. Alternate disposition of tetrads in peripheral couplings of skeletal muscle. J. Muscle Res. Cell Motil. 16:319–324. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong, C., and F. Protasi. 1997. The ryanodine receptor of striated muscles, a complex channel capable of multiple interactions. Physiol. Rev. 77:699–729. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong, C., V. Ramesh, and F. Protasi. 1999. Shapes, sizes and distributions of Ca2+ release units and couplons in a variety of skeletal and cardiac muscles. Biophys. J. 77:1528–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabella, G. 1971. Caveolae intracellulares and sarcoplasmic reticulum in smooth muscle. J. Cell Sci. 8:601–609. [DOI] [PubMed] [Google Scholar]
- Gabella, G. 1972. The arrangement of sarcoplasmic reticulum in smooth muscle. Experientia. 28:948–949. [DOI] [PubMed] [Google Scholar]
- Ganitkevich, V. Y., and G. Isenberg. 1990. Contribution of two types of calcium channels to membrane conductance of single myocytes from guinea-pig coronary arteries. J. Physiol. 426:19–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich, V. Y., and G. Isenberg. 1991. Depolarization-mediated intracellular calcium transients in isolated smooth muscle cells of guinea-pig urinary bladder. J. Physiol. 435:187–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich, V. Y., and G. Isenberg. 1992. Contribution of Ca2+-induced Ca2+ release to the [Ca2+]I transients in myocytes from guinea-pig urinary bladder. J. Physiol. 458:119–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerster, U., B. Neuhuber, K. Groschner, J. Striessnig, and B. E. Flucher. 1999. Current modulation and membrane targeting of the calcium channel alpha1C subunit are independent functions of the beta subunit. J. Physiol. 517:353–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordienko, D. V., T. B. Bolton, and M. B. Cannell. 1998. Variability in spontaneous subcellular calcium release in guinea-pig ileum smooth muscle cells. J. Physiol. 507:707–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordienko, D. V., I. A. Greenwood, and T. B. Bolton. 2001. Direct visualization of sarcoplasmic reticulum regions discharging Ca2+ sparks in vascular myocytes. Cell Calcium. 29:13–28. [DOI] [PubMed] [Google Scholar]
- Gregg, R. G., A. Messing, C. Strube, M. Beurg, R. Moss, M. Behan, M. Sukhareva, S. Haynes, J. A. Powell, R. Coronado, and P. A. Powers. 1996. Absence of the β subunit (cchb1) of the skeletal muscle dihydropyridine receptor alters expression of the α1 subunit and eliminates excitation-contraction coupling. Proc. Natl. Acad Sci. USA. 93:13961–13966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera, G. M., T. J. Heppner, and M. T. Nelson. 2001. Voltage dependence of the coupling of Ca2+ sparks to BkCa channels in urinary bladder smooth muscle. Am. J. Physiol. Cell Physiol. 280:C481–C489. [DOI] [PubMed] [Google Scholar]
- Herrmann-Frank, A., E. Darling, and G. Meissner. 1991. Functional characterization of the Ca(2+)-gated Ca2+ release channel of vascular smooth muscle sarcoplasmic reticulum. Pflugers Archiv. 418:353–359. [DOI] [PubMed] [Google Scholar]
- Hohaus, A., M. Poteser, C. Romanin, N. Klugbauer, F. Hofmann, I. Morano, H. Haase, and K. Groschner. 2000. Modulation of the smooth-muscle L-type Ca2+ channel alpha1 subunit (alpha1C-b) by the beta2a subunit: a peptide which inhibits binding of beta to the I–II linker of alpha1 induces functional uncoupling. Biochem. J. 348:657–665. [PMC free article] [PubMed] [Google Scholar]
- Isenberg, G., M.-F. Wendt-Gallitelli, and V. Y. Ganitkevich. 1992. Contribution of Ca2+-induced Ca2+-release to depolarization-induced Ca2+ transients of myocytes from guinea-pig urinary bladder myocytes. Jpn. J. Pharmacol. 59:81–86. [PubMed] [Google Scholar]
- Jaggar. J. H., V. A. Porter, W. J. Lederer, and M. T. Nelson. 2000. Calcium sparks in smooth muscle. Am. J. Physiol. 278:235–256. [DOI] [PubMed] [Google Scholar]
