Abstract
Neomycin is a large, positively charged, aminoglycoside antibiotic that has previously been shown to induce a voltage-dependent substate block in the cardiac isoform of the ryanodine receptor (RyR2). It was proposed that block involved an electrostatic interaction between neomycin and putative regions of negative charge in both the cytosolic and luminal mouths of the pore. In this study, we have attempted to screen charge by increasing potassium concentration in single-channel experiments. Neomycin block is apparent at both cytosolic and luminal faces of the channel in all K+ concentrations tested and alterations in K+ concentration have no effect on the amplitudes of the neomycin-induced substates. However, the kinetics of both cytosolic and luminal block are sensitive to changes in K+ concentration. In both cases increasing the K+ concentration leads to an increase in dissociation constant (KD). Underlying these changes are marked increases in rates of dissociation (koff), with little change in rates of association (kon). The increase in koff is more marked at the luminal face of the channel. Changes in K+ concentration also result in alterations in the voltage dependence of block. We have interpreted these data as supporting the proposal that neomycin block of RyR2 involves electrostatic interactions with the polycation forming a poorly fitting “plug” in the mouths of the conduction pathway. These observations emphasize the usefulness of neomycin as a probe for regions of charge in both the cytosolic and luminal mouths of the RyR2 pore.
INTRODUCTION
The ryanodine receptor (RyR) is a ligand-gated cation channel with a high conductance for calcium and provides a pathway for the rapid translocation of large quantities of Ca2+ from the sarcoplasmic reticulum. It has been suggested that the preferential translocation of divalent cations over monovalents through RyR may be achieved via regions of negative charge in the pore region of the channel (Tinker et al., 1992a). Subsequent studies have indicated that there may be significant regions of negative charge at both the cytosolic (Mead et al., 1998) and luminal (Tu et al., 1994) mouths of the channel. We have previously reported work examining the block of K+ translocation in RyR2 by the polyamine neomycin (Mead and Williams, 2002a,b). This is of particular interest for studies of the RyR pore region, as neomycin is capable of blocking at both the cytosolic and luminal mouths of the channel, unlike other RyR pore blockers (Tinker et al., 1992b; Tinker and Williams, 1993; Mead et al., 1998). Neomycin block has been shown to be voltage-dependent and there is evidence that not only does neomycin interact with sites within the voltage drop at either channel mouth, but that it can also pass through the pore under certain conditions (Mead and Williams, 2002a). As a result, we have suggested that block was due to a pore occlusion influenced by an electrostatic interaction between the positive charge of the blocker and negative charges in the pore region. Neomycin, therefore, is potentially an invaluable probe of the RyR pore. However, to exploit this probe, it is important to further understand the mechanism of block.
Electrostatic charge interactions between blockers and cation channels have been investigated using several techniques. MacKinnon and Miller (1989) reported reduced charybdotoxin (CTX) binding affinity for Ca2+-activated K+ channels after chemical modification of the channels with trimethyloxonium, which neutralizes carboxyl groups. An alternative to chemical modification, point mutation of the channel protein, has also been effective for illustrating the importance of a charge interaction between CTX and K+ channels (Myers and Stampe, 2000). Modification of blocking peptides has allowed detailed investigation of charge-charge interactions involved in the N-type inactivation mechanism of Shaker K+ channels (Murrell-Lagnado and Aldrich, 1993a), as well as providing important information about electrostatic interactions involved in CTX block of K+ channels (Park and Miller, 1992; Goldstein and Miller, 1993). However, the simplest approach to studying the potential involvement of electrostatic mechanisms in block is by the screening of charge. This has been achieved by altering pH (Pietrobon et al., 1989), increasing permeant ion concentration (Green et al., 1987; MacKinnon and Miller, 1988; Giangiacomo et al., 1992; Murrell-Lagnado and Aldrich, 1993b) or by introducing, and altering the concentration of, an “inert” cation (Anderson et al., 1988; Lucchesi and Moczydlowski, 1991). In this study, we have adopted the charge screening approach. This has been limited to increasing the concentration of the permeant ion due to the lack of a truly inert cation in RyR. However, as will be demonstrated, this approach has successfully shown that neomycin block of RyR is largely dependent upon electrostatic interactions that result in partial pore occlusion. This study reinforces previous work suggesting the existence of negative charge at both ends of the RyR pore, provides novel information on the mechanisms involved in block, and highlights the potential of neomycin as a probe in the pore region of this channel.
MATERIALS AND METHODS
Preparation of purified cardiac ryanodine receptor channels
Purified RyR2 channels were prepared from heavy sarcoplasmic reticulum (HSR) vesicles isolated from sheep heart and solubilized using CHAPS as described previously (Sitsapesan and Williams, 1990). In brief, the left ventricle and septum of a sheep heart were homogenized and the membranes isolated by centrifugation. HSR was then extracted by discontinuous density gradient centrifugation in sucrose. HSR was solubilized using 0.4% CHAPS and RyR2 isolated using continuous density gradient centrifugation in sucrose (Lindsay and Williams, 1991). After separation of the sucrose gradients into 2-ml fractions, RyR2 was identified using [3H]-ryanodine binding, and the relevant RyR2-containing fraction was dialyzed to remove CHAPS and to reconstitute RyR2 into vesicles. These vesicles were snap-frozen in liquid nitrogen and stored at −80°C.
Planar lipid bilayer methods
Phosphatidylethanolamine bilayers (35 mg/ml) were painted across a 200-μm diameter hole in a partition separating the cis (0.5 ml) and trans (1.0 ml) chambers. The trans chamber was held at ground, and the cis chamber was held at various holding potentials relative to ground. Current flow was measured using an operational amplifier as a current-voltage converter (Miller, 1982). Bilayers were formed in symmetrical solutions of 100 mM, 210 mM, 410 mM, or 610 mM KCl with 20 mM HEPES, pH 7.4. Vesicles were added to the cis chamber and fusion with the bilayer was stimulated by the addition of a KCl osmotic gradient to the cis chamber. The cis chamber was perfused with the appropriate K+ solution after single-channel incorporation. Single channels were used in all experiments as multiple channels could not be analyzed effectively. Each channel was modified by addition of 100–200 nM ryanodine to the cis chamber, and excess ryanodine was removed by perfusion after ryanodine modification. Ryanodine modification resulted in the opening of the channel to a reduced conductance state with an open probability (Po) approaching 1.0 (Fig. 1), allowing the study of neomycin interaction with the channel without interference from normal closing events. Ryanodine modification provided the only method of raising Po consistently for the prolonged periods necessary for these experiments, especially in low salt conditions.
FIGURE 1.
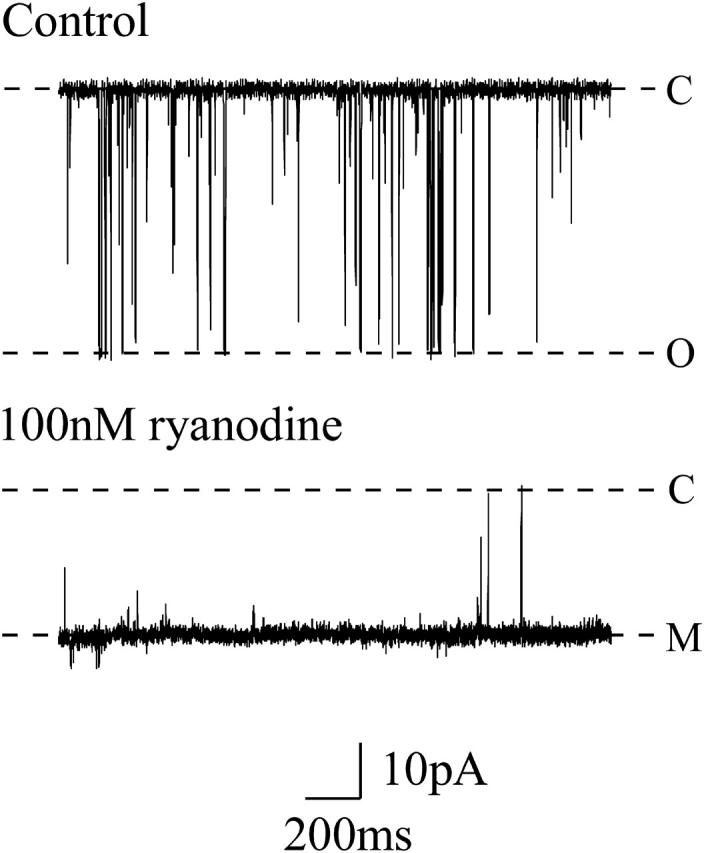
Representative channel recordings showing a single purified RyR2 channel recorded at +60 mV in 210 mM symmetrical KCl. The upper trace shows normal channel openings as downward deflections. The lower trace shows the effect of channel modification by 100 nM ryanodine. Ryanodine modification was used in all experiments reported in this study. C, closed-channel level; O, open-channel level; M, modified-open level.
