Abstract
Recent evidence suggests that circulating leukocytes respond to physiological levels of fluid shear stress. This study was designed to examine the shear stress response of individual leukocytes adhering passively to a glass surface. Human leukocytes were exposed to a step fluid shear stress with amplitude between 0.2 and 4 dyn/cm2 and duration between 1 and 20 min. The response of the cells was determined in the form of projected cell area measurements by high-resolution observation before, during, and after fluid shear application. All cells selected initially had a round morphology. After application of fluid shear many cells projected pseudopodia and spread on the glass surface. The number of leukocytes responding with pseudopod projection and the extent of cell spreading increased with increasing amplitude and duration of fluid shear stress. Pseudopod projection after exposure to a step fluid shear occurs following a delay that is insensitive to the shear stress amplitude and duration. Leukocytes that did not project pseudopodia and spread in response to low shear stress could be shown to respond to a second shear step of higher amplitude. The spreading response requires an intact actin network and activated myosin molecules. Depleting the cell glycocalyx with protease treatment enhances the spreading response in sheared leukocytes. These results indicate that passive leukocytes respond to fluid shear stress with active pseudopod projection and cell spreading. This behavior may contribute to cell spreading on endothelium and other cells as well as to transendothelial migration of leukocytes in the microcirculation.
INTRODUCTION
Mobilization of leukocytes from the microcirculation into tissues is an early step in the response to injury, infection, or inflammation. The accumulation of neutrophils in tissues has been described as a sequence of events (Springer, 1994) including leukocyte capture from the bloodstream, transmigration across the endothelium, active motion to the site of injury or infection, and microbial function. The processes underlying cell behavior during these steps are under precise cellular control. Uncontrolled accumulation of leukocytes in tissues can cause excessive inflammation and tissue injury. Impaired function is the cause of several clinical situations characterized by recurrent infection or chronic inflammation.
There is accumulating evidence suggesting that mechanical signals are important regulators of leukocyte function. Specifically, leukocytes sense and respond to fluid shear stress. Leukocytes exposed to fluid shear exhibit increased adhesion (McIntire et al., 1976; Dewitz et al., 1977; Okuyama et al., 1996), increased level of motility (Tomczok et al., 1996; Kitayama et al., 2000), transformation from random to directed migration (Rainger et al., 1999; Kitayama et al., 2000), enhanced endothelial transmigration (Weber et al., 1999; Kitayama et al., 2000; Cinamon et al., 2001a,b; Cuvelier and Patel, 2001), and altered phagocytic ability (Rosenson-Schloss et al., 1999; Shive et al., 2000) compared to cells maintained in a static environment. Each of these behaviors is required to bring circulating cells to a site of injury or infection and to destroy invading pathogens.
The objective of the current investigation was to examine the response of passive leukocytes after exposure to a defined fluid shear stress. Leukocytes gently isolated from whole blood were allowed to nonspecifically bind to a glass surface. Fluid flow from a pipette generated a shear field on the membrane of single cells. The cell morphology was monitored in response to multiple amplitudes and durations of fluid shear stress. While this in vitro approach to investigate the response of leukocytes to fluid shear stress does not completely mimic the more complex shear field experienced by leukocytes in vivo, it does permit a detailed investigation of the time course and extent of the leukocyte response to precisely controlled fluid flow. It was observed that passive leukocytes respond to fluid shear stress by projecting pseudopodia and spreading on the glass. Cells were responsive to low levels of shear stress, and the extent of spreading increased with amplitude and duration of fluid shear. While the spreading behavior requires an intact actin cytoskeleton and active myosin in the cytoskeleton, key components underlying the spreading response may lie at the cell surface. Enzymatic degradation of the main component of the neutrophil glycocalyx enhanced the spreading response in cells exposed to shear stress.
METHODS
Cell preparation and visualization
The experimental protocol was designed to examine individual leukocytes under high-resolution brightfield microscopy during exposure to well-defined fluid shear stress (Moazzam et al., 1997). Blood was collected from healthy volunteers by venous puncture into heparinized vacutainers (Monoject, Kendall Health Care, Totowa, NJ) or by finger prick into microhematocrit capillary tubes (Fisherbrand, Heusenstamm, Germany). Venous blood was transferred from vacutainers to microhematocrit capillary tubes. Following red-cell sedimentation in capillary tubes for 30 min, 5–10 μl of supernatant was diluted in 1 ml media (Plasma-Lyte A (Baxter, Deerfield, IL) supplemented with 2.5 mM Ca2+, adjusted to pH 7.4, and filtered (0.22 μm, Fisherbrand, Fisher Scientific, Fair Lawn, NJ)). The diluted blood was deposited on a coverglass that was previously rinsed with isopropyl alcohol, scrubbed with soapy water, and thoroughly flushed with ion-purified water. Individual cells were viewed under brightfield using an inverted microscope (Leitz Diavert, Rockleigh, NJ) with 50× objective (N.A. 1.4) and 25×-projection eyepiece. Experiments were recorded on videotape (VHS, Panasonic, Secaucus, NJ) with a CCD camera (Javelin Systems, Irvine, CA) for subsequent analysis.
