Connections between weather and disease are well established, with many diseases occurring during certain seasons or erupting from unseasonable flood or drought conditions. With new concerns about global warming, accompanied by greater climate variability, many recent studies have focused on disease fluctuations related to short-term or interannual climate oscillations (e.g., from weather extremes driven by El Niño). Yet, the nagging question remains as to whether or not there has been any documented change in human disease trends in response to long-term climate change, since warming has already occurred over the last century (1, 2).
This study likely represents the first piece of evidence that warming trends over the last century are affecting human disease.
This trend analysis has been elusive because of the scarcity or inconsistent quality of health databases over long periods. Additionally, strong confounding factors especially complicate long-term trend analysis. Some of these include increasing trends in travel, trade and migration, erratic disease control efforts, emerging drug or pesticide resistance, human population growth, urban sprawl, agricultural development, and variable reporting biases. But Rodo et al. (3) have now succeeded in finding a robust relationship between progressively stronger El Niño events and cholera prevalence in Bangladesh, spanning a 70-year period; their use of a uniquely high quality extensive cholera database and innovative statistical methods were key. This study likely represents the first piece of evidence that warming trends over the last century are affecting human disease.
The investigators used innovative statistical methods to conduct a time-series analysis of historical cholera data dating back to 1893 to examine the effect of nonstationary interannual variability possibly associated with climate change. In the last two decades, the El Niño Southern Oscillation (ENSO) differed from previous decades (3). Since the 1980's, there has been a marked intensification of the ENSO beyond that expected from the known shift in the Pacific basin temperature regime that began in the mid 1970s. The authors found that the association of cholera incidence in the earlier half of the century (1893–1940) is weak and uncorrelated with ENSO, whereas late in the century (1980–2001), the relationship is strong and consistent with ENSO. Past climate change, therefore, may have already affected cholera trends in the region through intensified ENSO events.
Recent Climate Change and Trends in El Niño
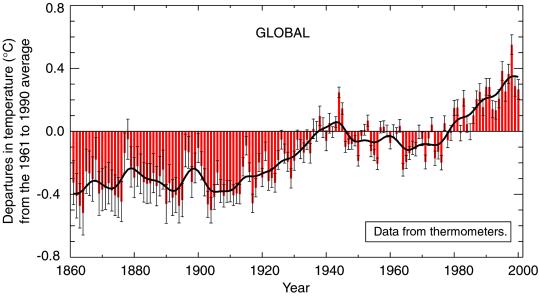
According to the United Nations Intergovernmental Panel on Climate Change (IPCC), evidence of past warming is building. Since the late 1950s, global average surface temperature has increased by 0.6°C (Fig. 1), snow cover and ice extent have diminished, and ocean heat content has increased (2). Also, sea level has risen on average by 10–20 cm during the past century. Relevant to Bangladesh, the Indian ocean has progressively warmed since 1960 (4), and the subcontinent has warmed by 2–3°C over the last century (5). Rodo et al. found an increase over time in the frequency and amplitude in the ENSO; although debate remains about the relationship between climate change and ENSO intensification, the observations of Rodo et al. are consistent with model projections of greenhouse gas warming (6). The rate of change in climate is faster now than in any period in the last thousand years (2), making the findings of Rodo et al. of progressively more intense ENSO in the region pertinent to climate scientists as well as health professionals.
Figure 1.
Average global surface temperatures (in centigrade) from 1860–2000, showing deviation from the baseline 1961–1990 average temperature. [Reproduced with permission from ref. 19 (Copyright 2001, Intergovernmental Panel on Climate Change).]
Human Disease and Short-Term Climate Variability
Seasonality in disease incidence can often infer an association with weather factors. Epidemics of meningococcal meningitis in subSaharan Africa consistently erupt during the hot dry season and subside soon after the onset of the rainy season (7). Mosquito-borne diseases, such as dengue fever, show strong seasonal patterns; transmission is highest in the months of heavy rainfall and humidity. Enteric diseases also show significant seasonal fluctuations. In the U.S., Rotavirus peaks in the winter, and, in Scotland, Campylobacter infections are characterized by short peaks in the spring (8). In Peru, Cyclospora infections peak in the summer and subside in the winter months (9). Also, in Peru, Checkley et al. (10) used harmonic regression to control for seasonality, and found childhood diarrheal disease to be significantly affected by elevated El Niño temperatures; the number of daily admissions for diarrhea increased by more than twofold in winter, compared with expected trends based on the prior 5 years. For each degree centigrade of increase in mean annual temperature, the number of admissions increased by 8%.