Neomycin was added to the solution in either the cis or trans chambers as required. RyR2 incorporates into the bilayer in a fixed orientation in such a way that the cis chamber corresponds to the cytosolic face of the channel and the trans chamber corresponds to the luminal face (Sitsapesan and Williams, 1994a). The contaminating free-Ca2+ concentration of solutions was monitored using a calcium-sensitive electrode as described previously (Sitsapesan and Williams, 1994b) and found to be ∼10 μM. The experiments were performed at room temperature (21 ± 2°C).
Data acquisition and analysis
Single-channel current fluctuations were displayed on an oscilloscope and recorded on digital audio tape. For analysis, data were replayed, low-pass filtered with an 8-pole Bessel filter at 1 kHz and digitized at 4 kHz using an AT-based computer system (Intracel, Cambridge, U.K.).
We have used two approaches to monitor neomycin block of RyR2 in this article, with the choice of method dictated by the kinetics of the interaction. In the majority of experiments, channel block by either cytosolic or luminal neomycin is manifest as clearly resolved transitions from the ryanodine-modified open state to a subconductance state. Under these conditions we have monitored Po using 50% threshold analysis having placed cursors at these two levels, and block is expressed as 1 − Po. This approach also yields dwell times in the open and blocked states. Analysis of dwell times revealed that, in all cases examined, both open and blocked lifetimes were best described by single exponential distributions (data not shown) and as a consequence mean open and mean block times were used to ascertain, respectively, rate constants for blocker association (kon) and dissociation (koff).
As will be evident from an inspection of the data presented in the Results section of this article, the characteristics of neomycin block at both the cytosolic and luminal faces of the channel are altered when experiments are conducted in high K+ concentrations. Under these conditions individual blocking events are no longer clearly resolved and block is manifest as a noisy state of reduced conductance. Block of this form was quantified by expressing the amplitude of the reduced conductance state (γ) as a proportion of the full open conductance (γ0).
Block by both cytosolic and luminal neomycin was fully reversed on wash-out. Data are presented as mean ± SEM. Fits of the data to various schemes were obtained by linear and nonlinear regression analysis using GraphPad Prism (GraphPad Software, San Diego, CA).
Materials
Neomycin is an aminoglycosidic antibiotic comprised of a hexose ring surrounded by three amino sugars and has a net charge of +4.4 at pH 7.4 (Haws et al., 1996). The amino groups are distributed evenly across the molecule with no concentration of positive charge at any particular location (Mead and Williams, 2002a).
All solutions were prepared using deionized water. [3H]-ryanodine was obtained from Amersham Biosciences (Little Chalfont, Buckinghamshire, UK); neomycin was obtained from Sigma-Aldrich (Poole, Dorset, UK), and phosphatidylethanolamine was obtained from Avanti Polar Lipids (Alabaster, AL). All other chemicals were obtained from VWR International (Poole, Dorset, UK).
RESULTS
The aim of these studies is to test the hypothesis that electrostatic interactions between neomycin and putative regions of negative charge at the mouths of the RyR2 channel contribute to block K+ translocation. Potential charge-charge interactions can be investigated by studying the effects of altering charge on either the blocker (Park and Miller, 1992; Murrell-Lagnado and Aldrich, 1993a; Mead et al., 1998; Mullmann et al., 1999), or the channel (MacKinnon and Miller, 1989; Myers and Stampe, 2000), or by charge screening (Anderson et al., 1988; MacKinnon and Miller, 1988; Lucchesi and Moczydlowski, 1991; Giangiacomo et al., 1992, Murrell-Lagnado and Aldrich, 1993b). We have chosen to adopt the latter approach, which has been used in many previous investigations, and have monitored both cytosolic and luminal block in the presence of increasing concentrations of KCl. By changing K+ concentration in the recording solution we have observed notable variations in the effectiveness of neomycin as a blocker of RyR2.
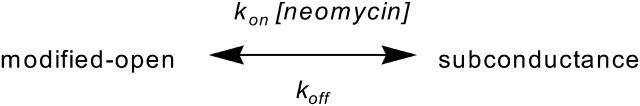
Our previous investigations of RyR2 block by neomycin have established that the polycation interacts with separate sites at the cytosolic and luminal faces of the open channel and that both forms of block can be described by simple bimolecular schemes (Scheme 1).
SCHEME 1.
Bimolecular scheme describing block of RyR by neomycin. kon and koff are, respectively, rate constants for the association of neomycin with and dissociation of neomycin from the modified-open RyR2 channel. In such a scheme the probability of occurrence of the subconductance state should be dependent upon the concentration of neomycin ([neomycin]).
The interaction of a single molecule of neomycin with the cytosolic or luminal faces of the open channel results in the occurrence of a reduced conductance state as the result of partial block of the pore (Mead and Williams, 2002a,b). In this study we have used these schemes to investigate the influence of changing K+ concentration on the kinetics of cytosolic and luminal block of RyR2 by neomycin. Some of the data reported for neomycin block in 210 mM KCl has been reported previously (Mead and Williams, 2002b), but is included here to aid comparison with the other permeant ion concentrations used in this study.
Neomycin-induced block at the cytosolic face of the channel
A quantitative assessment of the concentration dependence of cytosolic block
We have monitored the influence of a range of concentrations of neomycin in the presence of 100, 210, 410, and 610 mM K+. Within the context of the simple kinetic scheme set out above, variations in probability of block (1 − Po) with neomycin concentration will be described by the following relationship:
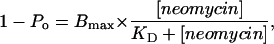 |
(1) |
in which Bmax is the maximum probability of block and KD is the concentration of neomycin at which the probability of block is 0.5.
Cytosolic block in 100 mM K+
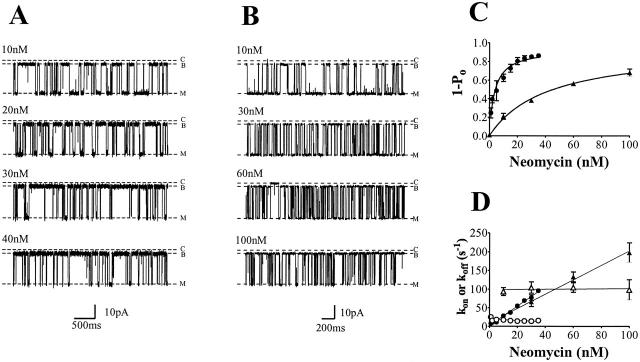
The interaction of cytosolic neomycin with RyR2 at 100 mM K+ results in the occurrence of blocking events to a subconductance state. As shown in Fig. 2 A, the amplitude of the blocked subconductance state is independent of neomycin concentration and has a value of 10.6 ± 1.1% of the modified-open level. The probability of block clearly increases as the concentration of neomycin is increased.