Micropipettes
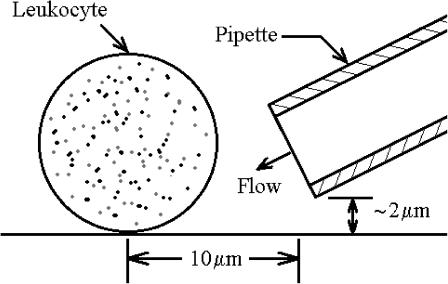
Pipettes used to expose leukocytes to a controlled fluid flow were fabricated from borosil capillary tubing (Frederick Haer and Co., Bowdoinham, ME) with a vertical pipette puller (David Kopf Instruments, Tujunga, CA). Pipette tips were manually broken to size (mean diameter 4.58 ± 0.41 μm, n = 40). Pipettes were filled with media, mounted on a micromanipulator (Narishige, Tokyo, Japan), connected to a hydrostatic pressure control system, and positioned adjacent to the cell. The pipette tip was inclined 30° to the horizontal plane, raised ∼2 μm above the glass substrate, and maintained a distance of ∼10 μm from the cell center (Fig. 1).
FIGURE 1.
Schematic diagram of a glass adherent cell exposed to shear stress from fluid discharge out of a pipette. The distance between the cell center and pipette tip was maintained at ∼10 μm. The pipette was held at an angle of 30° to the substrate and raised a distance of ∼2 μm above the substrate.
Application of fluid shear stress
Individual leukocytes were selected that adhered to the coverglass and exhibited no motility before exposure to fluid shear stress. Only adherent cells with spherical morphology were chosen for fluid shear exposure. No subclasses of leukocytes were investigated in this study because cell isolation techniques may influence the fluid shear response (Fukuda and Schmid-Schönbein, 2002). The flow rate out of the pipette was adjusted by hydrostatic pressure ΔP in a fluid reservoir connected to the pipette. The protocol for shearing each cell was composed of three intervals: 1), an initial 5-min observation of the cell shape without fluid shear stress; 2), a period of shear stress application for several preselected time intervals; and 3), a 5-min observation period without fluid shear stress. To examine the amplitude dependence of the leukocyte shear response, reservoir fluid pressures ΔP = 0, 1, 5, 10, and 20 cmH2O were maintained for a shear duration tτ = 5 min. These pressures correspond to maximum fluid shear stresses on the cell surface of approximately τ = 0, 0.2, 1, 2, and 4 dyn/cm2, respectively (Moazzam et al., 1997). To examine how the duration of shear application influenced the leukocyte shear response, a constant shear stress of τ = 2 dyn/cm2 was applied to the cell surface for tτ = 1, 5, and 20 min. Cell morphology was carefully monitored before, during, and after shear stress application.
Cell shape analysis
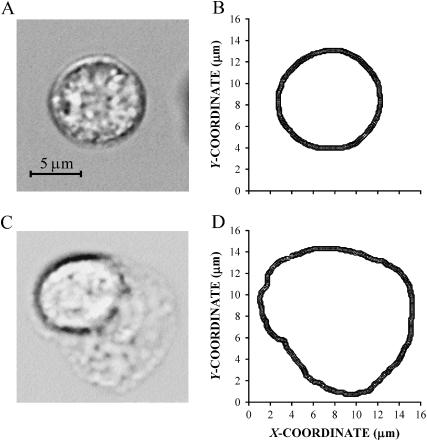
Individual video frames were digitized (Xceed Technology, Chesterfield, MI) and analyzed using image analysis software (NIH Image, v. 1.61). A discrete representation of the cell shape was obtained from digitized frames (Fig. 2, A and C) by using the mouse and the freehand selection tool to manually outline the cells (Fig. 2, B and D). The coordinates of the cell outlines were used to compute the projected cell area, A. The cell area A was normalized by its initial value, A0, defined as the mean area measured over the initial 5-min observation period preceding the application of shear stress. If the cell underwent no shape change, the time interval between analyzed images was 1 min or less. As the cell began to change its shape, the time interval between frames was reduced. For cells undergoing rapid shape changes, the time interval was typically 5 s, and in selected cases it was even shorter.
FIGURE 2.
Digitized images of a round (A) and spreading (C) cell and the corresponding discrete representations of the cell outline (B and D, respectively).
The normalized area A/A0 was used to identify cells undergoing a shape change in response to the applied fluid shear (responding cells) from cells exhibiting no shape change (nonresponding cells). To distinguish these populations of cells, a critical normalized area AC was defined such that any cell in which A/A0 > AC was categorized as a responding cell, otherwise it was categorized as a nonresponding cell. The critical area AC was defined by
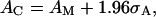 |
(1) |
where AM is the maximum normalized area measured from cells in the absence of shear stress and σA is the variance in the measurement of cell area. The maximum normalized area AM was determined from all measurements of normalized cell area in the absence of shear stress. As such, AM includes the measurements of cell area from the initial observation of cell shape that precedes the application of shear stress and the measurements of cell area from the entire record of control cells not exposed to a shear stress, τ = 0 dyn/cm2. The advantage of this particular choice of AC was to insure with high confidence that if A/A0 > AC, then the behavior of any particular cell in response to fluid shear differed from the behavior of cells in the absence of shear. The first term on the right-hand side of Eq. 1 is the maximum area observed in cells under static no-shear conditions. This term insures that the normalized area A/A0 of responding cells exceeds the maximum A/A0 measured in cells in the absence of shear stress. The second term on the right-hand side of Eq. 1 represents the ∼95% confidence interval in the measurement of the normalized cell area A/A0. This term is included to account for the variability in measuring A/A0 from digitized images of cells. Thus, the second term insures that when a measured A/A0 > AC, it is because the cell has undergone substantial shape change that can be measured by the current method, which relies on the detection of the projected cell contour.
Two parameters were used to quantitatively characterize the response to shear stress among the population of responding leukocytes. The response time, TR, defined as the time elapsed from the onset of shear application to the time when A/A0 reached AC, was used as a measure of how rapidly a cell undergoes morphological changes following the onset of fluid shear. The maximum normalized area AMAX, defined as the maximum achieved A/A0 during the time course of the experiment, was used as a measure of the extent of cell shape change following the onset of fluid shear exposure.
