Abstract
Recent studies have indicated that the tip links and kinocilial links of sensory hair bundles in the inner ear have similar properties and share a common epitope, and that cadherin 23 may also be a component of each link type. Transmission electron microscopy was therefore used to study and compare the fine structure of the tip links and kinocilial links in avian sensory hair bundles. Tannic acid treatment revealed a thin strand, 150–200 nm long and 8–11 nm thick, present in both link types. Fourier analysis of link images showed that the strand of both link types is formed from two filaments coiled in a helix-like arrangement with an axial period of 20–25 nm, with each filament composed of globular structures that are ∼4 nm in diameter. Differences in the radius and period of the helix-like structure may underlie the observed variation in the length of tip and kinocilial links. The similar helix-like structure of the tip links and kinocilial links is in accord with the presence of a common cell-surface antigen (TLA antigen) and similarities in the physical and chemical properties of the two link types. The spacing of the globular structures comprising each filament of the two link types is similar to the 4.3 nm center-to-center spacing reported for the globular cadherin repeat, and is consistent with the suggestion that cadherin 23 is the tip link.
INTRODUCTION
Several types of extracellular stereocilia links, tip links, horizontal top connectors, shaft connectors, ankle links, and kinocilial links have been described in the sensory hair bundles of birds (Pickles et al., 1989; Richardson et al., 1990; Goodyear and Richardson, 1992, 1999, 2003). Deflection of hair-cell stereocilia results in the opening and closing of mechanoelectrical transduction channels and the tip links are thought to gate these channels (Hudspeth, 1989; Pickles and Corey, 1992). In electron micrographs, the tip link appears as a thin strand that extends from the tip of a shorter stereocilium and attaches to an adjacent taller stereocilium (Osborne et al., 1984, 1988; Pickles et al., 1984, Furness and Hackney, 1985). The diameter of the tip links reported by different authors varies from 5.5 ± 1.7 nm (Osborne et al., 1988) to 10.3 ± 0.3 nm (Kachar et al., 2000), and may depend on the fixation and preparation techniques used to visualize them and how the boundaries of the link are determined. A helical structure of thin, coiled filaments composed of globular repeated elements has been previously described for the tip-link strand in mammals (Kachar et al., 2000; Tsuprun and Santi, 2000). The filaments appear to separate at their ends and form multiple insertion points into the membrane of the stereocilium (Kachar et al., 2000).
Kinocilial links, a class of link that connects the kinocilium to the tallest stereocilia, are also composed of individual thin strands similar to those of the tip links; however, these strands can aggregate laterally to form thicker structures (Goodyear and Richardson, 2003). Individual kinocilial links may, like tip links (Kachar et al., 2000; Tsuprun and Santi, 2000), be formed from thinner helically-wound filaments (Goodyear and Richardson, 2003). The tip-link antigen (TLA) is a novel antigen associated with both tip links and kinocilial links, and it has been suggested that it may be a member of the cadherin superfamily (Goodyear and Richardson, 2003). It has been recently shown that antibodies against Cdh23 label tip links and kinocilial links in the frog saccule (Siemens et al., 2004), and that tip links are absent in zebrafish homozygous for mutations in cdh23 (Sollner et al., 2004). The cadherins are a superfamily of transmembrane proteins that mediate Ca2+-dependent cell-cell adhesion (Yagi and Takeichi, 2000). All cadherins are made up of an extracellular domain, a transmembrane domain, and a cytoplasmic domain. Cadherins differ in the number of repeat sequences, the so-called cadherin repeats or cadherin motifs, located in their extracellular region. The extracellular region of the classical cadherins, such as E-, N-, P-, and C-cadherins, comprises five similar cadherin repeats. Cdh23 is closely related to the subgroup of nonclassical cadherins defined by the Drosophila cadherins, Dachsous, and Fat, which are characterized by a large number of similar cadherin repeats: 27 for Dachsous and 34 for Fat (Nollet et al., 2000; Yagi and Takeichi, 2000). The extracellular region of Cdh23 is composed of 27 tandemly arranged cadherin repeats that account for 87% of the overall mass of the protein (Di Palma et al., 2001).
