Abstract
High-sensitivity differential scanning calorimetry and Fourier transform infrared spectroscopy were used to study the interaction of a cationic α-helical transmembrane peptide, acetyl-Lys2-Leu24-Lys2-amide (L24), and members of the homologous series of zwitterionic n-saturated diacyl phosphatidylethanolamines (PEs). Analogs of L24, in which the lysine residues were replaced by 2,3-diaminopropionic acid (acetyl-DAP2-Leu24-DAP2-amide (L24DAP)) or in which a leucine residue at each end of the polyleucine sequence was replaced by a tryptophan (Ac-K2-W-L22-W-K2-amide (WL22W)), were also studied to investigate the roles of lysine side-chain snorkeling and aromatic side-chain interactions with the interfacial region of phospholipid bilayers. The gel/liquid-crystalline phase transition temperature of the PE bilayers is altered by these peptides in a hydrophobic mismatch-independent manner, in contrast to the hydrophobic mismatch-dependent manner observed previously with zwitterionic phosphatidylcholine (PC) and anionic phosphatidylglycerol (PG) bilayers. Moreover, all three peptides reduce the phase transition temperature to a greater extent in PE bilayers than in PC and PG bilayers, indicating a greater disruption of PE gel-phase bilayer organization. Moreover, the lysine-anchored L24 reduces the phase transition temperature, enthalpy, and the cooperativity of PE bilayers to a much greater extent than DAP-anchored L24DAP, whereas replacement of the terminal leucines by tryptophan residues (Ac-K2-W-L22-W-K2-amide) only slightly attenuates the effects of this peptide on the chain-melting phase transition of the host PE bilayers. All three peptides form very stable α-helices in PE bilayers, but small conformational changes occur in response to mismatch between peptide hydrophobic length and gel-state lipid bilayer hydrophobic thickness. These results suggest that the lysine snorkeling plays a significant role in the peptide-PE interactions and that cation-π-interactions between lysine and tryptophan residues may modulate these interactions. Altogether, these results suggest that the lipid-peptide interactions are affected not only by the hydrophobic mismatch between these peptides and the host lipid bilayer but also by the electrostatic and hydrogen-bonding interactions between the positively charged lysine residues at the termini of these peptides and the polar headgroups of PE bilayers.
INTRODUCTION
All biological membranes consist of lipid and protein molecules held together by noncovalent interactions (Yeagle, 1993). The fundamental structural element of all biological membranes is a lipid bilayer, which also functions as the major permeability barrier. The zwitterionic phospholipids phosphatidylcholine (PC) and phosphatidylethanolamine (PE) are the major lipid components in animal cell membranes, and PE is often a major component of bacterial cell membranes as well (Daum, 1985; Devaux and Seigneuret, 1985). In addition, eukaryotic cell membranes exhibit an asymmetry in lipid distribution across the lipid bilayer. For example, in human erythrocyte membranes, PC and sphingomyelin are mainly found in the outer leaflet, whereas PE and phosphatidylserine (PS) are mainly found in the inner leaflet of the bilayer (Verkleij et al., 1973). The basic functions of biological membranes are carried out by membrane proteins that may associate loosely with the membrane surface (peripheral proteins) or traverse the lipid bilayer (integral membrane proteins). Most of the integral membrane proteins are composed of tightly packed bundles of transmembrane α-helical segments that contain ∼20 hydrophobic residues (Kleinschmidt, 2003). Recent studies have shown that PE can assist in the correct folding of integral membrane proteins such as the lactose permease and the high affinity phenylalanine permease of Escherichia coli (Bogdanov and Dowhan, 1998; Zhang et al., 2003). A number of x-ray crystal structures also indicate that integral membrane proteins such as cytochrome c oxidase from Rhodobacter sphaeroides (Svensson-Ek et al., 2002) and the purple bacterial reaction center from Thermochromatium tepidum (Jones et al., 2002) may contain bound PE or other phospholipids. It was also found that aminophospholipids such as PE in the cytoplasmic leaflet of Golgi, endosome, and plasma membranes contribute to the protein-mediated vesicle budding competence of these membrane systems (Pomorski et al., 2003).
Although the mutual interactions of lipids and proteins are fundamentally important to both the structure and the function of all biological membranes, our understanding of the physical principles underlying lipid-protein interactions remains incomplete, and the actual molecular mechanisms whereby associated lipids alter the activity, and presumably also the structure and dynamics, of integral membrane proteins are largely unknown. This is due in part to the fact that most transmembrane proteins are relatively large, multidomain macromolecules with complex and often unknown three-dimensional structure and topology. Despite the fact that about one-third of all proteins are integral membrane proteins, less than 100 unique membrane protein structures have been reported to date, compared with the huge number (over 14,000) of soluble proteins in the Protein Data Bank. Moreover, membrane proteins can interact with lipid bilayers in complex, multifaceted ways (Gennis, 1989; Selinsky, 1992; Marsh and Horváth, 1998). To overcome this problem, a number of workers have designed and synthesized peptide models of specific regions of natural membrane proteins and have studied their interactions with model membranes of defined lipid composition (Davis et al., 1983; Ren et al., 1997; de Planque and Killian, 2003).
The synthetic peptide acetyl-K2-G-L24-K2-A-amide (P24) and its analogs such as acetyl-Lys-2-Leu-24-Lys-2-amide (L24) have been successfully utilized as a model of the hydrophobic transmembrane α-helical segments of integral membrane proteins (Davis et al., 1983). These peptides contain a long sequence of hydrophobic leucine residues capped at both the N- and C-termini with two positively charged, relatively polar lysine residues. Moreover, the normally positively charged N-terminus and the negatively charged C-terminus are blocked to provide a symmetrical tetracationic peptide that will more faithfully mimic the transbilayer region of natural membrane proteins. The central polyleucine region of these peptides was designed to form a maximally stable α-helix, particularly in the hydrophobic environment of the lipid bilayer core, whereas the dilysine caps were designed to anchor the ends of these peptides to the polar surface of the lipid bilayer and to inhibit the lateral aggregation of these peptides. In fact, circular dichroism (CD) (Davis et al., 1983) and Fourier transform infrared (FTIR) (Zhang et al., 1992a,b; Axelsen et al., 1995) spectroscopic studies of P24 have shown that it adopts a very stable α-helical conformation both in solution and in lipid bilayers, and x-ray diffraction (Huschilt et al., 1989), fluorescence quenching (Bolen and Holloway, 1990), and FTIR spectroscopic (Zhang et al., 1992a,b; Axelsen et al., 1995) studies have confirmed that P24 and its analogs assume a transbilayer orientation with the N- and C-termini exposed to the aqueous environment and the hydrophobic polyleucine core embedded in the hydrocarbon core of the lipid bilayer when reconstituted with various PCs. DSC (Huschilt et al., 1985; Morrow et al., 1985; Zhang et al., 1992b) and 2H NMR spectroscopy (Huschilt et al., 1985; Morrow et al., 1985; Paré et al., 2001) studies have shown that P24 and L24 broaden the gel/liquid-crystalline phase transition of the host phospholipid bilayer and reduces its enthalpy. The phase transition temperatures (Tms ) of PC and phosphatidylglycerol (PG) bilayers are shifted either upward or downward by P24 or L24, mainly depending on the degree of mismatch between the peptide hydrophobic length and the lipid hydrophobic thickness (Zhang et al., 1992b; Liu et al., 2002, 2004). In contrast, the phase transition temperatures of PE bilayers are substantially decreased by the presence of P24 in a manner essentially independent on the peptide-lipid hydrophobic mismatch (Zhang et al., 1995a). 2H NMR (Pauls et al., 1985) and electron spin resonance (ESR) (Subczynski et al., 1998, 2003) spectroscopic studies have shown that the rotational diffusion of P24 about its long axis perpendicular to the membrane plane is rapid in the liquid-crystalline state of the bilayer and that the closely related peptides L24 and acetyl-Lys-2-(Leu-Ala)-12-Lys-2-amide ((LA)12) exist at least primarily as monomers in liquid-crystalline POPC bilayers, even at relatively high peptide concentrations.
