Abstract
Muscarinic K+ (KACh) channels are key determinants of the inhibitory synaptic transmission in the heart. These channels are heterotetramers consisting of two homologous subunits, G-protein-gated inwardly rectifying K+ (GIRK)1 and GIRK4, and have unitary conductance of ∼35 pS with symmetrical 150 mM KCl solutions. Activation of atrial KACh channels, however, is often accompanied by the appearance of openings with a lower conductance, suggesting a functional heterogeneity of G-protein-sensitive ion channels in the heart. Here we report the characterization of a small conductance GIRK (scGIRK) channel present in rat atria. This channel is directly activated by Gβγ subunits and has a unitary conductance of 16 pS. The scGIRK and KACh channels display similar affinities for Gβγ binding and are frequently found in the same membrane patches. Furthermore, Gβγ-activated scGIRK channels—like their KACh counterparts—exhibit complex gating behavior, fluctuating among four functional modes conferred by the apparent binding of a different number of Gβγ subunits to the channel. The electrogenic efficacy of the scGIRK channels, however, is negligible compared to that of KACh channels. Thus, Gβγ subunits employ the same signaling strategy to regulate two ion channels that are apparently endowed with very different functions in the atrial membrane.
INTRODUCTION
Autonomic regulation of the heart rate is a continuous process integrating multiple cellular events controlled by sympathetic and parasympathetic signals to the heart. Both branches of the autonomic nervous system rely on G-protein-coupled receptors and their downstream effectors to accomplish the control of cardiovascular function. Among these effectors, the KACh channels of the sinoatrial node and atria play a prominent role in the inhibitory synaptic transmission in the heart and slowing of the heart rate (Wickman et al., 1998; Yamada, 2002).
The cardiac KACh channels consist of two homologous subunits, G-protein-gated inwardly rectifying K+ (GIRK)1 (Dascal et al., 1993; Kubo et al., 1993) and GIRK4 (Krapivinsky et al., 1995a). The GIRK1 and GIRK4 subunits form heterotetrameric channels with a (GIRK1)2(GIRK4)2 stoichiometry (Silverman et al., 1996, Tucker et al., 1996) and pore conductance of ∼35 pS with symmetrical 150-mM KCl solutions. Topologically consistent with the bacterial KcsA K+ channel (Doyle et al., 1998), each GIRK subunit comprises two transmembrane helixes (TM1, TM2) interrupted by a P region, and cytoplasmic NH2- and COOH-terminal domains. The transmembrane helixes and the P regions form an integral transmembrane pore (Doyle et al., 1998), which is significantly extended by a tetrameric cytoplasmic pore formed by the NH2- and COOH-terminal domains of the GIRK subunits (Nishida and MacKinnon, 2002). The activation of the KACh channels is conferred by direct Gβγ binding, apparently to multiple domains, on the intracellular surface of the channel (for reviews see Yamada et al., 1998, and Sadja et al., 2003). Gβγ-activated KACh channels exhibit complex gating behavior, fluctuating among four functional modes conferred by the apparent binding of a different number of Gβγ subunits to four identical and independent sensors in the protein complex (Ivanova-Nikolova et al., 1998). This Gβγ-dependent KACh channel gating is further complicated by slow modal transitions (on a timescale of minutes), which are independent of Gβγ concentration and probably involve some membrane-delimited processes (Yakubovich et al., 2000). At this time, the structural principles governing such complex KACh channel gating are not fully understood. Mutational studies of the transmembrane helixes of GIRK suggest that upon gating the TM2 helixes undergo both rotation (Yi et al., 2001) and bending at a conserved glycine residue (Jin et al., 2002, Jiang et al., 2002). Surprisingly, the structure of the cytoplasmic GIRK pore connects more realistically to an open conformation of the transmembrane pore than to a closed one (Nishida and MacKinnon, 2002). This structure identifies an additional possibility for channel activation—gating at the cytoplasmic pore—in the presence of a permanently open transmembrane pore of the GIRK channels.
Whereas the Gβγ subunits govern the gating phenomenon, the Gαi subunits have significant ramifications for the fidelity of coupling between different G-protein-coupled receptors and GIRK channels (Leaney et al., 2000). In addition, the Gαi subunits are responsible for termination of the G-protein signaling to the KACh channel (Doupnik et al., 1997; Saitoh et al., 1997; Peleg et al., 2002).
In the atria, the heterotetrameric GIRK1/GIRK4 channels coexist with GIRK4 homomultimers of yet unidentified biological function (Corey and Clapham, 1998). The biochemical heterogeneity of atrial GIRK multimers is accompanied by a functional variability of G-protein-sensitive ion channels. Our examination of receptor-activated KACh channels in neonatal rat atrial myocytes frequently revealed the presence of two types of channel openings, one with a conductance of 34 pS and another one with a conductance of ∼16 pS (with symmetrical 150-mM KCl solutions). Although these two conductance levels could be distinguished in KAch channel recordings from different species in a number of studies from other laboratories (Ito et al., 1991; Sui et al., 1996), the relation between the 34-pS and 16-pS events has never been investigated. These observations prompted us to investigate the 16-pS channel openings and their dependence on Gβγ. Here we define the properties of a previously uncharacterized Gβγ-sensitive inwardly rectifying K+ channel observed in the membrane of rat atrial myocytes. This channel exhibits a small conductance (13–17 pS), fast kinetics, and, like the KACh channel, is directly activated by the Gβγ subunits. On the basis of the small conductance and mode of activation, we designated this channel scGIRK channel. Mechanistically, the activation of the scGIRK channels by Gβγ replicates the activation of the KACh channels, strengthening the notion that Gβγ-driven control of multiple functional states, evolving from the same effector molecule, is a common mechanism of signal transduction. The electrogenic efficacy of the scGIRK channel, however, is negligible compared to that of the KACh channel. Thus, these two channels are most probably endowed with very different physiological functions in the membrane of the atrial myocytes.
MATERIALS AND METHODS
Isolation and cell culture of neonatal cardiac myocytes
Experiments were performed on Sprague-Dawley rat atrial cells. Neonatal animals were cryoanaesthetized on ice and then decapitated. The heart was rapidly excised and the atrial tissue was removed for further dissociation. Primary cultures of rat atrial myocytes were obtained according to previously established procedures (Foster et al., 1990). Briefly, the atrial tissue was minced and treated with a mixture of trypsin, chymotrypsin, and elastase at 37°C. The suspension of dissociated cells was centrifuged through Percoll step gradients to obtain cell preparations consisting of >94% myocytes. The myocytes were suspended in modified Eagle's medium containing 5% newborn calf serum and 0.1 mM 5-bromo-2′-deoxyuridine and were plated on round (7 mm in diameter) gelatin-coated coverslips in culture dishes at a density of 1 × 105 cells/cm2. After an overnight incubation, the cells were washed to remove nonadherent cells and cultured in a defined serum-free media 4 additional days.
Expression and purification of G-protein β1γ7 dimers followed the method developed by Kozasa and Gilman (1995) for purification of β1γ2 and has been previously described (Ivanova-Nikolova et al., 1998).
Electrophysiology
Single-channel currents through KACh and scGIRK channels were recorded from cell-attached and inside-out patches of atrial myocytes using the standard high-resolution patch-clamp method (Hamill et al., 1981). The membrane potential was zeroed with the following bath solution (in mM): 150 KCl, 5 EGTA, 5 glucose, 1.6 MgCl2, 5 HEPES (pH 7.4). Pipette solution contained (in mM): 150 KCl, 1 CaCl2, 1.6 MgCl2, 5 HEPES (pH 7.4). Patch pipettes were made from borosilicate glass (World Precision Instruments, Sarasota, FL) on a Flaming Brown micropipette puller (Sutter Instrument, Novato, CA) and firepolished on a microforge (Narishige Scientific Instrument Lab, Tokyo, Japan). The resistance of the patch pipettes (when filled with pipette solution) was 10–20 MΩ. To estimate the number of KACh channels present in each patch, the atrial m2-muscarinic receptors were activated with 1 μM acetylcholine added to the pipette solution, and the KACh channel activity was monitored in cell-attached configuration for 3–6 min. Patches with superimposed 34 pS-openings were not subjected to further investigation. Single-channel currents were recorded with a Patch Clamp List-Medical EPC-7 amplifier (ALA Scientific Instruments, Westbury, NY) using a DigiData 1200 series interface and pCLAMP 6.0 software, both from Axon Instruments (Foster City, CA). Data were digitized at 5 kHz and stored on a computer for further analysis. Some of the data were additionally filtered with an 8-pole Bessel filter (Frequency Devices, Haverhill, MA) at 2 kHz. The fast and slow time constants, τo1 = 0.29 ± 0.07 ms and τo2 = 1.1 ± 0.3 ms (n = 9 cells), derived from the open-time distributions of the filtered data were not significantly different from the time constants, τo1 = 0.31 ± 0.05 ms and τo2 = 1.3 ± 0.2 ms (n = 8 cells), estimated from unfiltered recordings of the elementary currents at the same membrane potential.
Only recordings with current transitions to a single 16-pS and/or a single 35-pS level (monitored for at least 10 min in the presence of Gβγ) were included in the analyses of the amplitude of channel openings, open dwell times, and gating modes. All-points amplitude histograms were generated using pCLAMP 6.0 software and fitted with the sum of several Gaussian functions to determine the mean current amplitude for the scGIRK and KACh channels. Lists of durations of single-channel events were generated by the FETCHAN Events List function using the half-amplitude threshold-crossing algorithm. Cumulative histograms of open dwell times were generated and fitted with the sum of two exponential components.
Examination of long episodes of single-channel data recordings at a constant Gβ1γ7 concentration frequently revealed the presence of slow modal transitions in scGIRK and of KACh channel gating, analogous to the slow gating transitions described for heterologously expressed GIRK1/4 channels (Yakubovich et al., 2000) but on a much slower timescale. Such transitions between different phases of channel activity were always associated with a change in the frequency of channel gating. Therefore, each recording was divided into 400-ms consecutive segments, and the frequency of apparent openings, f, was calculated for each segment. The 400-ms duration of the individual data segments was selected to reflect the mean burst duration of KACh channels exhibiting well-delineated bursting behavior; the same time interval was used for our analysis of the scGIRK channels. The mean frequency, F, was averaged over 30-s data recordings (from 75 consecutive 400-ms data segments) and plotted versus time (see Fig. 10, A and B). The resulting F-t plots accurately identified the apparently homogeneous sections of each recording. A data recording (usually longer than 6–7 min) was judged as homogeneous if the mean F values (denoted by the horizontal lines in Fig. 10, A and B) calculated from the first and the second halves of this recording were statistically indistinguishable.