The purpose of this study was to compare the structure of tip links and kinocilial links in avian sensory hair bundles, and to determine to what extent the structures observed are consistent with the hypothesis that Cdh23 is the major component of these two link types.
MATERIALS AND METHODS
Transmission electron microscopy
Utricles from 1–2 day posthatch chickens were dissected in HEPES-buffered (10 mM, pH 7.2) HBSS, fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.2, containing 1% tannic acid for 2 h at room temperature, rinsed three times in sodium cacodylate buffer, and postfixed with cacodylate-buffered 1% osmium tetroxide for 1 h. Tissue pieces were finally washed three times in cacodylate buffer and once with H2O, dehydrated through a series of ascending concentrations of ethanol, equilibrated in propylene oxide, and embedded in Taab 812 resin. Tissue blocks were cured at 60°C for 24–48 h. Ultrathin sections (100 and 200 nm thick) were cut on a Reichert Ultracut E microtome (Leica, Milton Keynts, UK), mounted on copper grids, counterstained with 1% aqueous uranyl acetate followed by lead citrate, and viewed in a Hitachi (Tokyo, Japan) 7100 transmission electron microscope operating at 75 kV.
Image analysis
Noise filtering and adjustment of the brightness and contrast of the entire image field were used to enhance the images and accentuate their details. The low spatial frequencies (∼1/10 nm−1 and below as estimated from the diffraction pattern), which normally do not contribute to the structure, were set to reduced values using an Adobe Photoshop high-pass filter. All distinct strands of tip links and kinocilial links, which did not overlap with other strands, were selected interactively from digitized micrographs. The digitized images of the links were Fourier transformed to study the helical structure of the links. The interpretation of diffraction patterns derived from electron micrographs of helical structures has been discussed previously by DeRosier and Moore (1970). Image-Pro Plus (version 4.1) for Windows (Media Cybernetics, Silver Spring, MD) or NIH Image (version 1.60) software was used for Fourier transformation (128 × 128 pixels size) and calculating the amplitudes and phases of the diffraction peaks in the link images. The selected area of the link image, smaller than 128 × 128 pixels with a pixel size of ∼1.6 nm at the specimen level, was brought to a standard 128 × 128 pixel size box with addition of densities of zero magnitude outside the image. The amplitudes and phases of the diffraction patterns were calculated using Image-Pro Plus software and displayed as bitmap (128 × 128) images. The transform sampling interval was (1.6 × 64)−1 nm−1 in the X and Y directions. The periodicity of the structure was measured from the position of the diffraction peaks in the Fourier transform of the image using structures of known periodicity for calibration. The first zero of the diffraction area corresponded to resolution of ∼3-nm in the link images. Five tip-link images and five kinocilial link images showing relatively symmetric intensities corresponding to a periodicity of ∼20 nm, and a near-meridianal intensity with a periodicity of ∼4-nm, were analyzed. Three separate micrographs showing multiple examples of ankle links were also subjected to image analysis.
RESULTS
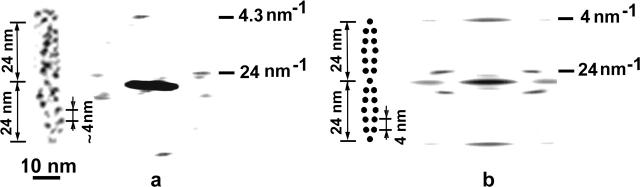
In our micrographs, the tip link is a fine strand ∼8–11 nm in diameter and with a length of 150–200 nm that extends obliquely between the tip of a shorter stereocilium and the side of an adjacent taller stereocilium of the next row. Typical images of tip links (Fig. 1, a–e) show small, rounded particles (Fig. 1, b and d). To study the periodic distribution of these particles, the noise-filtered images of tip links were Fourier transformed. Analysis of five typical Fourier transforms of tip-link images shows two peaks located on the layer-line corresponding to a periodicity of 22.5 ± 2 nm, and a near-meridianal peak on the layer-line corresponding to a periodicity of 4.2 ± 0.2 nm (Fig. 1, b and d). The near-symmetric location of the diffraction peaks relative to the meridian on the horizontal layer-lines is characteristic of a structure that has very close to helical symmetry. High-power images of the tip links frequently display two filaments composed of small globular units (Fig. 1 e).
