Abstract
The interaction of colicins with target cells is a paradigm for protein import. To enter cells, bactericidal colicins parasitize Escherichia coli outer membrane receptors whose physiological purpose is the import of essential metabolites. Colicins E1 and E3 initially bind to the BtuB receptor, whose β-barrel pore is occluded by an N-terminal globular “plug”. The x-ray structure of a complex of BtuB with the coiled-coil BtuB-binding domain of colicin E3 did not reveal displacement of the BtuB plug that would allow passage of the colicin (Kurisu, G., S. D. Zakharov, M. V. Zhalnina, S. Bano, V. Y. Eroukova, T. I. Rokitskaya, Y. N. Antonenko, M. C. Wiener, and W. A. Cramer. 2003. Nat. Struct. Biol. 10:948–954). This correlates with the inability of BtuB to form ion channels in planar bilayers, shown in this work, suggesting that an additional outer membrane protein(s) is required for colicin import across the outer membrane. The identity and interaction properties of this OMP were analyzed in planar bilayer experiments.OmpF and TolC channels in planar bilayers were occluded by colicins E3 and E1, respectively, from the trans-side of the membrane. Occlusion was dependent upon a cis-negative transmembrane potential. A positive potential reversibly opened OmpF and TolC channels. Colicin N, which uses only OmpF for entry, occludes OmpF in planar bilayers with the same orientation constraints as colicins E1 and E3. The OmpF recognition sites of colicins E3 and N, and the TolC recognition site of colicin E1, were found to reside in the N-terminal translocation domains. These data are considered in the context of a two-receptor translocon model for colicin entry into cells.
INTRODUCTION
Cellular import of cytotoxic colicins requires translocation across the outer membrane and periplasmic space of Escherichia coli and insertion into, or transfer across, the cytoplasmic membrane, as discussed and reviewed elsewhere (Lazdunski et al., 1998; James et al., 2002; Zakharov and Cramer, 2004). In this pathway, the multidomain colicins of groups A and B use the Tol and Ton translocation routes, respectively (Braun et al., 2002; Lazzaroni et al., 2002). The initial step of the import is recognition by and binding to outer membrane receptors. For this purpose, most colicins of group A use the vitamin B12 (cobalamin) receptor, BtuB (Fig. 1 A) (Chimento et al., 2003; Kurisu et al., 2003). It belongs to the family of OM receptors of gram-negative bacteria that are 22-strand β-barrel proteins, whose physiological purpose is the accumulation of metals (e.g., Fe, Co) and metabolites (e.g., vitamin B12). The β-barrels of these receptors are “plugged” by 130–150 residue N-terminal globular domains that have extensive interactions with the strands of the β-barrel (Ferguson and Deisenhofer, 2002).
FIGURE 1.
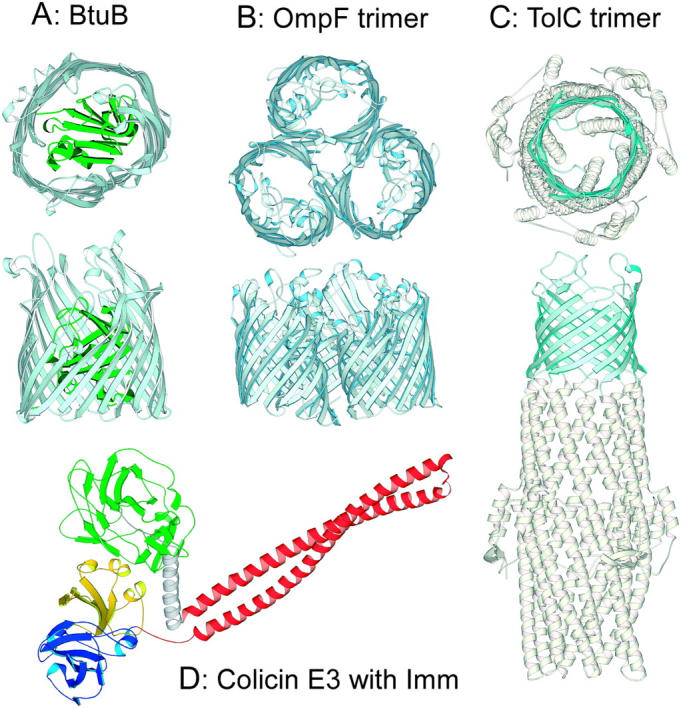
Ribbon diagrams of E. coli outer membrane proteins and colicin E3. (A) Cobalamin receptor, BtuB (Chimento et al., 2003). (B) OmpF trimer (Cowan et al., 1992). (C) TolC trimer (Koronakis et al., 2000). Top and side views are shown with the β-barrel colored light blue, and the N-terminal 132 residue plug domain of BtuB colored green. (D) Colicin E3 with bound immunity protein (Soelaiman et al., 2001). N-terminal translocation (T), central coiled-coil BtuB-binding (R), and C-terminal endonuclease (C) domains are colored green, red, and blue, respectively. Imm protein, yellow.
OmpF (Fig. 1 B) and TolC (Fig. 1 C) form stable trimers in the bacterial outer membrane. Every monomer in the OmpF trimer is capable of forming an ion-permeable channel that is slightly cation-selective (Benz et al., 1985; Alcaraz et al., 2004). OmpF is a 16-stranded β-barrel protein with its pore partially occluded by a long external loop L3 (Fig. 1 B; Cowan et al., 1992). It is required, in addition to BtuB, for the activity of many group A colicins (Cavard and Lazdunski, 1981; Chai et al., 1982; Benedetti et al., 1989). The combined contribution of BtuB and TolC is required for the activity of colicin E1 (Nagel de Zwaig and Luria, 1967; Davies and Reeves, 1975). OmpF alone is required for the activity of colicin N (Bourdineaud et al., 1990; Fourel et al., 1990; El Kouhen et al., 1993). TolC is a trimeric protein embedded in the outer membrane by its β-barrel (Fig. 1 C), and spans the periplasmic space as an α-helical “tunnel” (Koronakis et al., 2000; Sharff et al., 2001). Each monomer of TolC contributes four β-strands to formation of a 12-stranded β-barrel in the outer membrane. Entrance to the TolC tunnel from the periplasmic space is regulated by the iris-like aperture of the three sets of α-helices (Andersen et al., 2002b).
The atomic structure of colicin E3 has been solved at 3.0 Å resolution (Fig. 1 D; Soelaiman et al., 2001). The N-terminal (T), central coiled-coil (R), and C-terminal (C) domains are responsible for translocation across the outer membrane and periplasmic space, for interaction with the BtuB receptor, and for cytotoxic activity, respectively (Fig. 1 D). An elongate (160 Å and 100 Å) coiled-coil associated with the R-domain is a prominent feature of the structures of colicins Ia and E3 (Wiener et al., 1997; Soelaiman et al., 2001). The recently solved structure of the complex of BtuB with the colicin E3 135 residue receptor-binding coiled-coil domain (Kurisu et al., 2003) did not show plug displacement from the BtuB β-barrel, implying that colicin does not cross the outer membrane through the BtuB barrel. The extended length of the R-domain coiled-coil, and its oblique orientation in the 2.75 Å x-ray structure suggest that the function of its initial interaction with BtuB is to deliver the colicin T- and C-domains (Fig. 1 D) to a secondary receptor-translocator, which was implied to be OmpF (Benedetti et al., 1989). It was suggested that OmpF provides the channel through which unfolded domains of the colicin can pass into the periplasm (Kurisu et al., 2003).
Translocation of colicins through OmpF channels should cause occlusion of the channels that would be manifested by a decrease in their ion conductance. In this study, such a decrease in the conductance of OmpF channels in planar bilayer membranes caused by colicins E3 and N was observed. The asymmetry of this conductance decrease with respect to the side of colicin addition, the polarity of a required membrane potential, and localization of the interaction site in the translocation domain of the colicins, implies that the occlusion is specific to one side of the membrane-inserted OmpF receptor. Similar specific occlusion of TolC channels by colicin E1 was also observed. These data imply that BtuB and OmpF or TolC are involved in a two-receptor mechanism for colicin import through the E. coli outer membrane.