FIGURE 2.
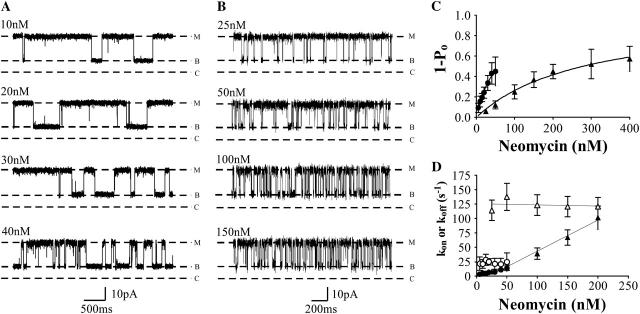
Cytosolic neomycin block of ryanodine modified RyR2 channels. Channels were recorded in (A) 100 mM or (B) 210 mM symmetrical KCl at +60 mV. Neomycin induces blocking events to a subconductance level (B), distinct from the closed-channel level (C). Neither the amplitude of the modified open state (M) nor the blocked subconductance state change with increasing neomycin concentration. Increasing neomycin concentration in 100 mM (•) or 210 mM (▴) KCl is plotted against (C) probability of block (1 − Po) and (D) rates of association (kon, solid symbols) and dissociation (koff, open symbols). The curves in graph C are nonlinear regression-derived from a single-site binding scheme (Scheme 1). Lines in graph D are linear regression. All graph data obtained from four or more experiments.
Neomycin-induced blocking events in RyR2 in the presence of 100 mM K+ are fully resolved at all concentrations of the polycation examined and Po was quantified from dwell times in the open and blocked states as described in Materials and Methods. The relationship between probability of block and neomycin concentration is shown in Fig. 2 C. Probability of block rises and saturates as neomycin concentration is increased. The curve shown in Fig. 2 C is the best fit to Eq. 1 obtained by nonlinear regression with a Bmax of 0.93 ± 0.04 and KD of 3.7 ± 0.7 nM.
Mechanisms underlying the block of RyR2 by cytosolic neomycin at 100 mM K+ were investigated by determining variations in rates of blocker association and dissociation, monitored as kon = mean open time−1 and koff = mean blocked time−1 (see Materials and Methods), with neomycin concentration. These data are plotted in Fig. 2 D. Consistent with the proposed bimolecular scheme for block, the rate of neomycin association with RyR2 increases linearly as concentration of the polycation is raised, whereas the rate of blocker dissociation is independent of concentration. Values of kon and koff determined from Fig. 2 D are 2.6 ± 0.12 nM−1 s−1 and 19.3 ± 1.7 s−1, respectively. The dissociation constant for this interaction, calculated as
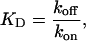 |
(2) |
is 7.4 nM and is in good agreement with the value calculated from the measurement of 1 − Po versus neomycin concentration (see above).
Cytosolic block in 210 mM K+
In the presence of 210 mM K+ cytosolic neomycin induces blocking events to a subconductance state with an amplitude similar to that observed at 100 mM K+ (15.0 ± 3.0%)(Fig. 2 B). As is the case at the lower K+ concentration, increasing concentrations of neomycin increase the probability of block and individual blocking events are well resolved at all neomycin concentrations examined. However, durations of events in the blocked state appear to be shorter than those occurring in the lower K+ concentration. As before we have used dwell times in the open and blocked states to determine the probability of block. Data from the 1 − Po calculations are plotted in Fig. 2 C and values of Bmax and KD were obtained by nonlinear regression of fits to Eq. 1. Under these conditions Bmax is 0.96 ± 0.071 and KD 41.6 ± 7.4 nM, a value ∼10-fold greater than that determined for cytosolic neomycin block in 100 mM K+.
Variations in rates of neomycin association and dissociation with varying neomycin concentration at 210 mM K+ are shown in Fig. 2 D. As is the case at 100 mM K+, kon varies linearly with neomycin concentration, whereas koff is independent of blocker concentration. Values determined from the plots in Fig. 2 D are 1.9 ± 0.1 nM−1 s−1 for kon and 98.8 ± 5.6 s−1 for koff. It should be noted that the rate of association shows a modest 27% decrease when the bath K+ concentration is increased. However, koff increases much more dramatically by ∼5-fold with an increase in K+ from 100 to 210 mM. KD calculated from these rate constants is 52 nM, which is again comparable to the equivalent value obtained from the variation in 1 − Po with neomycin concentration.
Cytosolic block in 410 mM K+
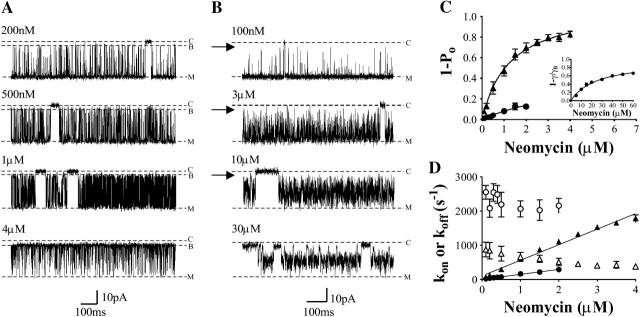
Examples of block induced by increasing concentrations of neomycin at 410 mM K+ are shown in Fig. 3 A. As is the case for the lower K+ concentrations, block is manifest as transitions from the open state of the channel to a subconductance state. The amplitude of this state is similar to that observed for block in 100 and 210 mM K+ (14.1 ± 1.6%) and as is observed at lower ionic concentrations, the probability of channel block increases as the concentration of neomycin is raised. Measurements of variation of probability of block with increasing neomycin concentration at 410 mM K+ are shown in Fig. 3 C. Values of Bmax and KD obtained for these data are 1.11 ± 0.03 and 1.3 ± 0.21 μM, respectively; an ∼4-fold increase in bath K+ concentration produces an ∼350-fold decrease in the affinity of the RyR2 cytosolic site of interaction for neomycin.
FIGURE 3.
Cytosolic neomycin block of channels recorded in (A) 410 mM or (B) 610 mM symmetrical KCl at +60 mV. Closed-channel level indicated by C, modified-open level by M. Blocked subconductance level is indicated by B. In 610 mM KCl, blocking events become less well-defined. The trace recorded with 10 μM neomycin would appear to show blocking events to a substate (indicated by the arrow) equivalent in amplitude to that seen in lower salt concentrations. Graph C shows the effect of increasing neomycin concentration in 410 mM (▴) or 610 mM (•) KCl on probability of block (1 − Po), and block observed as a reduction in current amplitude in 610 mM KCl (1 − γ/γ0, inset). Graph D represents the effect of increasing neomycin concentration in 410 mM (▴) or 610 mM (•) KCl on kon (solid symbols) and koff (open symbols).
Again, information on the mechanisms underlying this alteration can be gained from an inspection of the rates of neomycin association and dissociation. It is immediately apparent from a comparison of the data in Fig. 3 A with equivalent data at 100 and 210 mM K+ that the duration of neomycin-induced blocking events decreases as K+ concentration is raised. Analysis of mean dwell times in the open and blocked states at 410 mM K+ (Fig. 3 D) yields values of 0.459 ± 0.014 nM−1 s−1 for kon and 816.6 ± 69.14 s−1 for koff. In comparison with the data obtained at 100 mM K+ there is a fivefold decrease in the rate of neomycin association but a 43-fold increase in the rate of dissociation. The value of KD calculated from these rate constants is 1.78 μM, which is again comparable to the value determined from the variation in 1 − Po with neomycin concentration.