- Jaggar, J. H., G. C. Wellman, T. J. Heppner, V. A. Porter, G. J. Perez, M. Gollasch, T. Kleppisch, M. Rubart, A. S. Stevenson, W. J. Lederer, H. J. Knot, A. D. Bonev, and M. T. Nelson. 1998. Ca2+ channels, ryanodine receptors and Ca(2+)-activated K+ channels: a functional unit for regulating arterial tone. Acta Physiol. Scand. 164:577–578. [DOI] [PubMed] [Google Scholar]
- Jiang, D., B. Xiao, X. Li, and S. R. W. Chen. 2003. Smooth muscle tissues express a major dominant-negative splice variant of the type 3 Ca2+ release channel (Ryanodine receptor). J. Biol. Chem. 278:4763–4769. [DOI] [PubMed] [Google Scholar]
- Jorgensen, A. O., A. C. Shen, K. P. Campbell, and D. H. MacLennan. 1983. Ultrastructural localization of calsequestrin in rat skeletal muscle by immunoferritin labeling of ultrathin frozen sections. J. Cell Biol. 97:1573–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker, J., J. R. Sommer, M. Sar, and G. Meissner. 1994. Extended junctional sarcoplasmic reticulum of avian cardiac muscle contains functional ryanodine receptors. J. Biol. Chem. 269:1627–1634. [PubMed] [Google Scholar]
- Katayama, E., H. Funahashi, T. Michikawa, T. Shiraishi, T. Ikemoto, M. Iino, and K. Mikoshiba. 1996. Native structure and arrangement of inositol-1,4,5-trisphopshate receptor molecules in bovine cerebellar Purkinje cells as studied by quick-freeze deep-etch electron microscopy. EMBO J. 15:4844–4851. [PMC free article] [PubMed] [Google Scholar]
- Kirber, M. T., E. F. Etter, K. A. Bellve, L. M. Lifshitz, R. A. Tuft, F. S. Fay, J. V. Walsh, and K. E. Fogarty. 2001. Relationship of Ca2+ sparks to STOCs studied with 2D and 3D imaging in feline esophageal smooth muscle cells. J. Physiol. 531:315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöckner, U., and G. Isenberg. 1985a. Action potentials and net membrane currents in isolated smooth muscle cells (urinary bladder of the guinea-pig). Pflügers. Archiv. 405:329–339. [DOI] [PubMed] [Google Scholar]
- Klöckner, U., and G. Isenberg. 1985b. Calcium currents of cesium loaded isolated smooth muscle cells (urinary bladder of the guinea-pig). Pflügers. Archiv. 405:340–348. [DOI] [PubMed] [Google Scholar]
- Klöckner, U., and G. Isenberg. 1991. Myocytes isolated from porcine coronary arteries: reduction of currents through L-type Ca-channels by verapamil-type Ca-antagonists. J. Physiol. Pharmacol. 42:163–179. [PubMed] [Google Scholar]
- Klöckner, U., and G. Isenberg. 1992. ATP suppresses activity of Ca2+-activated K+ channels by Ca2+ chelation. Pflügers. Archiv. 420:101–105. [DOI] [PubMed] [Google Scholar]
- Klöckner, U., and G. Isenberg. 1994. Intracellular pH modulates the availability of vascular L type Ca2+ channels. J. Gen. Physiol. 103:647–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kockskämper, J., K. A. Sheehan, D. J. Bare, S. L. Lipsius, G. A. Mignery, and L. A. Blatter. 2001. Activation and Propagation of Ca2+ Release during Excitation-Contraction Coupling in Atrial Myocytes. Biophys. J. 81:2590–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, F. A., Q. Y. Liu, L. Xu, A. el-Hashem, N. R. Kramarcy, R. Sealock, and G. Meissner. 1992. Amphibian ryanodine receptor isoforms are related to those of mammalian skeletal or cardiac muscle. Am. J. Physiol. 263:C545–C550. [DOI] [PubMed] [Google Scholar]
- Lesh, R. E., G. F. Nixon, S. Fleischer, J. A. Airey, A. P. Somlyo, and A. V. Somlyo. 1998. Localization of ryanodine receptors in smooth muscle. Circ. Res. 8:175–185. [DOI] [PubMed] [Google Scholar]
- Leung, A. T., T. Imagawa, B. Block, C. Franzini-Armstrong, and K. P. Campbell. 1988. Biochemical and ultrastructural characterization of the 1,4-Dihydropyridine receptor from rabbit skeletal muscle. Evidence for a 52,000 subunit. J. Biol. Chem. 263: 994–1001. [PubMed] [Google Scholar]
- MacLennan, D. H., and Wong, P. T. 1971. Isolation of a calcium-sequestering protein from sarcoplasmic reticulum. Proc. Natl. Acad. Sci. USA. 68:1231–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahony, L., and L. R. Jones. 1986. Developmental changes in cardiac sarcoplasmic reticulum in sheep. J. Biol. Chem. 261:15257–15265. [PubMed] [Google Scholar]