MATERIALS AND METHODS
Materials
Synthetic phospholipids, DOPC and DOPE (Avanti Polar Lipids, Alabaster, AL), used for planar bilayer formation, were dissolved in chloroform.
Outer membrane proteins
BtuB was expressed in E. coli strain TNEO12 (pJC3) and purified as described (Taylor et al., 1998; Kurisu et al., 2003), using 1.5% OG for extraction and 0.1% LDAO during purification. The same protocol was successfully used for OmpF purification from E. coli strain MH225 (pPR272) (Misra and Reeves, 1987). Purity and activity of OmpF (LPS content was not measured) were comparable with protein purified according to a protocol based on multiple extraction of membranes and purification in octyl-POE (Garavito and Rosenbusch, 1986; Hitscherich et al., 2000). OmpF purified in LDAO was transferred to 1% octyl-POE before it was used for planar bilayer formation.
TolC was expressed in E. coli strain AG100 with the plasmid pTrc99A-TolC under a strong IPTG-inducible promoter (Vakharia et al., 2001), and purified by ion-exchange chromatography on Q Sepharose FF followed by gel filtration on Sephacryl S-300 as described previously (Koronakis et al., 1997).
Colicins and fragments
Colicins E1 (Zhang and Cramer, 1992), E3 (Herschman and Helinski, 1967), and N (Cavard and Lazdunski, 1979; Izard et al., 1994) were prepared according to existing protocols.
Point and deletion mutations in colicin E3 were made by cloning respective PCR amplified products into the Nde1/XhoH1 sites of pET41b vector DNA. Proteins were expressed in BL21 (DE3) cells and purified using Ni-charged IDA-agarose column. Recombinant R-domain of colicin E3, R135 (Kurisu et al., 2003); thermolytic fragments of colicin E1, P178, and P342 (Ohno-Iwashita and Imahori, 1982); and colicin N, P205, and P322 (Massotte and Pattus, 1989; Evans et al., 1996b) were prepared as described. The C-terminal domain of colicin E3 with bound immunity (Imm) protein was kindly provided by K. Jakes (Albert Einstein College of Medicine, Bronx, NY). Truncated colicin E3 T-domain fragments were obtained by digestion with thermolysin for 60 min at 37°C at thermolysin:colicin E3, 1:1000 (w/w). The C-terminal fragments of colicin N, 35 and 22 kDa, were obtained by digestion for 60 and 300 min at 37°C at thermolysin:colicin N ratios, 1:400 and 1:200 (w/w), respectively. The N-terminal sequences and molecular mass of proteolytic fragments were verified by the MALDI-MS analysis.
Planar bilayer membranes
Planar bilayer lipid membranes were formed on a 0.2 or 0.45 mm diameter aperture in a partition separating two 1-ml compartments of the experimental setup, using a 1:1 mixture (10 mg/ml) of DOPC and DOPE in n-decane, by a brush technique (Mueller et al., 1962). The use of lipids dissolved in n-decane for membrane formation was based on extension of other studies on porins (Benz et al., 1985; Song et al., 1998). The aqueous solutions in both compartments were 5 mM NaPi, pH 7.0, 0.1 M KCl, 23°C. 0.1–2 μl OmpF, 0.001–1 ng/ml in the buffer with 1% octyl-POE were added to the cis-compartment (less protein was added for membranes of larger area), and the solution was magnetically stirred for 5–20 min until channels appeared. 1–2 μl TolC, 50 μg/ml, was added from the buffer with 1% octylglucoside. For single-channel measurements, after OmpF channels were recorded, the cis-chamber was perfused by 10 ml of the buffer solution to prevent new channel incorporation into the membrane. The membrane electrical resistance was not altered by addition of 1% OG or octyl-POE (10 μl). Colicins were added to the cis- or trans-side of the membrane, and solutions in both chambers were magnetically stirred for 5 min. The membrane current was measured in voltage-clamp mode with Ag/AgCl electrodes, using a WARNER BC-525C amplifier (Warner Instruments, Hamden, CT). The transmembrane potential was applied to the electrode on the cis-side of the membrane.
The efficiency of occlusion of OmpF channels by colicin E3 was characterized by two parameters: τocc, duration of channel transition from open to occluded state, measured as the interval of time between application of the transmembrane potential and attainment of the steady-state of occlusion; Focc, the fraction of time that channels are in the closed state measured using channel histograms for t > τocc.
RESULTS
Binding of colicin E3 to BtuB does not displace the plug domain from the β-barrel
To test for the existence of a significant colicin-induced ion conductance through BtuB that could be an indicator of a polypeptide translocation channel, a planar bilayer membrane was incubated with BtuB (5 μg/ml in the cis-compartment) for 30 min. Addition of BtuB to the bilayer membrane increased the current (transmembrane potential, 80 mV) from 0.23 ± 0.40 pA to 0.55 ± 0.39 pA at a positive potential, and from −0.19 ± 0.43 pA to −0.48 ± 0.33 pA at negative potentials. These changes in current, corresponding to conductance changes of ± 4 pS, are within the range of current fluctuations in the apparatus, and can be attributed to an increase in ion leakage through the lipid bilayer due to the presence of detergent in the BtuB stock solution. Subsequent addition of colicin E3 or vitamin B12 from either side of the membrane did not affect the bilayer conductance.
Nonfacilitated diffusion of the large (50–60 kDa) and water-soluble colicin molecules through the phospholipid-lipopolysaccharide bilayer of the bacterial outer membrane seems unlikely. The most probable candidates for colicin translocators through the bacterial outer membrane would be those outer membrane proteins for which there is structural and/or electrophysiological evidence for a transmembrane channel of adequate size. Two such proteins are OmpF (Cowan et al., 1992) and TolC (Koronakis et al., 2000). Mutation studies indicated a requirement of these proteins for import of the group A colicins (Benedetti et al., 1989; Lazzaroni et al., 2002).
Occlusion of OmpF by colicin E3
In 0.1 M KCl, OmpF incorporated into planar bilayers yielded a single channel conductance of 300 ± 20 pS at negative and 260 ± 20 pS at positive transmembrane potentials per a monomer (Fig. 2, A and E). To prevent channel closing in the presence of high transmembrane potentials (voltage-gating) (Schindler and Rosenbusch, 1978; Phale et al., 1997), the applied transmembrane potentials were not allowed to exceed a magnitude of 50 mV. All three channels of the single OmpF trimer are mostly in an open state with occasional closings, which were more frequent at negative than at positive potentials (Fig. 2 A).
FIGURE 2.