Cytosolic block in 610 mM K+
An inspection of the parameters obtained for neomycin-induced block of RyR2 at 100, 210, and 410 mM K+ reveals that the efficiency of the polycation as a blocker is markedly reduced as K+ concentration is increased and that this decreased affinity results, predominantly, from a dramatic increase in the rate of dissociation of the blocker. These observations are confirmed by the measurement of neomycin block at 610 mM K+. Representative traces are shown in Fig. 3 B. As is the case at the lower K+ concentrations used in this study the addition of neomycin to the solution at the cytosolic face of the channel results in the occurrence of blocking events. At the lower K+ concentrations examined neomycin interaction could be clearly resolved as well-defined events to a subconductance state. At 610 mM K+ dwell times in the neomycin-induced blocking events are very short and, as a result, it is not possible to state unequivocally that block is to a subconductance state. Nevertheless, individual blocking events are clearly evident at low concentrations of neomycin (see 100 nM and 3 μM in Fig. 3 B) and, as at the lower K+ concentrations examined, we have quantified block at neomycin concentrations up to and including 2 μM by monitoring lifetimes of open and blocked events. Although this range of neomycin concentrations is insufficient to allow for an accurate assessment of KD from a plot of 1 − Po (Fig. 3 C) we can obtain this information from the rates of blocker association and dissociation determined from dwell times in the open and blocked states. As is evident in Fig. 3 D, and in keeping with data obtained at lower K+ concentrations, raising cytosolic neomycin concentration at 610 mM K+ results in a linear increase in the rate of blocker association with a value of kon of 0.141 ± 0.003 nM−1 s−1. Within this range of neomycin concentrations koff is essentially independent of blocker concentration with a value of 2411.00 ± 107.3 s−1 and KD calculated from these rate constants is 17.09 μM. A comparison of these parameters with those obtained at 100 mM K+ reveals a 2300-fold decrease in affinity of RyR2 for cytosolic neomycin on elevation of K+ concentration resulting from a 20-fold decrease in the rate of blocker association and a 125-fold increase in the rate of blocker dissociation.
Consistent with the observed dependence of kon on neomycin concentration, increasing blocker concentration above 3 μM results in a loss of resolution of individual blocking events (Fig. 3 B). At 10 μM neomycin block is manifest as a very noisy open state with a clear reduction in open-state amplitude characteristic of poorly resolved block. As expected, further increases in blocker concentration result in more marked reductions in the amplitude of the open state and a reduction in noise (30 μM in Fig. 3 B). Fig. 3 C (inset) shows the relationship between block, expressed as 1 − γ/γ0, and neomycin concentration. In this analysis γ is measured by placing a cursor through the center of the noisy reduced state and γ0 is the amplitude of the modified-open state in the absence of neomycin. Although not providing a complete description of block, the best fit of Eq. 1 to these data gives a realistic estimate of the affinity of the cytosolic neomycin site at 610 mM K+ over a much wider range of neomycin concentrations than can be determined by monitoring dwell times, yielding values of KD of 25.51 ± 2.6 μM and Bmax of 0.96 ± 0.05.
Voltage dependence of block by cytosolic neomycin
We have previously ascertained that the interaction of cytosolic neomycin with RyR2 is voltage dependent (Mead and Williams, 2002a,b). This observation led us to propose that block of the channel by cytosolic neomycin involves an electrostatic interaction between positive charge on the neomycin molecule and negatively charged residues of RyR2, with at least some of the neomycin charge entering the voltage drop across the pore. In this section of the article, we have assessed the influence of changing K+ concentration on the voltage dependence of the interaction of cytosolic neomycin with RyR2 by monitoring block at a range of positive holding potentials. Particular care was taken to use concentrations of neomycin at which blocking events could be unambiguously resolved at the holding potentials investigated to permit accurate determinations of Po.
In all cases we have investigated block within the context of the simple model proposed by Woodhull (1973) that envisages a single site of interaction within the voltage drop across the pore accessible to the blocker from only one side of the channel. In this scheme the probability of block (expressed as relative open probability PoRel = Po in the presence of neomycin as a proportion of Po in the absence of neomycin) will vary with holding potential so that:
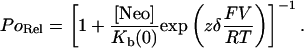 |
(3) |
This equation is used to derive two important parameters. Kb(0) is the dissociation constant for neomycin at 0 mV and zδ is the effective valence of the interaction, where z is the valence of the blocker and δ is the electrical distance into the voltage drop across the pore at which the interaction occurs. F, R, and T have their usual meanings and RT/F is 25.2 mV at 20°C.
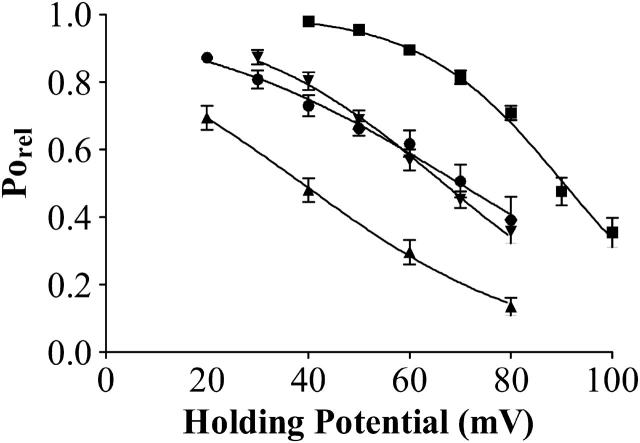
The relationships between PoRel and holding potential for 5 nM, 100 nM, 1.0 μM, and 2.0 μM neomycin in, respectively, 100, 210, 410, and 610 mM K+ are shown in Fig. 4 together with lines of best fit to Eq. 3 obtained by nonlinear regression. Blocking parameters obtained from these curves are as follows: at 100 mM K+ Kb(0) is 64.47 ± 14.81 nM and zδ is 0.923 ± 0.096; at 210 mM K+ Kb(0) is 534.9 ± 35.17 nM and zδ is 1.09 ± 0.035; at 410 mM K+ Kb(0) is 27.91 ± 6.174 μM and zδ is 1.257 ± 0.09; and at 610 mM K+ Kb(0) is 1.474 ± 0.479 mM and zδ is 1.8 ± 0.11. In agreement with data obtained by varying neomycin concentration at a holding potential of +60 mV, these data demonstrate that the affinity of the cytosolic site for neomycin interaction on RyR2 decreases dramatically as the bathing K+ concentration is increased. It is also apparent that the effective valence of block by neomycin increases as the bathing K+ concentration is raised. However, it should be noted that the interpretation of zδ in this context is confounded by the fact that neomycin has a charge of at least +4 in these experimental conditions, but it is as yet unknown how much of this charge is directly involved in the blocking interaction.
FIGURE 4.
Relationship between holding potential and relative Po over the range of permeant ion concentrations. Po was measured in the presence of 5 nM neomycin in 100 mM KCl (•), 100 nM neomycin in 210 mM KCl (▴), 1 μM neomycin in 410 mM KCl (▾) and 2 μM neomycin in 610 mM KCl (▪). Curves are nonlinear regression derived from Woodhull (1973), giving values for Kb(0) and zδ, which are given in the text. Data obtained from four or more experiments.
Neomycin-induced block at the luminal face of the channel
A quantitative assessment of the concentration dependence of luminal block
Although several classes of cationic ligand are effective blockers of RyR2 when present at the cytosolic face of the channel (Tinker et al., 1992b; Tinker and Williams, 1993; Mead and Williams, 1998), neomycin is unusual in that it is capable of inducing comparable block when present at both the cytosolic and luminal faces of RyR2. Luminal block is observed at negative holding potentials and is apparent at all four bath K+ concentrations tested.
Luminal block in 100 mM K+
Luminal neomycin block observed in 100 mM K+ is induced over a similar blocker concentration range to that seen when neomycin is applied to the cytosolic face of the channel. Representative traces are shown for a single channel in Fig. 5 A. Block occurs as very well-defined events to a subconductance state with an amplitude of 27.6 ± 2.0% of the normal ryanodine-modified open state. The probability of occurrence of block increases as neomycin is raised but the amplitude of the neomycin-induced subconductance state is independent of blocker concentration. As outlined at the beginning of the Results section of this article, we interpret these observations as an interaction of the polycation with regions of negative charge at the luminal entrance to the RyR2 pore that results in partial block of K+ translocation.