Modulation of cell adhesion
To examine the biophysical mechanisms underlying the leukocyte response to shear stress application, the cells were pretreated with several agents that modify their ability to adhere to a substrate. The approach was guided by a theoretical framework for cell adhesion (Bell et al., 1984; Dembo and Bell, 1987) in which the adhesive state of the cell is regarded as a competition between forces promoting and preventing contact with the substrate.
The main cellular component responsible for preventing adhesion to a substrate is the hydrated polymer coating of the glycocalyx (Bell et al., 1984). On blood cells, the main constituent of the glycocalyx is the heavily O-glycosylated and sialylated surface protein CD43. The ability of CD43 to prevent surface interactions has been demonstrated in neutrophils (Nathan et al., 1993) and other leukocytes (Ardman et al., 1992). To decrease the ability of the glycocalyx to prevent adhesion, cells in this study were treated with 5 and 17 μg/ml human neutrophil elastase (HNE) for at least 10 min before shear stress application. Flow cytometric analysis has shown that HNE is remarkably specific for CD43, causing loss of the distal ∼40% of its extracellular region (Remold-O'Donnell and Parent, 1995). Other prominent surface proteins on neutrophils associated with adhesion (CD11a, CD11b, CD18, and others) are unaffected by HNE (Remold-O'Donnell and Parent, 1995). A concentration of 5 and 17 μg/ml HNE cleaves ∼50% and ∼25% of the surface CD43 (Remold-O'Donnell and Parent, 1995).
The forces responsible for keeping the cell in a round configuration are primarily attributed to the cytoskeleton. To decrease the ability of the cell to maintain a round configuration, cells were treated with the actin-disrupting agent cytochalasin D (1 and 10 μM) and the myosin inhibitor ML-7 (5 and 50 μM). Cytochalasin D disrupts the network of actin filaments by preventing polymerization at the fast-growing end. At a concentration of 1 μM, cytochalasin D decreases the apparent cortical tension of human neutrophils by 43% as assessed by micropipette aspiration (Ting-Beal et al., 1995) and decreases the apparent cell stiffness by ∼30% as assessed by cell poking (Worthen et al., 1989). Treatment with 10 μM cytochalasin D is so damaging to the actin network that human neutrophils are unable to maintain a spherical morphology (Keller and Niggli, 1995; Ting-Beal et al., 1995). ML-7 impairs force generation by myosin molecules by specifically and potently inhibiting myosin light chain kinase. At concentrations of 5 and 50 μM, ML-7 inhibits leukocyte phagocytosis by ∼60% and ∼100%, respectively (Mansfield et al., 2000), and decreases neutrophil motility by ∼70 and ∼100%, respectively (Eddy et al., 2000).
The ability of the cell to maintain a round configuration was enhanced by treatment of cells with sodium azide (10 mM). Sodium azide depletes ATP and induces a rigor state between actin filaments and myosin molecules. Lymphocyte stiffness increases threefold with 10 mM sodium azide as assessed by cell poking (Pasternak et al., 1989).
Cells were incubated in the presence of each treatment for at least 10 min on the coverslip before the shear stress was applied. After each intervention, the cells were exposed for 5 min to fluid shear at 2 dyn/cm2, as described above. The intervention studies and the data analysis were performed blindly.
RESULTS
After placement on the coverglass, the majority of the freshly harvested leukocytes were round (∼94%). Few cells exhibited pseudopod projection (∼4%) or spontaneous spreading (∼2%). This ratio of passive to active cells is consistent with in vivo observations (Ohashi et al., 1996) showing that most circulating leukocytes are in a round or passive state. Many of the round cells adhered to the glass (∼41%), whereas the remaining round cells contacted the glass but did not adhere. The adherent cells remained in place if exposed to fluid flow from the pipette. In the current study, individual cells from this passive, glass-adherent population were exposed to fluid shear stress.
Under static conditions, the cells maintained a round morphology, and the normalized area was nearly constant (A/A0 = 1.000 ± 0.023) for all observations (n = 1276). The maximum measured value of the normalized area in the absence of shear stress was AM = 1.06. The variability in measuring A/A0 for a single responding cell was σA = 4.4% in nine independent measurements by several observers. These values give a critical area AC = 1.15 to distinguish responding from nonresponding cells.
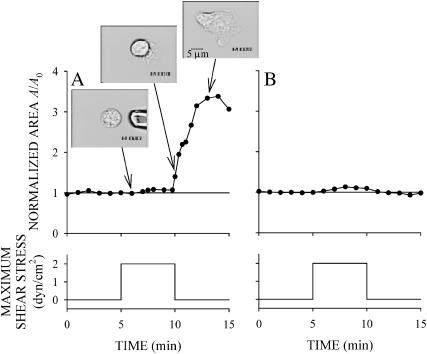
Some of the passive, glass-adherent leukocytes undergo a dramatic shape change after application of a sustained fluid flow from the pipette. An example of a responding cell and a nonresponding cell are shown in Fig. 3. Before application of fluid flow the cells are round and A/A0 is close to one. Immediately after shear application both cells remain round, indicating that the subsequent shape change is not due to a passive cell deformation (Fig. 3, A first inset, and B). The responding cell projects pseudopodia and spreads on the coverglass (Fig. 3 A, insets). The cell continues spreading on the substrate after the shear flow is stopped. The fluid shear response of passive leukocytes was not uniform among all cells. Some cells assumed a round shape after initially projecting pseudopodia and spreading in response to fluid shear stress. Other cells showed no detectable response during or after fluid shear application (Fig. 3 B).