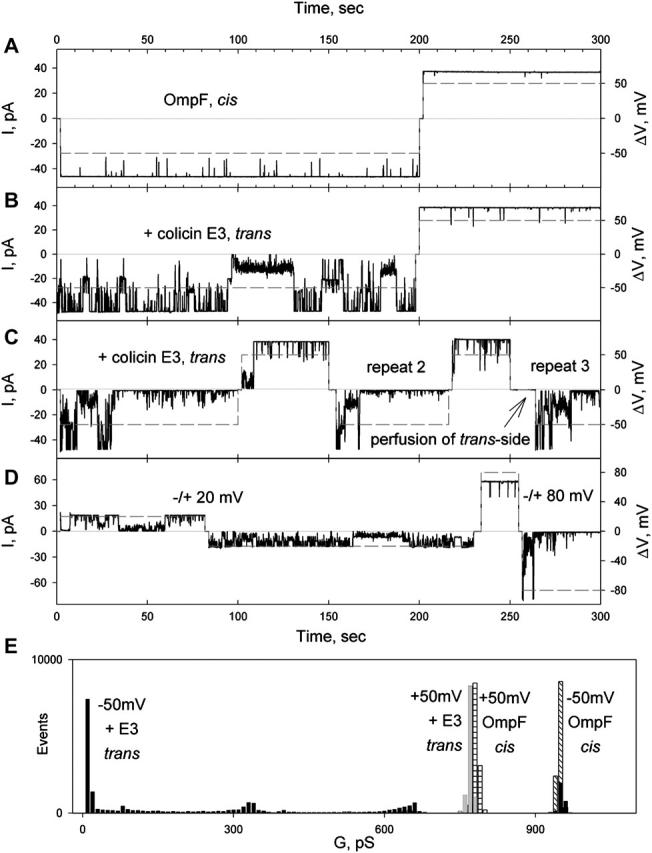
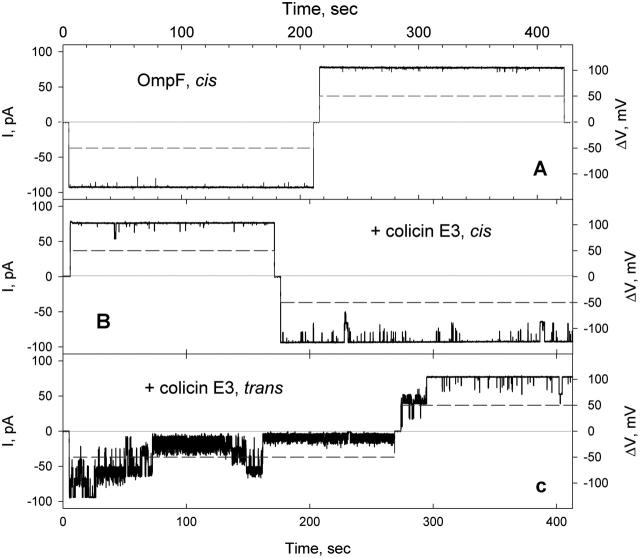
Occlusion of OmpF channels by colicin E3 from the trans-side of the membrane, driven by cis-negative potential. (A) Transmembrane current (solid) through OmpF channels (one trimer incorporated) at a potential of 50 mV (dashed). OmpF, 10 pg/ml, added to the cis-compartment. Solution, 5 mM NaPi, pH 7.0, 0.1 M KCl, was stirred until channels were detected (∼5 min), and then replaced by perfusion, as described in Methods. The sign of the potential refers to the cis-side of membrane. (B) Colicin E3, 50 ng/ml, was added to the trans-compartment, and the record started after 5 min stirring. (C) Increasing the colicin E3 concentration to 500 ng/ml increased rate of occlusion. Perfusion of trans-compartment at 0 mV (after repeat 2) or +50 mV (not shown) does not abolish occlusion. (D) Dependence of efficiency of occlusion and opening of occluded OmpF channels on the magnitude of the transmembrane potential. (E) Histogram of OmpF channel conductance as a function of transmembrane potential polarity in the absence and presence of colicin E3 on the trans-side. Maximum conductance of OmpF was 780 and 950 pS at +50 and −50 mV in the absence of colicin, and 780 and 10 pS at +50 and −50 mV in the presence of colicin E3, 500 ng/ml, on the trans-side of membrane.
Colicin E3 induced the closing of the OmpF channels only when it was added from the trans-side of the planar bilayer, the side opposite to that of addition of OmpF (Fig. 2, B, C, and E). This channel closing occurred at cis-negative potentials. The rate and magnitude of the effect were dependent on colicin E3 concentration in the bathing solution. With a colicin concentration of 50 ng/ml (τocc > 100 s; Focc 24 ± 11%, n = 3; Table 1), the occlusion of the OmpF channels was incomplete with a pattern of flickering (Fig. 2 B). Increasing the colicin E3 concentration to 500 ng/ml resulted in complete occlusion of all or most channels, with τocc = 43 ± 26 s; Focc = 83 ± 14%, n = 11 (Fig. 2 C; Table 1). Changing the sign of the potential to cis-positive opened all channels, and switching back to a negative potential again resulted in channel closing (Fig. 2 C, repeat 2), implying that the voltage-induced occlusion is reversible. Removal of unbound colicin E3 by perfusion of the trans-compartment after application of the positive potential did not eliminate channel occlusion (Fig. 2 C, repeat 3), demonstrating that the colicin, which occludes OmpF at negative potentials, continues to be bound at positive potentials.
TABLE 1.
Occlusion of OmpF and TolC by colicins occurs from the trans-side of membrane
| Receptor Colicin side concentration | τocc s | Focc (n) % |
|---|---|---|
| OmpF | >100 | 24 ± 11 (3) |
| Colicin E3 trans 50 ng/ml | 43 ± 26 | 83 ± 14 (11) |
| 500 ng/ml | 12 ± 9 | 0.3 |
| cis 2.4 μg/ml | 3 ± 2 | 76 ± 8 (4) |
| E3 T-domain trans 250 ng/ml | 74 ± 39 | 84 ± 7 (4) |
| Colicin N trans 1 μg/ml | 55 ± 15 (7) | |
| TolC | ||
| Colicin E1 trans 1 μg/ml |
Transmembrane potential, −50 mV. Buffer, 5 mM NaPi, pH 7.0, 0.1 M KCl.
The efficiency of occlusion by negative potentials, and the opening of occluded channels by positive, depends on the magnitude of the potential (Fig. 2 D), as only partial closing was observed at −20 mV; however, fast and complete channel closing or opening takes place at −80 mV (Focc = 39% and 98% at −20 and −80 mV, respectively).
To elucidate the role of transmembrane potentials in colicin E3 occlusion of OmpF, i.e., whether i), it facilitates colicin binding to OmpF; or ii), a transmembrane potential is required to translocate OmpF-bound colicin into the channel, the trans-compartment was perfused after incubation for 5 min with colicin E3, (as in Fig. 2 C, but without application of a transmembrane potential). This did not abolish subsequent occlusion of OmpF channels by the previously added colicin in the presence of the cis-negative potential (not shown), implying that binding of the colicin does not require the transmembrane potential. It was concluded that the role of the potential is to translocate the bound colicin E3 into the OmpF channel lumen.
Colicin E3 added to the cis-compartment (2.4 μg/ml) did not affect the OmpF channel conductance at positive or negative transmembrane potentials (Focc = 0.3%; Fig. 3, A and B; Table 1). Subsequent addition of colicin E3 (400 ng/ml) to the trans-compartment induced OmpF channel closing at cis-negative potentials (τocc = 67 ± 20 s; Focc = 71 ± 12%, n = 3, Fig. 3 C), showing that the presence of colicin E3 on the cis-side does not perturb occlusion of OmpF from the trans-side.
FIGURE 3.
Colicin E3 does not occlude OmpF channels from the cis-side of the membrane. (A). Transmembrane current through OmpF channels (two trimers are incorporated) at a transmembrane potential of 50 mV (dashed). OmpF added to cis-compartment. (B) Colicin E3 (2.4 μg/ml) was added to the cis-compartment. Record started after 5 min stirring, and a transmembrane potential of 50 mV was applied. (C) Colicin E3 added to the trans-side (400 ng/ml) in the presence of cis-side colicin.
OmpF-occlusion site of colicin E3
It has been proposed that the N-terminal T-domain of colicins (Fig. 1 D, green) is responsible for interactions with the Omp and Tol proteins that allow or facilitate colicins to cross the outer membrane and periplasmic space (Bouveret et al., 2002; Lazzaroni et al., 2002; Kurisu et al., 2003). To locate the putative OmpF-binding site on the colicin, recombinant and proteolytic fragments of colicin E3 (Fig. 4, A and B) were examined for occlusion competence. Recombinant E3 fragments containing residues 313–447 (R-domain), 455–551 (C-domain with bound Imm protein), and 289–551 (R- and C-domains with bound Imm protein) added at concentrations of 5–10 μg/ml from either side of the membrane, in the presence of either sign of the transmembrane potential, did not diminish or perturb the OmpF channel conductance (not shown). However, the N-terminal segment of colicin E3, residues 1–285 (T-domain, Fig. 4, A and B), showed efficient occlusion when added from the trans-side of membrane at a cis-negative potential (τocc = 12 ± 9 s; Focc = 76 ± 8%, n = 4; Fig. 4 C, red histogram; Table 1), implying that the OmpF-binding site of colicin E3 is located in the translocation domain. Addition of T-domain from the cis-side caused only a transient closing of 1–2 OmpF channels (Focc ≈ 6%, Fig. 4 D).
FIGURE 4.