Statistical analysis shows that the hydrophobic core of the transmembrane α-helices of many integral membrane proteins are normally flanked by aromatic residues like trytophan or tyrosine and that positively charged lysine or arginine residues are found adjacent to these aromatic residues (Killian and von Heijne, 2000). To study the roles of tryptophan and lysine residues in lipid-protein interactions, we have previously synthesized three analogs of P24, Ac-K2-L24-K2-amide (L24), Ac-DAP2-L24-DAP2-amide (L24DAP; DAP is diaminopropionic acid), and Ac-K2-W-L22-W-K2-amide (WL22W), and studied their interactions with zwitterionic PC and anionic PG bilayers (Liu et al., 2002, 2004). First, in the peptide L24DAP, the two pairs of capping lysine residues at the N- and C-termini of L24 have been replaced with the lysine analogs DAP, in which three of the four side-chain methylene groups have been removed. This peptide was used to test the so-called snorkel model first suggested by Segrest et al. (1990) to explain the behavior of positively charged residues in the amphipathic helices present at the surfaces of blood lipoproteins and later extended to transmembrane α-helices by von Heijne et al. (Monne et al., 1998). According to the transmembrane peptide version of the snorkel model, the long, flexible hydrophobic side chains of lysine or arginine residues could extend along the transmembrane helix so that the terminal charged moiety can reside in the lipid polar headgroup region whereas the α-carbon of the amino acid residue remains well below (or possibly above) the polar/apolar interfacial region of the phospholipids molecule, even when the hydrophobic length of the peptide is considerably different from that of the host lipid bilayer. Because of the shorter spacer arms between the charged group and the α-carbon in DAP, L24-DAP is expected to be less accommodating to the peptide-lipid hydrophobic mismatch than L24, and any effects of such mismatch on the thermotropic phase behavior of the host lipid bilayer should thus be exaggerated. Second, in the peptide WL22W, the Leu-3 and Leu-26 residues of L24 are replaced with tryptophans. The preference of aromatic tryptophan or tyrosine residues for the polar/apolar interfacial region of the host lipid bilayer has been found to be one of the common features of natural membrane proteins (von Heijne, 1994). Since tryptophan residues have been proposed to anchor the ends of α-helical transmembrane peptides to the polar/apolar interface of the lipid bilayer, we also expect that WL22W would be less accommodating to hydrophobic mismatch between the peptides and host lipid bilayer than would L24.
To further clarify the role of interfacially localized tryptophan and lysine residues in lipid-protein interactions, we have used DSC and FTIR spectroscopy to study the interactions of L24, L24DAP and WL22W with a series of zwitterionic PEs with different hydrocarbon chain lengths and have compared these results with those obtained previously for a homologous series of zwitterionic PCs (Liu et al., 2002) and anionic PGs (Liu et al., 2004). Our results demonstrate that the snorkeling of the terminal lysine residues at the end of the model peptides is required for optimization of the hydrogen-bonding and/or electrostatic interactions between the lysine residues at the termini of these peptides and the polar headgroups of the PE. Our results also indicate that the presence of interfacially localized tryptophan residues only slightly affects the interactions of these model peptides with the zwitterionic phospholipids PE and PC (Liu et al., 2002), although tyryptophan residues strongly modulate such interactions in anionic PG bilayers (Liu et al., 2004).
MATERIALS AND METHODS
The phospholipids used in this study were obtained from Avanti Polar Lipids (Alabaster, AL) and were used without further purification. Commercially supplied solvents of at least analytical grade quality were redistilled before use. Peptides were synthesized and purified as trifluoroacetic acid (TFA) salts using previously published solid-phase synthesis and reversed phase high-performance liquid chromatographic procedures (Mantsch et al., 1985).
Samples were prepared for DSC as follows. Appropriate quantities of peptides and PE were codissolved in methanol to obtain the desired lipid/peptide ratio, and the solvent was removed in a stream of nitrogen at temperatures near 60–70°C to ensure sample homogeneity. Then the sample was redissolved in chloroform/methanol (1:1), and the solvent was again removed in a stream of nitrogen at temperatures near 60–70°C. Later the sample was redissolved in benzene and lyophilized overnight to form a white powder. Samples containing 0.5 mg lipid were then hydrated by vigorous vortexing with water at temperatures some 10–15°C above the gel/liquid-crystalline phase transition temperature of the lipid. DSC thermograms were obtained from 0.5-ml samples with a high sensitivity Microcal VP-DSC instrument (Microcal, Northampton, MA), operating at heating and cooling rates of 10°C per hour. The data were analyzed and plotted with the Origin software package (OriginLab, Northampton, MA).
Peptide samples to be used in FTIR spectroscopic experiments were converted to the hydrochloride salt by two cycles of lyophilization from 10 mM hydrochloric acid. This procedure was necessary because the trifluoroacetate ion gives rise to a strong absorption band (∼1670 cm−1) that partially overlaps the amide I absorption band of the peptide (Zhang et al., 1992a,b). Typically, samples were prepared by codissolving lipid and peptide in methanol at a lipid/peptide ratio of near 30:1 (mol/mol). After removal of the solvent and drying of the film (see above), samples containing 2–3 mg lipid were hydrated by vigorous mixing with 75 μl D2O-based buffer (50 mM Tris, 150 mM NaCl, 1mM NaN3, pD 7.4). The paste obtained was then squeezed between the CaF2 windows of a heatable, demountable liquid cell (NSG Precision Cells, Farmingdale, NY) equipped with a 25-μm Teflon spacer. Once mounted in the sample holder of the spectrometer, the sample temperature could be varied between 20°C and 90°C by an external, computer-controlled water bath. Infrared spectra were acquired as a function of temperature with a Digilab FTS-40 Fourier-transform spectrometer (Bio-Rad, Digilab Division, Cambridge, MA) using data acquisition parameters similar to those described by Mantsch et al. (1985). The experiment involved a sequential series of 2°C temperature ramps with a 20-min interramp delay for thermal equilibration and was equivalent to a scanning rate of 4°C per hour. Spectra were analyzed with software supplied by the instrument manufacturers and other programs obtained from the National Research Council of Canada.
RESULTS
To evaluate the DSC and FTIR spectroscopic data presented below, we need to compare the intrinsic hydrophobic length of the model peptides L24, L24DAP, and WL22W with the intrinsic hydrophobic thickness of the various PE bilayers used in this study (see Table 1). On the basis of measurements of a molecular model of L24 in an ideal α-helical conformation, we estimate that the mean hydrophobic length of L24 (the average length of the leucine sequence measured at any point on the surface of the helix) is ∼30.6 Å. Note that the effective hydrophobic length measured in this manner is two helical half-turns shorter at each end of the hydrophobic helical core than the maximal hydrophobic length of this peptide, which would be 36.0 Å. Thus the mean hydrophobic length of L24 is intermediate between the mean hydrophobic thickness of 14:0 PE and 16:0 PE bilayers (where the number before the colon represents the total number of carbon atoms in the hydrocarbon chains and the number after the colon the number of double bonds). For the shorter chain 12:0 PE bilayers, L24 hydrophobic length exceeds bilayer hydrophobic thickness in both the gel and the liquid-crystalline phases, whereas for other three PEs, the hydrophobic length of L24 will be less than that of gel state but more than that of the liquid-crystalline bilayer. The same considerations also apply to L24DAP and WL22W, which are taken to have the same effective hydrophobic length as L24.
TABLE 1.
Hydrophobic thicknesses of the bilayer formed by various phosphatidylethanolamines
| Hydrophobic thickness (Å)*
|
|||
|---|---|---|---|
| PE | Gel phase | Liquid-crystalline phase | Mean† |
| 12:0 | 29.3 | 19.7 | 24.5 |
| 14:0 | 34.2 | 22.8 | 28.5 |
| 16:0 | 39.5 | 26.3 | 32.9 |
| 18:0 | 44.7 | 29.8 | 37.3 |
Hydrophobic thicknesses were estimated using the equations used by Sperotto and Mouritsen (1988) to calculate the hydrophobic thicknesses of PC bilayers.
Mean of the hydrophobic thicknesses of the gel and liquid-crystalline phases.