FIGURE 1.
(a and c) Micrographs of tannic-acid stained tip links connecting adjacent stereocilia. (b and d) High-power images of the tip links and their computer Fourier transforms. Two near-symmetric diffraction peaks and one near-meridianal peak have a periodicity of 22–24 nm and 4.2–4.3 nm, respectively. (e) Three examples showing the two fine filaments of each tip link, illustrating small globular repeats (arrowheads).
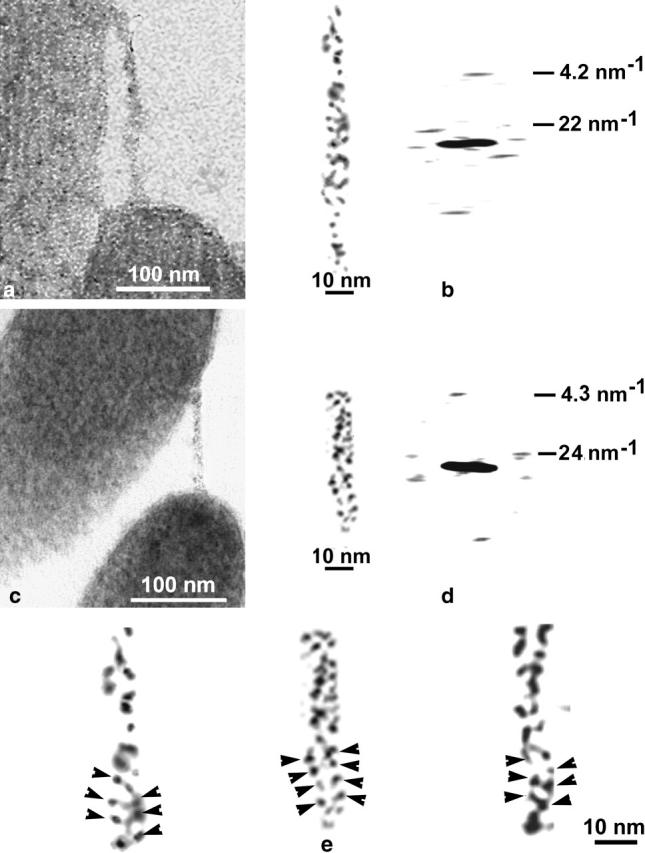
Numerous kinocilial links that connect the kinocilium to adjacent stereocilia were observed in longitudinal sections of the utricular hair bundles (Fig. 2 a). Kinocilial links are frequently laterally aggregated; however, individual strands having a diameter (8–11 nm) similar to that of tip links, may be seen occasionally (Fig. 2, b, c, e, and f). The length of kinocilial links (120–200 nm) depends on the spacing between the kinocilium and adjacent stereocilia. The characteristic Fourier transform (see Materials and Methods) obtained from five images of individual kinocilial-link strands showed two near-symmetric peaks located on the layer-line corresponding to a periodicity of 22 ± 2 nm, and a near-meridianal peak on the layer-line corresponding to a periodicity of 4.1 ± 0.2 nm (Fig. 2, b and d). This was similar to the diffraction patterns obtained from the images of tip links (Fig. 1, b and d). The data indicate that these two types of links have a similar helical structure composed of globular units, each ∼4 nm in diameter. Each layer-line in the Fourier transform of helical structure arising from a particular family of helices, and the position of each peak on layer lines, is related to the Bessel function ℐn(2πrR), where r is the helix radius (Cochran et al., 1952; Klug et al., 1958, DeRosier and Moore, 1970). The relative phase of the two peaks shows whether n (number of helices) is odd or even. If the phase origin is on the helical axes, two peaks should be in phase for an even value or out of phase for an odd value of n. Fig. 2 d shows the computer amplitudes and phase of the Fourier transform (Materials and Methods) on the 22-nm−1 layer line of the kinocilial link shown in Fig. 2 c. The radius of the helical structure and position of the two peaks on this layer line is such that n is between 2 and 3. The computed phases of these peaks are, with some approximation, in phase. This reduces this choice to an even number, or n = 