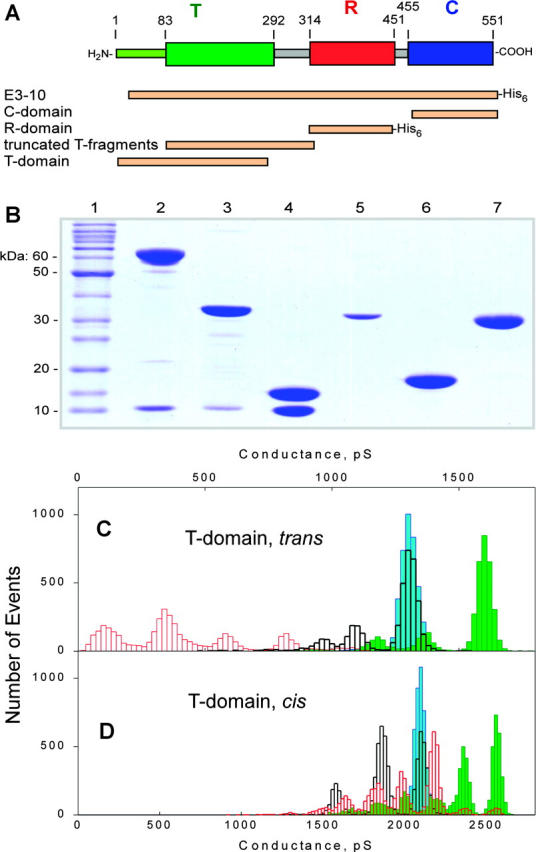
OmpF channel occlusion by the T domain of colicin E3. (A) Colicin E3 fragments used to infer location in colicin polypeptide of OmpF-binding site. “E3-10”, colicin E3 with N-terminal 10 residues deleted. Fragments labeled by His6 were His-tagged. (B) SDS-PAGE of colicin E3 and its recombinant fragments. (Lanes) 1, protein molecular weight standards; 2, intact colicin E3 with Imm; 3, RC-fragment (residues 289–551) with Imm; 4, C-fragment (residues 455–551) with Imm; 5, T-domain (residues 1–255); 6, R-domain (residues 313–447); and 7, truncated T-domain (residues 84–315). (C) Histograms of the OmpF channel conductance without added T-domain at +50 mV (blue) and −50mV (green), and with T-domain (residues 1–285), 0.1 μg/ml, in the trans-compartment at +50 mV (black) and −50mV (red). (D) Histograms of OmpF channel conductance without added T-domain at +50 mV (blue) and −50mV (green), and with T-domain (residues 1–285), 0.1 μg/ml, in the cis-compartment at +50 mV (black) and −50mV (red). Buffer, 5 mM NaPi, pH 7.0, 0.1 M KCl.
The N-terminal residues 1–83 of the colicin E3 T-domain, which are presumably disordered because of high content (42%) of glycine residues, were not resolved in the x-ray structure of colicin E3 (Soelaiman et al., 2001). The rest of the T-domain forms a compact globule (Soelaiman et al., 2001). Proteolytic digestion of colicin E3 by thermolysin yielded 23,930 and 24,680 Da fragments (residues 81–310 and 84–320), whose sequence was revealed by Edman degradation and MALDI-MS. These fragments did not occlude OmpF channels (tested at 10 μg/ml), implying that the OmpF binding site is located within residues 1–80 in the N-terminal segment.
The occlusion capability of colicin E3 was also lost in truncated colicins E3 in which the N-terminal 50, 25, and 10 residues were deleted. This localizes the OmpF-occlusion site of colicin E3 to residues 1–10, close to the N-terminus, and excludes the “TolB box” (residues 35–42; Escuyer and Mock, 1987; Bouveret et al., 1997) as a candidate for this function. The amino acid sequence of residues 1–10 is: Met-Ser-Gly-Gly-Asp-Gly-Arg-Gly-His-Asn. Taking into account that occlusion depends on transmembrane potential, N-terminal charged residues, Asp-5 and Arg-7, were mutated to Ala. The colicin E3 double mutant, D5A/R7A, was inactive in occlusion (not shown), implying that the OmpF-occlusion site of colicin E3 includes Asp-5 and/or Arg-7.
Occlusion of OmpF channels by colicin N
OmpF is the only outer membrane protein required for the cytotoxicity of colicin N, which acts as both a cell surface receptor and the translocation pathway (Benedetti et al., 1989).
Colicin N (1 μg/ml) added from the cis-side of the planar bilayer did not occlude OmpF channels (Fig. 5 A). However, like colicin E3, it occluded efficiently from the trans-side (τocc, 3 ± 2. s; Focc, 84 ± 8%, n = 7; Fig. 5 B; Table 1), demonstrating fast closing of most OmpF channels at negative potentials. These channels opened at positive potentials. However, for complete opening of all occluded OmpF channels, a larger potential, (+80 mV) was required (not shown).
FIGURE 5.
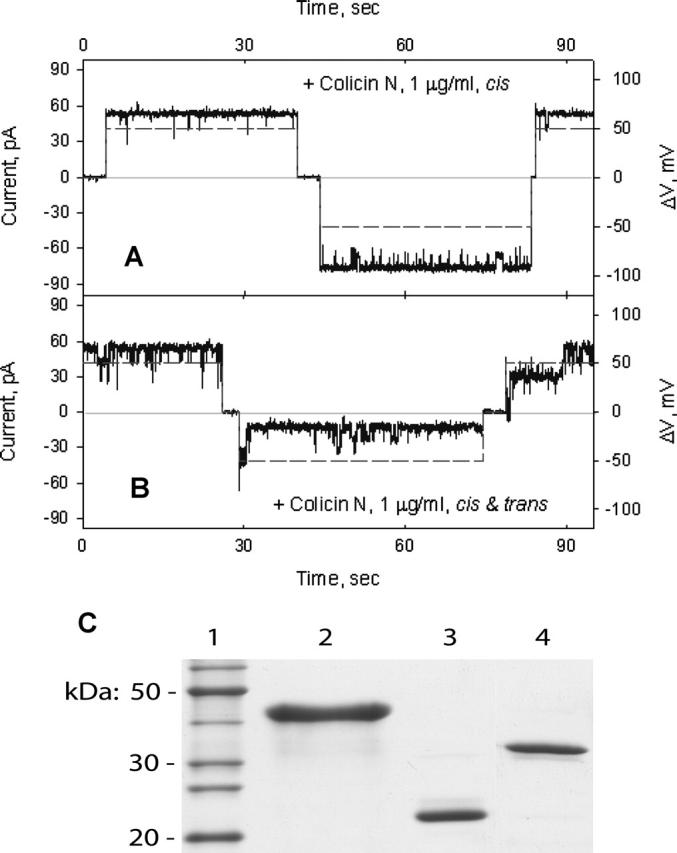
Occlusion of OmpF channels by colicin N. (A) Colicin N in the cis-compartment, 1 μg/ml, does not affect the current through OmpF channels. (B) Subsequent addition of colicin N in the trans-compartment induces fast OmpF channel closings at the negative transmembrane potential. The change in the magnitude of transmembrane potential is shown by dashed line. (C) SDS-PAGE of colicin N fragments obtained by proteolytic digestion by thermolysin. (Lanes) 1, molecular weight protein standards; 2, colicin N; 3, 22,320 Da fragment; and 4, 35,250 Da fragment. Molecular mass of fragments was determined by MALDI mass-spectroscopy.
Proteolytic digestion of colicin N by thermolysin produces two fragments, 35,250 Da (residues 66–387) and 22,320 Da (residues 183–387) (Fig. 5 C), which contain the R-C and C-domains, respectively (Massotte and Pattus, 1989; Evans et al., 1996c; Vetter et al., 1998). These fragments did not affect OmpF channel conductance from either side of the planar bilayer (not shown). This implies that i), the OmpF binding site of colicin N is located in an N-terminal peptide, residues 1–65, that contains the T-domain; and ii), any possible binding of colicin N through the C- or R-domains (Evans et al., 1996a; Stora et al., 1999; Dover et al., 2000) is not associated with OmpF channel occlusion.
