Abstract
The three important characteristics of the output signal of mitogen activated protein kinase (MAPK) cascade are time delay between stimulus and response, amplitude gain, and duration of the output signal. In this study, we performed a sensitivity analysis on the computational model of epidermal growth factor receptor (EGFR) activated MAPK cascade developed by Schoeberl and co-workers (1) to identify the sensitive steps of the pathway affecting these characteristics. We show that the signaling network is sensitive in a decoupled manner, which provides the ability to control its output amplitude and duration one at a time. Signal duration is found sensitive only to the phosphatase reactions at the MEK level. In contrast, signal amplitude is found most sensitive to the phosphatase reactions at the ERK level. Time delay is found to be a robust characteristic of the system.
Signaling pathways sense, process, and transmit the extracellular information inside the cell. Signaling initiated by the phosphorylation of epidermal growth factor (EGF) receptor (EGFR) leading to the activation of ERK via the mitogen activated protein kinase (MAPK) cascade has been of interest because mutations or aberrations in these pathways have been identified as one of the major factors involved in human cancer. Like most biological systems, signaling pathways typically have a complex topology and many regulatory interactions, and together the topology and regulation lead to the functional operation of the pathway. These complex interconnections result in manifestations of systems-level properties such as robustness, sensitivity, and flexibility. Computational methods are needed to elucidate these complex systems-level characteristics of the signaling pathways (2).
The three-tier MAPK cascade (Raf → MEK → ERK) is a highly conserved module in signaling pathways. Recently, multi-level signaling cascades have been shown to play a key role in the attenuation of input signal noise (3), and in the amplification of signal transduction specificity (4). The importance of the cascade structure in MAPK has also been associated with the amplification and switch-like behavior of signal transduction pathways (5–7). Herein, we investigated the characteristics of the MAPK cascade that help it in controlling its output.
In this study, we used a comprehensive computational model of the EGF signaling pathway activating MAPK developed by Schoeberl et al. (1). Their model consists of 103 species and 83 biochemical parameters. The large dimension and multi-timescale nature of the system make it difficult to gain an understanding of the signaling pathway without a systems level perspective. Herein, we have used sensitivity analysis to understand the systems-level characteristics of the MAPK cascade activated by EGFR.
All of the 83 parameters were changed one at a time to ±20% of their nominal values. We studied the effect of individually changing the 83 parameters on doubly phosphorylated ERK (ERKPP). The total area under the ERKPP versus time curve is used as a measure of the output signal, and this area is called the proliferation potential. The effect of varying each of the parameters on the proliferation potential was calculated (see supplementary material). The parameters that have a significant effect on the proliferation potential belong to the MAPK cascade. The proliferation potential was robust to variations in all non-MAPK parameters (±20%). The robustness of the system to parameter variations in non-MAPK parameters is provided by the timescale decoupling between the MAPK cascade and the set of pathways that lead to activation of Raf (8).
The dynamic response of the ERKPP has been hypothesized to play an important role in the further-downstream signaling process (9). Therefore, a more detailed approach for understanding the parameter sensitivity is to study the effect of parameter variations on the features of the ERKPP concentration profile. Analyzing the details of the dynamic response of the ERKPP profile is more informative than solely looking at the proliferation potential. The three key dynamic characteristics of the ERKPP dynamic response are delay time between stimulus (RasGTP) and response (ERKPP), amplitude gain from stimulus to response, and decay time or duration of the response (8). Herein, we have studied the changes in these three dynamic characteristics of the ERKPP response as a function of kinetic parameters. These characteristics can have a significant impact on physiological outcomes, providing specificity to MAPK function (9).