We stress here that the pattern of matching of lipid bilayer thickness and peptide hydrophobic length just described applies only if these model peptides adopt an ideal α-helical conformation that is not influenced by lipid bilayer thickness. In fact, our spectroscopic studies show that in PE bilayers these model peptides do adopt a predominantly α-helical conformation that is, however, affected by gel-state PE bilayer thickness (see below). Moreover, the hydrocarbon chains of the PE molecules do change their degree of conformational order, and thus the effective hydrophobic thickness of the PE bilayer, in response to the presence of these model peptides. Thus the actual degree of hydrophobic mismatch between these model peptides and the various PE bilayers, in both the gel and the liquid-crystalline states, may be somewhat different than indicated by the intrinsic effective hydrophobic length of this peptide and the hydrophobic thickness of PE bilayers in the absence of peptide.
Thermotropic phase behavior of PE bilayers in the absence of peptides
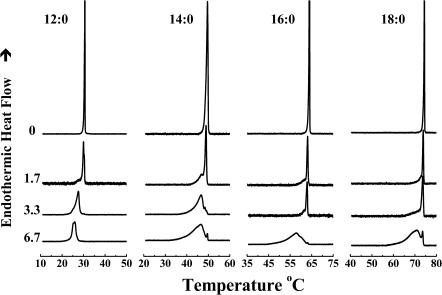
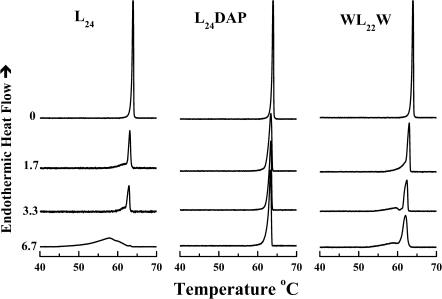
As illustrated in Fig. 1, in the absence of peptides the DSC thermograms of unannealed aqueous dispersions of each of four n-saturated diacyl PEs studied here exhibit a single asymmetric fairly energetic and highly cooperative lamellar gel/lamellar liquid-crystalline phase transition on heating. This transition is freely reversible, as shown by the absence of significant cooling hysteresis (data not shown). In addition, the temperatures of the phase transitions of these PEs increase progressively but nonlinearly, and the transition enthalpy increases linearly, with increases in the hydrocarbon chain length, as expected. For a thorough discussion of the thermotropic phase behavior of the complete homologous series of n-saturated diacyl PEs, see Lewis and McElhaney (1993) and references cited therein.
FIGURE 1.
Effect of L24 on the DSC thermograms of a series of n-saturated diacyl-PEs. Thermograms are shown as a function of the acyl chain length (N:0) of the lipids, at the peptide concentrations (mol %) indicated.
Thermotropic phase behavior of various PE bilayers in the presence of L24
The effects of the incorporation of L24 on the main phase transition of PE bilayers are also illustrated in Fig. 1. In all cases the incorporation of increasing quantities of L24 produces an apparently two-component DSC endotherm, consisting of a broad, lower-temperature component and a sharp, higher-temperature component. The relative contribution of the sharp component of the DSC endotherm, which initially possesses a phase transition temperature, enthalpy, and cooperatively similar to that of the PE alone, decreases in magnitude as the concentration of L24 increases. In fact, the sharp component of the DSC endotherm is barely detectable at a peptide concentration of 3.3 mol % in 12:0 PE bilayers and a concentration of 6.7 mol % in the three longer chain PE bilayers studied. In contrast, the relative contribution of the broad component increases as the peptide concentration increases, and it is the only or at least the major component that persists at the highest peptide concentrations tested. Moreover, our FTIR spectroscopic studies of the temperature dependence of the methylene stretching frequency changes indicate that both the sharp and broad components of the DSC endotherms are accompanied by increases in the rotational conformational disorder of the PE hydrocarbon chains (data not shown). Using the rationale provided in our previous DSC studies of the interaction of P24 and related peptides with PC, PE, and PG bilayers (Zhang et al., 1992b, 1995a; Liu et al., 2002), we thus assign the sharp component of our DSC endotherms to the hydrocarbon chain-melting phase transition of peptide-poor PE domains and the broad component to the melting of peptide-rich PE domains. Note that these characteristic effects of the incorporation of L24 and its analogs on the sharp component of the DSC endotherm are noted in all the PEs studied and are essentially hydrocarbon chain length independent.
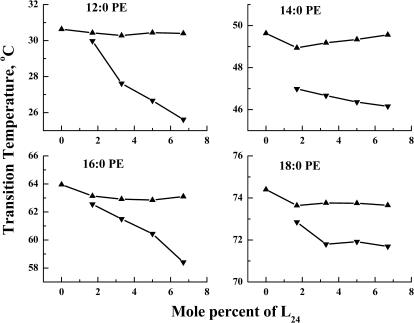
The effects of L24 incorporation on the temperatures of the sharp and broad components of the DSC heating endotherms for all four PEs are illustrated in Fig. 2. As noted above, the temperature of the sharp component changes little with increases in L24 concentration in each of the four PE bilayers examined. However, in all cases there is a gradual decrease in the temperature of the broad component with increasing peptide concentration, resulting in a gradual increase in ΔT (the downward shift of the phase transition temperature of the broad component relative to that of the sharp component). Moreover, at all peptide concentrations, the phase transition temperature of the broad component of the DSC endotherm always occurs at a lower temperature than that of the sharp component, regardless of PE hydrocarbon chain length. At 6.7 mol % L24 concentration, the ΔT is largest in 12:0 PE and smallest in 18:0 PE bilayers, but the variation with hydrocarbon chain length is small (<1°C). Qualitatively similar results were observed when L24 DAP and WL22W are incorporated into PE bilayers.
FIGURE 2.
Effect of L24 concentration on the peak temperatures of the two components of the DSC thermograms exhibited by the mixtures of L24 and the n-saturated diacyl-PEs. Data are presented for the sharp (▴) and broad (▾) components of the DSC endotherms.
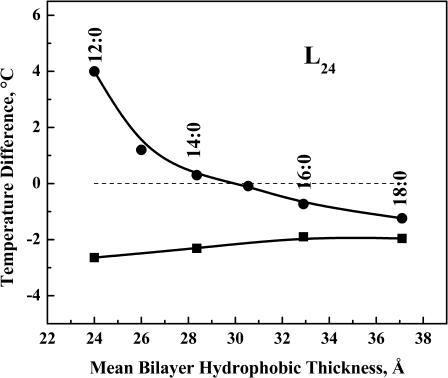
The effects of the lipid acyl chain length on the ΔT values observed with PE-peptide mixtures containing 3.3 mol % L24 are illustrated in Fig. 3. With all of the PEs examined, the ΔT values obtained are all negative and only weakly dependent on lipid hydrocarbon chain length, with the absolute ΔT values diminishing only slightly with increasing hydrocarbon chain length (from −2.6°C for 12:0 PE to −2.0°C for 18:0 PE). As illustrated in Fig. 3, these observations contrast sharply from those that occur when L24 is incorporated into PC bilayers. With the latter, the sign and magnitude of ΔT is strongly hydrocarbon chain length dependent in a manner consistent with the sign and degree of the hydrophobic mismatch between peptide hydrophobic length and lipid bilayer hydrophobic thickness. With the shorter chain PC bilayers (≤14:0 PC), the temperature of the broad component of the DSC endotherm is progressively increased relative to that of the sharp component as hydrocarbon chain length decreases, and vice versa in the longer chain PC bilayers (≥16:0 PC), whereas the ΔT value is near zero for 15:0 PC bilayers. As noted previously (Liu et al., 2002), the effective hydrophobic length of P24 (or L24) match the mean thickness of 15:0 PC bilayers. Thus the ΔT value becomes increasing positive in PC bilayers with mean hydrophobic thicknesses that are progressively less than the effective hydrophobic length of the peptide and vice versa. Qualitatively similar results are also obtained when L24 is incorporated into in anionic PG bilayers (Liu et al., 2004). The possible molecular basis for these results will be discussed later.
FIGURE 3.
Hydrocarbon chain length dependence of the differences (ΔT) between the transition temperatures of the peptide-rich and the peptide-poor PE components of DSC thermograms exhibited by L24/PE mixtures at a peptide concentration of 3.3 mol % (▪). To facilate comparison, comparable data obtained from studies of L24 with PC bilayers (•) are also shown.