Interaction of colicins E3 and N with OmpF, resulting in channel occlusion, is dependent upon electrostatic forces
All of the experiments described above were carried out in 0.1 M KCl on both sides of planar bilayer. Increasing the salt concentration to 1.0 M entirely prevents occlusion of OmpF channels by colicins E3 and N (Table 2). At 0.3 M salt, only transient closing was observed in the presence of colicin N (Table 2). This implies that electrostatic interactions between colicins E3 or N and OmpF are necessary for channel occlusion.
TABLE 2.
Occlusion of OmpF channels by colicins is abolished by high salt concentration in bath solution
|
Focc (n = 3) %
|
||
|---|---|---|
| KCl, M | Colicin E3 | Colicin N |
| 0.1 | 91 ± 6 | 88 ± 5 |
| 0.3 | ca. 0.1 | 4 ± 3 |
| 1.0 | <0.1 | <0.1 |
Colicins, 1 μg/ml, were added to the trans-compartment. Transmembrane potential, −50 mV. Solutions in both compartments were buffered with 5 mM NaPi, pH 7.0.
Colicin E1 (20 μg/ml) did not affect the OmpF channel conductance (tested at 20 μg/ml; not shown). This is in agreement with previous studies that showed import of this colicin is not dependent on OmpF, but on the outer membrane proteins, BtuB (Di Masi et al., 1973; Bradbeer et al., 1976) and TolC (Nagel de Zwaig and Luria, 1967; Davies and Reeves, 1975).
TolC channels in planar bilayers are occluded by colicin E1, but not colicin E3
In the planar bilayer experiments, TolC shows an asymmetric channel conductance that is dependent on transmembrane potential polarity, with a main conductance state of 80 pS at cis-positive potentials in 1 M KCl, pH 7.0, and 20 pS in 0.1M KCl (Andersen et al., 2002a). In addition to the dominant 80 pS state, substates with conductance of 10, 20, and 30 pS at 1M KCl have been reported (Andersen et al., 2002a).
The effect of colicin E1 on TolC channel conductance was tested in multichannel experiments (Fig. 6). TolC was added to the cis-side of the planar bilayer, and after stirring for 20–30 min, current was measured in the presence of positive or negative transmembrane potentials (Fig. 6 A). Colicin E1 added from the trans-side of the membrane decreased the TolC channel current in the presence of negative potentials (τocc = 74 ± 39 s; Focc = 55 ± 15%, n = 7; Table 1; Fig. 6, A, B, and D), and did not have any effect at positive potentials. The same extent of channel closing was observed after the potential was switched to positive, and then to negative potential (Fig. 6 B). Perfusion of the trans-compartment abolishes the occlusion of TolC channels (Fig. 6 B), implying that, in contrast to the colicin E3 interaction with OmpF, colicin E1 dissociates from TolC in the absence of a cis-negative potential.
FIGURE 6.
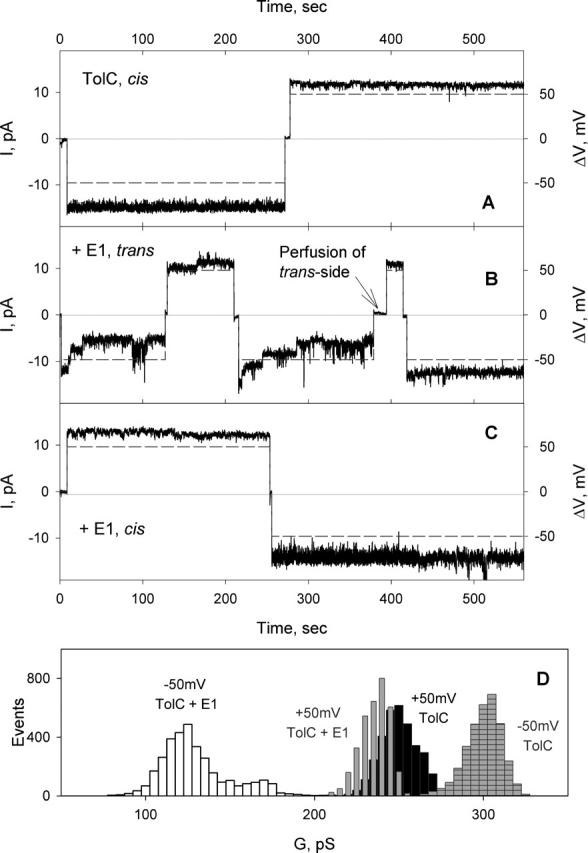
Effect of colicin E1 on TolC channels. (A) TolC (0.5 ng/ml) was added to the cis-side of the planar membrane, and solutions were stirred for 20 min. Channel current was measured after perfusion of the cis-compartment with 5 ml of buffer at transmembrane potentials of 50mV (dashed line). Buffer in both compartments, 5 mM KPi, pH 7.0, 0.1 M KCl, 23°C. (B) After colicin E1 (1 μg/ml) was added to the trans-side, solutions were stirred for 5 min, and channel current measured again. Perfusion of trans-compartment resulted in abolishment of TolC channel occlusion at negative potentials. (C) Colicin E1, 5 μg/ml, in the cis-compartment does not occlude TolC channels. (D) TolC channel conductance histograms before and after addition of colicin E1. Conductance values for the histogram were measured when the current of occluded channels was stabilized (t > τocc), ∼70 s after switching the potential to −50 mV.
Colicin E1 added to the cis-side of the membrane bilayer did not occlude TolC channels at negative or positive potentials (Fig. 6 C). Colicins E3 (16 μg/ml) or N (4 μg/ml) added to the cis- or trans-side of the membrane bilayer did not occlude TolC channels at any transmembrane potential (not shown).
To find out which domain of the colicin E1 is responsible for interactions with TolC, proteolytic fragments of colicin E1 were tested for their ability to occlude. Thermolysin digests colicin E1 from the N-terminus leaving the C-terminal domain intact (Dankert et al., 1982). Thermolytic fragments, P178 (residues 345–522) and P342 (residues 181–522) that include C-domain and CR-domains, respectively, were characterized previously (Dankert et al., 1982; Ohno-Iwashita and Imahori, 1982; Griko et al., 2000). Addition of these fragments (10 μg/ml) to either bilayer compartment did not occlude TolC channels at either polarity of the transmembrane potential (not shown). This implies that the N-terminal segment, residues 1–180, containing the T-domain, mediates the interaction of colicin E1 with TolC.
DISCUSSION
Components of the colicin cellular import system
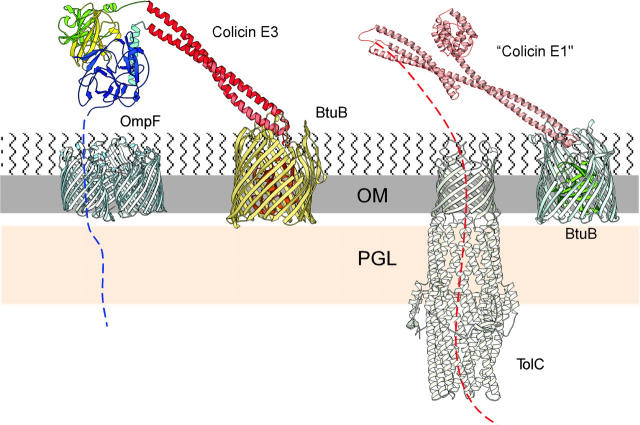
The BtuB receptor for colicins E3 and E1 in the outer membrane (Fig. 1 A) is a β-barrel protein plugged by a 132 residue globular N-terminal domain (Chimento et al., 2003). The major axes of the β-barrel pore are 27–33 Å, which would be sufficient, if not plugged, for translocation of unfolded and partially folded domains of colicin molecules. Taking into account the highly elongate shape of colicins E3 (Fig. 1 D) and Ia (Wiener et al., 1997), this initially suggested the possibility of colicin entry into the cell through the pore of the BtuB barrel (the “nail” hypothesis, reviewed in Cao and Klebba, 2002). This hypothesis has been abandoned because: i), the x-ray structure of the R135-BtuB complex showed that the receptor binding domain of colicin E3 hardly penetrates the extracellular loop region of BtuB (Kurisu et al., 2003); and ii), electrophysiological measurements provided no evidence for colicin translocation of BtuB. Alternatively, it was proposed that BtuB functions as the initial high affinity receptor for colicin E3 (Kd ≈ 10−9 M for the engineered R135 receptor-binding domain (Kurisu et al., 2003); Kd ≈ 10−11 M for the intact colicin (Imajoh et al., 1982)). The initial binding converts the three-dimensional search by the colicin for cell entry into a 2-dimensional search on the membrane surface for a second outer membrane protein that can function as a translocator. The extended coiled-coil anchored on BtuB (Fig. 1 D), and the disordered 83 residues at the N-terminus of colicin E3 (Soelaiman et al., 2001) provide the equipment needed to “fish” for the translocator (Fig. 7).