El Niño is a natural interannual climate phenomenon that originates in the Pacific ocean every 3–7 years and, next to the seasons, is the strongest short-term driving force of climate throughout many regions of the world. The 1997–98 El Niño event was one of the two strongest this century. The accompanying absence of the monsoon brought extremely dry conditions resulting in devastating fires and hazardous air pollution in Southeast Asia (11). At the other extreme, the same El Niño event caused severe flooding in East Africa, triggering a mosquito-borne Rift Valley fever epidemic (12). Because the mosquitoes that transmit Rift Valley fever lay their eggs at the tops of grasses, only during periods of flooding are the eggs submersed, allowing for development. The link between malaria and extreme climatic episodes also has long been the subject of study in the Indian subcontinent. Historical analyses have shown that the risk of a malaria epidemic is increased approximately fivefold during the year after an El Niño in this region (13, 14).
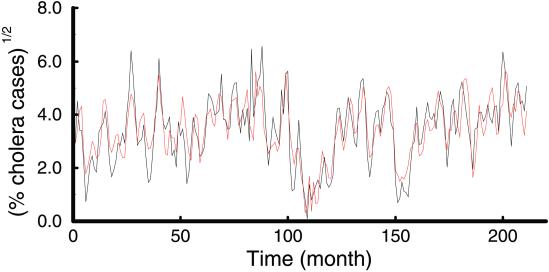
The long-term ENSO trend analysis of Rodo et al. expands upon previous work by Pascual et al. (15), who showed a strong relationship between ENSO and cholera by using 18 years of data from the ICDDR,B (International Center for Diarrhoeal Disease Research, Bangladesh; Fig. 2). Colwell (16) discovered the biological basis for a link between sea surface temperature (SST), marine ecology, and human cholera. Copepods (or zooplankton), which feed on algae, serve as reservoirs for Vibrio cholerae and other enteric pathogens. This observation may explain why cholera follows seasonal warming of SST that can enhance plankton blooms. Vibrio spp. in general are influenced by temperature and salinity (17), which, along with SST, is consistent with the role played by sea surface height (18).
Figure 2.
Model relationship between ENSO and cholera in Bangladesh. The (square-root-transformed) cholera data (black line) and the 2-months-ahead prediction of the fitted model incorporating both seasonality and ENSO at a lag f = 11 (red line). [Reproduced with permission from ref. 15 (Copyright 2000, AAAS).]
Difficulties in Determining Human Disease Indicators Linked to Long-Term Climate Trends
Many physical and biological indicators of long-term climate change effects have been documented. Some examples include the thawing of permafrost, later freezing and earlier break-up of ice on rivers and lakes, poleward and altitudinal shifts in the ranges of a variety of plants and animals, earlier flowering of trees, emergence of insects, and egg-laying of birds (19).
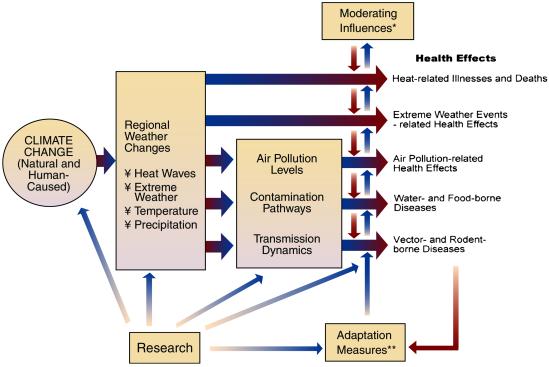
However, human health outcomes depend on many “upstream” physical and biological systems. Adding complexity to disease analyses is the potential for numerous response options by human populations to reduce risk (ref. 20; Fig. 3). Even if disease does occur, variability in detection and/or reporting remain major obstacles to determining valid trends in human disease incidence. Rodo et al. studied cholera percentages (rather than cases) to reduce varying denominators and minimize reporting bias.
Figure 3.
Potential health effects of climate variability and change. *, moderating influences include nonclimate factors that affect climate-related health outcomes, such as population growth and demographic change, standards of living, access to health care, improvements in health care, and public health infrastructure. **, adaptation measures include actions to reduce risks of adverse health outcomes, such as vaccination programs, disease surveillance, monitoring, use of protective technologies (e.g., air conditioning, pesticides, water filtration/treatment), use of climate forecasts, development of weather warning systems, emergency management and disaster preparedness programs, and public education. (Reproduced with permission from ref. 20.)