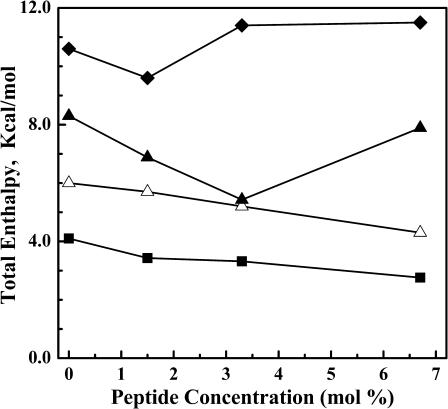
The variation in the enthalpy of the overall phase transition as a function of L24 concentration in each of the four PE bilayers studied is shown in Fig. 4. As noted earlier, in all cases the contribution of the sharp component to the total enthalpy change measured decreases steeply with increasing peptide concentration with little dependence on PE hydrocarbon chain length (see Fig. 1). However, the variation in the enthalpy of the overall and broad components of the DSC endotherms depends strongly on PE hydrocarbon chain length. With the shorter chain PEs (i.e., 12:0 and 14:0 PE), the transition enthalpies of both components decrease as L24 concentration increases. However, in the longer chain PE bilayers, these transition enthalpies first decrease and then increase again as peptide concentration increases, with this effect becoming more pronounced as PE hydrocarbon chain length increases. A similar pattern of behavior was observed in our previous studies of P24/PE systems (Zhang et al., 1995a), in which case the apparent increase in the overall phase transition enthalpy, and that of the broad component of the DSC endotherm, was ascribed to the release of heat due to the disaggregation of peptide multimers in the gel states of the longer chain PE bilayers as the gel/liquid-crystalline phase transition is approached on heating. It is noteworthy, however, that similar behavior is not observed when L24 is incorporated into PC and PG bilayers (Liu et al., 2002, 2004). With the latter lipids the overall phase transition enthalpy decreases linearly with increasing L24 concentration over a similar range of hydrocarbon chain lengths, indicating that significant peptide aggregation does not occur in the more loosely packed gel states of these phospholipids.
FIGURE 4.
Peptide-concentration dependent changes in the enthalpy of the thermotropic transitions exhibited by L24-containing PE bilayers. The data presented represents the total enthalpy change measured for mixtures of L24 with 12:0 PE (__▪__), 14:0 PE(__▵__), 16:0 PE (__▴__), and 18:0 PE (__♦__).
A closer examination of Fig. 1 also shows that the width of the broad component of the DSC endotherms increases modestly with increases in peptide concentration but is almost independent of lipid hydrocarbon chain length. For example, in 12:0 PE bilayers, the width of the broad component (as measured from the starting to completion temperatures) exhibits an increase from 8°C to 11°C as peptide concentrations increases from 1.7 to 6.7 mol %. Similar behavior occurs when the peptide P24 is incorporated into the same PE bilayers (Zhang et al., 1995a). However, markedly different behavior occurs when L24 is incorporated into either PC or PG bilayers (Liu et al., 2002, 2004). With either L24-containing PC or L24-containing PG bilayers, the widths of the broad component in the DSC thermograms are always considerably larger at comparable peptide concentrations and decrease markedly with increases in lipid hydrocarbon chain length. The possible basis of these experimental observations will be explored in the Discussion.
Thermotropic phase behavior of PE bilayers in the presence of various peptides
To examine the effect of truncating the lysine side chains of L24 or of replacing the terminal leucines with aromatic tryptophan residues on the peptide-PE interactions, we have also studied the effects of L24DAP and WL22W incorporation on the thermotropic phase behavior of various PE bilayers by high-sensitivity DSC. A comparison of the effects of the incorporation of various amounts of L24, L24DAP, and WL22W on the phase behavior of 16:0 PE bilayers is shown in Fig. 5. The patterns of peptide concentration-dependent changes in PE thermotropic phase behavior shown therein are typical of our observations of the interactions of these peptides with all of the other PEs examined. We will therefore present the overall effects of these peptides on the thermotropic phase behavior of 16:0 PE bilayers as a qualitative example of what occurs with all of the PE bilayers studied. As noted earlier, the DSC thermograms exhibited by the L24/16:0 PE mixtures (left panel) are composed of overlapping short and broad components with the former decreasing and the latter increasing with increases in L24 concentration. At the 6.7 mol % peptide, the DSC thermogram exhibited by the L24/16:0 PE mixture is mainly composed of a broad component centered near 57.8°C with only traces of the sharp component centered near 63.2°C. The DSC thermogram exhibited by the corresponding WL22W/16:0 PE mixtures (right panel) is also composed of overlapping sharp and broad components, but in this case the sharp component of the DSC endotherm predominates over the broad component, even at the highest peptide concentration tested. In contrast to L24 and WL22W, the incorporation of L24DAP has only a small effect on the gel/liquid-crystalline phase transition of 16:0 PE bilayers and does not appear to induce clearly resolvable sharp and broad components in the DSC endotherm. At 6.7 mol % peptide concentration, the DSC thermogram exhibited by the L24DAP/16:0 PE mixture is only slightly broader than that of the pure 16:0 PE. This marked difference between L24DAP and L24 or WL22W is also observed when these peptides are incorporated into 12:0, 14:0, and 18:0 PE bilayers and suggests that the L24DAP is not interacting extensively with the host PE bilayers.
FIGURE 5.
A comparison of the effects of L24, L24DAP, and WL22W on the DSC thermograms of 16:0 PE. The thermograms shown were obtained at the peptide concentrations (mol %) indicated.
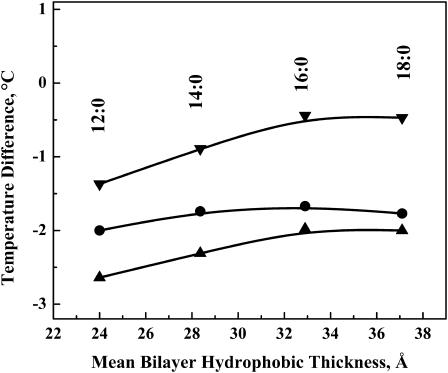
The plot of ΔT versus the hydrocarbon chain length of the PE bilayers for L24, L24DAP, and WL22W are shown in Fig. 6. Although ΔT remains negative in all of the peptide/PE mixtures studied, the magnitude of the decrease in the temperature of the broad component is largest for L24, moderate for WL24W, and smallest for L24DAP, especially in the longer chain PEs. For all the three model peptides, only a very small increase in ΔT is observed when the hydrocarbon chain length of PE bilayers is increased from 12 to 18 carbons. This is markedly different from the results of the same three model peptides in PC and PG bilayers, in which the ΔT initially decreases significantly with an increase of the lipid hydrocarbon chain length and is strongly positive in very short chain phospholipids. It is also interesting to note that in all four hydrocarbon chain lengths studied, the ΔT values exhibited by L24/PE and WL22W/PE mixtures are ∼1.5°C and 1.0°C lower, respectively, than that theΔT exhibited by L24DAP/PE mixtures, again indicating less extensive interactions between the latter peptide its host PE bilayer.
FIGURE 6.
Hydrocarbon chain length dependence of the differences (ΔT) between the transition temperatures of the peptide-rich and the peptide-poor PE components of DSC thermograms exhibited by peptide/PE mixtures at a peptide concentration of 3.3 mol %. Data are presented for mixtures of PE with L24 (▴), WL22W (•), and L24DAP (▾).
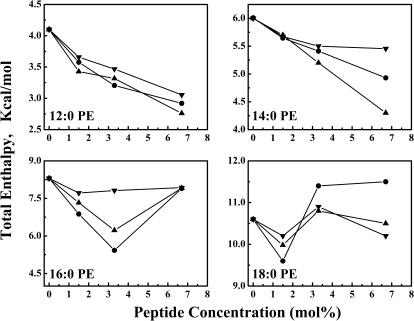
Illustrated in Fig. 7 is a comparison of the peptide concentration-dependent changes in the enthalpy of the thermotropic phase transitions observed when the peptides L24, L24DAP, and WL22W are incorporated into the various PE bilayers examined. With the shorter chain lipids 12:0 PE and 14:0 PE, incremental increases in peptide content are accompanied by a progressive decrease in the enthalpy values throughout the peptide concentration range examined. Also, the magnitude of this effect varies with the nature of the peptide and decreases in the order L24 > WL22W > L24DAP. With the longer chain PEs, the measured enthalpy values also decrease at low peptide concentrations, in a manner and magnitude comparable to what occurs with the shorter chain lipids. Unlike the shorter chain PEs, however, at higher peptide concentrations a marked increase in enthalpy values is observed. Similar behavior has been observed previously in studies of P24- and (LA)12-containing PE membranes (Zhang et al., 1995a, 2001) and have been ascribed to additional energy arising from increased dispersal of the aggregated peptide at temperatures below the onset of the lipid chain-melting phase transition. Here, we also find that the magnitude of this phenomenon is dependent on the nature of the peptide and decreases in the same order described above (i.e., L24 > WL22W ≫ L24DAP). We also note that across the range of PE hydrocarbon chain lengths examined, the effects of L24-DAP on the enthalpy of the lipid phase transition are considerably smaller than observed with peptides L24 and WL22W. These results suggest either that L24DAP is intrinsically less disordering to PE bilayers than are L24 and WL22W, or that PE-L24DAP interactions are being minimized by the lateral aggregation of L24DAP in PE bilayers.