FIGURE 7.
Two-receptor model of colicin cellular import. X-ray structures of colicin E3 (Soelaiman et al., 2001), and of its 135 residue receptor-binding domain (red) in complex with BtuB (Kurisu et al., 2003), are superimposed to show the position of the translocation domain (blue) proposed to extend its N-terminus through the OmpF trimer (Cowan et al., 1992). The x-ray structure of colicin Ia (Wiener et al., 1997) was used to model colicin E1 in its interaction with TolC (Koronakis et al., 2000).
The OmpF porin is a prominent candidate for the second outer membrane protein in the colicin translocation pathway of colicin E3 because of the known requirement of the OmpF gene product for cytotoxicity (Benedetti et al., 1989), its high abundance in the OM (Nikaido and Vaara, 1987; Nikaido, 2003), and its relatively large ion channel (Cowan et al., 1992).
Occlusion of OmpF and TolC channels by colicins of group A
Colicins E3 and N occluded OmpF (Figs. 2 and 5), and colicin E1 occluded TolC channels (Fig. 6). The properties of these occlusion effects were: i), they were unidirectional, i.e. each colicin occluded only from the trans-side of the planar bilayer relative to the side (cis) of OmpF addition to the bilayer; ii), the occlusion required a transmembrane potential, negative on the side opposite to that of colicin addition; iii), occluded channels were opened by a positive potential; and iv), an increase in salt concentration of the buffer solution prevented occlusion (Table 2), implying that the colicin-porin interactions that cause occlusion are primarily electrostatic.
Occlusion of OmpF by colicins is similar to alteration of porin channels by polyamines that resulted in the stabilization of channel closed states (Iyer and Delcour, 1997). Effects of polyamines are concentration- and potential-dependent, but unlike colicins, they modulate porin channels from both sides of membranes, interacting with the porin eyelet (Samartzidou and Delcour, 1999; Samartzidou et al., 2003). The polyamines protect susceptible cells from colicin action (Bredin et al., 2003).
Interaction of the colicin translocation domain with the outer membrane receptor-translocator
Presently, a detailed understanding of the functions of the N-terminal translocation domain of colicins that enable their import across the cell envelope is not available. It is known that the N-terminal domains of E3 and E1 interact with the periplasmic TolB (Escuyer and Mock, 1987; Bouveret et al., 1997) and TolA (Benedetti et al., 1991; Derouiche et al., 1997) proteins, respectively. This study shows that one function is to interact with the outer membrane OmpF (colicins E3 and N) and TolC (colicin E1) receptor-translocators, as shown by occlusion of the ion channel conductance of these OM proteins. In the case of colicin E3, occlusion of OmpF conductance has been localized to the N-terminal residues 5–7. The “TolB box” of colicin E3, located between residues 35 and 42, which interacts with TolB in the periplasm, and which is thereby required for colicin E3 translocation (Escuyer and Mock, 1987; Bouveret et al., 1997), is not required for occlusion of OmpF channels.
Requirement of negative transmembrane potential
The requirement of a negative transmembrane potential for occlusion seems to contradict the view that there is no significant electrical potential gradient across the bacterial outer membrane (Nikaido and Vaara, 1987). This view is based on the abundance (>105 molecules of OmpF per E. coli cell) of highly conductive porins in these membranes. However, occlusion of OmpF and TolC by colicins, unlike voltage-gating of OmpF, does not requires a high potential and occurs at −20 mV (Fig. 2 D).
An additional source of the electrical field necessary for colicin occlusion could be the asymmetric distribution of phospholipid and LPS in the outer membrane. Phospholipid forms the periplasmic leaflet of OM, and the outer layer consists mostly of LPS (Kamio and Nikaido, 1976; Nikaido, 2003). This may generate surface and dipole potentials of significant magnitude in the outer membrane that results in a potential difference between the phospholipid and LPS monolayers of 40–80 mV, negative on the side (periplasmic) of the phospholipid. Such a potential was measured in asymmetric planar LPS/phospholipid bilayers (Seydel et al., 1992; Wiese et al., 1994, 1996). Cationic species could be driven through pores in the electrical field generated by such a potential gradient. In case of long polypeptides, this would be possible only if they first bind specifically and then enter the pore.
Translocon model of colicin import
The oblique orientation (40° relative to the membrane plane (Kurisu et al., 2003) of the 135 residue 100 Å-long R-domain bound to BtuB implies that when the entire colicin E3 is bound to BtuB, its C-terminal cytotoxicity (C) and N-terminal translocation (T) domains are well separated from BtuB, and the T-domain projects close the membrane surface, 50–100 Å from BtuB (Fig. 7). The abundance of OmpF in the E. coli outer membrane, >105 molecules per cell (Nikaido and Vaara, 1987), implies that ∼1 OmpF will be found on the surface of a 1-μm diameter cell at 100-Å spacings. The disordered N-terminal T-domain of colicin E3 (Fig. 7) can then search for a thermodynamically favorable binding site at the entrance to the aperture of its channel lumen (7 Å × 11 Å; Cowan et al., 1992). BtuB, OmpF, and colicin E3 could form a translocon to move the colicin T- and C-domains across the outer membrane. A similar translocon could be formed by BtuB, TolC, and colicin E1 (Fig. 7). This is in agreement with genetic studies showing that cellular import of colicin E1 on the one hand, and colicins A, E3, and N on the other, is dependent on the presence of tolC and ompF gene products (Nagel de Zwaig and Luria, 1967; Davies and Reeves, 1975; Benedetti et al., 1989; Bourdineaud et al., 1990; Koronakis et al., 1997).
Translocation of colicins through porin receptors would require unfolding of the translocated domains of colicins. In this context, the partial unwinding of the coiled-coil termini of receptor-binding domain of colicin E3, which was detected in the x-ray structure of the BtuB-R-domain structure (Kurisu et al., 2003), could be considered as an initial step in colicin unfolding which is triggered by the colicin-BtuB interaction. The interactions of components of the periplasmic and inner membrane Tol system with porins or directly with the inserted colicins could provide another source of energy, as could potential-dependent threading of the colicin across the outer membrane through OmpF or TolC.
Acknowledgments
We thank G. Jarori and K. Wood for MALDI-MS analyses, E. Bouveret (LISM, CNRS, Marseille, France), and K. Jakes (Albert Einstein College of Medicine) for the contribution of useful materials.
These studies were supported by grants to W.A.C. from the National Institutes of Health, GM18457, and Fogarty TW01235.
Abbreviations used: DOPC, dioleoyl-phosphatidylcholine; DOPE, dioleoyl-phosphatidylethanolamine; LDAO, lauryl-dimethylamine-oxide; LPS, lipopolysaccharide; MALDI-MS, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry; P178, P342, 178 and 342 residue C-terminal fragments of colicin E1; R135, recombinant 135 residue segment of colicin E3 containing receptor-binding domain; OG, octylglucoside; octyl-POE, octyl-oligo-oxyethylene; OMP; outer membrane protein; PGL, peptidoglycan layer; T-, R- and C-domains, N-terminal translocation, receptor-binding and C-terminal cytotoxic domains of colicins.