FIGURE 3.
Examples of the response of leukocytes exposed to shear stress of 2 dyn/cm2 for 5 min. (A) A responding cell that spread extensively on the substrate. Notice that the cell continued to spread after the shear stress was returned to zero. (B) A cell that failed to show any detectable shear stress response.
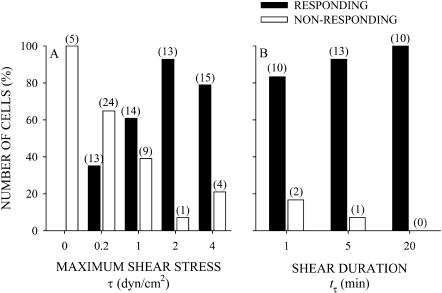
The number of responding cells increased and nonresponding cells decreased with increasing shear stress amplitude τ and duration tτ (Fig. 4, A and B). In the absence of shear stress, τ = 0, all of the cells were nonresponding (Fig. 4 A). For the lowest shear stress of τ = 0.2 dyn/cm2 applied for tτ = 5 min, some cells underwent sufficient shape change to be classified as responding cells (33%); however, most were nonresponding (67%). For each τ ≥ 1 dyn/cm2 and tτ = 5 min, a majority of cells responded to shear stress (>60%) by projecting pseudopodia and spreading on the coverglass. The shorter duration of shear application at τ = 2 dyn/cm2 of 1 min was still sufficient to induce pseudopod projection and spreading (Fig. 4 B). During 20 min of shear at τ = 2 dyn/cm2, all cells were responding. These results show that leukocytes are more likely to respond at the higher shear stress amplitudes and longer shear stress duration.
FIGURE 4.
The number of responding cells as a function of the (A) maximum shear stress for cells exposed to a 5-min shear application, and (B) duration of shear exposure for cells sheared with a maximum shear stress of 2 dyn/cm2. The filled bars correspond to the number of responding cells in each group. The open bars correspond to the number of nonresponding cells. The numbers over each bar correspond to the number of observations.
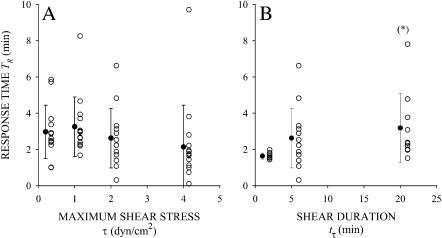
Among responding cells, the response time TR was insensitive with respect to the amplitude of shear stress (Fig. 5 A). On average, TR slightly increased with the duration of fluid shear stress exposure (Fig. 5 B). For tτ = 5 min and all shear stresses τ, TR was ∼3 min (Fig. 5 A), indicating that passive leukocytes require minutes to respond with a shape change to the shear stress. For τ = 2 dyn/cm2, TR was significantly higher (p < 0.05) for tτ = 20 min than for tτ = 1 min (Fig. 5 B), whereas TR was not significantly different for tτ = 1 and 5 min. The reason TR was higher in cells sheared for 20 min compared to 1 or 5 min may be that the longer shear duration induces more cells to respond. That is, a longer shear duration may cause cells to respond that otherwise would not respond to short shear exposure. For the shortest duration of shear exposure, tτ = 1 min, TR ≥ 1 min for all the responding cells. This indicates that all the cells respond to fluid shear stress after the shear stress is removed (Fig. 5 B). The delay between the cell response and the application of shear stress is evidence that human leukocytes have a memory of their past mechanical exposure.
FIGURE 5.
The response time of responding cells as a function of the (A) maximum shear stress for cells exposed to a 5-min shear application, and (B) duration of shear exposure for cells sheared with a maximum shear stress of 2 dyn/cm2. Solid symbols and error bars correspond to the mean ± SD of the observations (open symbols). In B, (*) indicates statistical significance (p < 0.05) compared to τ = 1 min.
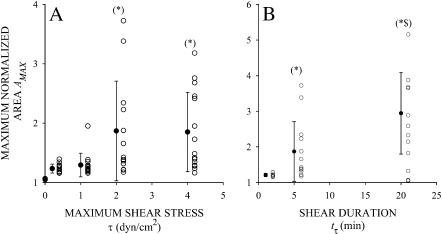
Among responding cells, the maximum normalized area AMAX increased with increasing τ and tτ (Fig. 6, A and B). The mean values for AMAX at each τ and tτ were significantly higher than the mean value for AMAX observed in cells under static conditions. For the two lowest shear stresses τ = 0.2 and 1.0 dyn/cm2 and tτ = 5 min, AMAX was slightly higher than AC, indicating that these cells respond to shear stress with a moderate level of spreading. For the lowest shear stress, τ = 0.2 dyn/cm2, no cell projected extensive pseudopodia or flattened on the substrate. In contrast, at higher shear stresses of τ = 2 and 4 dyn/cm2, while maintaining tτ = 5 min, many cells responded to shear application by completely and irreversibly flattening on the glass substrate. Similarly, extensive spreading in responding cells was observed for τ = 2 dyn/cm2 and tτ > 1 min (Fig. 6 B). Increasing tτ from 1 to 5 and 20 min resulted in a systematic and significant increase in AMAX. The duration of shear exposure, tτ, appears to be more important than the amplitude, τ, in stimulating the spreading response. Some cells exposed to 20 min of shear stress at τ = 2 dyn/cm2 spread even more extensively (Fig. 6 B) than cells exposed to higher shear stress of τ = 4 dyn/cm2 for only tτ = 5 min (Fig. 6 A).
FIGURE 6.