2, for the number of helical filaments in the kinocilial link. As for the tip link, two filaments consisting of small globular units can be directly seen in the images of individual kinocilial links (Fig. 2 f). The two peaks located on the ∼22-nm−1 layer line and the ∼4.0-nm−1 near-meridianal peak, appear to correspond to an axial period of ∼22 nm for the helical array and an ∼4 nm axial shift for the globular units, respectively. The diffraction intensity on the ∼4-nm−1 layer line is located around the meridian as a single peak with very little variation in phase (Fig. 2 e). It appears to correspond to the zero order of the Bessel function and indicates that the globular units of the two filaments are related by twofold rotational symmetry rather than showing axial staggering. Local bending of the links is observed in some images and may cause a small deflection of the ∼4-nm−1 diffraction peak away from the meridian. These deflections are unlikely to correspond to the first order of the Bessel function (one-start helix) that would be expected if the link were composed of two staggered filaments without twofold symmetry. A tentative model for tip and kinocilial links having a two-start helical structure is proposed that approximates these diffraction data (Fig. 3). We suggest that the arrangement of globular units in both tip and kinocilial links is close to a two-start helical array with 10–12 units per ∼22-nm period of helical structure (i.e., equivalent to 20–24 units per ∼44-nm helical pitch for each of two filaments related by twofold rotational symmetry).
FIGURE 2.
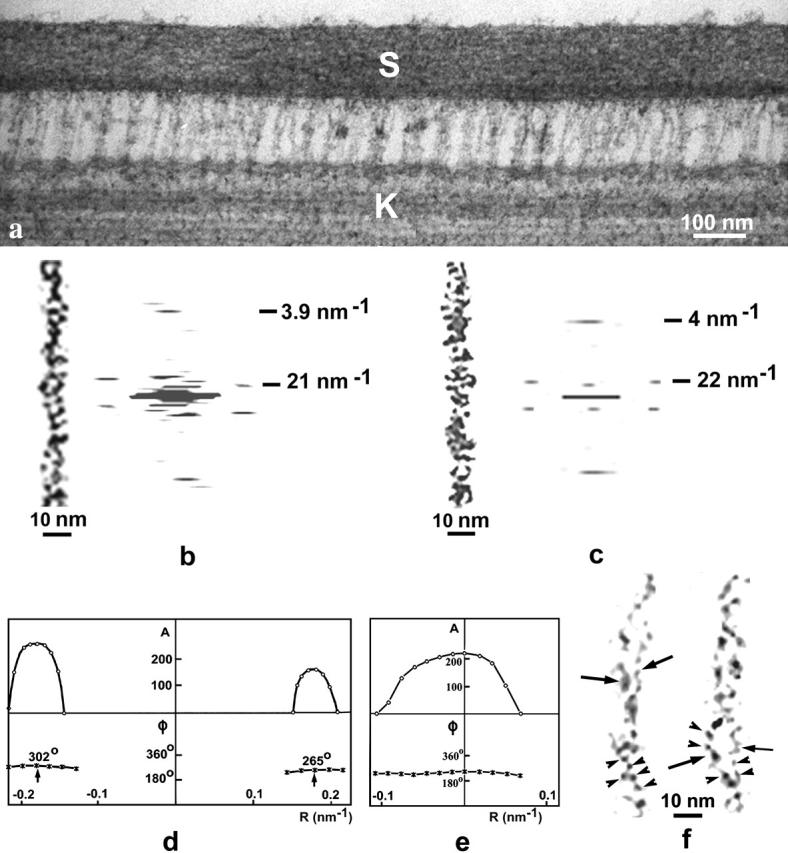
(a) Micrograph of tannic-acid stained kinocilial links. (b and c) Noise-filtered images of kinocilial links and their computer Fourier transforms. Two, strong, near-symmetric diffraction peaks and one near-meridianal peak correspond to a periodicity of 21–22 nm and 3.9–4 nm, respectively. (d and e) Digital amplitudes (A) and phases (φ) for the 22-nm−1 (d) and 4-nm−1 (e) layer lines of the diffraction pattern shown in c. The computed phases of the two 22-nm−1 peaks are approximately in phase (d), indicating an even number of helical filaments in the link. The 4-nm−1 intensity function shows one peak with a small variation in phase (e) corresponding to the zero order of the Bessel function. (f) Two fine filaments (arrows) of the kinocilial link showing small globular protein units (arrowheads).