References
- Alcaraz, A., E. M. Nestorovich, M. Aguilella-Arzo, V. M. Aguilella, and S. M. Bezrukov. 2004. Salting out the ionic selectivity of a wide channel: the asymmetry of OmpF. Biophys. J. 87:943–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen, C., C. Hughes, and V. Koronakis. 2002a. Electrophysiological behavior of the TolC channel-tunnel in planar lipid bilayers. J. Membr. Biol. 185:83–92. [DOI] [PubMed] [Google Scholar]
- Andersen, C., E. Koronakis, E. Bokma, J. Eswaran, D. Humphreys, C. Hughes, and V. Koronakis. 2002b. Transition to the open state of the TolC periplasmic tunnel entrance. Proc. Natl. Acad. Sci. USA. 99:11103–11108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti, H., M. Frenette, D. Baty, R. Lloubes, V. Geli, and C. Lazdunski. 1989. Comparison of the uptake systems for the entry of various BtuB groups colicins into Escherichia coli. J. Gen. Microbiol. 135:3413–3420. [DOI] [PubMed] [Google Scholar]
- Benedetti, H., C. Lazdunski, and R. Lloubes. 1991. Protein import into Escherichia coli: colicins A and E1 interact with a component of their translocation system. EMBO J. 10:1989–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz, R., A. Schmid, and R. E. W. Hancock. 1985. Ion selectivity of gram-negative bacterial porins. J. Bacteriol. 162:722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdineaud, J.-P., H.-P. Fierobe, C. Lazdunski, and J.-M. Pages. 1990. Involvement of OmpF during reception and translocation steps of colicin N entry. Mol. Microbiol. 4:1737–1743. [DOI] [PubMed] [Google Scholar]
- Bouveret, E., L. Journet, A. Walburger, E. Cascales, H. Benedetti, and R. Lloubes. 2002. Analysis of the Escherichia coli Tol-Pal and TonB systems by periplasmic production of Tol, TonB, colicin, or phage capsid soluble domains. Biochimie. 84:413–421. [DOI] [PubMed] [Google Scholar]
- Bouveret, E., A. Rigal, C. Lazdunski, and H. Benedetti. 1997. The N-terminal domain of colicin E3 interacts with TolB which is involved in the colicin translocation step. Mol. Microbiol. 23:909–920. [DOI] [PubMed] [Google Scholar]
- Bradbeer, C., M. L. Woodrow, and L. I. Khalifah. 1976. Transport of vitamin B12 in Escherichia coli: common receptor system for vitamin B12 and bacteriophage BF23 on the outer membrane of the cell envelope. J. Bacteriol. 125:1032–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun, V., S. I. Patzer, and K. Hantke. 2002. Ton-dependent colicins and microcins: modular design and evolution. Biochimie. 84:365–380. [DOI] [PubMed] [Google Scholar]
- Bredin, J., V. Simonet, R. Iyer, A. Delcour, and J.-M. Pages. 2003. Colicins, spermine and cephalosporins: a competitive interaction with the OmpF eyelet. Biochem. J. 376:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, Z., and P. E. Klebba. 2002. Mechanisms of colicin binding and transport through outer membrane porins. Biochimie. 84:399–412. [DOI] [PubMed] [Google Scholar]
- Cavard, D., and C. Lazdunski. 1981. Involvement of BtuB and OmpF proteins in binding and uptake of colicin A. FEMS Microbiol. Lett. 12:311–316. [Google Scholar]
- Cavard, D., and C. J. Lazdunski. 1979. Purification and molecular properties of a new colicin. Eur. J. Biochem. 96:519–524. [DOI] [PubMed] [Google Scholar]
- Chai, T. J., V. Wu, and J. Foulds. 1982. Colicin A receptor: role of two Escherichia coli outer membrane proteins (OmpF protein and btuB gene product) and lipoolysacchararide. J. Bacteriol. 151:983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimento, D. P., A. K. Mohanty, R. J. Kadner, and M. Wiener. 2003. Substrate-induced transmembrane signaling in the cobalamin transporter BtuB. Nat. Struct. Biol. 10:394–401. [DOI] [PubMed] [Google Scholar]
- Cowan, S. W., T. Schirmer, G. Rumel, M. Steiert, R. Ghosh, R. A. Pauptit, J. N. Jansonius, and J. Rosenbusch. 1992. Crystal structures explain functional properties of two E. coli porins. Nature. 358:727–733. [DOI] [PubMed] [Google Scholar]
- Dankert, J. R., Y. Uratani, C. Grabau, W. A. Cramer, and M. Hermodson. 1982. On a domain structure of colicin E1: a COOH-terminal peptide fragment active in membrane depolarization. J. Biol. Chem. 257:3857–3863. [PubMed] [Google Scholar]
- Davies, J. K., and P. Reeves. 1975. Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group A. J. Bacteriol. 123:102–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derouiche, R., G. Zeder-Lutz, H. Benedetti, M. Gavioli, A. Rigal, C. Lazdunski, and R. Lloubes. 1997. Binding of colicin A and E1 to TolA. Microbiol. 143:3185–3192. [DOI] [PubMed] [Google Scholar]
- Di Masi, D. R., J. C. White, C. A. Schnaitman, and C. Bradbeer. 1973. Transport of vitamin B12 in Escherichia coli: common receptor sites for vitamin B12 and the E colicins on the outer membrane of the cell envelope. J. Bacteriol. 115:506–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dover, L. G., D. F. Evans, S. L. Fridd, G. Bainbridge, E. M. Raggett, and J. H. Lakey. 2000. Colicin pore-forming domains bind to Escherichia coli trimeric porins. Biochemistry. 39:8632–8637. [DOI] [PubMed] [Google Scholar]
- El Kouhen, R., H.-P. Fierobe, S. Scianimanico, M. Steiert, and F. Pattus. 1993. Characterization of the receptor and translocator domains of colicin N. Eur. J. Biochem. 214:635–639. [DOI] [PubMed] [Google Scholar]
- Escuyer, V., and M. Mock. 1987. DNA sequence analysis of three missense mutations affecting colicin E3 bactericidial activity. Mol. Microbiol. 1:82–85. [DOI] [PubMed] [Google Scholar]
- Evans, D. F., S. Labeit, A. Cooper, L. H. Bond, and J. H. Lakey. 1996a. The central domain of colicin N possess the receptor recognition site but not the binding affinity of the whole toxin. Biochemistry. 35:15143–15148. [DOI] [PubMed] [Google Scholar]
- Evans, L. J. A., A. Cooper, and J. H. Lakey. 1996b. Direct measuremnt of the association of a protein with a family of membrane receptors. J. Mol. Biol. 255:559–563. [DOI] [PubMed] [Google Scholar]
- Evans, L. J. A., M. L. Goble, K. A. Hales, and J. H. Lakey. 1996c. Different sensitivity to acid denaturation within a family of proteins: implications for acid unfolding and membrane translocation. Biochemistry. 35:13180–13185. [DOI] [PubMed] [Google Scholar]
- Ferguson, A. D., and J. Deisenhofer. 2002. TonB-dependent receptors—structural perspectives. Biochim. Biophys. Acta. 1565:318–332. [DOI] [PubMed] [Google Scholar]
- Fourel, D., C. Hikita, J. M. Bolla, S. Mizushima, and J.-M. Pages. 1990. Characterization of OmpF domains involved in Escherichia coli K-12 sensitivity to colicins A and N. J. Bacteriol. 172:3675–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavito, R. M., and J. P. Rosenbusch. 1986. Isolation and crystallization of bacterial porin. Methods Enzymol. 125:309–328. [DOI] [PubMed] [Google Scholar]
- Griko, Y. V., S. D. Zakharov, and W. A. Cramer. 2000. Structural stability and domain organization of colicin E1. J. Mol. Biol. 302:941–953. [DOI] [PubMed] [Google Scholar]
- Herschman, H. R., and D. R. Helinski. 1967. Purification and characterization of colicin E2 and colicin E3. J. Biol. Chem. 242:5360–5368. [PubMed] [Google Scholar]
- Hitscherich, C. J., J. Kaplan, M. Allaman, J. Wiencek, and P. J. Loll. 2000. Static light scattering studies of OmpF porin: implications for integral membrane protein crystallization. Protein Sci. 9:1559–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imajoh, S., Y. Ohno-Iwashita, and K. Imahori. 1982. The receptor for colicin E3. Isolation and some properties. J. Biol. Chem. 257:6481–6487. [PubMed] [Google Scholar]