FIGURE 10.
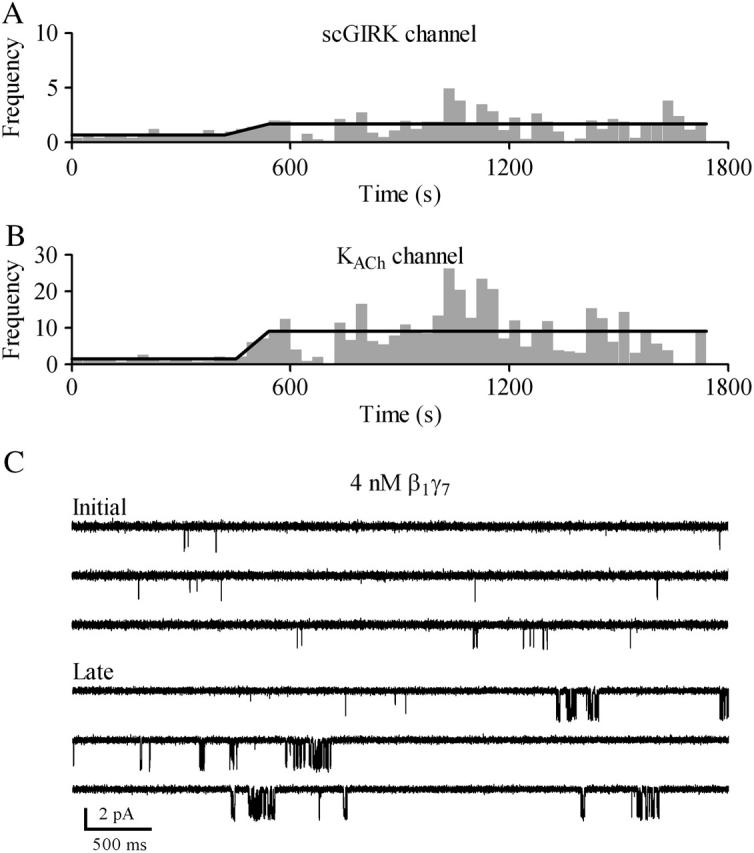
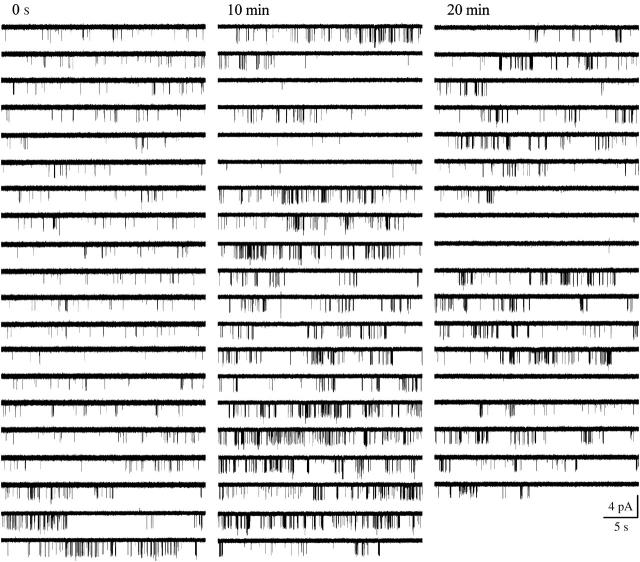
Biphasic activation of GIRK channels. Time courses of Gβγ activation for the scGIRK (A) and the KACh (B) channels shown in Fig. 9. To identify the different phases of scGIRK and KACh channel activation, the F-t plots were generated as described in Materials and Methods. The horizontal lines indicate the mean F values obtained within apparently homogeneous sections of channel activity. After an initial period (∼7 min) of steady gating, the activity of both channels increased simultaneously. (C) Two representative 15-s segments of single-channel recordings are shown to illustrate the changes in the channel behavior associated with different phases of this experiment.
RESULTS
Evidence for the presence of scGIRK channels in neonatal rat atrial myocytes
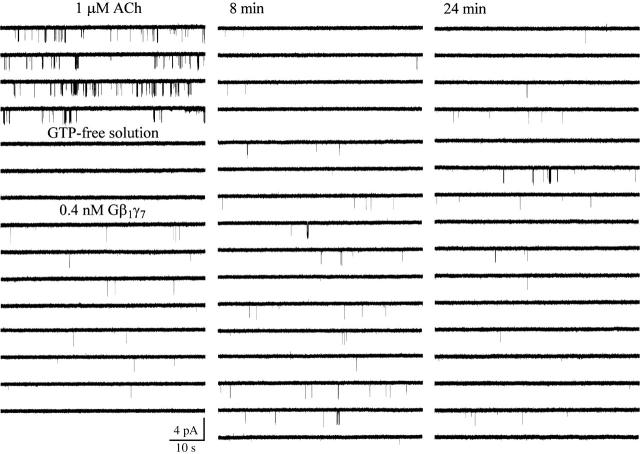
Inspection of individual atrial KACh channels activated in cell-attached patches by 1 μM ACh revealed the presence of two populations of single-channel openings as illustrated in Fig. 1. One population exhibited a unitary conductance of 34 ± 1 pS (n = 14), characteristic of classical KACh channels, whereas the other population had a unitary conductance of 16 ± 1 pS (n = 14).
FIGURE 1.
Two populations of G-protein-activated single-channel openings in neonatal rat atrial myocytes. Activation of a single 34-pS KACh channel in a cell-attached patch is accompanied by the appearance of 16-pS openings. In this and all following figures, the downward deflections correspond to inward currents. Membrane potential: −90 mV; 1 μM ACh is added to the pipette solution. Excision of the membrane patch in GTP-free solution instantaneously and completely eliminated both 16- and 34-pS events. The membrane potential continued to be clamped at −90 mV during the entire experiment. Perfusion of the experimental chamber with 0.4 nM Gβ1γ7 restored both the 16- and 34-pS single-channel openings. The single-channel recording in the presence of Gβ1γ7 begins 10 min after Gβ1γ7 application.
Patch excision in GTP-free solution completely abolished the activity of both the 16-pS and 34-pS events (Figs. 1 and 2), indicating the absence of an obligatory component required for channel gating in the cell-free membrane. Subsequent perfusion of the cytoplasmic side of the membrane with nanomolar concentrations of purified recombinant Gβ1γ7 restored the activity of both channels. Thus, a sole elevation in free membrane concentration of Gβγ was sufficient to activate not only the KACh channels, but the 16-pS events as well.
FIGURE 2.
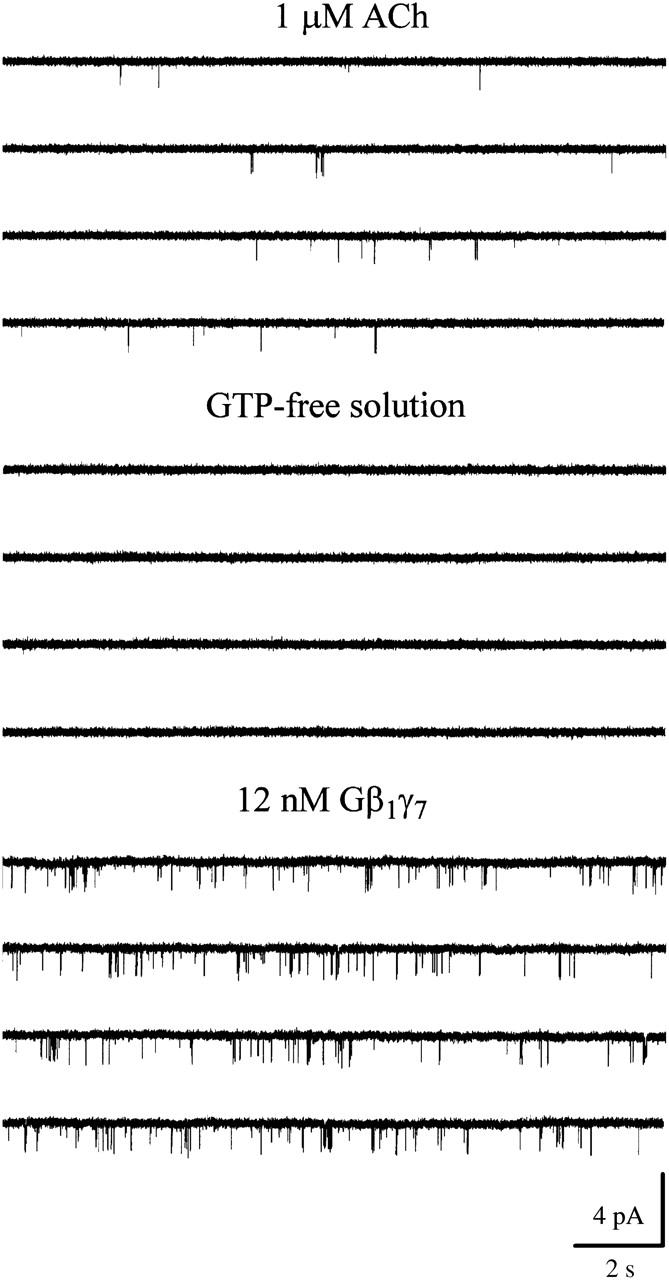
Small conductance atrial GIRK channel. Single-channel activity recorded in cell-attached configuration from an atrial myocyte in the presence of 1 μM ACh. Channel activity ceased after patch excision in GTP-free solution. Application of 12 nM Gβ1γ7 restored the channel activity. Membrane potential: −90 mV.