FIGURE 5.
Luminal neomycin block recorded in (A) 100 mM or (B) 210 mM symmetrical KCl at −60 mV. Blocking events are induced to a well-defined subconductance level (B), distinguishable from the closed state (C). The level of the closed state was assessed from control experiments (not shown). Modified open-state (M) and subconductance-state amplitudes do not change with increasing neomycin concentration. Increasing luminal neomycin concentration in 100 mM (•) or 210 mM (▴) KCl is plotted against (C) 1 − Po and (D) kon (solid symbols) and koff (open symbols).
Luminal neomycin block was assessed in 100 mM K+ by monitoring dwell times in the open and blocked conductance states at a holding potential of −60 mV. A plot showing variations in probability of block (1 − Po) for several channels at a range of neomycin concentrations is shown in Fig. 5 C. Values of Bmax and KD obtained from best fits to the single site scheme (Eq. 1) by nonlinear regression are 0.96 ± 0.12 and 62.3 ± 5.6 nM, respectively.
We have obtained information on the mechanisms underlying the variation in probability of block with increasing luminal neomycin concentration by determining rates of blocker association and dissociation for the data shown in Fig. 5 C. Variations of these parameters with changing neomycin concentration are shown in Fig. 5 D. Consistent with the proposed bimolecular scheme for block, kon rises linearly with increasing concentrations of neomycin and has a value of 0.21 ± 0.04 nM−1 s−1, whereas koff is independent of neomycin concentration and has a value of 25.27 ± 6.9 s−1. KD calculated from koff/kon is 120.3 nM.
Luminal block in 210 mM K+
Representative traces of luminal neomycin block of RyR2 at 210 mM K+ are shown in Fig. 5 B. Under these conditions individual blocking events to a subconductance state, similar to that seen at 100 mM K+ (32.6 ± 1.4%), are clearly resolved; however, it is immediately apparent that the durations of the blocked events are considerably shorter than those seen at the lower K+ concentration. As expected, the probability of occurrence of block is increased as the concentration of blocker is raised. These observations are confirmed by plots showing the variation in probability of block at a range of neomycin concentrations for several channels (Fig. 5 C). Values of Bmax and KD obtained from this relationship are 1.01 ± 0.12 and 284.1 ± 60.84 nM, respectively. A twofold increase in K+ concentration results in an ∼5-fold decrease in the affinity of the luminal neomycin site of interaction.
Variations in rates of luminal neomycin association and dissociation with blocker concentration at 210 mM K+ are shown in Fig. 5 D. In keeping with the proposed mechanism, kon is determined by blocker concentration with a value of 0.54 ± 0.08 nM−1 s−1, whereas koff is independent of concentration and has a value of 126.6 ± 15.99 s−1. The value of KD calculated from these rate constants is 234.4 nM. These measurements demonstrate that, as is the case for the cytosolic site of neomycin interaction, alterations in affinity of the luminal neomycin site with changing K+ concentration arise predominantly as the consequence of increased rates of blocker dissociation.
Luminal block in 410 mM K+
Representative traces of current fluctuations of a single channel in symmetrical 410 mM K+ with increasing concentrations of luminal neomycin at a holding potential of −60 mV are shown in Fig. 6. Block, under these conditions, is again characterized by transitions from the modified-open state to a subconductance state at 28.50 ± 0.47% of the open state. However, individual blocking events are not well resolved and as the concentration of neomycin is increased block is characterized by very rapid fluctuations in current around a poorly defined reduced-conductance state. The amplitude of this state is reduced by an increase in neomycin concentration from 3 to 6 μM.
FIGURE 6.
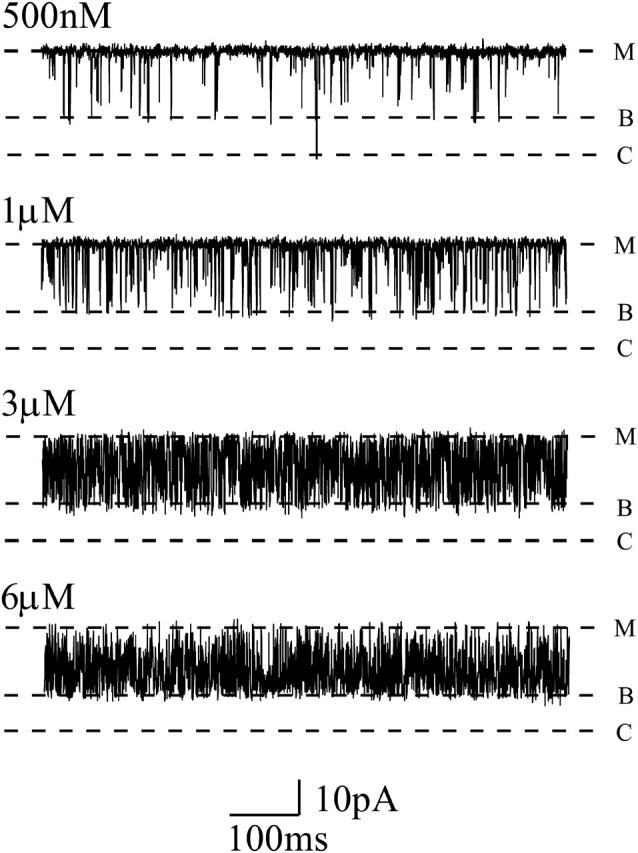
Representative single-channel recordings of increasing luminal neomycin concentration in 410 mM symmetrical KCl. Blocking events are less well-defined than those observed in 100 mM or 210 mM KCl, but the blocked substate is still apparent (B). C, closed, M, modified. Data recorded at −60 mV.
In keeping with the data obtained on raising K+ concentration from 100 to 210 mM, a very obvious consequence of increasing the bath K+ concentration to 410 mM is a marked reduction in the duration of neomycin-induced blocking events. Consistent with a simple bimolecular reaction mechanism, increases in neomycin concentration result in an augmented rate of blocker association and a consequent loss of resolution of individual blocking events.
Whereas the data for block of RyR by luminal neomycin at 410 mM K+ are in qualitative agreement with our results at lower K+ concentrations we have not been able to quantify variations in block with increasing neomycin concentration under these conditions. The particular combination of short durations of both open and blocked events at this K+ concentration make 50% threshold analysis inaccurate. As an alternative approach we have attempted to resolve block as a time-averaged reduction in current amplitude by severely low-pass filtering data; however, this too proved unsuccessful. At corner frequencies as low as 400 Hz block is visualized as a noisy state of variable amplitude.