- Meissner, G. 1975. Isolation and characterization of two types of sarcoplasmic reticulum vesicles. Biochim. Biophys. Acta. 389:51–68. [DOI] [PubMed] [Google Scholar]
- Mironneau, J., S. Arnaudeau, N. Macrez-Lepretre, and F. X. Boittin. 1996. Ca2+ sparks and Ca2+ waves activate different Ca2+-dependent ion channels in single myocytes from rat portal vein. Cell Calcium. 20:153–160. [DOI] [PubMed] [Google Scholar]
- Moore, E. D. W., E. F. Etter, K. D. Philipson, W. A. Carrington, K. E. Fogarty, L. M. Lifshitz, and F. S. Fay. 1993. Coupling of the Na+/Ca2+ exchanger, Na+/K+ pump in smooth muscle. Nature. 365:657–660. [DOI] [PubMed] [Google Scholar]
- Morad, M., and L. Cleemann. 1987. Role of Ca2+ channels in development of tension in heart muscle. J. Mol. Cell. Cardiol. 19:527–533. [DOI] [PubMed] [Google Scholar]
- Nakai, J., T. Tanabe, T. Konno, B. Adams, and K. G. B. Beam. 1998. Localization within the II–III loop of the dihydropyridine receptor of a sequence critical for excitation-contraction coupling. J. Biol. Chem. 273:24983–24986. [DOI] [PubMed] [Google Scholar]
- Nelson, M. T., H. Cheng, M. Rubart, F. S. Santana, A. D. Bonev, H. J. Knot, and W. J. Lederer. 1995. Relaxation of arterial smooth muscle by calcium sparks. Science. 270:633–637. [DOI] [PubMed] [Google Scholar]
- Neuhuber, B., U. Gerster, F. Doring, H. Glossmann, T. Tanabe, and B. E. Flucher. 1998. Association of calcium channel α1s and β1a subunits is required for the targeting of beta1a but not of alpha1S into skeletal muscle triads. Proc. Natl. Acad. Sci. USA. 95:5015–5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon, G. F., G. A. Mignery, and A. V. Somlyo. 1994. Immunogold localization of inositol 1,4,5-trisphosphate receptors and characterization of ultrastructural features of the sarcoplasmic reticulum in phasic and tonic smooth muscle. J. Muscle Res. Cell Motil. 15:682–700. [DOI] [PubMed] [Google Scholar]
- North, A. J., B. Galazkiewicz, T. J. Byers, J. R. Glenney, and J. V. Small. 1993. Complementary distributions of vinculin and dystrophin define two distinct sarcolemma domains in smooth muscle. J. Cell Biol. 120:1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi, Y., H. Yamamura, N. Nagno, M. Katsuhico, M. Watanabe, and Y. Imaizumi. 2003. Local Ca2+ transients and distribution of BK channels and ryanodine receptors in smooth muscle cells of guinea pig vas derens and urinary bladder. J. Physiol. 534:313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez, G. J., A. D. Bonev, J. B. Patlak, and M. T. Nelson. 1999. Functional coupling of ryanodinereceptors to KCa channels in smooth muscle cells from rat cerebral arteries. J. Gen. Physiol. 113:229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pragnell, M., J. Sakamoto, S. D. Jay, and K. P. Campbell. 1991. Cloning and tissue-specific expression of the brain calcium channel beta-subunit. FEBS Lett. 291:253–258. [DOI] [PubMed] [Google Scholar]
- Protasi, F., C. Franzini-Armstrong, and P. D. Allen. 1998. Role of ryanodine receptors in the assembly of calcium release units in skeletal muscle. J. Cell Biol. 140:831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer, D., I. G. Huber, M. L. Garcia, H. Haase, and J. Striessnig. 2000. Beta subunit heterogeneity of L-type Ca2+ channels in smooth muscle tissues. FEBS Lett. 467:65–69. [DOI] [PubMed] [Google Scholar]
- Sato, T. 1968. A modified method for lead staining of thin sections. J. Electron Microsc. 17:158–159. [PubMed] [Google Scholar]
- Schneider, P., H. H. Hopp, and G. Isenberg. 1991. Ca2+ influx through ATP-gated channels increments [Ca2+]i and inactivates ICa in myocytes from guinea-pig urinary bladder. J. Physiol. 440:479–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scriven, D. R. L., P. Dan, and E. D. W. Moore. 2000. Distribution of proteins implicated in excitation-contraction coupling in rat ventricular myocytes. Biophys. J. 79:2682–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo, A. P., C. E. Devine, A. V. Somlyo, and S. R. North. 1971. Sarcoplasmic reticulum and the temperature-dependent contraction of smooth muscle in calcium-free solutions. J. Cell Biol. 51:722–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo, A. V., and C. Franzini-Armstrong. 1985. New views of smooth muscle structure using freezing, deep-etching and rotary shadowing. Experientia. 41:841–856. [DOI] [PubMed] [Google Scholar]