Examination of the gating pattern of the 16-pS events (Fig. 2) revealed a strong resemblance to the behavior of the canonical KACh channels. The 16-pS events, like their 34-pS counterparts, fluctuated between singular openings preceded and followed by long closed intervals and clusters of relatively closely spaced openings. Direct transitions between the 16-pS and the 34-pS conductance levels, however, were not detected even during long episodes (20–30 min) of single-channel data recordings. In addition, membrane patches harboring exclusively the 16-pS Gβγ-activated events were frequently found (Fig. 2). These observations suggest that the 16-pS openings might result from activation of a previously uncharacterized atrial channel rather than from transitions to a subconductance state of the KACh channel. Furthermore, to the best of our knowledge there are no published data describing subconductance states of KACh channels.
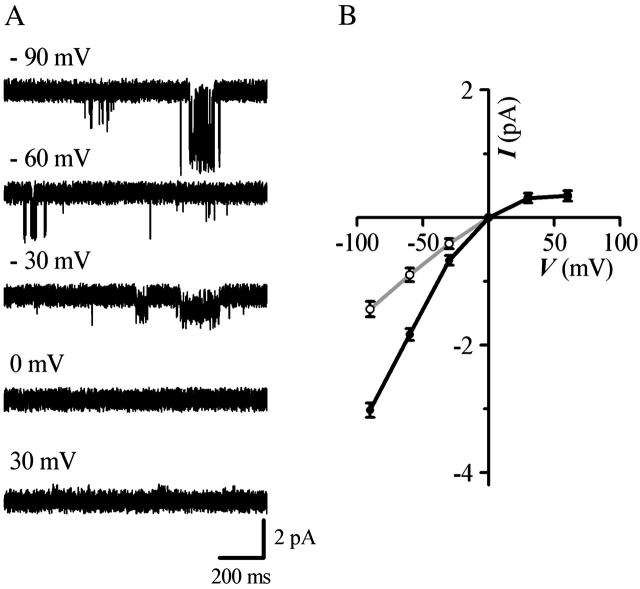
To determine the voltage dependence of the small conductance events, current amplitudes were measured in symmetrical 150-mM KCl solutions at different membrane potentials and compared to those of KACh channels found in the same membrane patches (Fig. 3 A). The current-voltage plot (Fig. 3 B) generated from such experiments demonstrates that the 16-pS events, like the KACh channels, display inward rectification. Although the molecular identity of the 16-pS events is unknown, on the basis of their small conductance, direct Gβγ activation, and inward rectification, we designated these events “small conductance (sc) GIRK channels” for clarity of presentation.
FIGURE 3.
Voltage dependence of scGIRK and KACh channels. (A) Gβ1γ7-activated single-channel currents were recorded from the same membrane patch at the indicated potentials. All-points histograms were generated from the recordings and fitted by a sum of Gaussian functions to determine the mean current amplitudes. (B) I−V curves for the scGIRK (gray) and KACh (black) channels were constructed from the single-channel current amplitudes determined at each membrane potential. Each data point represents the mean ± SEM of currents recorded from 5 to 14 channels.
To identify the elementary open states entered by the scGIRK channels, we generated distributions of open-interval durations at different (1.6–12 nM) Gβ1γ7 concentrations. The adequate fit of the open-time distributions (Fig. 4 A) invariably required a sum of two exponential components characterized by mean time constants, τo1 and τo2, of 0.31 ms and 1.3 ms, respectively. Both τo1 and τo2 were independent of the Gβγ concentration. Similar analysis of KACh channels, activated in the same range of Gβ1γ7 concentration, yielded fast and slow open-time constants that were threefold greater (τo1 = 0.93 ± 0.14 ms and τo2 = 4.3 ± 0.6 ms, n = 10) than those estimated for the scGIRK channel.
FIGURE 4.
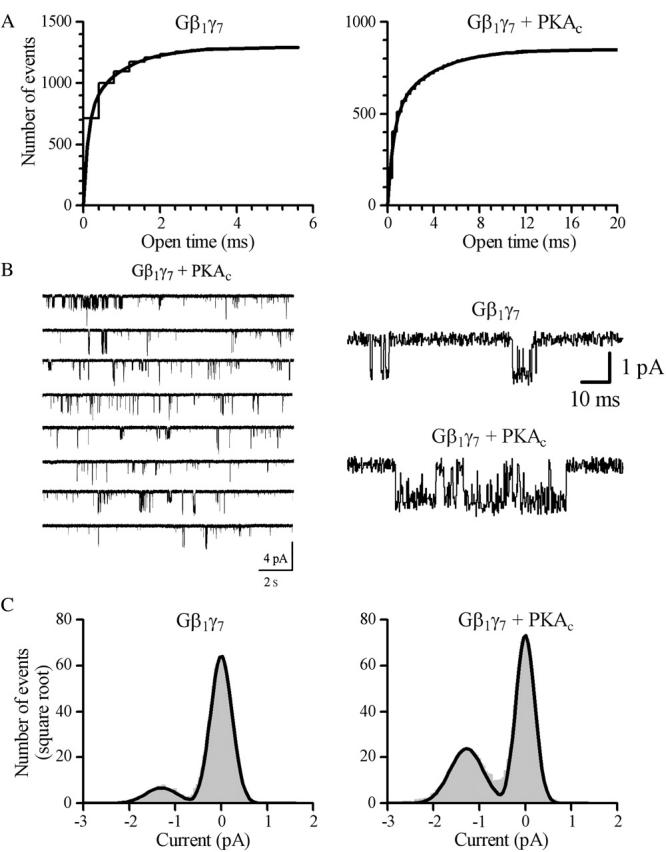
Characteristics of scGIRK channels. (A) Cumulative open-time distributions with superimposed fits are shown for two different scGIRK channels activated by 4 nM Gβ1γ7 in the absence (left panel) and presence (right panel) of Mg-ATP/PKAc (the scGIRK channel shown in B). Membrane potential: −90 mV. Two exponential components were readily identified in both histograms. The time constants of the fast and slow components are τo1 = 0.12 ms (57.7%) and τo2 = 0.94 ms (42.3%) in the presence of Gβ1γ7 alone, and τo1 = 0.49 ms (55.3%) and τo2 = 3.49 ms (44.7%) in the presence of Gβ1γ7/Mg-ATP/PKAc. The relative areas of individual components are given in parentheses. (B) Effect of PKA on scGIRK channel gating. A pair of an scGIRK and a KACh channel residing in the same membrane patch was activated in the presence of 4 nM Gβ1γ7, and then PKAc was applied to the cytoplasmic side of the patch in the presence of Mg-ATP. Application of PKA triggered clustering of scGIRK channel openings into long bursts of activity. Expanded current traces recorded from the same patch before and after application of PKA are shown in the right panel to illustrate the kinetic behavior of the channel in greater detail. (C) All-points histograms generated from data recorded from the same scGIRK channel in the presence of Gβγ (left panel) and Gβγ/Mg-ATP/PKAc (right panel). The histograms were fitted by the sum of two Gaussian functions to determine the unitary conductance of scGIRK channel (14.4 pS and 14.2 pS, respectively).
The fast open-time constants estimated for the Gβγ-activated scGIRK channels give rise to an important concern that the 16-pS events might represent distorted brief 34-pS openings rather than genuine events. To rule out this possibility we investigated the effect of cyclic adenosine monophosphate-dependent protein kinase (PKA) on scGIRK channel activation by Gβγ. Since PKA phosphorylation significantly increases KACh channel open times (Kim, 1990; Mullner et al., 2003), it is expected to eliminate the 16-pS events from our recordings if they were poorly resolved, brief 34-pS events. Pairs of scGIRK and KACh channels residing in the same membrane patch were activated by purified recombinant Gβγ, and then purified PKA catalytic subunit was applied to the cytoplasmic side of the patch in the presence of Mg-ATP. PKAc application (200 pg/μl) caused a substantial upregulation of Gβγ-activated scGIRK channels, which is illustrated in Fig. 4 (n = 4). The effect of PKAc on scGIRK channel activity was observed within 3–6 min after PKA application and was sustained for the duration of our experiments. In control experiments, perfusion of Gβ1γ7-activated scGIRK and KACh channels with either Mg-ATP or heat inactivated PKAc had no effect on the channel activity (data not shown). Comparison of the open-time distributions revealed that PKA potentiation of the scGIRK channel activity was associated with a twofold increase in both the fast and slow open-time constants (τo1 = 0.66 ± 0.08 ms and τo2 = 3.08 ± 0.52 ms, n = 4). Furthermore, PKA induced significant shift in the scGIRK channel gating toward a high open probability mode that was infrequently accessible in the presence of Gβ1γ7 alone (see Gating mechanism of scGIRK channels). At the same time, the unitary conductance of scGIRK channels was not affected in the presence of PKAc (Fig. 4), thus eliminating the possibility that the 16-pS events result from very brief distorted KACh channel openings.
In summary, our analysis of unitary currents recorded from membrane patches either after m2-receptor stimulation, or application of purified Gβ1γ7 subunits revealed the presence of ∼16-pS channel openings with open durations threefold briefer than those of the canonical KACh channels activated under the same conditions. Our data also indicate that the activity of the scGIRK channels and their sensitivity to G-protein stimulation is efficiently regulated by PKA.
Gating mechanism of scGIRK channels
To understand the gating mechanism of scGIRK channels, we analyzed channel behavior at Gβ1γ7 concentrations ranging from 0.4 to 12 nM. Consistent with our previous observations using Gβ1γ5, the activation of both scGIRK and KACh channels by Gβ1γ7 developed gradually over the course of 6–10 min before reaching a sustained level of activity. To provide sufficient time for Gβ1γ7 incorporation in the membrane and to ensure a consistency of experimental conditions, the analysis of single-channel recordings was started at the same time point in each experiment, 10 min after Gβ1γ7 application.
Gβ1γ7 activated the scGIRK channels in a concentration-dependent manner. At 0.4 nM of Gβ1γ7, only a few singular openings could be recorded within a 40-min interval (Fig. 1). As Gβ1γ7 concentration was raised, the probability of the channel being open increased (Fig. 2). This increase in the channel open probability, however, was also associated with a shift from exclusively singular openings to more complex patterns of channel activity.