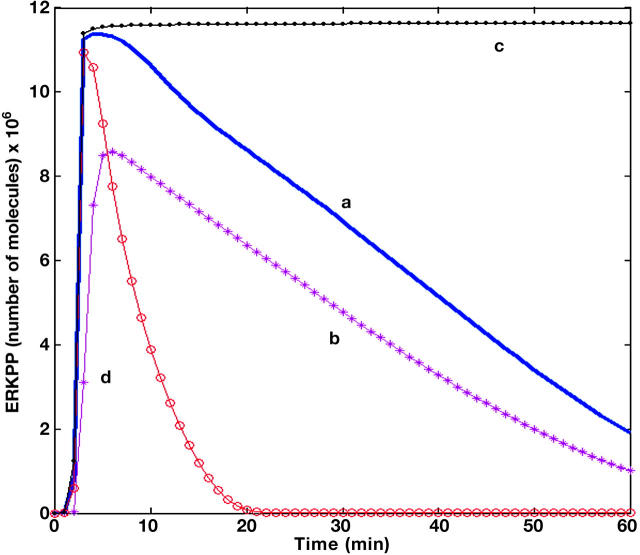
Our sensitivity analysis results indicate that the parametric variations in the system influence the decay time and the amplitude gain of the output signal. Additionally, the pathway is organized such that there is a decoupling between the kinetic parameters that influence the signal amplitude gain and the decay time (see Fig. 1). We found that the decay time was most sensitive to the phosphatase reactions at the MEK level as is shown by curves c and d in Fig. 1, which correspond to a 20% decrease and a 20% increase in the kinetic constant of MEKPP → MEKPP-phosphatase, respectively. In contrast, amplitude gain was found to be most sensitive to the phosphatase reactions at the ERK level. Amplitude gain sensitivity is shown by curve b in Fig. 1, which corresponds to a 20% increase in the kinetic constant of ERKPP → ERKPP-phosphatase. Also, the phosphatase reaction at the MEK level does not have a significant influence on the output signal amplitude gain, and same is the case with the phosphatase reaction at ERK level, which does not influence the signal decay time. The time taken to reach the peak of the output signal does not vary significantly with the kinetic parameters. Therefore, delay time is shown to be a robust property of the signaling pathway with respect to kinetic parameter variations. Furthermore, we found that output signal decay time is the most sensitive property of the MAPK cascade, which can be varied from very small values to sustained activity (Fig. 1).
FIGURE 1.
Effects of parameter changes on ERKPP profile that cause significant deviations in amplitude and duration. (a) Nominal parameters, (b) change in amplitude caused by 20% increase in phosphatase activity at the ERK level, (c) sustained ERKPP activity caused by 20% decrease in phosphatase activity at the MEK level, and (d) decrease in ERKPP signal duration caused by 20% increase in phosphatase activity at the MEK level.
In conclusion, we have identified built-in features of the MAPK cascade that provide the cascade the ability to control the signal amplitude and signal duration one at a time. The decoupled control provides more flexibility of the MAPK cascade to control its output, which is crucial as MAPKs regulate a large variety of cellular processes. Furthermore, this feature can play a useful role in designing treatments for conditions such as cancer.
SUPPLEMENTARY MATERIAL
An online supplement to this article can be found by visiting BJ Online at http://www.biophysj.org.
Supplementary Material
Acknowledgments
This work was funded by a grant from the United States Department of Energy.
Kapil Mayawala and Claudio A. Gelmi contributed equally to this work.
REFERENCES
- (1).Schoeberl, B., C. Eichler-Jonsson, E. D. Gilles, and G. Müller. 2002. Computational modeling of the dynamics of the MAP kinase cascade activated by surface and internalized EGF receptors. Nat. Biotechnol. 20:370–375. [DOI] [PubMed] [Google Scholar]
- (2).Neves, S. R., and R. Iyengar. 2002. Modeling of signaling networks. Bioessays. 24:1110–1117. [DOI] [PubMed] [Google Scholar]
- (3).Thattai, M., and A. van Oudenaarden. 2002. Attenuation of noise in ultrasensitive signaling cascades. Biophys. J. 82:2943–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Swain, P. S., and E. D. Siggia. 2002. The role of proofreading in signal transduction specificity. Biophys. J. 82:2928–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Koshland, D. E., A. Goldbeter, and J. B. Stock. 1982. Amplification and adaptation in regulatory and sensory systems. Science. 217:220–225. [DOI] [PubMed] [Google Scholar]
- (6).Huang, C. Y. F., and J. E. Ferrell. 1996. Ultrasensitivity in the mitogen-activated protein kinase cascade. Proc. Natl. Acad. Sci. USA. 93:10078–10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Ferrell, J. E. 1996. Tripping the switch fantastic: how a protein kinase cascade can convert graded inputs into switch-like outputs. Trends Biochem. Sci. 21:460–466. [DOI] [PubMed] [Google Scholar]
- (8).Mayawala, K., A. P. Singh, and J. S. Edwards. Submitted for publication.
- (9).Marshall, C. J. 1995. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-related kinase activation. Cell. 80:179–185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.