The maximum normalized area of responding cells as a function of the (A) maximum shear stress for cells exposed to a 5-min shear application, and (B) duration of shear exposure for cells sheared with a maximum shear stress of 2 dyn/cm2. Solid symbols and error bars correspond to the mean ± SD of the observations (open symbols). Mean values were significantly different from control observations. In A, (*) indicates statistical significance (p < 0.05) compared to τ = 1 and 5 dyn/cm2. In B, (*) indicates statistical significance compared to tτ = 1 min; ($) indicates statistical significance (p < 0.05) compared to tτ = 5 min.
Since Plasma-Lyte has no buffering capacity, it is possible that shear stress-induced local pH fluctuations influence the cell spreading response. To test this possibility, AMAX of responding cells in Plasma-Lyte was compared to AMAX of responding cells in phosphate-buffered saline (PBS). The average AMAX of responding cells in Plasma-Lyte (AMAX = 1.87 ± 0.84) was greater than in PBS (AMAX = 1.50 ± 0.50), but the difference was not significant (p = 0.24).
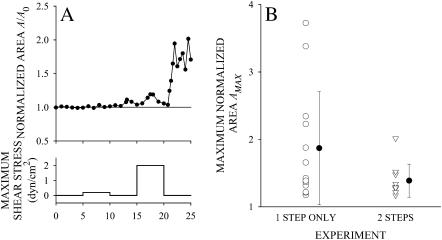
To further investigate the trigger mechanisms for the spreading response of leukocytes, cells that did not respond to a 5-min exposure to low fluid shear stress, τ = 0.2 dyn/cm2, were exposed to a second period of 5 min fluid shear stress at τ = 2 dyn/cm2. Among cells that did not respond to the first shear period of τ = 0.2 dyn/cm2, 66% responded to a second shear application of τ = 2 dyn/cm2 with an increase in A/A0 above AC. A typical example of a cell that responded to shear at τ = 2 dyn/cm2 but not at τ = 0.2 dyn/cm2 is shown in Fig. 7 A. This observation suggests that nonresponding cells maintain the ability to respond to shear application but require a sufficiently strong signal to initiate pseudopod projecting and spreading. Among the cells that responded to a second step at 2 dyn/cm2 for 5 min, the extent of pseudopod projection and cell spreading was attenuated compared to cells not exposed to the initial step (Fig. 7 B). This result also indicates that the fluid shear history influences the cell response.
FIGURE 7.
(A) Typical example of a leukocyte that failed to respond to a shear of 0.2 dyn/cm2 for 5 min but did respond to a second shear application at 2 dyn/cm2 for 5 min. (B) The maximum normalized area for cells exposed to a single fluid shear exposure of 2 dyn/cm2 for 5 min (1 STEP ONLY) and cells exposed to fluid shear of 0.2 dyn/cm2 for 5 min followed by a second shear application of 2 dyn/cm2 for 5 min (2 STEPS).
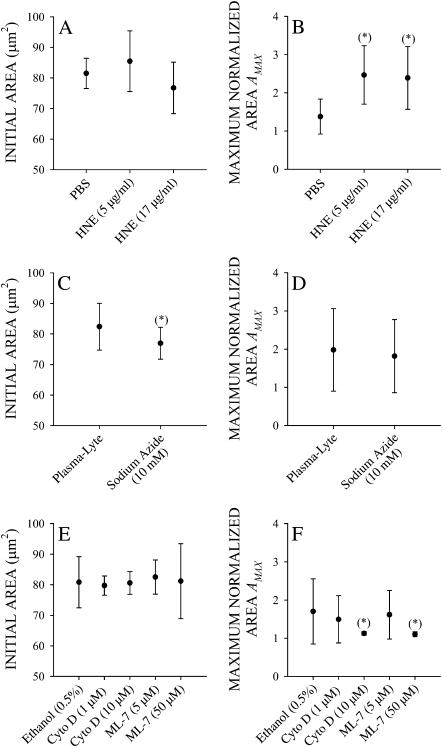
Cells pretreated with 5 or 17 μg/ml HNE in PBS caused no significant change in the initial adhesive state of the cell compared to controls as judged by the cell area (Fig. 8 A). However, in responding cells pretreatment with HNE significantly increased AMAX compared to cells pretreated with only PBS (Fig. 8 B). This enhanced spreading response to shear stress is consistent with the possibility that shear stress acts through the glycocalyx to induce a spreading response.
FIGURE 8.
Initial projected area before application of shear stress and maximum normalized area with application of fluid shear stress at 2 dyn/cm2 for 5 min of leukocytes pretreated with 5 and 17 μg/ml HNE (A and B, respectively), 10 mM sodium azide (C and D, respectively), and 1 and 10 mM cytochalasin D (Cyto D) and 5 and 50 mM ML-7 (E and F, respectively).
Pretreatment with 10 mM sodium azide (NaN3) in Plasma-Lyte significantly shrunk the cell in the initial state as indicated by the decrease in cell area (Fig. 8 C). Nevertheless, these cells still exhibited a spreading response with no difference in AMAX in NaN3-treated and untreated cells (Fig. 8 D).
Pretreatment with low doses of cytochalasin D or ML-7 in ethanol did not change the initial projected cell area (Fig. 8 E). Responding cells exhibited less spreading following shear stress application (Fig. 8 F). Higher doses of these agents completely obliterated the spreading response (Fig. 8 F). Although these cells still responded to shear application, i.e., A/A0 > AC, none of the cells projected broad pseudopodia or became extensively spread. Thus, the spreading response to shear stress application requires an intact actin cytoskeleton and active myosin molecules.