To assess the gating of the scGIRK channels at different Gβ1γ7 concentrations, we implemented the routine developed previously by us for the analysis of heterogeneous KACh channel behavior (Ivanova-Nikolova et al., 1998). The homogeneous sections of continuous recordings were divided into consecutive 400-ms segments and two parameters—frequency of openings, f, and open probability, po—were calculated for each data segment and plotted versus time.
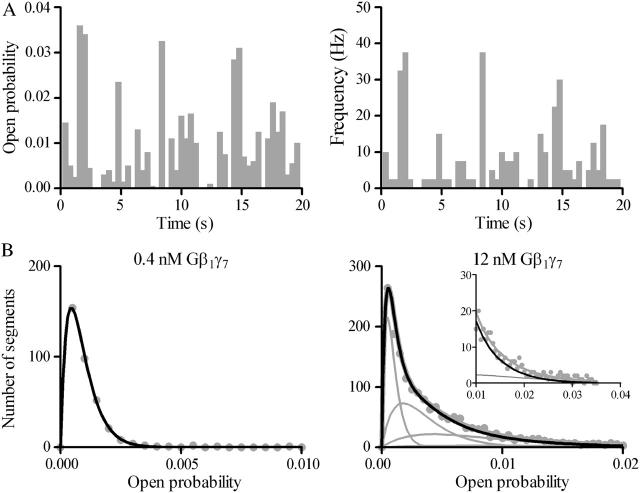
The po and f plots presented in Fig. 5 A illustrate the fluctuations in the open probability and the frequency of openings for the initial 20 s of the scGIRK channel recording shown in Fig. 2. The po and f values calculated from the individual 400-ms data segments were further used to generate f and po histograms. These histograms were used to determine the number of conducting conformations accessible to the scGIRK channel and to quantify the equilibrium among different channel conformations (Colquhoun and Hawkes 1981, 1983; Colquhoun and Sakmann, 1985).
FIGURE 5.
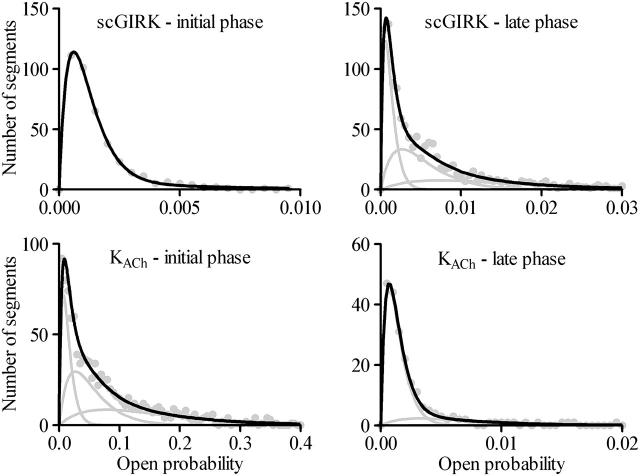
Classification of the scGIRK channel gating. (A) Continuous single-channel recordings were divided into consecutive 400-ms segments and the channel open probability and the frequency of openings were determined for the individual segments. Plots of open probability (left panel) and frequency of gating (right panel) versus time, derived from the initial 20 s of the scGIRK channel recording illustrated in Fig. 2, are shown to illustrate the heterogeneous channel behavior. (B) Modal classification of scGIRK channel gating. Open probability histograms generated from scGIRK channel activity recorded at two different Gβ1γ7 concentrations. The histogram shown at left was generated from pooled data recorded from four different scGIRK channels activated in the presence of 0.4 nM Gβ1γ7. The histogram shown at right was generated from data recordings from a single scGIRK channel activated in the presence of 12 nM Gβ1γ7. The histograms shown in this and the following figures leave out the blank 400-ms data segments and include the active data segments only. The po histograms were fitted by a sum of gamma components 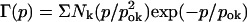 to determine the mean po values and the relative occupancy of different gating components. The mean po values and relative areas (given in parentheses) of different components are po1 = 0.00045 (100%) in the presence of 0.4 nM Gβ1γ7; and po1 = 0.00046 (29.3%), po2 = 0.0018 (37.7%), po3 = 0.0043 (27.2%), and po4 = 0.0085 (5.8%) in the presence of 12 nM Gβ1γ7. The inset shows the right part of the histogram on a different scale to illustrate the minor contribution of the component representing mode 4 (gray line) to the total fit of the histogram. This component was frequently too small to be accurately distinguished from the component representing mode 3, and therefore, modes 3 and 4 were analyzed together in the po histograms.
to determine the mean po values and the relative occupancy of different gating components. The mean po values and relative areas (given in parentheses) of different components are po1 = 0.00045 (100%) in the presence of 0.4 nM Gβ1γ7; and po1 = 0.00046 (29.3%), po2 = 0.0018 (37.7%), po3 = 0.0043 (27.2%), and po4 = 0.0085 (5.8%) in the presence of 12 nM Gβ1γ7. The inset shows the right part of the histogram on a different scale to illustrate the minor contribution of the component representing mode 4 (gray line) to the total fit of the histogram. This component was frequently too small to be accurately distinguished from the component representing mode 3, and therefore, modes 3 and 4 were analyzed together in the po histograms.
Fig. 5 B provides a comparison of two po distributions obtained at different Gβ1γ7 concentrations. In the presence of 0.4 nM Gβ1γ7, a single gamma distribution Γ (p) for the sum of two variables with the same exponential distribution, 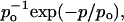 and mean po (Colquhoun and Sakmann, 1985) was sufficient to fit the po histogram, whereas the adequate fit of the po histogram generated in the presence of 12 nM Gβ1γ7 required a sum of four gamma components. Since each conducting conformation of an ion channel contributes a single kinetic component to the po and f histograms, our data suggest the presence of four conducting conformations or gating modes for each scGIRK channel. However, in the absence of PKAc in the experimental chamber, even at saturating Gβ1γ7 concentrations the occupancy of gating mode 4 was very low (Fig. 5 B, inset). We therefore analyzed the gating in modes 3 and 4 together and the parameters estimated from the third gamma component in the po and f histograms were interpreted as a combined representation of the channel performance within these two modes.
and mean po (Colquhoun and Sakmann, 1985) was sufficient to fit the po histogram, whereas the adequate fit of the po histogram generated in the presence of 12 nM Gβ1γ7 required a sum of four gamma components. Since each conducting conformation of an ion channel contributes a single kinetic component to the po and f histograms, our data suggest the presence of four conducting conformations or gating modes for each scGIRK channel. However, in the absence of PKAc in the experimental chamber, even at saturating Gβ1γ7 concentrations the occupancy of gating mode 4 was very low (Fig. 5 B, inset). We therefore analyzed the gating in modes 3 and 4 together and the parameters estimated from the third gamma component in the po and f histograms were interpreted as a combined representation of the channel performance within these two modes.
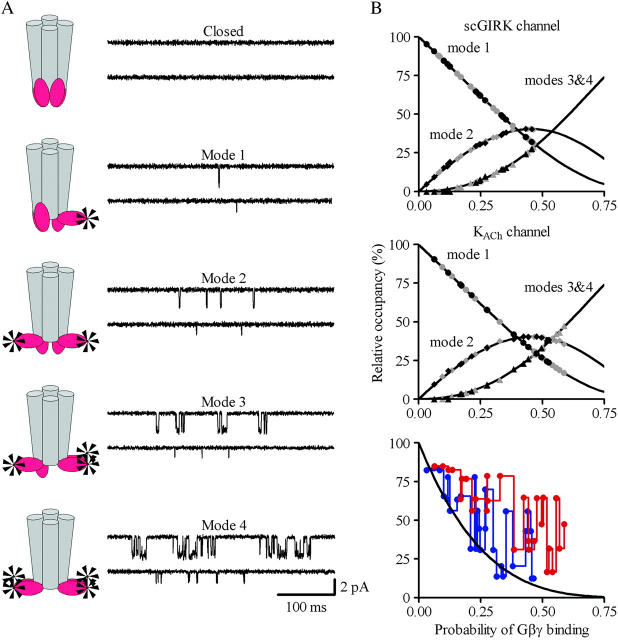
The analysis of po and f histograms was also used to quantify the equilibrium probability (or relative occupancy) of individual gating modes. Different biochemical mechanisms may underline the complex gating behavior of an ion channel regulated by Gβγ binding. Previously, for the interpretation of heterogeneous KACh channel gating, we suggested that each gating mode, k, arises from binding of k Gβγ subunits to four equivalent and independent Gβγ sensors on a single tetrameric KACh channel (Ivanova-Nikolova et al., 1998). In this case, the probability of observing mode k should follow the binomial distribution,
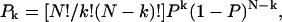 |
(1) |
where P is the probability of Gβγ binding to one of the G-protein sensors, and N = 4. The equilibrium between the four functional modes of Gβ1γ5-activated KACh channels is in excellent agreement with the predictions of this model (Ivanova-Nikolova et al., 1998). Therefore, in this study we tested the possibility that different gating modes of the scGIRK channel arise from binding of one to four Gβγ subunits to four equivalent and independent Gβγ sensors in the scGIRK channel (Fig. 6 A). A simple test of this assumption would be to determine the correlation between the occupancies of individual gating modes. For each po and f histogram, the probability of Gβγ binding, P, was computed from the relative occupancy of mode 1, F1, according to the equation
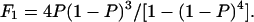 |
(2) |
FIGURE 6.
Gating equilibrium among four functional modes of GIRK channels. (A) A model assuming four functional modes of GIRK channels rendered active by the independent binding of an increasing number of Gβγ subunits to four equivalent Gβγ sensors in the channel structure. (B) For each channel, the relative occupancy of different gating modes was estimated from the fraction of the po (gray symbols) and f (black symbols) histograms fitted by the corresponding gamma components. The sojourns of the channels to gating modes 3 and 4 are jointly represented. In each experiment, the probability of Gβ1γ7-binding to one of the Gβγ sensors, P, was computed from the relative occupancy of mode 1, F1, according to Eq. 2. The solid lines represent the predicted occupancy of the individual gating modes. The symbols represent the occupancy of mode 1 (circles), mode 2 (diamonds), and modes 3 and 4 (triangles) determined from the individual po and f distributions for different scGIRK (n = 14) and KACh (n = 14) channels. The graph in panel B, bottom, illustrates the discrepancy between the predicted occupancy of mode 0 and the experimentally determined fractions of blank data segments for the scGIRK (blue) and KACh (red) channels.