Luminal block in 610 mM K+
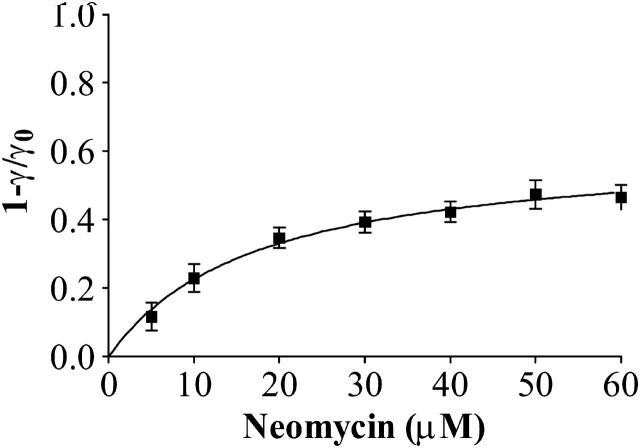
Block of an individual RyR2 channel by increasing concentrations of luminal neomycin in symmetrical 610 mM K+ at a holding potential of −60 mV is shown in Fig. 7. Individual blocking events are rarely resolved at this K+ concentration and block is seen as a reduction in open-state amplitude to a noisy reduced-conductance state. The amplitude of this reduced-conductance state decreases with increasing neomycin concentration. These observations indicate that, consistent with the trend observed at lower K+ concentrations, an increase of bath K+ from 410 to 610 mM produces a further increase in the rate of neomycin dissociation. As a consequence durations of individual blocking events are too short to be resolved and block approaches a time-averaged reduction in current amplitude. Under these conditions a rise in neomycin concentration will increase the rate of blocker association and will result in a reduction in the time-averaged current amplitude. As we are unable to resolve individual blocking events, we have obtained an estimate of block by luminal neomycin at 610 mM K+ as a reduction in open-state amplitude (1 − γ/γ0). In this analysis, γ is measured by placing a cursor through the center of the noisy reduced state and γ0 is the amplitude of the modified-open state in the absence of neomycin. The concentration dependence of luminal neomycin block at 610 mM K+ for several channels is shown in Fig. 8. Although not providing a complete description of block under these conditions, the value of KD obtained from the best fit of Eq. 1 to the data in Fig. 8 gives a useful estimate of the affinity of the luminal neomycin site at 610 mM K+. Values of Bmax and KD obtained for the best fit of the single-site binding scheme to these data are 0.62 ± 0.03 and 17.42 ± 2.11 μM.
FIGURE 7.
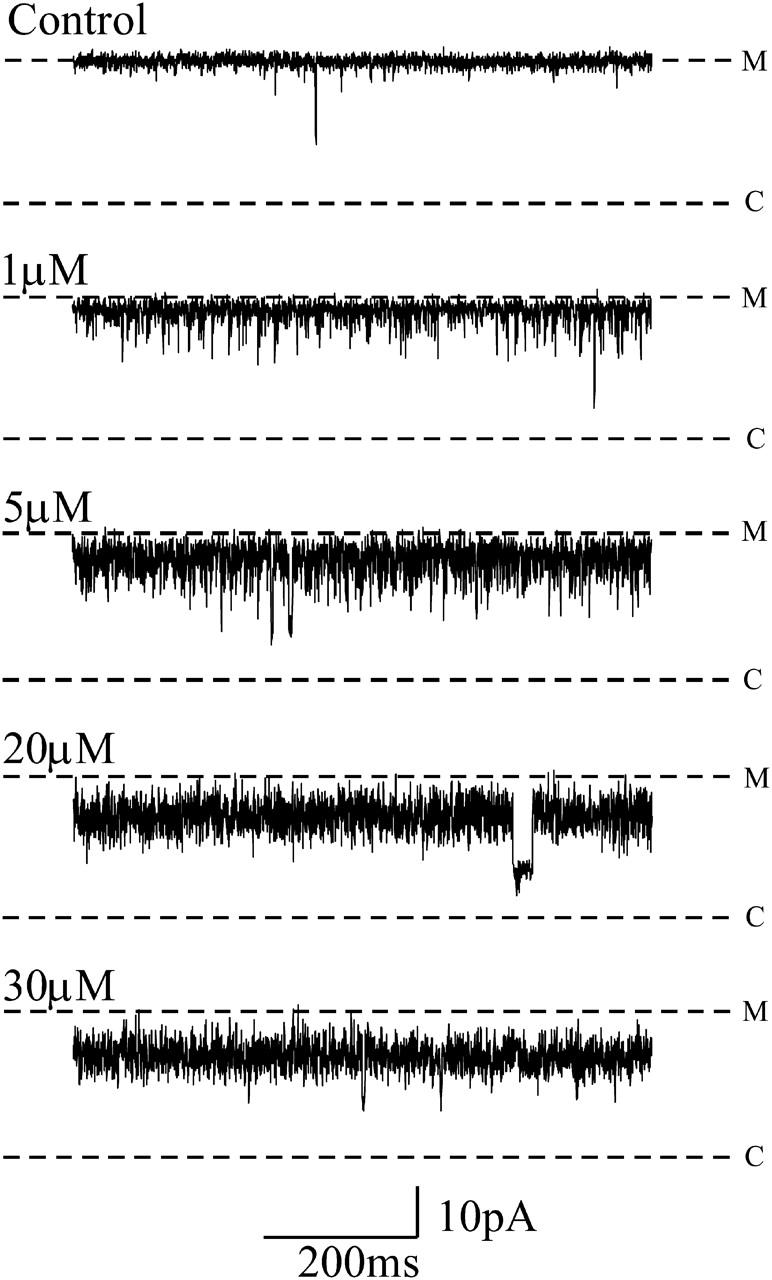
Representative single-channel recordings showing the effect of increasing luminal neomycin concentration in symmetrical 610 mM KCl. Blocking events are poorly defined, although the occurrence of a blocked substate is occasionally apparent, notably in the traces recorded with 5 μM and 20 μM.
FIGURE 8.
Graph representing the change in current amplitude induced by the addition of increasing concentrations of luminal neomycin in 610 mM KCl. Curve calculated by nonlinear regression based on Scheme 1.
Voltage dependence of block by luminal neomycin
We have not carried out a quantitative assessment of the influence of varying K+ concentration on the voltage dependence of block by luminal neomycin. The range of potentials at which we could monitor block at 100 mM K+ was limited as we observed a tendency for ryanodine to dissociate at high negative holding potentials under these conditions. Experiments in 410 and 610 mM K+ were not attempted due to the limits in resolution described above. Blocking parameters for luminal neomycin at 210 mM K+ were determined previously in Mead and Williams (2002b), and are Kb(0), 971.52 ± 66.62 nM, and zδ, −0.57 ± 0.03.
DISCUSSION
In previous studies (Mead and Williams 2002a,b), we have shown that the polycation neomycin is capable of inducing a concentration- and voltage-dependent partial block of K+ translocation in RyR2. The interaction of neomycin with the channel is unusual in that it is capable of inducing blocking events when applied to both the cytosolic and luminal sides of the channel. In our earlier work, we proposed that neomycin reduced channel conductance to a subconductance state by partially occluding the pore of the channel. It was suggested that this interaction was governed by an electrostatic interaction between the positive charge of the blocker and proposed regions of negative charge at both mouths of the conduction pathway (Tu et al., 1994; Mead and Williams, 1998). The observation of voltage-dependent block suggests an interaction within the voltage drop, which is further supported by the occurrence of voltage-dependent relief of neomycin block under certain conditions (Mead and Williams, 2002a).
In this study, we have investigated the hypothesis that partial block of RyR2 by neomycin is achieved by pore occlusion, and that this pore occlusion is governed by an electrostatic interaction between the blocker and the channel. One approach to investigating charge interactions governing block is to screen regions of charge thought to be involved in the blocking interaction by increasing the ionic strength of the recording solution. Previous investigators have changed ionic strength to screen charge to study the interaction of various toxin blockers with Ca2+-activated K+ (KCa) channels (MacKinnon and Miller, 1988; Anderson et al., 1988; Lucchesi and Moczydlowski, 1991; Giangiacomo et al., 1992) and the block induced by tetrodotoxin and saxitoxin in Na+ channels (Moczydlowski et al., 1984; Green et al., 1987). In these examples, screening negative charge at the mouth of the conduction pathway results in reduction of blocker affinity due to a reduction in the rate of blocker association. However, rates of dissociation are rarely altered. In the case of charybdotoxin block of KCa, charge screening disrupts the initial through-space electrostatic interaction of the positively charged blocker (Park and Miller, 1992) with negative charge of the channel (MacKinnon and Miller, 1989). If charge is screened at the site of blocker interaction, rates of dissociation are unaffected (Anderson et al., 1988). This is because electrostatic interactions are not the sole mechanism governing the binding of CTX (Park and Miller, 1992). In this study, we have altered the ionic strength of the permeant ion to investigate the nature of the proposed electrostatic interaction of neomycin with RyR2. However, our results suggest that the neomycin blocking interaction is more complex than those described above.