DISCUSSION
The observations presented here provide direct evidence that fluid shear stress has a profound and fundamental influence on the activities of circulating leukocytes. Although isolation techniques to achieve purified cell populations were avoided, the current study focused mostly on neutrophils. Other leukocyte types, like lymphocytes (Cinamon et al., 2001a,b) and monocytes (Weber et al., 1999), also exhibit a fluid shear response.
The aim of the current study was to systematically examine the response of adherent but otherwise passive human leukocytes to a defined fluid shear. A key observation in this study is that leukocytes, which initially show no signs of active movement and remain adhered to their substrate with almost perfect spherical cell outline, are induced to project pseudopodia by fluid shear stresses as encountered in the microcirculation. Such cells are able to sense a remarkably low shear stress, τ = 0.2 dyn/cm2 (1 dyn/cm2 = ∼1/981 cm H2O), as well as higher shear stresses, τ = 4 dyn/cm2, that are sufficient to peel some cells off the substrate. The maximum shear stress on the surface of an adherent spherical leukocyte in the microcirculation has been estimated at 100 dyn/cm2 (Gaver and Kute, 1998). A majority of passive leukocytes respond to shear by projecting pseudopodia and spreading on a glass substrate. The response in the form of pseudopod projection and spreading is manifested within minutes of shear application (Fig. 5). The extent of the spreading response increases with increasing shear amplitude and duration (Fig. 6). Exposing leukocytes to a relatively short duration (tτ = 1 min) of shear showed that the cells maintain the ability to respond after the shear stimulus is removed (Fig. 5 B). Thus, human leukocytes have a memory for the history of their fluid shear stress exposure. This memory may underlie some of the variability in the current observations. While the cells were collected from laboratory volunteers without symptoms and under standardized conditions, the fact that leukocytes in the circulation are at different stages of their life cycle and have experienced different fluid shear histories may contribute to the variability in the shear responses observed in this study.
The biophysical mechanism that leads to spreading of leukocytes under fluid shear stress remains unknown. Partial cleavage of the glycocalyx enhances the spreading response to shear stress. It is possible that shear stress alone acts by a similar mechanism. That is, shear stress on the cell surface may mimic the proteolytic action of human neutrophil elastase and reduce the efficacy of the glycocalyx. Shear stress-induced shedding of components of the glycocalyx may enhance spreading by two mechanisms. First, loss of glycocalyx proteins reveals previously hidden receptors and sites for adhesion. Cell spreading progresses by engaging new receptors to complementary sites on the substrate. Since integrin receptors are particularly important for firm adhesion and diapedesis of leukocytes in vivo, shear stress-induced shedding of the glycocalyx may reveal integrin receptors on the cell surface that can participate in cell adhesion and spreading on the endothelium. Second, the main components of the glycocalyx are often rigid, extended molecules that bear anionic groups. Consequently, loss of glycocalyx reduces the electrostatic, osmotic, and stearic forces between the cell and opposing surface. Loss of repulsive interactions enhances spreading.
Another possible mechanism for the spreading response in passive neutrophils is activation of the GTPase rac. Rac has been identified as an important regulator of actin filament organization during formation of lamellipodia in adherent cells. Shear stress activates rac in endothelial cells, and activated rac enhances endothelial cell surface protrusions and motility (Hu et al., 2001). Importantly, rac causes the formation of actin-rich broad pseudopodia and spreading in human neutrophils (Bird et al., 2003). The spreading response reported here also requires an intact actin cytoskeleton. High concentrations of cytochalasin D completely obliterated the spreading response to shear stress (Fig. 8 F). Moreover, the delay between the application of fluid shear stress and the leukocyte spreading response (∼3 min, Fig. 5) is consistent with the time course of the increase in rac activity (∼2–5 min) in endothelial cells subjected to fluid shear stress.
The current results are consistent with other studies showing that fluid shear stress enhances motility in leukocytes (Tomczok et al., 1996; Rainger et al., 1999; Weber et al., 1999; Kitayama et al., 2000; Cinamon et al., 2001a,b; Cuvelier and Patel, 2001) and other cell types (Décave et al., 2003; Shiu et al., 2004). However, using the same pipette technique motile leukocytes instead retract all pseudopodia, approach a more spherical shape, and may even detach from the substrate with shear stress exposure (Moazzam et al., 1997). The results from this study indicate that the retraction response is rarely observed in the current population of passive adherent cells selected for investigation. Of the 10 cells that actively projected pseudopodia during the longest shear application of 20 min at 2 dyn/cm2, only one became round while exposed to shear stress. The remaining nine cells continued projecting pseudopodia and kept spreading.
In the current group of cells induced to project pseudopodia and spread by shear stress, the signal for retraction of pseudopodia seemed to be provided by the removal of shear stress. A majority (28 out of 42) of the cells exposed to 5 or 20 min shear duration that were spread at the end of the shear application (i.e., had A/A0 > AC when the shear stress was removed) quickly started rounding again after removing the shear stress. In those 28 cells, it took an average of 56 s for A/A0 to reach or drop below AC, indicating that the pseudopod retraction occurred rapidly once the shear stress was removed.
The two main differences between the previous (Moazzam et al., 1997) and the current study was the choice of parameter to characterize cell shape changes and the initial state of the cells being exposed to shear stress. In the current study, cell shape was characterized by the projected cell area computed from the cell outlines instead of the maximum cell length measured from videotape images as was previously done (Moazzam et al., 1997). Several geometric parameters were examined as candidate measures of cell shape, including projected area, maximum length (i.e., maximum distance between two points on the cell outline), maximum diameter (i.e., distance between points on the cell outline passing through the cell centroid), maximum radius, and cell circumference. Cell projected area has the advantage over other measures in that it closely corresponds to what is observed in watching a cell subjected to shear stress. Since each of these particular parameters are scalar measures of cell shape, they all gave similar results in terms of number of responding versus nonresponding cells, extent of cell shape change, and time course of cell shape change (data not shown). Thus, the choice of geometric parameter does not explain pseudopod retraction in one study and pseudopod projection found here. The other difference between the current and previous investigations of the leukocyte fluid shear response was the initial state of the cell. In this study, the cells were passively adhering to a glass substrate, whereas in Moazzam et al. (1997) the cells were actively projecting pseudopodia. The initial state of the cell may dictate whether fluid shear stress results in pseudopod retraction or projection. The underlying mechanisms remain to be further examined.