Then the relative occupancy of the remaining k modes, Fk, was examined as a function of the parameter P. Fig. 6 B illustrates the results from this test for scGIRK and KACh channels activated in the same range of Gβ1γ7 concentrations.
The equilibrium analysis indicated that the theoretically derived binomial distributions for the probability of observing gating modes 2, and 3 and 4 (Fig. 6 B, solid lines) superimposed upon the experimental Fk estimates (symbols) for both GIRK channels. We next investigated whether selecting a different gating mode as the basis for calculation of parameter P might influence its value. To test this possibility the probability of Gβ1γ7 binding to one of the Gβγ sensors, P, was computed from the relative occupancy of mode 2 in each experiment, F2, according to the equation: F2 = 6P2(1 − P)2/[1 − (1 − P)4]. Unlike the occupancy of mode 1, which provides a single P value for each F1, the occupancy of mode 2 provides two P estimates for each F2 value. To avoid this ambiguity in the interpretation of the occupancy of mode 2, for each histogram we selected the P value corresponding to the smaller difference, 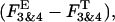 where
where 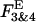 and
and 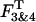 are the experimentally determined and the theoretical occupancies of modes 3 and 4, respectively. This analysis revealed that the P value derived from each histogram was independent of the mode selected for its calculation, and thus confirmed the excellent compatibility between the model illustrated in Fig. 6 A and the gating equilibria of GIRK channels. In most recordings, however, the proportion of blank data segments significantly exceeded the probability of observing gating mode 0 (no Gβγ bound to the channel) calculated from the binomial distribution (Fig. 6 B). One possible reason for this deviation from the theoretical curve could be a Gβγ-dependent desensitization of GIRK channels. Consistent with this possibility is our recent finding that the native KACh channels form large signaling complexes with several regulatory proteins in the atrial membrane (Nikolov and Ivanova-Nikolova, 2004). Two of the proteins found in these complexes, RACK1 (receptor for activated C kinase) and βARK (β-adrenergic receptor kinase) can function as Gβγ scavengers and, thus, might contribute to GIRK channel desensitization.
are the experimentally determined and the theoretical occupancies of modes 3 and 4, respectively. This analysis revealed that the P value derived from each histogram was independent of the mode selected for its calculation, and thus confirmed the excellent compatibility between the model illustrated in Fig. 6 A and the gating equilibria of GIRK channels. In most recordings, however, the proportion of blank data segments significantly exceeded the probability of observing gating mode 0 (no Gβγ bound to the channel) calculated from the binomial distribution (Fig. 6 B). One possible reason for this deviation from the theoretical curve could be a Gβγ-dependent desensitization of GIRK channels. Consistent with this possibility is our recent finding that the native KACh channels form large signaling complexes with several regulatory proteins in the atrial membrane (Nikolov and Ivanova-Nikolova, 2004). Two of the proteins found in these complexes, RACK1 (receptor for activated C kinase) and βARK (β-adrenergic receptor kinase) can function as Gβγ scavengers and, thus, might contribute to GIRK channel desensitization.
Alternatively, the discrepancy between the predicted and experimentally determined fractions of blank data segments in our experiments might be attributed to the presence of an additional nonconducting conformation of the GIRK channels. Thus, we considered a paradigm in which both the vacant and the singly occupied GIRK channels are silent (Fig. 7 A). To test this model, we once again examined the relative occupancy of the gating modes 1, 2, and 3 as a function of the probability of Gβγ binding, P. In this case, the parameter P, however, was computed from the relative occupancy of mode 0, F0, according to the equation: F0 = 4P(1 − P)3 + (1 − P)4. The results from this analysis (Fig. 7 B) indicate that such a model reasonably well predicts the occupancy of all gating modes in the low Gβ1γ7 concentration range (0.4–1.6 nM) used in our experiments. At higher Gβ1γ7 concentrations, however, the relative occupancy of individual gating modes significantly deviated from the predicted binomial distribution.
FIGURE 7.
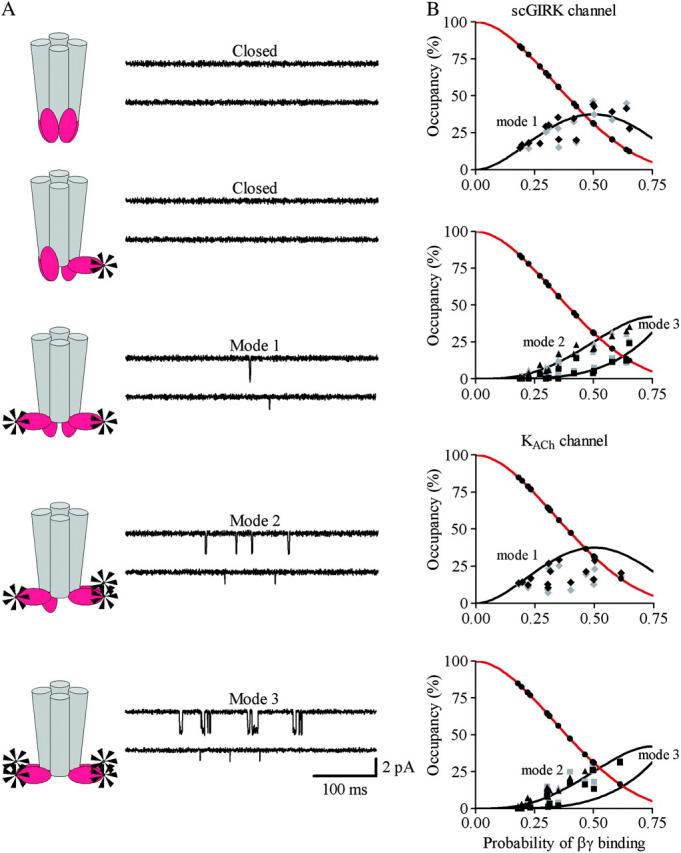
Gating equilibrium among three functional modes of GIRK channels. (A) A model assuming three functional modes of GIRK channels rendered active by the independent binding of two, three, and four Gβγ subunits to four equivalent Gβγ sensors in the channel structure. (B) For each channel, the relative occupancy of different gating modes was estimated from the fraction of the po (gray symbols) and f (black symbols) histograms fitted by the corresponding gamma components. In each experiment, the probability of Gβ1γ7-binding to one of the Gβγ sensors, P, was computed from the relative occupancy of mode 0, F0, according to the equation: F0 = 4P(1 − P)3 + (1 − P)4 (red line). The solid lines represent the predicted occupancy of the individual gating modes. The symbols represent the occupancy of mode 1 (diamonds), mode 2 (triangles), and mode 3 (squares) determined from the individual po and f distributions for different scGIRK (n = 14) and KACh (n = 14) channels.
Further, we sought to determine whether selecting a different gating mode for computation of the P values in the alternative model of GIRK channel gating shown in Fig. 7 A would improve the correlation between the theoretical distributions and the experimental Fk values. We therefore computed the P values from the occupancy of mode 1, F1, according to the equation F1 = 6P2(1 − P)2, and examined the relative occupancy of modes 0, 2, and 3 as a function of the parameter P. Our analysis indicated that the selection of a different mode for calculating the probability of Gβγ binding, P, did not improve the compatibility of our data with this model (data not shown). Nevertheless, the existence of nonconducting conformations of the singly occupied GIRK channels might be a viable option in the interpretation of channel behavior in the presence of different signaling partners of the GIRK channels and will be further tested.
Thus, the overall analysis of scGIRK channel activation by purified recombinant Gβ1γ7 supports the concept of channel gating in four conformations rendered active by the binding of an increasing number of Gβγ subunits to four seemingly identical and independent G-protein sensors on this channel. At the same time, we cannot rule out the possibility that the biochemical mechanisms underlying this heterogeneous GIRK channel behavior are much more complex, yet their consequences could be adequately explained by the simple idea entertained in this work.
Affinity of scGIRK and KACh channels for Gβ1γ7 binding
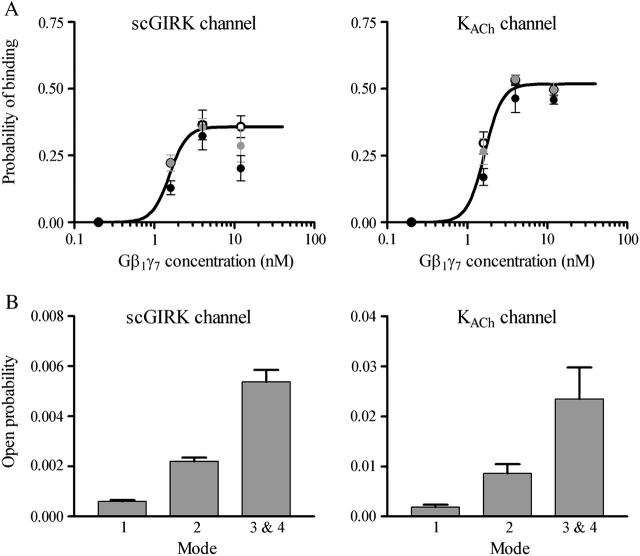
Membrane colocalization of two GIRK channels with different abilities to generate hyperpolarization responses would provide the atrial myocyte with a powerful means to control the membrane sensitivity to m2-muscarinic receptor stimulation by adjusting the affinity of these channels to Gβγ. To compare the Gβγ dissociation constants (Kd) of scGIRK and KACh channels in the atrial membrane, we analyzed the concentration dependence of the probability, P, of Gβγ binding to one of the putative Gβγ sensors. In each experiment we estimated parameter P at three standard time points—10, 20, and 30 min after the Gβ1γ7 perfusion. The mean value of the three P estimates was used to generate a dose-response curve for scGIRK and KACh channels, respectively (Fig. 8 A).
FIGURE 8.