FIGURE 7.
Effects of L24 (▾), WL22W (•), and L24DAP (▾) concentration on the total transition enthalpies of 12:0. 14:0, 16:0, and 18:0 PE bilayers.
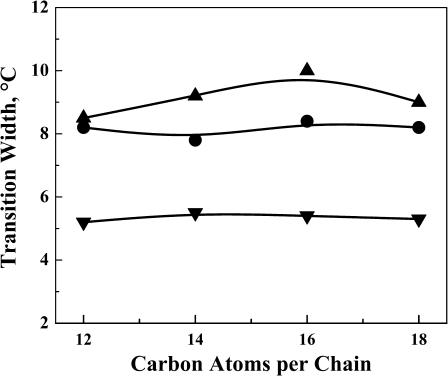
A comparison of the effects of L24, L24DAP, and WL22W on the transition widths of PE bilayers is shown in Fig. 8. The transition widths were measured from the starting temperature to the ending temperature of the broad component of the DSC endotherms at 3.3 mol % peptide concentration. For all three model peptides, there is only slight change in the transition widths of the broad component of the peptide/PE mixtures when the acyl chain length of PE bilayers is increased from 12 to 18 carbons. Moreover, in all four PEs examined, the transition widths of the broad component of L24DAP/PE mixtures (∼5.2°C) is much smaller than that of L24/PE mixtures and WL22W/PE mixtures (∼9.2°C and 8.4°C, respectively). These results again show that the incorporation of L24 or WL22W has much larger effects on the organization of PE bilayers than does the incorporation of L24DAP.
FIGURE 8.
Plot of the transition width versus the hydrophobic chain length of the lipid bilayer at a peptide concentration of 3.3 mol %. Data are presented for mixtures of PE with L24 (▴), WL22W (•), and L24DAP (▾).
Fourier transform infrared spectroscopic studies of peptide-containing PE bilayers
In these studies, infrared spectra of mixtures of the peptide with each of the four PEs studied were recorded as a function of temperature and as a function of the mol fraction of the peptide. The use of FTIR spectroscopy permits a noninvasive monitoring of both the structural organization of the lipid bilayer and the conformation of the incorporated peptide (for a more detailed description of this application of IR spectroscopy, see Lewis and McElhaney, 1996, 2002; Tamm and Tatulian, 1997; and references cited therein). Here, changes in the degree of hydrocarbon chain rotational isomeric disorder coincident with the lipid gel/liquid-crystalline phase transition were monitored by an examination of the methylene symmetric stretching band near 2850 cm−1, and the conformational stability of the embedded peptide was monitored by an examination of the contours of the amide I band centered near 1650 cm−1. Our studies (data not presented) showed that the incorporation of these peptides into PE bilayers had no discernable effect on the ester carbonyl stretching band near 1735 cm−1, nor on the O-P-O asymmetrical stretching bands near 1230 cm−1, suggesting that peptide incorporation at the levels studied here (∼3.3 mol %) did not have a major effect on the hydration of either the phosphate polar headgroups or the polar/apolar interfacial regions of these lipid bilayers.
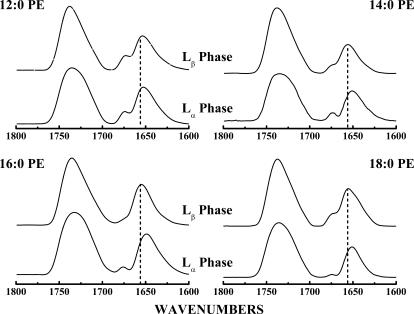
Illustrated in Fig. 9 are the amide I and C=O stretching regions of the FTIR spectra exhibited by mixtures of L24 with the four PEs examined here. The spectra shown were acquired at temperatures where the host lipids are in their gel (Lβ) and liquid-crystalline (Lα) phases. Under all conditions examined, the amide I absorption band of L24 is dominated by sharp absorption components centered near 1655–1657 cm−1 and near 1647–1650 cm−1. These absorption bands can be ascribed to the amide I vibrations of unexchanged- and deuterium-exchanged α-helical peptide domains (Chirgadze and Brazhnikov, 1974; Rabolt et al., 1977), and their prominence is therefore consistent with L24 maintaining a predominantly α-helical conformation under all conditions examined. However, Fig. 9 also shows that the gel to liquid-crystalline phase transition of the host PE bilayer is accompanied by a small downward shift in the frequency of the absorption maximum of the amide I band envelope. This frequency shift is minimal (<2 cm−1) with L24-containing 12:0 PE mixtures but increases with increases in lipid hydrocarbon chain length and approaches values near 5–6 cm−1 with 18:0 PE-based mixtures. A similar pattern of behavior has been reported in our studies of mixtures of L24 with PC and PG bilayers (Liu et al., 2002, 2004) and in studies of the interaction of the peptides P24 and (LA)12 with PC and PE bilayers (Zhang et al., 1992b, 1995a,b, 2001).
FIGURE 9.
The C=O stretching and amide I bands of the FTIR spectra exhibited by L24-containing PE bilayers. The absorbance spectra shown were acquired with mixtures containing 3.3 mol % L24 under conditions where the lipid bilayer hosts are in the Lβ-phase and Lα-phase states. The dashed lines mark the absorption maxima (1656 cm−1) of the amide I absorption bands arising from fully proteated α-helical peptide domains. The small absorption bands centered near 1670 cm−1 arise from small amounts of trifluoroacetate counterions that were not completely removed during sample preparation.
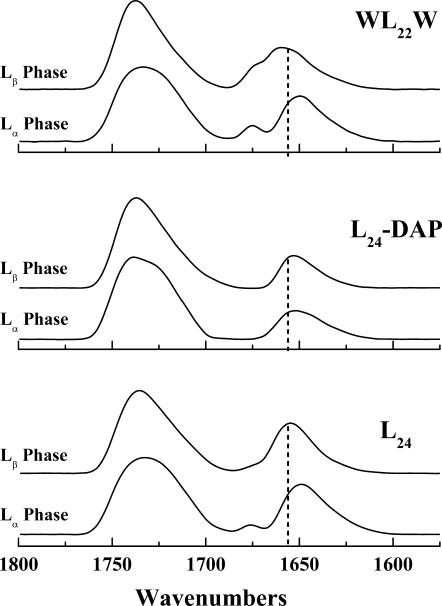
A comparison of the lipid phase state-induced changes in the amide I band maxima observed with mixtures of peptides L24, L24DAP, and WL22W with 16:0 PE is shown in Fig. 10. These peptides all exhibit a pattern of incremental hydrocarbon chain length-dependent decreases in amide I band maxima that is qualitatively similar to that described for L24 above. However, as is evident from Fig. 10, the magnitude of this effect is markedly smaller with the peptide L24DAP, for which the maximal chain length-dependent frequency shifts observed never exceeds 2 cm−1, indicating that the coupling between the conformation of L24DAP and changes in the hydrophobic thickness of these PE bilayers is attenuated compared with the other two peptides. Interestingly, our studies of the interactions of these same peptides with PC and PG bilayers (Liu et al., 2002, 2004) also show a similar pattern in which lipid phase state-induced decreases in the amide I band maxima of L24DAP are smaller than in L24 and WL22W, although in those cases this affect is smaller in magnitude than observed here with PE bilayers. The molecular basis of these experimental observations are explored in the Discussion below.
FIGURE 10.