The experimental approach in this study using a fluid flow from a pipette on single cells has several advantages and limitations. The approach allows continuous observation of individual leukocytes at high optical resolution so that the morphological response can be observed continuously. The interaction between the cell and the glass substrate was nonspecific. In the presence of plasma, glass surfaces tend to become coated with plasma proteins. Several adhesion receptors participate in signal transduction events and the subsequent spreading behavior. To investigate this aspect further, we blocked β1 integrin receptors with mouse antihuman CD29, clone 6S6 (Chemicon International, Temecula, CA) and β2 integrin receptors with mouse antihuman CD18 (IgG1 clone M2M48, Research Diagnostic, Flanders, NJ). The presence of the antibodies resulted in extensive cell activation. In this study, such a shift in the baseline cell behavior would have prevented characterization of the shear response of passive cells.
The pipette system used to deliver a fluid shear stress introduces several challenges over other methods (e.g., flow chambers or viscometers). Most noticeable is the uncertainty in velocity field in the region of the cell. A quantitative estimate of the shear stress on the cell surface was obtained from an analysis of the velocity field around a cell produced by flow out of a pipette (Moazzam et al., 1997). The Stokes approximation for the equation of motion of a Newtonian fluid (buffer solution) was numerically solved assuming a simplified disk-shaped cell geometry. Order of magnitude estimates of the shear stress over the cell surface were obtained. A key feature of this flow field is the kinematic linearity, i.e., doubling the pipette pressure doubles the shear stress on the cell surface. An advantage of the pipette system is that only a single cell is sheared during each experiment. It is possible to observe individual cells at high magnification for various shear stress histories, and repeat the process for many cells from a single blood sample. In contrast, a flow chamber or viscometer simultaneously exposes all cells in a sample to shear stress. Thus, either one cell per sample can be observed, or multiple cells are observed simultaneously but at lower magnification. Both situations are less desirable for the purpose of this investigation.
In the microcirculation, the spreading response of human leukocytes may have a number of physiological consequences. For example, at a site of injury or infection, the endothelium expresses a number of membrane adhesion receptors to capture circulating leukocytes. The majority of circulating leukocytes are in a nonactivated state without pseudopods. A circulating leukocyte traveling with the flowing blood experiences a sudden and substantial increase in fluid shear stress on its surface after capture and adhesion to the endothelium. The leukocyte spreading response may then provide a key signal to the cell to initiate migration on the endothelium and transendothelial extravasation. No soluble messenger is required for such a response.
Acknowledgments
This research was supported by the United States Public Health Service Program Project grant HL43024.
References
- Ardman, B., M. A. Sikorski, and D. E. Staunton. 1992. CD43 interferes with T-lymphocyte adhesion. Proc. Natl. Acad. Sci. USA. 89:5001–5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, G. I., M. Dembo, and P. Bongrand. 1984. Cell adhesion. Competition between nonspecific repulsion and specific bonding. Biophys. J. 45:1051–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird, M. M., G. Lopez-Lluch, A. J. Ridley, and A. W. Segal. 2003. Effects of microinjected small GTPases on the actin cytoskeleton of human neutrophils. J. Anat. 203:379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinamon, G., V. Grabovsky, E. Winter, S. Franitza, S. Feigelson, R. Shamri, O. Dwir, and R. Alon. 2001a. Novel chemokine functions in lymphocyte migration through vascular endothelium under shear flow. J. Leukoc. Biol. 69:860–866. [PubMed] [Google Scholar]
- Cinamon, G., V. Shinder, and R. Alon. 2001b. Shear forces promote lymphocyte migration across vascular endothelium bearing apical chemokines. Nat. Immunol. 2:515–522. [DOI] [PubMed] [Google Scholar]
- Cuvelier, S. L., and K. D. Patel. 2001. Shear-dependent eosinophil transmigration on interleukin 4-stimulated endothelial cells: a role for endothelium-associated eotaxin-3. J. Exp. Med. 194:1699–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Décave, E., D. Rieu, J. Dalous, S. Fache, Y. Bréchet, B. Fourcade, M. Satre, and F. Bruckert. 2003. Shear flow-induced motility of Dictostelium discoideum cells on solid substrate. J. Cell Sci. 116:4331–4343. [DOI] [PubMed] [Google Scholar]
- Dembo, M., and G. I. Bell. 1987. The thermodynamics of cell adhesion. Curr. Topics Memb. Trans. 79:71–89. [Google Scholar]
- Dewitz, T. S., T. C. Hung, R. R. Martin, and L. V. McIntire. 1977. Mechanical trauma in leukocytes. J. Lab. Clin. Med. 90:728–736. [PubMed] [Google Scholar]