Comparison of the Gβ1γ7 affinity and electrogenic efficacy of the scGIRK and KACh channels. (A) Gβ1γ7 binding to scGIRK and KACh channels. The probability of Gβ1γ7 binding, P, is plotted against Gβ1γ7 concentration for the scGIRK (left panel) and KACh (right panel) channels. The parameter P was estimated at three standard time points of each experiment: 10 (black circle), 20 (gray circle) and 30 (empty circle) min after Gβ1γ7 perfusion. The points and error bars represent the mean ± SEM of four to six separate experiments. The solid lines through the data represent the least-squares fit with the Hill equation (Eq. 3), with a Hill coefficient, N, of 4 for both channels. The Pmax constant is 0.52 for the KACh channel and 0.36 for the scGIRK channel, whereas the dissociation constant Kd is 1.65 nM for the KACh channel and 1.55 nM for the scGIRK channel. (B) Open probability of different gating modes. The mean open probabilities determined from the analysis of scGIRK po histograms (left panel) are po1 = 0.00060 ± 0.00006, po2 = 0.0022 ± 0.0002, and po3&4 = 0.0054 ± 0.0005. The values for the KACh channel functional modes (right panel) are po1 = 0.0019 ± 0.0005, po2 = 0.0086 ± 0.0018, and po3&4 = 0.0234 ± 0.0063. Data are mean ± SEM (n = 10–14).
The probability of Gβγ binding, P, is a sigmoidal function of Gβγ concentration, and a fit with the Hill equation
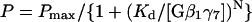 |
(3) |
yields a Hill coefficient, N, of 4 for both channels. The dissociation constant, Kd, was 1.55 nM for the scGIRK and 1.65 nM for the KACh channels. The latter value is similar to the Kd value reported earlier for native KACh channels and the same Gβγ combination (Wickman et al., 1994). Although the scGIRK and KACh channels had virtually equal Gβγ affinity, the mean open probability of each gating mode was ∼3.8-fold smaller for the scGIRKs as compared to the KACh channels (Fig. 8 B). In addition, the maximum probability of Gβγ binding, Pmax, declined from 0.52 for the KACh channels to 0.36 for the scGIRKs, thus defining different gating boundaries for the two channels and further limiting the efficacy of the scGIRK channels. Altogether, the analysis of atrial scGIRK channel, in the presence of Gβγ alone, provided a picture of an ion channel with a significant Gβγ-binding capacity but with very limited electrogenic efficacy.
Relationship between KACh and scGIRK channels in the membrane of atrial myocytes
The presence of KACh and scGIRK channels with identical Gβγ affinities in the same myocytes raises the possibility that these channels might compete for binding to the free Gβγ available in the membrane. To test this possibility we investigated the relationship between scGIRK and KACh channels found in the same membrane patch. Examination of 14 GIRK channel pairs demonstrated that the scGIRK and KACh channels behaved as individual entities and the gating equilibrium of each channel was not affected by the presence of the other channel in the same patch.
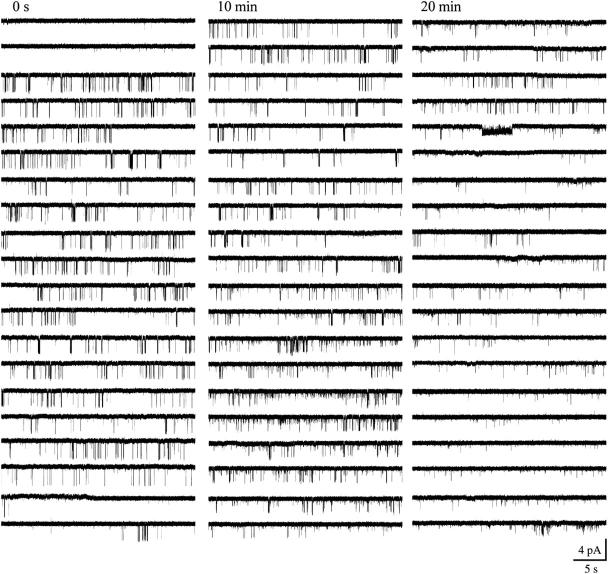
On the other hand, the inspection of long episodes of single-channel data revealed the presence of slow modal transitions (Yakubovich et al., 2000) in the gating of scGIRK and KACh channels, and these slow modal transitions were frequently linked for the two channels (Figs. 9; 10, A and B; and see Fig. 12). For 13 out of the 14 individual scGIRK channels monitored at a constant Gβ1γ7 concentration for at least 20 min each, an initial period of infrequent but stable scGIRK gating was followed by a period of more appreciable channel activity (Fig. 10 A). Such transitions in scGIRK channel gating from low to high levels of activity were frequently coupled to slow modal transitions in KACh channel gating. Intriguingly, the KACh channels were able to undergo transitions in both directions: from low to high (Fig. 9) and from high to low level of activity (Fig. 12). In the set of 14 patches, seven displayed a synchronized increase in the activity of both channels (Fig. 10, A and B), whereas five patches displayed a reduction of KACh channel activity after the upregulation of scGIRK channels. In the remaining two patches the activity of the KACh channels was stable for the duration of the experiments.
FIGURE 9.
Synchronized slow modal transitions in GIRK channel gating. Single-channel recordings from a pair of scGIRK and KACh channel activated by 4 nM Gβ1γ7. After an initial period (∼7 min) of steady gating, the activity of both channels increased simultaneously.
FIGURE 12.
Opposing regulation of scGIRK and KACh channels. Single-channel activity recorded in the presence of 12 nM Gβ1γ7 from a membrane patch harboring a scGIRK and a KACh channel. In this experiment, an initial phase of efficient KACh channel gating and infrequent scGIRK openings was replaced with a second phase, characterized by increased scGIRK activity and infrequent KACh channel gating.
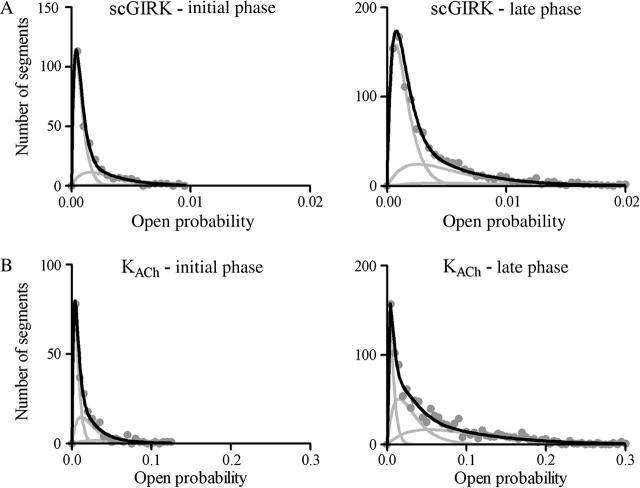
The slow modal transitions in the gating of GIRK channels led to changes in the equilibrium between the four functional modes of these channels. Fig. 11 provides a direct comparison of the po histograms generated from the different phases of the experiment illustrated in Fig. 9, whereas the po histograms generated from the different phases of the experiment illustrated in Fig. 12 are shown in Fig. 13. The analysis of these po distributions revealed significant changes in the equilibrium probability of different functional modes, consistent with changes in the probability of Gβγ binding to the protein complexes. In addition, in some of our experiments, the slow modal transitions in GIRK channel gating affected the open probability of the individual gating modes (Figs. 11 and 13).
FIGURE 11.
The slow modal transitions in scGIRK and KACh channel gating are associated with changes in the equilibrium among the four functional modes of these channels. (A) Open probability histograms generated from the initial (left panel) and late (right panel) phases of scGIRK activity shown in Fig. 9. The mean po values and the relative areas of different gamma components (shown in parentheses) are po1 = 0.00037 (65.5%), po2 = 0.0015 (28.7%), and po3 = 0.0032 (5.8%) for the initial phase; and po1 = 0.00072 (58.7%), po2 = 0.0026 (32.8%), and po3 = 0.0045 (8.5%) for the late phase of channel activation. The P values computed from these histograms are Pinitial = 0.225, and Plate = 0.270. In this experiment, the changes in the kinetic behavior of the scGIRK were associated with both a slight shift in modal equilibrium and a significant increase in the open probability of different functional modes. (B) Open probability histograms generated from the initial (left panel) and late (right panel) phases of KACh channel activity shown in Fig. 9. The mean po values and the relative areas of different components are po1 = 0.0033 (50.0%), po2 = 0.012 (37.5%), and po3 = 0.031 (12.5%) for the initial phase; and po1 = 0.0033 (20.3%), po2 = 0.015 (37.4%), and po3 = 0.053 (42.3%) for the late phase of channel activation. The P values computed from these histograms are Pinitial = 0.328 and Plate = 0.555 for the KACh channel. Thus, the changes in the gating behavior for this KACh channel were mostly associated with a shift in the modal equilibrium toward gating modes 3 and 4.
FIGURE 13.
Modal distributions of scGIRK and KACh channels associated with the opposing regulation of these channels. Open probability histograms generated from the homogeneous phases of scGIRK (top panels) and KACh (bottom panels) channel gating shown in Fig. 12. For the scGIRK channel the mean po values and relative areas (given in parentheses) of different components are po1 = 0.00060 (87.2%) and po2 = 0.0030 (12.8%) for the initial phase; and po1 = 0.00061 (34.3%), po2 = 0.0026 (40.3%), and po3 = 0.0072 (25.4%) for the late phase of channel activation. For the KACh channel the mean po values and the relative areas of different components are po1 = 0.0073 (26.3%), po2 = 0.0268 (40.0%), and po3 = 0.0780 (33.7%) for the initial phase; and po1 = 0.00068 (79.4%), po2 = 0.0032 (18.7%), and po3 = 0.0080 (1.9%) for the late phase of channel activation. The P values, determined from these distributions, are Pinitial = 0.084 and Plate = 0.440 for the scGIRK channel; and Pinitial = 0.503, and Plate = 0.130 for the KACh channel. The KACh channel gating during the late phase is impaired not only by its confinement within the low-efficient modes 1 and 2, but also by the significant reduction in the open probability of individual kinetic components contributing to channel activity.
Two basic conclusions can be drawn from these observations. First, the Gβγ sensitivity of both channels appears to be a rather dynamic parameter controlled by yet unrecognized membrane components. As a result, the amplitude of hyperpolarization response of atrial myocytes to uniform receptor stimulation would greatly vary, depending not only on the relative density of the KACh and scGIRK channels in the membrane, but also on their relative Gβγ affinity at any particular moment. Second, there are at least two different membrane-delimited factors (or combinations of factors) that might modify the sensitivity of GIRK channels to Gβγ stimulation. In our experiments, one of these factors was apparently capable of amplifying the activity of both channels, whereas the other factor upregulated the scGIRK channels while inhibiting the KACh channel activity. Further studies are needed to identify these factors and to delineate their precise mechanism of action.