- Somlyo, A. P., and A. V. Somlyo. 1970. Vascular smooth muscle. II. Pharmacology of normal and hypotensive vessels. Pharmacol. Rev. 22:249–353. [PubMed] [Google Scholar]
- Somlyo, A. P., and A. V. Somlyo. 1994. Signal transduction and regulation in smooth muscle. Nature. 372:231–236. [DOI] [PubMed] [Google Scholar]
- Somlyo, A. P., J. W. Walker, Y. E. Goldman, D. R. Trentham, S. Kobayashi, T. Kitazawa, and A. V. Somlyo. 1988. Inositol trisphosphate, calcium and muscle contraction. Phil. Trans. Roy. Soc. London B. 320:399–414. [DOI] [PubMed] [Google Scholar]
- Sun, X.-H., F. Protasi, M. Takahashi, H. Takeshima, D. G. Ferguson, and C. Franzini-Armstrong. 1995. Molecular architecture of membranes involved in excitation-contraction coupling of cardiac muscle. J. Cell Biol. 129:659–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutko, J. L., and J. A. Airey. 1996. Ryanodine receptor Ca2+ release channels: does diversity in form equal diversity in function? Physiol. Rev. 76:1027–1071. [DOI] [PubMed] [Google Scholar]
- Takekura, H., L. Bennett, T. Tanabe, K. G. Beam, and C. Franzini-Armstrong. 1994. Restoration of junctional tetrads in dysgenic myotubes by dihydropyridine receptor cDNA. Biophys. J. 67:793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijskens, P., G. Meissner, and C. Franzini-Armstrong. 2003. Location of ryanodine and dihydropyridine receptors in frog myocardium. Biophys. J. 84:1079–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa, A., P. Podini, M. C. Panzeri, H. D. Soling, P. Volpe, and J. Meldolesi. 1993. The endoplasmic-sarcoplasmic reticulum of smooth muscle: immunocytochemistry of vas deferens fibers reveals specialized subcompartments differently equipped for the control of calcium homeostasis. J. Cell Biol. 121:1041–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S. Q., M. D. Stern, E. Rios, and H. Cheng. 2004. The quantal nature of Ca2+ sparks and in situ operation of ryanodine receptor array in cardiac cells. Proc. Natl. Acad. Sci USA. 101:3979–3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel, E. R. 1979. Stereological Methods, Vol.1: Practical Methods for Biological Morphometry. Academic Press, New York.
- Wuytack, F., L. Raeymaekers, J. Verbist, L. R. Jones, and R. Casteels. 1987. Smooth-muscle endoplasmic reticulum contains a cardiac-like form of calsequestrin. Biochim. Biophys. Acta. 899:151–158. [DOI] [PubMed] [Google Scholar]
- Xu, L., F. A. Lai, A. Cohn, E. Etter, A. Guerrero, F. S. Fay, and G. Meissner. 1994. Evidence for a Ca(2+)-gated ryanodine-sensitive Ca2+ release channel in visceral smooth muscle. Proc. Natl. Acad. Sci. USA. 91:3294–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZhuGe, R., K. E. Fogarty, R. A. Tuft, and J. V. Walsh Jr. 2002. Spontaneous transient outward currents arise from microdomains where BK channels are exposed to a mean Ca2+ concentration on the order of 10 microM during a Ca2+ spark. J. Gen. Physiol. 120:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZhuGe, R., S. M. Sims, R. A. Tuft, K. E. Fogarty, and J. V. Walsh Jr. 1998. Ca2+ sparks activate K+ and Cl- channels, resulting in spontaneous transient currents in guinea-pig tracheal myocytes. J. Physiol. 513:711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZhuGe, R., R. A. Tuft, K. E. Fogarty, K. Bellve, F. S. Fay, and J. V. Walsh Jr. 1999. The influence of sarcoplasmic reticulum Ca2+ concentration on Ca2+ sparks and spontaneous transient outward currents in single smooth muscle. J. Gen. Physiol. 113:215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]