- Eddy, R. J., L. M. Pierini, F. Matsumura, and F. R. Maxfield. 2000. Ca2+-dependent myosin II activation is required for uropod retraction during neutrophil migration. J. Cell Sci. 113:1287–1298. [DOI] [PubMed] [Google Scholar]
- Fukuda, S., and G. W. Schmid-Schönbein. 2002. Centrifugation attenuates the fluid shear response of circulating leukocytes. J. Leukoc. Biol. 72:133–139. [PubMed] [Google Scholar]
- Gaver, III, D. P., and S. M. Kute. 1998. A theoretical model study of the influence of fluid stresses on a cell adhering to a microchannel wall. Biophys. J. 75:721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, Y.-L., S. Li, H. Miao, T.-C. Tsou, M. Angel del Pozo, and S. Chien. 2002. Roles of microtubule dynamics and small GTPase rac in endothelial cell migration and lamellipodium formation under flow. J. Vasc. Res. 39:465–476. [DOI] [PubMed] [Google Scholar]
- Keller, H., and V. Niggli. 1995. Effects of cytochalasin D on shape and fluid pinocytosis in human neutrophils as related to cytoskeletal changes (actin, α-actinin and microtubules). Eur. J. Cell Biol. 66:157–164. [PubMed] [Google Scholar]
- Kitayama, J., A. Hidemura, H. Saito, and H. Nagawa. 2000. Shear stress affects migration behavior of polymorphonuclear cells arrested on endothelium. Cell Immunol. 203:39–46. [DOI] [PubMed] [Google Scholar]
- Mansfield, P. J., J. A. Shayman, and L. A. Boxer. 2000. Regulation of polymorphonuclear leukocyte phagocytosis by myosin light chain kinase after activation of mitogen-activated protein kinase. Blood. 95:2407–2412. [PubMed] [Google Scholar]
- McIntire, L. V., T. S. Dewitz, and R. R. Martin. 1976. Mechanical trauma effects on leukocytes. Trans. Am. Soc. Artif. Intern. Organs. 22:444–449. [PubMed] [Google Scholar]
- Moazzam, F., F. A. DeLano, B. W. Zweifach, and G. W. Schmid-Schönbein. 1997. The leukocyte response to fluid stress. Proc. Natl. Acad. Sci. USA. 94:5338–5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan, C., Q.-W. Xie, L. Halbwachs-Mecarelli, and W. W. Jin. 1993. Albumin inhibits neutrophil spreading and hydrogen peroxide release by blocking the shedding of CD43 (sialophorin, leukosialin). J. Cell Biol. 122:243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi, K. L., D. K.-L. Tung, J. Wilson, B. W. Zweifach, and G. W. Schmid-Schönbein. 1996. Transvascular and interstitial migration of neutrophils in rat mesentery. Microcirculation. 3:199–210. [DOI] [PubMed] [Google Scholar]
- Okuyama, M., J. Kambayashi, M. Sakon, and M. Monden. 1996. LFA-1/ICAM-3 mediates neutrophil homotypic aggregation under fluid shear stress. J. Cell. Biochem. 60: 550–559. [DOI] [PubMed] [Google Scholar]
- Pasternak, C., J. A. Spudich, and E. L. Elson. 1989. Capping of surface receptors and concomitant cortical tension are generated by conventional myosin. Nature. 341:549–551. [DOI] [PubMed] [Google Scholar]
- Rainger, G. E., C. D. Buckley, D. L. Simmons, and G. B. Nash. 1999. Neutrophils sense flow-generated stress and direct their migration through αVβ3-integrin. Am. J. Physiol. 276:H858–H864. [DOI] [PubMed] [Google Scholar]
- Remold-O'Donnell, E., and D. Parent. 1995. Specific sensitivity of CD43 to neutrophil elastase. Blood. 86:2395–2402. [PubMed] [Google Scholar]
- Rosenson-Schloss, R. S., J. L. Vitolo and P. V. Moghe. 1999. Flow-mediated cell stress induction in adherent leukocytes is accompanied by modulation of morphology and phagocytic function. Med. Biol. Eng. Comput. 37:257–263. [DOI] [PubMed] [Google Scholar]
- Shiu, Y.-T., S. Li, W. A. Marganski, S. Usami, M. A. Schwartz, Y.-L. Wang, M. Dembo, and S. Chien. 2004. Rho mediates the shear-enhancement of endothelial cell migration and traction force generation. Biophys. J. 86:2558–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shive, M. S., M. L. Salloum, and J. M. Anderson. 2000. Shear stress-induced apoptosis of adherent neutrophils: A mechanism for persistence of cardiovascular device infections. Proc. Natl. Acad. Sci. USA. 97:6710–6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer, T. A. 1994. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 76:301–314. [DOI] [PubMed] [Google Scholar]
- Ting-Beal, H. P., A. S. Lee, and R. M. Hochmuth. 1995. Effect of cytochalasin D on the passive mechanical properties and morphology of passive human neutrophils. Ann. Biomed. Eng. 23:666–671. [DOI] [PubMed] [Google Scholar]
- Tomczok, J., W. Sliwa-Tomczok, C. L. Klein, T. G. van Kooten, and C. J. Kirkpatrick. 1996. Biomaterial-induced alterations of human neutrophils under fluid shear stress: scanning electron microscopical study in vitro. Biomaterials. 17:1359–1367. [DOI] [PubMed] [Google Scholar]
- Weber, K. S. C., P. von Hundelshausen, I. Clark-Lewis, P. C. Weber, and C. Weber. 1999. Differential immobilization and hierarchical involvement of chemokines in monocyte arrest and transmigration on inflamed endothelium in shear flow. Eur. J. Immunol. 29:700–712. [DOI] [PubMed] [Google Scholar]
- Worthen, G. S., B. Schwab III, E. L. Elson, and G. P. Downey. 1989. Mechanics of stimulated neutrophils: Cell stiffening induces retention in capillaries. Science. 245:183–186. [DOI] [PubMed] [Google Scholar]