FIGURE 3.
(a) Micrograph of a region of a tip link showing periodic widening and narrowing of the structure. A 24-nm periodicity between crossover points and ∼4.3-nm axial shift between globular units are seen in the Fourier transform. (b) Projected structure and Fourier transform of the link model. Two coiled helices of 12 globular units per 48-nm pitch are related by twofold rotational symmetry resulting in a 24-nm axial period for the helical array. The meridianal diffraction peak of 4.0-nm periodicity corresponds to the axial repeat of the globular units.
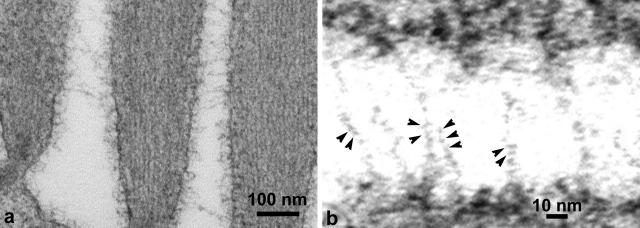
Ankle links are a distinct type of interstereocilary link found around the base of sensory hair bundles in the chicken utricle. These links are formed by thin filaments that are ∼4 nm in diameter and are composed of globular structures with a spacing of 3–5 nm (Fig. 4). However, these links do not show a double-helical structure like that observed for tip and kinocilial links.
FIGURE 4.
(a) Filamentous ankle links distributed between adjacent stereocilia near the base of the hair bundle. (b) High-power image showing small globular structures (arrowheads) regularly arranged along single ankle-link filaments.
DISCUSSION
Our data indicate that kinocilial links and tip links from chicken hair bundles are both likely to be helix-like arrays of two coiled filaments. Each of the coiled filaments consists of globular units, ∼4 nm in diameter, and the axial period of the helical structure varies from 20 to 25 nm. A double-helix structure for the tip link has been described previously in auditory (guinea-pig cochlea) and vestibular (guinea-pig utricle and bullfrog sacculus) hair cells by Kachar et al. (2000). Although these authors reported a 30-nm periodicity for the helical structure, the periodicity of the thinner and thicker regions of the double-helix structure measured from their published images of tip links is 22–25-nm. The same periodicity was observed for tip links in chinchilla auditory hair bundles (Tsuprun and Santi, 2000) indicating that these links in birds are likely to have two-start helix-like structure similar to auditory and vestibular links of other species.
The similarity in the structure of the tip and kinocilial links is consistent with the presence of a common cell-surface antigen (TLA antigen) associated with both links (Goodyear and Richardson, 2003). Although tip links and kinocilial links have common antigens (TLA and Cdh23) associated with them and have a similar structure, these two types of links may have different functional roles. Tip links are thought to gate the transducer channels during mechanical deflection of the stereocilia (Pickles et al., 1984; Assad et al., 1991). In the tip-link model for transduction, the tip links provide the tension for opening the mechanically sensitive ion channels that may lie at each end of the tip link (Denk et al., 1995). The kinocilium is not thought to be essential for mechanoelectrical transduction (Hudspeth and Jacobs, 1979). Unlike tip links, the kinocilial links are aggregated into large clusters between the kinocilium and the tallest stereocilia, and couple the kinocilium to the nearest stereocilia in the hair bundle. Side links (Osborne et al., 1984; Pickles et al., 1984; Furness and Hackney, 1985), like kinocilial links, are also aggregated into clusters and form bonds between each other that may help maintain the integrity and rigidity of the bundle (Tsuprun and Santi, 2002). A single side link is formed from two beaded filaments that extend from adjacent stereocilia. These filaments are folded at their distal ends to form a single globule in the center of the gap between the stereocilia (Tsuprun et al., 2003). Unlike tip links or kinocilial links, the filaments of the side link are shorter, not helically wound, and form a periodic “zipper-like” lattice between adjacent stereocilia (Tsuprun et al., 2003).