The C=O stretching and amide I bands of the FTIR spectra exhibited by peptide-containing 16:0 PE bilayers. The absorbance spectra shown were acquired with mixtures containing 3.3 mol % of the peptides indicated under conditions where the lipid bilayer hosts are in the Lβ-phase and Lα-phase states. The dashed lines mark the absorption maxima (1656 cm−1) of the amide I absorption bands arising from fully proteated α-helical peptide domains. The small absorption bands centered near 1670 cm−1 arise from small amounts of trifluoroacetate counterions that were not completely removed during sample preparation.
DISCUSSION
In this study we have shown that interactions of model peptides that mimic the hydrophobic transmembrane α-helical segments of integral membrane proteins with zwitterionic PE bilayers are quite different from their interactions with zwitterionic PC or anionic PG bilayers. Specifically, we demonstrate that all three model peptides reduce the Tms of PE bilayers to a grater extent than in PC and PG bilayers and that the peptide-induced reduction of the Tms of these PE bilayers is largely independent of the lipid bilayer thickness. The latter observations contrast sharply from those reported in our previous work, in which the direction and magnitude of the peptide-induced shift in the Tms of PC and PG bilayers was shown to be dependent on the thickness of the lipid bilayer (Liu et al., 2002, 2004). Evidently the nature and strength of the interactions of the somewhat polar and charged amino acid residues at the ends of these model peptides with the polar headgroups of zwitterionic PE and anionic PG or zwitterionic PC bilayers are quite different. We provide a possible molecular interpretation of these experimental observations below.
The Tms of saturated PCs are comparable to those of saturated PG bilayers and are 20–30°C lower than those of saturated PE bilayers of comparable hydrocarbon chain length (see Lewis et al., 1987; Zhang et al., 1997; and references cited therein). This observation can be rationalized on the basis of stronger electrostatic and H-bonding interactions between the polar headgroups at the surfaces of PE bilayers (see Boggs, 1980, 1986, 1987, and references cited therein). Under physiologically relevant conditions, PE amino groups are fully protonated and, being positively charged, are capable of both net attractive electrostatic interactions with negatively charged groups and H-bonding interactions with H-bonding acceptor groups. Thus, the intermolecular forces that constitute the basis of the relatively high transition temperatures of PEs are, in effect, a summation of contributions arising from electrostatic attraction between positively charged amino groups and negatively charged phosphate moieties, electrostatic repulsion arising from both amino-amino and phosphate-phosphate contacts, as well as H-bonding interactions between the headgroup amino protons and H-bonding acceptor groups in the headgroup and polar/apolar interfacial regions of the lipid bilayer. With anionic PG bilayers, their relatively low transition temperatures are largely attributable to electrostatic repulsion between the negatively charged headgroup phosphate moieties, though this effect will be partially mitigated by H-bonding interactions between the exchangeable protons of the headgroup glycerol moiety and H-bonding acceptor groups in the headgroup and polar/apolar interfacial regions of the lipid bilayer. Finally, with PC bilayers, there are no H-bonding donor groups on their polar headgroups, and as a result electrostatic attraction between positively charged choline groups and negatively charged phosphate moieties and electrostatic repulsion arising from both choline-choline and phosphate-phosphate contacts will predominate. However, the steric bulk of the N-methyl groups will markedly reduce the frequency of close contacts interactions between the positively charged choline nitrogen and the negatively charged phosphate groups, and thus the electrostatic attraction component will be markedly attenuated in magnitude. Thus, despite the zwitterionic character of PC polar headgroups, electrostatic repulsion between phosphate moieties at the surfaces of PC bilayers may well have as large an effect on bilayer Tm as occurs with PG bilayers. Largely because of these effects, the forces favoring the gel phase over liquid-crystalline phases are expected to decrease in the order PE ≫ PG ∼ PC, and the incorporation of peptides such as L24 into PE, PC, and PG bilayers may be expected to affect interactions between lipid headgroups in two main ways. First, the presence of these peptides will reduce the access of adjacent phospholipid molecules to potential hydrogen-bonding partners, thus leading to the disruption of part of the hydrogen-bonding networks of PG and PE bilayers. Second, the positively charged side-chain amino groups at the N- and C-termini of these peptides will compete with positively charged and H-bonding donor groups on adjacent lipid polar headgroups for electrostatic and/or H-bonding acceptor sites, thereby disrupting the pattern of lipid-lipid interactions at the surface of these bilayers. As noted above, the electrostatic and H-bonding network at the surface of a PE bilayer is quite strong and is largely responsible for the relatively high transition temperature. Consequently, any significant peptide-induced disruption of the surface electrostatic and H-bonding network of a PE bilayer will have a considerably greater effect on its Tm than will occur with PC and PG bilayers, where the corresponding surface networks are considerably weaker. Moreover, given the magnitude of the changes in Tm that are likely to occur upon disruption of the electrostatic and H-bonding network at the surface of a PE bilayer, such changes may well mask the additional changes arising from hydrophobic mismatch effects, which are thus expected to be reduced in magnitude.
A major finding of this study is that the replacement of lysine by DAP residues at the ends of the model peptides markedly affects their interactions with zwitterionic PE bilayers. Specifically, our studies show that L24DAP is much less effective than L24 at reducing the Tm and enthalpy and broadening the phase transition of PE bilayers. These results indicate that the capacity of L24DAP to disrupt lipid-lipid interactions in PE bilayers is either intrinsically smaller than that of L24, or that this peptide may be more prone to lateral aggregation in PE bilayers than L24, or both. Our experimental observations can be attributed to the different lengths of the methylene spacers between the α-carbons and side-chain amino groups of DAP (one CH2 spacer) and lysine (four CH2 groups). Thus, when lysine-capped peptides such as L24 are incorporated into lipid bilayers, their lysine side chains can extend away from their helical surfaces and the side-chain amino groups can thus compete effectively with positively charged and H-bonding donor groups on adjacent lipids for electrostatic and/or H-bonding acceptor sites. Also, because their lysine side chains are longer than those of the leucine residues of the peptide, lateral contacts between L24 molecules in lipid bilayers can result in intermolecular contacts between the positively side-chain amino groups at the peptide termini and, in turn, charge repulsion between these side-chain amino groups will reduce the probability of forming peptide aggregates under such conditions. However, with L24DAP, the DAP side chains do not extend beyond those of the leucine residues on the peptide surface. Thus, when such peptides are incorporated into lipid bilayers, the side chain amino groups cannot compete with positively charged and H-bonding donor groups on adjacent lipids for electrostatic and/or H-bonding acceptor sites as effectively as can those of L24. Moreover, because the DAP side chains are so short, lateral contacts between L24DAP molecules in a lipid bilayer will not result in the type of intermolecular close contacts between the positively side-chain amino groups at the peptide termini that are likely to occur with a lysine-capped peptide like L24. Consequently, the energetic cost of peptide lateral aggregation in L24DAP-containing lipid bilayers will be smaller than that of comparable L24-containing lipid bilayers. We therefore suggest that, in general, L24DAP will not be as perturbing of lipid thermotropic phase behavior as L24 because of a combination of its smaller capacity to disrupt the electrostatic and H-bonding network at the lipid bilayer surfaces and the smaller energetic penalties against its aggregating in lipid bilayers. Moreover, these factors may be especially important in PE bilayers, because the electrostatic and H-bonding networks at the surfaces of PE bilayers are quite strong and are thus more likely to favor PE-PE interactions over PE-peptide interactions unless peptide inclusion induces a significant disruption of this network. Thus L24DAP should be inherently less perturbing of PE bilayers than L24. Interestingly, our previous studies indicated no major differences between L24 and L24DAP on the thermotropic phase behavior of either or PC of PG bilayers (Liu et al., 2002, 2004). Most probably this is because the electrostatic and H-bonding networks at the surfaces of PC and PG bilayers are considerably weaker than in PE bilayers and the differential effects of lysine and DAP in modulating these forces are not so obvious as in PE bilayers.