Reported linear correlations between disease and climate variability typically have been low (15). Rodo et al. posit that standard statistical techniques will fail to reveal even a strong climate/health relationship, assuming nonlinearity or a discontinuous association. The authors implemented innovative statistical techniques, such as Singular Spectrum Analysis to decompose a “noisy” time series into a nonlinear trend, oscillatory components, and remaining noise, followed by Scale-Dependent Correlation analysis, a time-series method developed to isolate possible transient signals. The association between cholera and ENSO was strong but transient, suggesting a threshold phenomenon. A linear correlation, therefore, would be entirely uninformative in this case and illustrates the inadequacies of using conventional statistical methods to decipher long-term climate/health relationships. Given such a threshold effect, higher average global temperatures and more extreme climate variability would not bode well for controlling cholera under a scenario of climate change. On the optimistic side, this new knowledge improves the potential for prediction of and early response to cholera risk in the region.
While this study by Rodo et al. quantitatively analyzed a long human health dataset for a climate signal, shorter time series studies have been conducted. In Sweden, Lindgren et al. (21) studied whether the increasing incidence of tick-borne encephalitis (TBE) could be linked to changes in climate during the period 1960–1998. One conclusion of the study was that the increased incidence of TBE can be explained by climate changing toward milder winters and early spring arrival. For example, the highest rates of TBE—a threefold increase from the annual average—were reported for Sweden in the year 1994, which was preceded by five consecutive mild winters and seven early spring arrivals. But, growing human population, changing land use patterns, and increased reporting may confound these findings (22).
In addition to confounding factors, pitfalls can arise from a mismatch in the scale of climate vs. health databases. Hay et al. (23) found no statistically significant change in climate in four villages in the East African Highlands where an increase in malaria incidence has been documented over the last century. Therefore, the authors concluded a lack of a climate effect. However, results were derived from interpolating a broad-scale gridded regional climate data set based on very sparse historical weather station data; such data are intended for upward aggregation for regional and subcontinental climate trend analysis, not for individual village sites (24). Drug resistance of the malaria parasite has received much attribution for increasing malaria in the region; however, drug resistance may disproportionately affect disease severity more than incidence which, at a minimum, requires suitable climatic conditions. In short, the question of vectorborne diseases and long-term climate change remains unresolved; there is a lack of unequivocal quantitative evidence in either direction. Kovats et al. (25) describe this as an “absence of evidence, rather than evidence of absence of a (climate) effect.”
Kovats et al. also offer criteria for assessing the evidence for a causal association between infectious diseases and observed climate change: (i) evidence for biological sensitivity to climate, requiring both field and lab research on important vectors and pathogens; (ii) meteorological evidence of climate change, requiring sufficient measurements for specific study regions; and (iii) evidence for epidemiological or entomological change with climate change, accounting for potential confounding factors.
In conclusion, the study by Rodo et al. reported in this issue of PNAS meets these rigorous criteria by applying sophisticated statistical tools to one of the longest quality disease databases available. With this landmark climate/health analysis, we are much better positioned to enter a new phase of inquiry into the links between human disease incidence and realized long-term climate change.
Acknowledgments
I thank Dr. Diarmid Campbell-Lendrum, Disease Control and Vector Biology Unit, Infectious Diseases Department, London School of Hygiene and Tropical Medicine, for comments on the manuscript drafts.
Footnotes
See companion article on page 12901.