Recent immunological, genetic, and biochemical evidence indicates that Cdh23 may be a component of both the tip link and the kinocilial link (Siemens et al., 2004; Sollner et al., 2004). Cell adhesion mediated by cadherins is thought to be homophilic and involve Ca2+-dependent interactions between the extracellular cadherin domains (Nollet et al., 2000; Yagi and Takeichi, 2000). X-ray structural analysis suggests a molecular mechanism for adhesion between cells by classical cadherins involving cis-interactions forming same-cell dimers and trans-interactions between cadherins extending from opposing cells (Boggon et al., 2002). For classical cadherins, cis- and trans-interactions result in a “zipper-like” superstructure, and Ca2+-binding is essential for linearization, rigidification, and dimerization of the cadherin molecules (Nagar et al., 1996). Cdh23 has also been demonstrated to mediate Ca2+-dependent homophilic cell-cell adhesion (Siemens et al., 2004).
The structural organization of the single strand observed for the tip and kinocilial links could possibly be explained by the association of extracellular Cdh23 domains. The predicted extracellular domain of Cdh23 is composed of 27 similar cadherin repeats (Di Palma et al., 2001; Petit, 2001). X-ray crystallography has shown that cadherin repeats have a globular structure with a center-to-center distance of ∼4.3 nm along their extended chains (Boggon et al., 2002). The globular units observed at a regular spacing of ∼4 nm along the tip and kinocilial links may correspond to the cadherin repeats of Cdh23. Since a minimal center-to-center distance between the globular units of two adjacent filaments in the tip link is ≤ 4 nm (Figs. 1 e and 2 e) these globular units are unlikely to be each comprised of two aligned cadherin repeats, each of which has minimal size of ∼3 nm along its short axis and ∼5 nm along its long axis (Boggon et al., 2002; Corey and Sotomayor, 2004). A twofold symmetry between adjacent filaments, as observed for tip and kinocilial links in this study, has also been observed by x-ray analysis in classical cadherins (Boggon et al., 2002).
A typical single link strand is 8–11 nm thick and up to ∼200 nm in length, indicating the possible supramolecular organization of Cdh23 in the link. Our structural finding and the known properties of Cdh23 support the suggestion that the two coiled filaments in a double-helix-like single strand of the tip and kinocilial links are two, homophilically-associated Cdh23 extracellular regions, each composed of 27 repeating ectodomains that are distributed at regular intervals of ∼4 nm along these filaments. The tip or kinocilial link would then be formed from two such dimers connecting and interacting in trans at their distal ends (Fig. 5). Thus, the single strand of the tip and kinocilial links would contain the extracellular domains of four Cdh23 molecules arranged as a pair of homophilic dimers. The helix-like arrangement of two homophilic Cdh23 cis-dimers would presumably stabilize a long linear conformation of the link strand; it is not observed in small classical cadherins where cis- and trans-interactions in N-terminal ectodomains result in a “zipper-like” superstructure (Boggon et al., 2002). Although our data may be consistent with the idea that Cdh23 is the tip link, it is not clear whether interactions between the N-terminal cadherin repeats would withstand the forces the tip link is subjected to during hair bundle deflections.
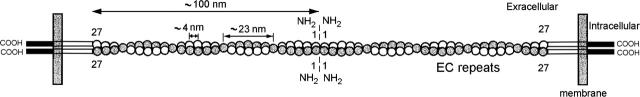
FIGURE 5.
Hypothetical model for the helix-like strand of the tip and kinocilial links. The strand is formed by the homophilic association of two in cis Cdh23 dimers that interact homophilically in trans via the first Cdh23 repeat (1) at the region indicated by the vertical broken line. Each Cdh23 molecule is composed of 27 similar EC repeats, regularly arranged with ∼4-nm spacing. The link contains four Cdh23 molecules arranged as two winding homophilic dimers.