DISCUSSION
In this work we characterized the function of a previously unidentified Gβγ-sensitive inwardly rectifying channel in the heart, which we designated the scGIRK channel. These channels are expressed in the membrane of atrial myocytes and are frequently localized in close proximity to the classical KACh channels. The scGIRK channels are characterized by a unitary conductance of ∼16 pS (with symmetrical 150-mM KCl solutions), very low open probability, and brief open states defined by two time constants, τo1 and τo2, of 0.31 ms and 1.3 ms, respectively. Apparently, the small conductance combined with the low open probability would prevent the scGIRK channels from generating significant levels of hyperpolarization current in the atrial membrane.
Common gating mechanism for atrial GIRK channels
Upon Gβγ activation the scGIRK and KACh channels exhibit a complex behavior, fluctuating between different patterns of gating. This behavior is dependent on the Gβγ concentration. At Gβ1γ7 concentrations <1 nM, GIRK channel gating is characterized by infrequent, mostly singular openings. However, the channel activity begins to oscillate between singular openings and heterogeneous bursts of openings as the Gβγ concentration increases. Such heterogeneity of channel gating is a major obstacle in the interpretation of the single-channel recordings from both scGIRK and KACh channels. To quantify the complex gating of Gβγ-activated KACh channels we previously developed a simplistic approach for classification of channel gating in functionally distinct modes (Ivanova-Nikolova et al., 1998). The recordings were divided into consecutive, equally spaced time intervals and the channel frequency of openings and the channel probability of being open were assessed within these intervals. The f and po values obtained from this analysis were then compiled to generate f and po histograms, and these histograms were used to evaluate the KACh channel-Gβγ interactions. In this study, the same approach was applied to classify the gating of scGIRK channels into individual functional modes. The results of our experiments indicated that although a single kinetic component was sufficient to approximate the f and po histograms at low Gβ1γ7 concentrations, a sum of up to four components was required for the adequate fit of the scGIRK channel data generated at saturating Gβ1γ7 concentrations. Thus, our analysis suggested the presence of four distinct gating modes not only for the KACh channels but for the scGIRKs as well.
In addition, the concentration dependence of the gating equilibrium is characteristic of an activation process driven by Gβγ concentration. Previously, for the KACh channels, we suggested that the multiple Gβγ binding sites identified in GIRK1 and GIRK4 subunits are arranged in a precise pattern to form four equivalent and independent Gβγ sensors in the channel structure (Ivanova-Nikolova et al., 1998). Additionally, we suggested that binding of one to four Gβγ subunits to these sensors gives rise to four distinct KACh channel conformations underlying the four gating modes. Several studies from different laboratories provided further support for the basic premises of this concept. The recent crystal structure of GIRK1 tetrameric cytoplasmic pore revealed the presence of four identical putative Gβγ sensors protruding from the pore (Nishida and MacKinnon, 2002). Similar structural organization is most probably conserved throughout the entire GIRK channel family. In addition, the existence of equilibrium between single-, double-, triple-, and quadruple-Gβγ occupied GIRK4 homotetramers was directly validated through biochemical experiments (Corey and Clapham, 2001). Furthermore, mutagenesis analysis of neuronal GIRK2 channels identified several mutations within GIRK2 transmembrane domains, which not only rendered the channels constitutively active but also confined their gating to individual gating modes (Yi et al., 2001). This last observation supports the notion that each gating mode represents a distinct configuration of the KACh channel protein.
In this study, we extended our concept of KACh channel gating to rationalize the modal behavior of the scGIRK channels. As long as the four gating modes can be viewed as manifestation of single-, double-, triple- and quadruple-Gβγ occupied scGIRK channels, the probability of observing each gating mode should be a function only of the probability of Gβγ binding to one of the four Gβγ sensors, P. To test this prediction we examined the gating equilibrium of scGIRK channels activated at different Gβγ concentrations. The parameter P was computed from the relative occupancy of mode 1, and the relative occupancy of remaining modes was examined as a function of P (Fig. 6). This examination demonstrated that the probability function of each of the remaining gating modes strictly followed the predicted binomial distribution (Eq. 1). Moreover, the selection of a different gating mode for the computation of parameter P did not influence the P values derived from the different f and po histograms, thus further confirming the excellent compatibility between the data and the model.
Analysis of the concentration dependence of gating equilibrium also demonstrated that the probability of one of the four Gβγ sensors being occupied, P, is a sigmoidal function of Gβγ concentration for both scGIRK and KACh channels. Remarkably, the two channels have identical Gβ1γ7 affinity (Kd of ∼1.6 nM). This observation implies that not only the four Gβγ sensors of each scGIRK and KACh channel behave as equivalent and independent modules, but also that these sensors are structurally similar in scGIRK and KACh channels. However, the molecular identity of the Gβγ sensors (for reviews, see Yamada et al., 1998, and Sadja et al., 2003), and the conformational changes elicited in them upon Gβγ binding remain to be defined.
Although the scGIRK and KACh channels exhibited identical Gβ1γ7 affinities (Fig. 8 A), the electrogenic efficacy of the two channels was markedly different (Fig. 8 B). Thus, the same mechanism of Gβγ control of multiple functional states of single effector molecule is used to achieve a robust hyperpolarization response in the case of the KACh channels, and to generate a mute response in the case of the scGIRK channels.
Altogether, classification of scGIRK and KACh channel gating into four functional modes and subsequent analysis of equilibrium between the different modes greatly simplified the kinetic analysis of the heterogeneous single-channel data. This method was also sensitive enough to capture slight changes in Gβγ regulation of the two channels. However, as we previously reported (Ivanova-Nikolova et al., 1998), this method did not accurately predict the amount of blank data segments in the single-channel recordings throughout the entire Gβγ range. In the majority of our experiments, the fraction of blank data segments considerably exceeded the probability of observing gating mode 0 (no Gβγ bound to the channel), calculated from the binomial distribution (Eq. 1) for both channels. This observation indicates the presence of Gβγ-dependent desensitization in addition to the Gβγ-dependent activation of GIRK channels. The mechanism of the desensitization process, however, was not investigated in this work.
Although no attempt was made here to determine the molecular composition of the scGIRK channels, several lines of evidence suggest that these channels might be functional GIRK4 homotetramers. The existence of GIRK4 homomultimers along with the GIRK1/GIRK4 heterotetramers has been demonstrated in the atria (Corey and Clapham, 1998). In addition, heterologous expression of GIRK4 homomultimers results in the formation of G-protein-regulated inwardly rectifying K+ channels (Krapivinsky et al., 1995a,b). In these studies, however, the fast gating of the recombinant GIRK4 channels impeded their analysis and prevented us from directly comparing the properties of GIRK4 homotetramers with those of atrial scGIRK channels. More recently, the properties of GIRK4 homomultimers were examined in Xenopus oocytes (He et al., 2002), and the analysis of GIRK4 channel openings resolved two components with time constants, τo1 and τo2, of 0.26 ms and 0.89 ms, respectively. These estimates are very similar to the open-time constants for the scGIRK channels reported here. Finally, ion channels with biophysical properties identical to those of the scGIRK channels were observed in the atrial myocytes of GIRK1 knockout mouse after m2-receptor activation (Bettahi et al., 2002). There are some differences, however, in the G-protein regulation of the atrial scGIRK channels and the residual GIRK4 homomultimers in the GIRK1 knockout mouse model. In the latter case, channel activity quickly ran down in cell-attached configuration after m2-receptor activation and could not be reliably restored in inside-out patches by addition of GTP and ATP. These differences suggest that the presence of heterotetrameric KACh channels in the atrial membrane might be important for the function of GIRK4 homomultimers.
Despite these functional similarities between the scGIRK channels and the GIRK4 homomultimers, we cannot completely rule out the possibility that the 16-pS openings might result from transitions to a subconductance state of the GIRK1/GIRK4 heterotetrameric channel that is accessed from the closed state only. An alternative explanation might be that the scGIRK channels represent a yet unidentified covalent modification of the KACh channels. In any case, the presence of the scGIRK channels in the atrial membrane could have a major effect on the cellular responses to m2-receptor stimulation.
Physiological role of scGIRK channels in the atrial membrane
The limited ability of the scGIRK channels to generate membrane current does not suggest a substantial role for these channels in the hyperpolarization of atrial myocytes. The examination of interactions between the scGIRK and canonical KACh channels, however, indicates that scGIRKs might create a gradient of hyperpolarization responses across atrial membrane. In fact, recent examination of acetylcholine-sensitive K+ currents in myocytes from adult mouse atrium revealed a significant gradient of IKACh current density across the supraventricular structures of the mouse heart (Lomax et al., 2003). The molecular mechanisms underlying this phenomenon are currently unknown but most likely involve different KACh channel densities in different supraventricular structures. At the same time, the interactions between scGIRK and KACh channels in the atrial membrane could be responsible for dynamic changes in the gradient of IKACh current density across the atria, and thus might have significant clinical repercussions. These interactions would be determined by the proximity of the two channels and their ability to compete for binding to the pool of Gβγ subunits released upon receptor activation. The analysis of scGIRK and KACh channel activation revealed similar Gβ1γ7 affinities for the two channels which supports the notion that the two channels might indeed compete for Gβγ binding. Furthermore, it is well established that KACh channel activity is modulated by a large variety of intracellular factors and processes. These include phosphatidylinositol-4,5-bisphosphate, Na+, Mg2+, oxidation-reduction, phosphorylation-dephosphorylation, and protonation (reviewed in Dascal, 1997, and Sadja et al., 2003). Our limited experiments with PKAc suggest that some of the same intracellular factors and processes might be involved in the regulation of the scGIRK channels too. Future studies will test this possibility.