Our studies also show that the effect of WL22W on the thermotropic phase behavior of PE bilayers is somewhat smaller than that of L24. Given the arguments presented above (see previous paragraph), it appears that the replacement of a leucine residue at each end of the hydrophobic core of L24 by tryptophan residues attenuates the capacity of the terminal lysine residues to interact with or otherwise perturb the electrostatic and H-bonding network at the surfaces of PE bilayers. This could be an indication that the tryptophan residues are forming cation-π-interactions with adjacent lysine residues, as suggested in our previous studies of the interaction of WL22W with anionic PG bilayers (Liu et al., 2004). Indeed, if these cation-π-interactions do occur between lysine and tryptophan residues, then the lysine residues may not be able to compete as effectively as those of L24 for positively charged and H-bonding donor groups on adjacent lipids for electrostatic and/or H-bonding acceptor sites. However, despite its having a smaller effect on the thermotropic phase behavior of PE bilayers than L24, it is clear that the effects of both peptides are of comparable magnitude and are both much greater than that of L24DAP. Thus, the effect of the cation-π-interactions between lysine and tryptophan residues is considerably less effective than the lysine snorkeling effect in regulating lipid-peptide interactions in lipid bilayers.
In these studies we also observe small decreases in the frequency of peptide amide I band maxima at the gel/liquid-crystalline phase transitions of their lipid bilayer hosts, and that the magnitude of these frequency shifts decrease with increases in lipid hydrocarbon chain length. Similar results have been reported in studies of these peptides with PC and PG bilayers (Liu et al., 2002, 2004) and in studies of the interaction of P24 with PC and PE bilayers (Zhang et al., 1992b, 1995a). As noted previously, these changes seem to arise from a lipid phase state-induced decline in populations giving rise to amide I absorption centered near 1660–1665 cm−1 and a concomitant increase in populations giving rise to amide I absorptions centered near 1645–1650 cm−1 and have been assigned to conformational changes in the deuterium-exchanged N- and C-termini of these peptides (Liu et al., 2002). However, we also find that these lipid phase state-induced decreases in peptide amide I band maxima are consistently smaller in L24DAP-containing PE membranes than observed with the either L24-containing or WL22W-containing membranes. This observation is consistent with the idea that interactions of L24DAP with PE bilayers are less extensive than those of the peptides L24 and WL22W and with the suggestion that, unlike L24- and WL22W-containing PE bilayers, significant peptide lateral aggregation occurs in L24DAP-containing PE bilayers.
In conclusion, our studies highlight the importance of the H-bond interactions and electrostatic attractions between the lipid polar headgroups and the terminal lysine and/or tryptophan residues in peptide-lipid interactions. Our studies also indicate an important role of the length and flexibility of the terminal lysine residues on the interactions of these model peptides with zwitterionic PE bilayers and extend our understanding of the role of interfacially localized lysine and tryptophan residues in natural membrane proteins. Further structural and computer modeling investigations are required to elucidate the detailed mechanism of how the terminal lysine and/or tryptophan residues may interact with adjacent lipid headgroups at the membrane polar/apolar interface.
Acknowledgments
Supported by operating grants from the Natural Sciences and Engineering Council of Canada and the Canadian Institutes of Health Research (R.N.M.) and major equipment grants from the Alberta Heritage Foundation for Medical Research (R.N.M.).
Robert S. Hodges' present address is Dept. of Biochemistry and Molecular Genetics, University of Colorado Health Sciences Center, Denver, CO 80262.
References
- Axelsen, P. H., B. K. Kaufman, R. N. McElhaney, and R. N. A. H. Lewis. 1995. The infrared dichroism of transmembrane helical polypeptides. Biophys. J. 69:2770–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanov, M., and W. Dowhan. 1998. Phospholipid-assisted protein folding: phosphatidyl-ethanolamine is required at a late step of the conformational maturation of the polytopic membrane protein lactose permease. EMBO J. 17:5255–5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs, J. M. 1980. Intermolecular hydrogen bonding between lipids: influence on organization and function of lipids in membranes. Can. J. Biochem. 58:755–770. [DOI] [PubMed] [Google Scholar]
- Boggs, J. M. 1986. Effect of lipid structural modifications on their intermolecular hydrogen bonding interactions and membrane function. Biochem. Cell Biol. 64:50–67. [DOI] [PubMed] [Google Scholar]
- Boggs, J. M. 1987. Lipid intermolecular hydrogen bonding: influence on structural organization and membrane function. Biochim. Biophys. Acta. 906:353–404. [DOI] [PubMed] [Google Scholar]
- Bolen, E. J., and P. W. Holloway. 1990. Quenching of tryptophan fluorescence by brominated phospholipid. Biochemistry. 29:9638–9643. [DOI] [PubMed] [Google Scholar]
- Chirgadze, Y. N., and E. V. Brazhnikov. 1974. Intensities and other spectral parameters of infrared amide bands of polypeptides in alpha helical form. Biopolymers. 13:1701–1712. [DOI] [PubMed] [Google Scholar]
- Daum, G. 1985. Lipids of mitochondria. Biochim. Biophys. Acta. 822:1–42. [DOI] [PubMed] [Google Scholar]
- Davis, J. H., D. M. Clare, R. S. Hodges, and M. Bloom. 1983. Interaction of a synthetic amphiphilic polypeptide and lipids in a bilayer structure. Biochemistry. 22:5298–5305. [Google Scholar]
- de Planque, M. R. R., and J. A. Killian. 2003. Protein-lipid interactions studied with designed transmembrane peptides: role of hydrophobic matching and interfacial anchoring. Mol. Membr. Biol. 20:271–284. [DOI] [PubMed] [Google Scholar]
- Devaux, P. F., and M. Seigneuret. 1985. Specificity of lipid-protein interaction as determined by spectroscopic techniques. Biochim. Biophys. Acta. 822:63–125. [DOI] [PubMed] [Google Scholar]
- Gennis, R. B. 1989. Membrane dynamics and protein-lipid interactions. In Biomembranes: Molecular Structure and Function. Springer-Verlag, New York. 166–198.
- Huschilt, J. C., R. S. Hodges, and J. H. Davis. 1985. Phase equilibria in an amphiphilic peptide-phospholipid model membrane by deuterium nuclear magnetic resonance difference spectroscopy. Biochemistry. 24:1377–1386. [Google Scholar]
- Huschilt, J. C., B. M. Millman, and J. H. Davis. 1989. Orientation of alpha-helical peptides in a lipid bilayer. Biochim. Biophys. Acta. 979:139–141. [DOI] [PubMed] [Google Scholar]
- Jones, M. R., P. K. Fyfe, A. W. Roszak, N. W. Isaacs, and R. J. Cogdell. 2002. Protein-lipid interactions in the purple bacterial reaction center. Biochim. Biophys. Acta. 1565:206–214. [DOI] [PubMed] [Google Scholar]
- Killian, J. A., and G. von Heijne. 2000. How proteins adapt to a membrane-water interface. Trends Biochem. Sci. 25:429–434. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt, J. H. 2003. Membrane proteins–introduction. Cell. Mol. Life Sci. 60:1527–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, R. N. A. H., N. Mak, and R. N. McElhaney. 1987. A differential scanning calorimetric study of the thermotropic phase behavior of model membranes composed of phosphatidylcholines containing linear saturated acyl chains. Biochemistry. 26:6118–6126. [DOI] [PubMed] [Google Scholar]
- Lewis, R. N. A. H., and R. N. McElhaney. 1993. Calorimetric and spectroscopic studies of the polymorphic phase behavior of a homologous series of n-saturated, 1,2-diacyl phosphatidyl-ethanolamines. Biophys. J. 64:1081–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, R. N. A. H., and R. N. McElhaney. 1996. FTIR spectroscopy in the study of hydrated lipids and lipid bilayer membranes. In Infrared Spectroscopy of Biomolecules. H. H. Mantsch and D. Chapman, editors. John Wiley & Sons, New York. 159–202.
- Lewis, R. N. A. H., and R. N. McElhaney. 2002. Vibrational spectroscopy of lipids. In The Handbook of Vibrational Spectroscopy, Vol. 5. J. M. Chalmers and P. R. Griffiths, editors. John Wiley & Sons, New York. 3447–3464.