The length of tip links varies in our micrographs from 150 to 200 nm. Since filaments are wound in helices, their length is less than their fully elongated length by approximately SQRT{1 + (2πr/c)2} times, where r is the helix radius and c is the period of the helical structure (∼22.5 nm for the tip link). For a helical structure composed of tightly-packed spherical domains 4 nm in diameter (r = 2 nm), the length of the helical structure will be ∼85% of its full, unwound length for c = 20 nm and ∼89% for c = 25 nm. For two helically-wound cadherin 23 cis-dimers interacting in trans the total length would be 0.87 × 2 × 27 × 4.0 nm ≃ 190 nm. However, cadherin domains are not identical and are not likely to form ideal helical arrays. We observed some variation in the distance between centers of globular units of two adjacent coiled filaments (∼4–7 nm) and even some partial separation of these filaments (Figs. 1 and 2). For helical structure with c = 22.5 nm, composed of spherical units 4 nm in diameter with ∼7 nm between the centers of units in two adjacent filaments (i.e., r ∼ 3.5 nm), the length of the helical structure will be only ∼71% of its full length or ∼150 nm. Thus the observed 150–200-nm range of the tip link length could be explained by the variation in the radius and period of the helix-like structure.
A helical array of structural units corresponding to cadherin domains may be stabilized by many repeating unit-unit interactions along each filament and between filaments that may provide a rigidity and stiffness to the tip and kinocilial links. Individual cadherin domains containing seven β-strands are not likely to stretch (Corey and Sotomayor, 2004), so the helix-like array of the tip link composed of nonelastic Cdh23 ectodomains will be more like a stiff cable than the elastic gating spring postulated to open the mechanotransducer channel. If the mammalian mechanotransducer channel is indeed similar to the invertebrate channel Nompc (Walker et al., 2000) and its zebrafish homolog TRPN1 (Sidi et al., 2003), the 29 ankyrin repeats present in these molecules may be elastic and provide the required gating spring (Howard and Bechstedt, 2004).
Acknowledgments
We are grateful to Drs. P. Schachern and M. Paparella for critical reading of the manuscript.
This research was supported by grants from the American Hearing Research Foundation, the International Hearing Foundation, National Institutes of Health-National Institute on Deafness and Other Communication Disorders grant P30 DC04660, and the Wellcome Trust (grant No. 05740/Z/99Z).
References
- Assad, J. A., G. M. Shepherd, and D. P. Corey. 1991. Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron. 7:985–994. [DOI] [PubMed] [Google Scholar]
- Boggon, T. J., J. Murray, S. Chappius-Flament, E. Wong, B. Gumbiner, and L. Shapiro. 2002. C-cadherin ectodomain and implication for cell adhesion mechanism. Science. 296:1308–1313. [DOI] [PubMed] [Google Scholar]
- Cochran, W., F. H. C. Crick, and V. Vand. 1952. The structure of synthetic polypeptides. I. Transform of atoms on a helix. Acta Crystallogr. 5:581–586. [Google Scholar]
- Corey, D. P., and M. Sotomayor. 2004. Hearing. Tightrope act. Nature. 428:901–903. [DOI] [PubMed] [Google Scholar]
- Denk, W., J. R. Holt, G. M. Shepherd, and D. P. Corey. 1995. Calcium imaging of single stereocilia in hair cells: localization of transduction channels at both ends of tip links. Neuron. 15:1311–1321. [DOI] [PubMed] [Google Scholar]
- Di Palma, F., R. Pellegrino, and K. Noben-Trauth. 2001. Genomic structure, alternative splice forms and normal mutant alleles of cadherin 23 (Chr23). Gene. 281:31–41. [DOI] [PubMed] [Google Scholar]