Cytosolic block is characterized by definable events to a subconductance state at lower K+ concentrations that become briefer with increasing K+ concentration until the recording system can no longer resolve them adequately. The same is true of luminal block, but the transition from well definable events to a noisy reduction in open-state amplitude is more marked. Assessment of the concentration dependence of block at each side, and in the presence of varying K+ (Table 1), shows that increasing concentrations of neomycin are required to induce block of the channel at both the cytosolic and luminal faces. In both cases, the amplitude of the substate is unchanged by increasing neomycin concentration or increasing K+ concentration. Although quantitative assessment using the same analysis technique is not possible across the range of differing conditions, this change in effective concentration range is apparent when channel recordings are studied. In low K+, nanomolar concentrations of neomycin are sufficient to induce blocking events at both cytosolic and luminal faces, but as K+ is increased to 610 mM, micromolar concentrations of neomycin are required to induce any form of block. This observation is supported by the dissociation constants calculated for block at each side in all K+ conditions. This increase in KD with increasing ionic strength may be expected if charge screening by this method is effective. Anderson et al. (1988) report an increase in KD for CTX blockade of KCa with increasing symmetrical K+ concentration.
TABLE 1.
Summary of data calculated for neomycin block of RyR2 in increasing symmetrical concentrations of KCl
| Cytosolic block
|
||||
|---|---|---|---|---|
| 100 mM | 210 mM | 410 mM | 610 mM | |
| kon | 2.6 ± 0.12 nM−1 s−1 | 1.9 ± 0.1 nM−1 s−1 | 459.2 ± 13.75 μM−1 s−1 | 141.1 ± 3.18 μM−1 s−1 |
| koff | 19.3 ± 1.7 s−1 | 98.8 ± 5.6 s−1 | 816.6 ± 69.14 s−1 | 2411 ± 107.3 s−1 |
| KD* (koff/kon) | 7.4 nM | 52 nM | 1.78 μM | 17.09 μM |
| KD† | 3.7 ± 0.7 nM | 41.6 ± 7.4 nM | 1.3 ± 0.213 μM | 25.51 ± 2.6 μM‡ |
| Kb(0) | 64.47 ± 14.81 nM | 534.9 ± 35.17 nM | 27.91 ± 6.174 μM | 1.474 ± 0.479 mM |
| zδ | 0.923 ± 0.096 | 1.09 ± 0.035 | 1.257 ± 0.09 | 1.8 ± 0.11 |
| Luminal block
|
|||
|---|---|---|---|
| 100 mM | 210 mM | 610 mM | |
| kon | 0.21 ± 0.04 nM−1 s−1 | 0.54 ± 0.08 nM−1 s−1 | Cannot calculate |
| koff | 25.27 ± 6.9 s−1 | 126.6 ± 15.99 s−1 | Cannot calculate |
| KD*(koff/kon) | 120.3 nM | 234.4 nM | Cannot calculate |
| KD† | 62.3 ± 56.3 nM | 284.1 ± 60.84 nM | 17.42 ± 2.114 μM‡ |
Due to the poor resolution of events induced by luminal neomycin in 410 mM KCl, it is not possible to calculate any parameters for that concentration.
KD values calculated from rates of association and dissociation.
KD values calculated from nonlinear regression of open probability data.
By determining the rates of association and dissociation for the neomycin interaction with RyR2 under different conditions, we can examine the underlying mechanism of block and the mechanisms that affect block when charge is screened. Rates of association and dissociation are summarized in Table 1. With each incremental increase in K+, there is a modest decrease in the rate of association for cytosolic neomycin block, but a surprisingly large increase in the rate of dissociation. Although it was not possible to assess luminal block by monitoring individual blocking events at greater K+ concentrations, it is apparent from the data obtained in 100 and 210 mM K+ that an increase in dissociation rate is primarily responsible for the decrease in neomycin affinity. It is not unreasonable to assume that the changing characteristics of luminal block observed in 410 and 610 mM K+ are due to a large increase in the rate of neomycin dissociation, given that cytosolic and luminal blockade of the channel are remarkably similar in all other respects.
The change in dissociation rate of both cytosolic and luminal neomycin in response to increasing ionic strength of the permeant ion is unexpected but, we believe, is still indicative of a charge screening effect and a major involvement of electrostatic interactions in block. Although MacKinnon and Miller (1988) reported an increase in dissociation rate of CTX from KCa channels, this only occurred when internal K+ was increased and was attributed to a repulsion effect of K+ ions in the multiion pore. This would not be applicable in RyR2. Although a change in dissociation characteristics would not be anticipated for a binding interaction that involves a specific physical interaction between a blocker and a binding site, it is important to stress at this point that the interaction of neomycin with RyR2 may not represent binding of the blocker to a conventional binding site. It may be more appropriate to consider neomycin block of RyR2 as a loose-fitting plug model, where the molecule interacts with the pore in such a way as to limit the flow of ions but without docking to a specific receptor site. If this is the case, then an increase in K+ in the vicinity of neomycin when in its blocking position could be sufficient to destabilize the interaction between the neomycin molecule and the channel. Park and Miller (1992) destabilized CTX block of KCa by neutralizing charged residues on the CTX molecule, and noted that there was a pronounced increase in koff, but little alteration in kon. Myers and Stampe (2000) showed that a single point mutation to introduce a glutamate residue to the pore region of dSlo could increase the affinity of CTX by decreasing koff. Therefore it is clear that electrostatic interactions can govern the rate of blocker dissociation. If the loose plug model is an accurate description of the neomycin blocking interaction, this could be achieved by an electrostatic interaction between the multivalent positive charge of neomycin and a region of negatively charged residues forming a ring near the pore mouth. In this case, the electrostatic interaction could be the sole basis for block, and neomycin may not form a bound complex with the channel involving interactions with specific residues in the pore mouth. The loose plug model of neomycin block is consistent with the observation that neomycin slows rates of association and dissociation of [3H]-ryanodine with RyR (Wang et al., 1996). If the ryanodine binding site is located within the pore (Wang et al., 2003), the presence of neomycin may obstruct the binding site and therefore alter [3H]-ryanodine binding in a noncompetitive manner.
This mechanism for subconductance-state induction by neomycin is supported by the observation that koff, as calculated from subconductance-state duration, is independent of blocker concentration. This would appear to rule out the possibility that the blocker-induced substate could be caused by a conformational change rather than a simple pore occlusion. Pore occlusion is consistent with Scheme 1, which describes an interaction with a single kinetic step. However, a conformational change, such as that described for Zn2+ block of Na+ channels (Schild et al., 1991), would require more than one kinetic step, which would include a concentration-dependent variation in koff.
As reported previously (Mead and Williams, 2002b), assessment of the voltage dependence of the blocking interaction is complicated by the fact that it is not known how much of the charge of neomycin is directly involved in an interaction within the voltage drop, although effective valence figures in excess of 1.0 suggest that more than one positive charge of the neomycin molecule encounters the voltage drop. Therefore, the interpretation of zδ is not straightforward. Effective valence of cytosolic neomycin increases as K+ concentration increases. For a monovalent blocker this may suggest that the site of interaction changes. However, Jordan (1986) notes that the position of the voltage drop relative to the bulk solution may change with altered ionic strength, and the proportion of neomycin charge sensed by the voltage drop in any of the tested conditions is unknown. As the blocked substate is the same proportion of the modified-open state in different K+ concentrations, it seems unlikely that neomycin induces block by interacting with the pore at an alternative site.