- Liu, F., R. N. A. H. Lewis, R. S. Hodges, and R. N. McElhaney. 2002. Effect of variations in the structure of a polyleucine-based α-helical transmembrane peptide on its interaction with phosphatidylcholine bilayers. Biochemistry. 41:9197–9207. [DOI] [PubMed] [Google Scholar]
- Liu, F., R. N. A. H. Lewis, R. S. Hodges, and R. N. McElhaney. 2004. Effect of variations in the structure of a polyleucine-based α-helical transmembrane peptide on its interaction with phosphatidylglycerol bilayers. Biochemistry. 43:3679–3687. [DOI] [PubMed] [Google Scholar]
- Mantsch, H. H., C. Madec, R. N. A. H. Lewis, and R. N. McElhaney. 1985. The thermotropic phase behavior of model membranes composed of phosphatidylcholines containing isobranched fatty acids. II. Infrared and 31P-NMR spectroscopic studies. Biochemistry. 24:2440–2446. [DOI] [PubMed] [Google Scholar]
- Marsh, D., and L. I. Horváth. 1998. Structure, dynamics and composition of the lipid-protein interface. Perspectives from spin-labelling. Biochim. Biophys. Acta. 1376:267–296. [DOI] [PubMed] [Google Scholar]
- Monne, M., I. Nilsson, M. Johansson, N. Elmhed, and G. von Heijne. 1998. Positively and negatively charged residues have different effects on the position in the membrane of a model transmembrane helix. J. Mol. Biol. 284:1177–1183. [DOI] [PubMed] [Google Scholar]
- Morrow, M. R., J. C. Huschilt, and J. H. Davis. 1985. Simultaneous modeling of phase and calorimetric behavior in an amphiphilic peptide/phospholipid model membrane. Biochemistry. 24:5396–5406. [DOI] [PubMed] [Google Scholar]
- Paré, C., M. Lafleur, F. Liu, R. N. A. H. Lewis, and R. N. McElhaney. 2001. Differential scanning calorimetry and 2H-nuclear magnetic resonance and Fourier transform infrared spectroscopy studies of the effects of transmembrane α-helical peptides on the organization of phosphatidylcholine bilayers. Biochim. Biophys. Acta. 1511:60–73. [DOI] [PubMed] [Google Scholar]
- Pauls, K. P., A. L. MacKay, O. Soderman, M. Bloom, A. K. Taneja, and R. S. Hodges. 1985. Dynamic properties of the backbone of an integral membrane polypeptide measured by 2H-NMR. Eur. Biophys. J. 12:1–11. [DOI] [PubMed] [Google Scholar]
- Pomorski, T., R. Lombardi, H. Riezman, P. F. Devaux, G. van Meer, and J. C. M. Holthuis. 2003. Drs2p-related P-type ATPases Dnf1p and Dnf2p are required for phospholipid translocation across the yeast plasma membrane and serve a role in endocytosis. Mol. Biol. Cell. 14:1240–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabolt, J. F., W. H. Moore, and S. Krimm. 1977. Vibrational analysis of peptides, polypeptides and proteins. 3. alpha-Poly(L-alaninr). Macromolecules. 10:1065–1074. [DOI] [PubMed] [Google Scholar]
- Ren, J., S. Lew, Z. Wang, and E. London. 1997. Transmembrane orientation of hydrophobic α-helices is regulated both by the relationship of helix length to bilayer thickness and by the cholesterol concentration. Biochemistry. 36:10213–10220. [DOI] [PubMed] [Google Scholar]
- Segrest, J. P., H. de Loof, J. G. Dohlman, C. G. Brouillette, and G. M. Anantharamaiah. 1990. Amphipathic helix motif: classes and properties. Proteins. 8:103–117. [DOI] [PubMed] [Google Scholar]
- Selinsky, B. S. 1992. Protein-lipid interactions and membrane function. In The Structure of Biological Membranes. P. Yeagle, editor. CRC Press, Boca Raton, FL. 603–651.
- Sperotto, M. M., and O. G. Mouritsen. 1988. Dependence of lipid-membrane phase-transition temperature on the mismatch of protein and lipid hydrophobic thickness. Eur. Biophys. J. 16:1–10. [Google Scholar]
- Subczynski, W. K., R. N. A. H. Lewis, R. N. McElhaney, R. S. Hodges, J. S. Hyde, and A. Kusumi. 1998. Molecular organization and dynamics of 1-palmitoyl-2-oleoyl-phosphatidylcholine bilayers containing a transmembrane α-helical peptide. Biochemistry. 37:3156–3164. [DOI] [PubMed] [Google Scholar]
- Subczynski, W. K., R. N. McElhaney, J. S. Hyde, and A. Kusumi. 2003. Molecular organization and dynamics of 1-palmitoyl-2-oleoyl phosphatidylcholine bilayer membranes containing a transmembrane α-helical peptide with hydrophobic surface roughness. Biochemistry. 42:3939–3948. [DOI] [PubMed] [Google Scholar]
- Svensson-Ek, M., J. Abramson, G. Larsson, S. Törnroth, P. Brzezinski, and S. Iwata. 2002. The x-ray crystal structures of wild-type and EQ (I-286) mutant cytochrome c oxidases from Rhodobacter sphaeroides. J. Mol. Biol. 257:3032–3038. [DOI] [PubMed] [Google Scholar]
- Tamm, L. K., and S. A. Tatulian. 1997. Infrared spectroscopy of proteins and peptides in lipid bilayers. Q. Rev. Biophys. 30:365–429. [DOI] [PubMed] [Google Scholar]
- Verkleij, A. J., R. F. Zwaal, B. Roelofsen, P. Comfurius, D. Kastelijn, and L. L. van Deenen. 1973. The asymmetric distribution of phospholipids in the human red cell membrane. Biochim. Biophys. Acta. 323:178–193. [DOI] [PubMed] [Google Scholar]
- von Heijne, G. 1994. Membrane proteins: from sequence to structure. Annu. Rev. Biophys. Biomol. Struct. 23:167–192. [DOI] [PubMed] [Google Scholar]
- Yeagle, P. L. 1993. The Membranes of Cells. Academic Press, San Diego.
- Zhang, W., M. Bogdanov, J. Pi, A. J. Pittard, and W. Dowhan. 2003. Reversible topological organization within a polytopic membrane protein is governed by a change in membrane phospholipid composition. J. Biol. Chem. 278:50128–50135. [DOI] [PubMed] [Google Scholar]
- Zhang, Y.-P., R. N. A. H. Lewis, R. S. Hodges, and R. N. McElhaney. 1992a. FTIR spectroscopic studies of the conformation and amide hydrogen exchange of a peptide model of the hydrophobic transmembrane α-helices of membrane proteins. Biochemistry. 31:11572–11578. [DOI] [PubMed] [Google Scholar]
- Zhang, Y.-P., R. N. A. H. Lewis, R. S. Hodges, and R. N. McElhaney. 1992b. Interaction of a peptide model of a hydrophobic transmembrane α-helical segment of a membrane protein with phosphatidylcholine bilayers: differential scanning calorimetric and FTIR spectroscopic studies. Biochemistry. 31:11579–11588. [DOI] [PubMed] [Google Scholar]
- Zhang, Y.-P., R. N. A. H. Lewis, R. S. Hodges, and R. N. McElhaney. 1995a. Interaction of a peptide model of a hydrophobic transmembrane α-helical segment of a membrane protein with phosphatidylethanolamine bilayers: differential scanning calorimetric and FTIR spectroscopic studies. Biophys. J. 68:847–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y.-P., R. N. A. H. Lewis, R. S. Hodges, and R. N. McElhaney. 1995b. Peptide models of hydrophobic transmembrane segments of membrane proteins. 2. Differential scanning calorimetric and FTIR spectroscopic studies of the interaction of Ac-K2-(LA)12-K2-amide with phosphatidylcholine bilayers. Biochemistry. 34:2362–2371. [DOI] [PubMed] [Google Scholar]
- Zhang, Y.-P., R. N. A. H. Lewis, R. S. Hodges, and R. N. McElhaney. 2001. Peptide models of the helical hydrophobic transmembrane segments of membrane proteins: interactions of acetyl-K2-(LA)12-K2-amide with phosphatidylethanolamine bilayer membranes. Biochemistry. 40:474–482. [DOI] [PubMed] [Google Scholar]
- Zhang, Y.-P., R. N. A. H. Lewis, and R. N. McElhaney. 1997. Calorimetric and spectroscopic studies of the thermotropic phase behavior of the n-saturated 1,2-diacylphosphatidylglycerols. Biophys. J. 72:779–793. [DOI] [PMC free article] [PubMed] [Google Scholar]