Acknowledgments
This work was made possible by the generosity of Dr. Janet D. Robishaw (Weis Center for Research, Geisinger Medical Center, Danville, PA), in whose lab the purification of Gβγ was carried out. We thank Dr. D. Logothetis and his colleagues for kindly providing the numerical τo1 and τo2 values for the GIRK4 homomeric channels. We are extremely grateful to the two reviewers for their insightful comments, which led to a significant improvement of this manuscript.
This work was supported by a faculty development grant from Brody School of Medicine, East Carolina University.
References
- Bettahi, I., C. L. Marker, M. I. Roman, and K. Wickman. 2002. Contribution of the Kir3.1 subunit to the muscarinic-gated atrial potassium channel IKACh. J. Biol. Chem. 277:48282–48288. [DOI] [PubMed] [Google Scholar]
- Colquhoun, D., and A. G. Hawkes. 1981. On the stochastic properties of single ion channels. Proc. R. Soc. B. 211:205–235. [DOI] [PubMed] [Google Scholar]
- Colquhoun, D., and A. G. Hawkes. 1983. The principles of the stochastic interpretation of ion-channel mechanisms. In Single Channel Recording. B. Sakmann, and E. Neher, editors. Plenum Press, New York. 135–175.
- Colquhoun, D., and B. Sakmann. 1985. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J. Physiol. 369:501–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey, S., and D. E. Clapham. 1998. Identification of native atrial G-protein-regulated inwardly rectifying K+ (GIRK4) channel homomultimers. J. Biol. Chem. 273:27499–27504. [DOI] [PubMed] [Google Scholar]
- Corey, S., and D. E. Clapham. 2001. The stoichiometry of Gβγ binding to G-protein-regulated inwardly rectifying K+ channels (GIRKs). J. Biol. Chem. 276:11409–11413. [DOI] [PubMed] [Google Scholar]
- Dascal, N. 1997. Signalling via the G protein-activated K+ channels. Cell. Signal. 9:551–573. [DOI] [PubMed] [Google Scholar]
- Dascal, N., W. Schreibmayer, N. F. Lim, W. Wang, C. Chavkin, L. Di-Magno, C. Labarca, B. L. Kieffer, C. Gaveriaux-Ruff, D. Trollinger, H. A. Lester, and N. Davidson. 1993. Atrial G protein-activated K+ channel: expression cloning and molecular properties. Proc. Natl. Acad. Sci. USA. 90:10235–10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupnik, C. A., N. Davidson, H. A. Lester, and P. Kofuji. 1997. RGS proteins reconstitute the rapid gating kinetics of Gβγ-activated inwardly rectifying K+ channels. Proc. Natl. Acad. Sci. USA. 94:10461–10466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle, D. A., J. H. Morais Cabral, R. A. Pfuetzner, A. Kuo, J. M. Gulbis, S. L. Cohen, B. T. Chait, and R. MacKinnon. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 280:69–77. [DOI] [PubMed] [Google Scholar]
- Foster, K. A., P. J. McDermott, and J. D. Robishaw. 1990. Expression of G proteins in rat cardiac myocytes: Effect of KCl depolarization. Am. J. Physiol. 28:H432–H441. [DOI] [PubMed] [Google Scholar]
- Hamill, O. P., A. Marty, E. Neher, B. Sakmann, and F. J. Sigworth. 1981. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 391:85–100. [DOI] [PubMed] [Google Scholar]
- He, C., X. Yan, H. Zhang, T. Mirshahi, T. Jin, A. Huang, and D. E. Logothetis. 2002. Identification of critical residues controlling G protein-gated inwardly rectifying K+ channel activity through interactions with the βγ subunits of G proteins. J. Biol. Chem. 277:6088–6096. [DOI] [PubMed] [Google Scholar]
- Ito, H., T. Sugimoto, I. Kobayashi, K. Takahashi, T. Katada, M. Ui, and Y. Kurachi. 1991. On the mechanism of basal and agonist-induced activation of the G protein-gated muscarinic K+ channel in atrial myocytes of guinea pig heart. J. Gen. Physiol. 98:517–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova-Nikolova, T. T., E. N. Nikolov, C. Hansen, and J. D. Robishaw. 1998. Muscarinic K+ channel in the heart: Modal regulation by G protein βγ subunits. J. Gen. Physiol. 112:199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y., A. Lee, J. Chen, M. Cadene, B. T. Chait, and R. MacKinnon. 2002. The open pore conformation of potassium channels. Nature. 417:523–526. [DOI] [PubMed] [Google Scholar]
- Jin, T., L. Peng, T. Mirshahi, T. Rohacs, K. W. Chan, R. Sanchez, and D. E. Logothetis. 2002. The βγ subunits of G proteins gate a K+ channel by pivoted bending of a transmembrane segment. 2002. Mol. Cell. 10:469–481. [DOI] [PubMed] [Google Scholar]
- Kozasa, T., and A. G. Gilman. 1995. Purification of recombinant G proteins from Sf9 cells by hexahistidine tagging of associated subunits. J. Biol. Chem. 270:1734–1741. [DOI] [PubMed] [Google Scholar]
- Kim, D. 1990. β-Adrenergic regulation of the muscarinic-gated K+ channel via cyclic AMP-dependent protein kinase in atrial cells. Circ. Res. 67:1292–1298. [DOI] [PubMed] [Google Scholar]
- Krapivinsky, G., E. A. Gordon, K. Wickman, B. Velimirovic, L. Krapivinsky, and D. E. Clapham. 1995a. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K+ channel proteins. Nature. 374:135–141. [DOI] [PubMed] [Google Scholar]
- Krapivinsky, G., L. Krapivinsky, B. Velimirovic, K. Wickman, B. Navarro, and D. E. Clapham. 1995b. The cardiac inward rectifier K+ channel subunit, CIR, does not comprise the ATP-sensitive K+ channel, IKATP. J. Biol. Chem. 270:28777–28779. [DOI] [PubMed] [Google Scholar]
- Kubo, Y., E. Reuveny, P. A. Slesinger, Y. N. Jan, and L. Y. Jan. 1993. Primary structure and functional expression of a rat G-protein-coupled muscarinic potassium channel. Nature. 364:802–806. [DOI] [PubMed] [Google Scholar]
- Leaney, J. L., G. Milligan, and A. Tinker. 2000. The G protein α subunit has a key role in determining the specificity of coupling to, but not the activation of, G protein-gated inwardly rectifying K+ channels. J. Biol. Chem. 275:921–929. [DOI] [PubMed] [Google Scholar]
- Lomax, A. E., R. A. Rose, and W. R. Giles. 2003. Electrophysiological evidence for a gradient of G protein-gated K+ current in adult mouse atria. Br. J. Pharmacol. 140:576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullner, C., D. Yakubovich, C. W. Dessauer, D. Platzer, and W. Schreibmayer. 2003. Single channel analysis of the regulation of GIRK1/GIRK4 channels by protein phosphorylation. Biophys. J. 84:1399–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolov, E. N., and T. I. Ivanova-Nikolova. 2004. Coordination of membrane excitability through a GIRK1 signaling complex in the atria. J. Biol. Chem. 279:23630–23636. [DOI] [PubMed] [Google Scholar]
- Nishida, M., and R. MacKinnon. 2002. Structural basis of inward rectification: Cytoplasmic pore of the G protein-gated inward rectifier GIRK1 at 1.8 A resolution. Cell. 111:957–965. [DOI] [PubMed] [Google Scholar]
- Peleg, S., D. Varon, T. Ivanina, C. W. Dessauer, and N. Dascal. 2002. GαI controls the gating of the G protein-activated K+ channel, GIRK. Neuron. 33:87–99. [DOI] [PubMed] [Google Scholar]
- Sadja, R., N. Alagem, and E. Reuveny. 2003. Gating of GIRK channels: Details of an intricate, membrane-delimited signaling complex. Neuron. 39:9–12. [DOI] [PubMed] [Google Scholar]
- Saitoh, O., Y. Kubo, Y. Miyatani, T. Asano, and H. Nakata. 1997. RGS8 accelerates G-protein-mediated modulation of K+ currents. Nature. 390:525–529. [DOI] [PubMed] [Google Scholar]
- Silverman, S. K., H. A. Lester, and D. A. Dougherty. 1996. Subunit stoichiometry of a heteromultimeric G protein-coupled inward-rectifier K+ channel. J. Biol. Chem. 266:19528–19535. [DOI] [PubMed] [Google Scholar]
- Sui, J. L., K. W. Chan, and D. E. Logothetis. 1996. Na+ activation of the muscarinic K+ channel by a G-protein-independent mechanism. J. Gen. Physiol. 108:381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker, S. J., M. Pessia, and J. P. Adelman. 1996. Muscarine-gated K+ channel: subunit stoichiometry and structural domains essential for G protein stimulation. Am. J. Physiol. 271:H379–H385. [DOI] [PubMed] [Google Scholar]
- Wickman, K. D., J. A. Inigues-Lluhi, P. A. Davenport, R. Taussing, G. B. Krapivinsky, M. E. Linder, A. G. Gilman, and D. E. Clapham. 1994. Recombinant G-protein βγ subunits activate the muscarinic-gated atrial potassium channel. Nature. 368:255–257. [DOI] [PubMed] [Google Scholar]
- Wickman, K., J. Nemec, S. J. Gendler, and D. E. Clapham. 1998. Abnormal heart rate regulation in GIRK4 knockout mice. Neuron. 20:103–114. [DOI] [PubMed] [Google Scholar]
- Yakubovich, D., V. Pastushenko, A. Bitler, C. W. Dessauer, and N. Dascal. 2000. Slow modal gating of single G-protein-activated K+ channels expressed in Xenopus oocytes. J. Physiol. 524:737–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, M. 2002. The role of muscarinic K+ channels in the negative chronotropic effect of a muscarinic agonist. J. Pharmacol. Exp. Ther. 300:681–687. [DOI] [PubMed] [Google Scholar]
- Yamada, M., A. Inanobe, and Y. Kurachi. 1998. G protein regulation of potassium ion channels. Pharmacol. Rev. 50:723–757. [PubMed] [Google Scholar]
- Yi, B. A., Y. F. Lin, Y. N. Jan, and L. Y. Jan. 2001. Yeast screen for constitutively active mutant G protein-activated potassium channels. Neuron. 29:657–667. [DOI] [PubMed] [Google Scholar]