- Iyer, R., and A. Delcour. 1997. Complex inhibition of OmpF and OmpC bacterial porins by polyamines. J. Biol. Chem. 272:18595–18601. [DOI] [PubMed] [Google Scholar]
- Izard, J., M. W. Parker, M. Chartier, D. Duche, and D. Baty. 1994. A single amino acid substitution can restore the solubility of aggregated colicin A mutants in Escherichia coli. Protein Eng. 7:1495–1500. [DOI] [PubMed] [Google Scholar]
- James, R., C. N. Penfold, G. R. Moore, and C. Kleanthous. 2002. Killing of E. coli cells by E group nuclease colicins. Biochimie. 84:381–389. [DOI] [PubMed] [Google Scholar]
- Kamio, Y., and H. Nikaido. 1976. Outer membrane of Salmonella typhimurium: accessibility of phospholipid headgroups to phospholipase C and cyanogen bromide activated dextran in the external medium. Biochemistry. 15:2561–2570. [DOI] [PubMed] [Google Scholar]
- Koronakis, V., J. Li, E. Koronakis, and K. Stauffer. 1997. Structure of TolC, the outer membrane component of the bacterial type I efflux system, derived from two-dimensional crystals. Mol. Microbiol. 23:617–626. [DOI] [PubMed] [Google Scholar]
- Koronakis, V., A. Sharff, E. Koronakis, B. Luisi, and C. Hughes. 2000. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature. 405:914–919. [DOI] [PubMed] [Google Scholar]
- Kurisu, G., S. D. Zakharov, M. V. Zhalnina, S. Bano, V. Y. Eroukova, T. I. Rokitskaya, Y. N. Antonenko, M. C. Wiener, and W. A. Cramer. 2003. The structure of BtuB with bound colicin E3 R-domain implies a translocon. Nat. Struct. Biol. 10:948–954. [DOI] [PubMed] [Google Scholar]
- Lazdunski, C., E. Bouveret, A. Rigal, L. Journet, R. Lloubes, and H. Benedetti. 1998. Colicin import into Escherichia coli cells. J. Bacteriol. 180:4993–5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzaroni, J.-C., J.-F. Dubuisson, and A. Vianney. 2002. The Tol proteins of Escherichia coli and their involvement in the translocation of group A colicins. Biochimie. 84:391–397. [DOI] [PubMed] [Google Scholar]
- Massotte, D., and F. Pattus. 1989. Colicin N and its thermolytic fragment induce phospholipid vesicle fusion. FEBS Lett. 257:447–450. [DOI] [PubMed] [Google Scholar]
- Misra, R., and P. R. Reeves. 1987. Role of micF in the tolC-mediated regulation of OmpF, a major outer membrane protein of Escherichia coli K-12. J. Bacteriol. 169:4722–4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, P., D. O. Rudin, H. T. Tien, and W. C. Wescott. 1962. Reconstitution of cell membrane structure in vitro and its transformation into an excitable system. Nature. 194:979–980. [DOI] [PubMed] [Google Scholar]
- Nagel de Zwaig, R., and S. E. Luria. 1967. Genetics and physiology of colicin-tolerant mutants of Escherichia coli. J. Bacteriol. 94:1112–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido, H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67:593–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido, H., and M. Vaara. 1987. Outer membrane. In Escherichia coli and Salmonella typhimurium. Cellular and Molecular Biology. F. C. Neidhardt, editor. Amercian Society for Microbiology. Washington, D.C. 7–22.
- Ohno-Iwashita, Y., and K. Imahori. 1982. Assignment of the functional loci in the colicin E1 molecule by characterization of its proteolytic fragments. J. Biol. Chem. 257:6446–6451. [PubMed] [Google Scholar]
- Phale, P. S., T. Schirmer, A. Prilipov, K.-L. Lou, A. Hardmeyer, and J. P. Rosenbusch. 1997. Voltage gating of Escherichia coli porin channels: role of the constriction loop. Proc. Natl. Acad. Sci. USA. 94:6741–6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samartzidou, H., and A. Delcour. 1999. Excretetion of endogenous cadaverine leads to a decrease in porin-mediated outer membrane permeability. J. Bacteriol. 181:791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samartzidou, H., M. Mehrazin, Z. Xu, M. J. Benedik, and A. Delcour. 2003. Cadaverine inhibition of porin plays a role in cell survival at acidic pH. J. Bacteriol. 185:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler, H., and J. P. Rosenbusch. 1978. Matrix protein from Escherichia coli outer membranes forms voltage-controlled channels in lipid bilayers. Proc. Natl. Acad. Sci. USA. 75:3751–3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydel, U., W. Eberstein, G. Schroder, and K. Brandenburg. 1992. Electrostatic potential in asymmetric planar lipopolysaccharide/phospholipid bilayers probed with the valinomycin-K+ complex. Z. Naturforsch. 47c:757–761. [DOI] [PubMed] [Google Scholar]
- Sharff, A., C. Fanutti, J. Shi, C. Calladine, and B. Luisi. 2001. The role of the TolC family in protein transport and multidrug efflux. From stereochemical certainty to mechanistic hypothesis. Eur. J. Biochem. 268:5011–5026. [DOI] [PubMed] [Google Scholar]
- Soelaiman, S., K. Jakes, N. Wu, C. M. Li, and M. Shoham. 2001. Crystal structure of colicin E3: implications for cell entry and ribosome inactivation. Mol. Cell. 8:1053–1062. [DOI] [PubMed] [Google Scholar]
- Song, J., C. A. Minetti, M. S. Blake, and M. Colombini. 1998. Successful recovery of the normal electrophysiological properties of PorB (class 3) porin from Neisseria meningitidis after expression in Escherichia coli and renaturation. Biochim. Biophys. Acta. 1370:289–298. [DOI] [PubMed] [Google Scholar]
- Stora, T., J. H. Lakey, and H. Vogel. 1999. Ion-channel gating in transmembrane receptor proteins: functional activity in tethered lipid membranes. Angew. Chem. Int. Ed. 38:389–392. [DOI] [PubMed] [Google Scholar]
- Taylor, R., J. W. Burgner, J. Clifton, and W. A. Cramer. 1998. Purification and characterization of monomeric Escherichia coli vitamin B12 receptor with high affinity for colicin E3. J. Biol. Chem. 273:31113–31118. [DOI] [PubMed] [Google Scholar]
- Vakharia, H., G. J. German, and R. J. Misra. 2001. Isolation and characterization of Escherichia coli tolC mutants defective in secreting enzymatically active alpha-hemolysin. J. Bacteriol. 183:6908–6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter, I. R., M. W. Parker, A. D. Tucker, J. H. Lakey, F. Pattus, and D. Tsernoglou. 1998. Crystal structure of a colicin N fragment suggests a model for toxicity. Structure. 6:863–874. [DOI] [PubMed] [Google Scholar]
- Wiener, M., D. Freymann, P. Ghosh, and R. M. Stroud. 1997. Crystal structure of colicin Ia. Nature. 385:461–464. [DOI] [PubMed] [Google Scholar]
- Wiese, A., J. O. Reiner, K. Brandenburg, K. Kawahara, U. Zahringer, and U. Seydel. 1996. Planar asymmetric lipid bilayers of glycosphingolipid or lipopolysaccharide on the one side and phospholipids on the other: membrane potential, porin function, and complement activation. Biophys. J. 70:321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese, A., G. Schroder, K. Brandenburg, A. Hirsch, W. Welte, and U. Seydel. 1994. Influence of the lipid matrix on incorporation and function of LPS-free porin from Paracoccus denitrificans. Biochim. Biophys. Acta. 1190:231–242. [DOI] [PubMed] [Google Scholar]
- Zakharov, S. D., and W. A. Cramer. 2004. On the mechanism and pathway of colicin import across the E. coli outer membrane. Front. Biosci. 9:1311–1317. [DOI] [PubMed] [Google Scholar]
- Zhang, Y.-L., and W. A. Cramer. 1992. Constraints imposed by protease accessibility on the transmembrane and surface topography of the colicin E1 channel. Protein Sci. 1:1666–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]