In conclusion, these studies have demonstrated that the block of K+ translocation in RyR2 by neomycin involves electrostatic interactions between the polycation blocker and areas of negative charge at both the cytosolic and luminal mouths of the channel pore. Neomycin, in conjunction with channels incorporating specific mutations of acidic residues, will be an invaluable probe in the investigation of the contribution of structural elements of the RyR2 pore to the processes of cation selection and translocation.
Acknowledgments
We are grateful to the British Heart Foundation for financial support and to Dr. Bill Welch for helpful discussions.
References
- Anderson, C. S., R. MacKinnon, C. Smith, and C. Miller. 1988. Charybdotoxin block of single Ca2+-activated K+ channels. Effects of channel gating, voltage, and ionic strength. J. Gen. Physiol. 91:317–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangiacomo, K. M., M. L. Garcia, and O. B. McManus. 1992. Mechanism of iberiotoxin block of the large-conductance calcium-activated potassium channel from bovine aortic smooth muscle. Biochemistry. 31:6719–6727. [DOI] [PubMed] [Google Scholar]
- Goldstein, S. A., and C. Miller. 1993. Mechanism of charybdotoxin block of a voltage-gated K+ channel. Biophys. J. 65:1613–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, W. N., L. B. Weiss, and O. S. Andersen. 1987. Batrachotoxin-modified sodium channels in planar lipid bilayers. Characterization of saxitoxin- and tetrodotoxin-induced channel closures. J. Gen. Physiol. 89:873–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haws, C. M., B. D. Winegar, and J. B. Lansman. 1996. Block of single L-type Ca2+ channels in skeletal muscle fibers by aminoglycoside antibiotics. J. Gen. Physiol. 107:421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan, P. C. 1986. Ion channel electrostatics and the shapes of channel proteins. In Ion Channel Reconstitution. C. Miller, editor. Plenum, New York. 37–55.
- Lindsay, A. R. G., and A. J. Williams. 1991. Functional characterisation of the ryanodine receptor purified from sheep cardiac muscle sarcoplasmic reticulum. Biochim. Biophys. Acta. 1064:89–102. [DOI] [PubMed] [Google Scholar]
- Lucchesi, K. J., and E. Moczydlowski. 1991. On the interaction of bovine pancreatic trypsin inhibitor with maxi Ca(2+)-activated K+ channels. A model system for analysis of peptide-induced subconductance states. J. Gen. Physiol. 97:1295–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon, R., and C. Miller. 1988. Mechanism of charybdotoxin block of the high-conductance, Ca2+-activated K+ channel. J. Gen. Physiol. 91:335–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon, R., and C. Miller. 1989. Functional modification of a Ca2+-activated K+ channel by trimethyloxonium. Biochemistry. 28:8087–8092. [DOI] [PubMed] [Google Scholar]
- Mead, F. C., D. Sullivan, and A. J. Williams. 1998. Evidence for negative charge in the conduction pathway of the cardiac ryanodine receptor channel provided by the interaction of K+ channel N-type inactivation peptides. J. Membr. Biol. 163:225–234. [DOI] [PubMed] [Google Scholar]
- Mead, F., and A. J. Williams. 2002a. Block of the ryanodine receptor channel by neomycin is relieved at high holding potentials. Biophys. J. 82:1953–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead, F., and A. J. Williams. 2002b. Ryanodine-induced structural alterations in the RyR channel suggested by neomycin block. Biophys. J. 82:1964–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, C. 1982. Open-state substructure of single chloride channels from Torpedo electroplax. Philos. Trans. R. Soc. Lond. B Biol. Sci. 299:401–411. [DOI] [PubMed] [Google Scholar]
- Moczydlowski, E., S. S. Garber, and C. Miller. 1984. Batrachotoxin-activated Na+ channels in planar lipid bilayers. Competition of tetrodotoxin block by Na+. J. Gen. Physiol. 84:665–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullmann, T. J., P. Munujos, M. L. Garcia, and K. M. Giangiacomo. 1999. Electrostatic mutations in iberiotoxin as a unique tool for probing the electrostatic structure of the maxi-K channel outer vestibule. Biochemistry. 38:2395–2402. [DOI] [PubMed] [Google Scholar]
- Murrell-Lagnado, R. D., and R. W. Aldrich. 1993a. Interactions of amino terminal domains of Shaker K channels with a pore blocking site studied with synthetic peptides. J. Gen. Physiol. 102:949–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell-Lagnado, R. D., and R. W. Aldrich. 1993b. Energetics of Shaker K channels block by inactivation peptides. J. Gen. Physiol. 102:977–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers, M. P., and P. Stampe. 2000. A point mutation in the maxi-K clone dSlo forms a high affinity site for charybdotoxin. Neuropharmacology. 39:11–20. [DOI] [PubMed] [Google Scholar]
- Park, C. S., and C. Miller. 1992. Mapping function to structure in a channel-blocking peptide: electrostatic mutants of charybdotoxin. Biochemistry. 31:7749–7755. [DOI] [PubMed] [Google Scholar]
- Pietrobon, D., B. Prod'hom, and P. Hess. 1989. Interactions of protons with single open L-type calcium channels. pH dependence of proton-induced current fluctuations with Cs+, K+, and Na+ as permeant ions. J. Gen. Physiol. 94:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild, L., A. Ravindran, and E. Moczydlowski. 1991. Zn2(+)-induced subconductance events in cardiac Na+ channels prolonged by batrachotoxin. Current-voltage behavior and single-channel kinetics. J. Gen. Physiol. 97:117–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitsapesan, R., and A. J. Williams. 1990. Mechanisms of caffeine activation of single calcium-release channels of sheep cardiac sarcoplasmic reticulum. J. Physiol. 423:425–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitsapesan, R., and A. J. Williams. 1994a. Gating of the native and purified cardiac SR Ca(2+)-release channel with monovalent cations as permeant species. Biophys. J. 67:1484–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitsapesan, R., and A. J. Williams. 1994b. Regulation of the gating of the sheep cardiac sarcoplasmic reticulum Ca(2+)-release channel by luminal Ca2+. J. Membr. Biol. 137:215–226. [DOI] [PubMed] [Google Scholar]
- Tinker, A., A. R. Lindsay, and A. J. Williams. 1992a. A model for ionic conduction in the ryanodine receptor channel of sheep cardiac muscle sarcoplasmic reticulum. J. Gen. Physiol. 100:495–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinker, A., A. R. Lindsay, and A. J. Williams. 1992b. Block of the sheep cardiac sarcoplasmic reticulum Ca(2+)-release channel by tetra-alkyl ammonium cations. J. Membr. Biol. 127:149–159. [DOI] [PubMed] [Google Scholar]
- Tinker, A., and A. J. Williams. 1993. Charged local anesthetics block ionic conduction in the sheep cardiac sarcoplasmic reticulum calcium release channel. Biophys. J. 65:852–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu, Q., P. Velez, M. Cortes-Gutierrez, and M. Fill. 1994. Surface charge potentiates conduction through the cardiac ryanodine receptor channel. J. Gen. Physiol. 103:853–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. P., D. H. Needleman, A. B. Seryshev, B. Aghdasi, K. J. Slavik, S. Q. Liu, S. E. Pedersen, and S. L. Hamilton. 1996. Interaction between ryanodine and neomycin binding sites on Ca2+ release channel from skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 271:8387–8393. [DOI] [PubMed] [Google Scholar]
- Wang, R., L. Zhang, J. Bolstad, N. Diao, C. Brown, L. Ruest, W. Welch, A. J. Williams, and S. R. W. Chen. 2003. Residue Gln4863 within a predicted transmembrane sequence of the Ca2+ release channel (ryanodine receptor) is critical for ryanodine interaction. J. Biol. Chem. 278:51557–51565. [DOI] [PubMed] [Google Scholar]
- Woodhull, A. M. 1973. Ionic blockage of sodium channels in nerve. J. Gen. Physiol. 61:687–708. [DOI] [PMC free article] [PubMed] [Google Scholar]