References
- 1.Folland C K, Karl T R. In: Climate Change 2001: The Scientific Basis. Houghton J, Ding Y, Griggs M, Noguer M, van der Linden P, Dai X, editors. Cambridge, U.K.: Cambridge Univ. Press; 2001. p. 881. [Google Scholar]
- 2.Easterling D R, Horton B, Jones P D, Peterson T C, Karl T R, Parker D E, Salinger M J, Razuvayev V, Plummer N, Jamason P, Folland C K. Science. 1997;277:364–367. [Google Scholar]
- 3.Rodó X, Pascual M, Fuchs G, Faruque A S G. Proc Natl Acad Sci USA. 2002;99:12901–12906. doi: 10.1073/pnas.182203999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levitus S, Antonov J I, Boyer T P. Science. 2000;287:2225–2229. [Google Scholar]
- 5.Kumar K K, Rajagopalan B, Cane M A. Science. 1999;284:2156–2159. doi: 10.1126/science.284.5423.2156. [DOI] [PubMed] [Google Scholar]
- 6.Timmermann A. Nature (London) 1999;398:694–697. [Google Scholar]
- 7.Moore P S. Clin Infect Dis. 1992;14:515–525. doi: 10.1093/clinids/14.2.515. [DOI] [PubMed] [Google Scholar]
- 8.Colwell R R, Patz J A. Climate, Infectious Disease and Human Health: An Interdisciplinary Perspective. Washington, DC: Am. Soc. Microbiol.; 1998. [PubMed] [Google Scholar]
- 9.Madico G, McDonald J, Gilman R H, Cabrera L, Sterling C R. Clin Infect Dis. 1997;24:977–981. doi: 10.1093/clinids/24.5.977. [DOI] [PubMed] [Google Scholar]
- 10.Checkley W, Epstein L D, Gilman R H, Figueroa D, Cama R I, Patz J A, Black R E. Lancet. 2000;355:442–450. doi: 10.1016/s0140-6736(00)82010-3. [DOI] [PubMed] [Google Scholar]
- 11.Patz J A, Engelberg D, Last J. Annu Rev Public Health. 2000;21:271–307. doi: 10.1146/annurev.publhealth.21.1.271. [DOI] [PubMed] [Google Scholar]
- 12.Linthicum K J, Anyamba A, Tucker C J, Kelley P W, Myers M F, Peters C J. Science. 1999;285:397–400. doi: 10.1126/science.285.5426.397. [DOI] [PubMed] [Google Scholar]
- 13.Bouma M J, van der Kaay H J. Lancet. 1994;344:1638–1639. doi: 10.1016/s0140-6736(94)90432-4. [DOI] [PubMed] [Google Scholar]
- 14.Bouma M, van der Kaay H. Trop Med Int Health. 1996;1:86–96. doi: 10.1046/j.1365-3156.1996.d01-7.x. [DOI] [PubMed] [Google Scholar]
- 15.Pascual M, Rodo X, Ellner S P, Colwell R, Bouma M J. Science. 2000;289:1766–1769. doi: 10.1126/science.289.5485.1766. [DOI] [PubMed] [Google Scholar]
- 16.Colwell R R. Science. 1996;274:2025–2031. doi: 10.1126/science.274.5295.2025. [DOI] [PubMed] [Google Scholar]
- 17.Lipp E K, Rose J B. Rev Sci Tech. 1997;16:620–640. doi: 10.20506/rst.16.2.1048. [DOI] [PubMed] [Google Scholar]
- 18.Lobitz B, Beck L, Huq A, Wood B, Fuchs G, Faruque A S, Colwell R. Proc Natl Acad Sci USA. 2000;97:1438–1443. doi: 10.1073/pnas.97.4.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canziani O, McCarthy J, editors. Intergovernmental Panel on Climate Change. Climate Change 2001: Impacts, Adaptation and Vulnerability. Cambridge, U.K.: Cambridge Univ. Press; 2001. [Google Scholar]
- 20.Patz J A, McGeehin M A, Bernard S M, Ebi K L, Epstein P R, Grambsch A, Gubler D J, Reiter P, Romieu I, Rose J B, et al. Environ Health Perspect. 2000;108:367–376. doi: 10.1289/ehp.00108367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindgren E, Gustafson R. Lancet. 2001;358:16–18. doi: 10.1016/S0140-6736(00)05250-8. [DOI] [PubMed] [Google Scholar]
- 22.Randolph S E. Philos Trans R Soc London B. 2001;356:1045–1056. doi: 10.1098/rstb.2001.0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hay S I, Cox J, Rogers D J, Randolph S E, Stern D I, Shanks G D, Myers M F, Snow R W. Nature (London) 2002;415:905–909. doi: 10.1038/415905a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patz, J. A., Hulme, M., Rosenzweig, C., Mitchell, T. D., Goldberg, R. A., Githeko, A. K., Lele, S., McMichael, A. J. & Le Sueur, D. (2002) Nature, in press. [DOI] [PubMed]
- 25.Kovats R S, Campbell-Lendrum D H, McMichael A J, Woodward A, Cox J S. Philos Trans R Soc London B. 2001;356:1057–1068. doi: 10.1098/rstb2001.0894. [DOI] [PMC free article] [PubMed] [Google Scholar]