- DeRosier, D. J., and P. B. Moore. 1970. Three dimensional images from electron micrographs of structures with helical symmetry. J. Mol. Biol. 52:355–359. [DOI] [PubMed] [Google Scholar]
- Furness, D. N., and C. M. Hackney. 1985. Cross-links between stereocilia in the guinea pig cochlea. Hear. Res. 18:177–188. [DOI] [PubMed] [Google Scholar]
- Goodyear, R., and G. Richardson. 1992. Distribution of the 275 kD hair cell antigen and cell surface specializations on auditory and vestibular hair bundles in the chicken inner ear. J. Comp. Neurol. 325:243–256. [DOI] [PubMed] [Google Scholar]
- Goodyear, R., and G. Richardson. 1999. The ankle-link antigen: an epitope sensitive to calcium chelation associated with the hair-cell surface and the calycal processes of photoreceptors. J. Neurosci. 19:3761–3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear, R. J., and G. P. Richardson. 2003. A novel antigen sensitive to calcium chelation that is associated with the tip links and kinocilial links of sensory hair bundles. J. Neurosci. 23:4878–4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard, J., and S. Bechstedt. 2004. Hypothesis: a helix of ankyrin repeats of NOMPC-TPR ion channel is the gating spring of mechanoreceptors. Curr. Biol. 114:R224–R226. [DOI] [PubMed] [Google Scholar]
- Hudspeth, A. J. 1989. How the ear's works work. Nature. 341:397–404. [DOI] [PubMed] [Google Scholar]
- Hudspeth, A. J., and R. Jacobs. 1979. Stereocilia mediate transduction in vertebrate hair cells. Proc. Natl. Acad. Sci. USA. 76:1506–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachar, B., M. Parrakal, M. Kurc, Y. Zhao, and P. G. Gillespie. 2000. High-resolution structure of hair-cell tip links. Proc. Natl. Acad. Sci. USA. 97:13336–13341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug, A., F. H. C. Crick, and H. W. Wyckoff. 1958. Diffraction by helical structures. Acta Crystallogr. 11:199–213. [Google Scholar]
- Nagar, M., M. Overduin, M. Ikura, and J. M. Rini. 1996. Structural basis of calcium-induced E-cadherin rigidification and dimerization. Nature. 380:360–364. [DOI] [PubMed] [Google Scholar]
- Nollet, F., P. Kools, and F. van Roy. 2000. Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. J. Mol. Biol. 299:551–572. [DOI] [PubMed] [Google Scholar]
- Osborne, M. P., S. D. Comis, and J. O. Pickles. 1984. Morphology and cross-linkage of stereocilia in the guinea-pig labyrinth examined without the use of osmium as a fixative. Cell Tissue Res. 237:43–48. [DOI] [PubMed] [Google Scholar]
- Osborne, M. P., S. D. Comis, and J. O. Pickles. 1988. Further observation on the fine structure of tip links between stereocilia of the guinea pig cochlea. Hear. Res. 35:99–108. [DOI] [PubMed] [Google Scholar]
- Petit, C. 2001. Usher syndrome: from genetics to pathogenesis. Annu. Rev. Genomics Hum. Genet. 2:271–279. [DOI] [PubMed] [Google Scholar]
- Pickles, J. O., J. Brix, S. D. Comis, O. Gleich, C. Koppl, G. A. Manley, and M. P. Osborne. 1989. The organization of the tip links and stereocilia on hair cells of bird and lizard basilar papillae. Hear. Res. 41:31–41. [DOI] [PubMed] [Google Scholar]
- Pickles, J. O., S. D. Comis, and M. P. Osborne. 1984. Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear. Res. 15:103–112. [DOI] [PubMed] [Google Scholar]
- Pickles, J. O., and D. P. Corey. 1992. Mechanoelectrical transduction by hair cells. Trends Neurosci. 15:254–259. [DOI] [PubMed] [Google Scholar]
- Richardson, G. P., S. Bartolami, and I. J. Russell. 1990. Identification of a 275 kDa protein associated with the apical surfaces of sensory hair cells in the avian inner ear. J. Cell Biol. 110:1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidi, S., R. W. Friedrich, and T. Nicolson. 2003. Gnomic TRP channel required for vertebrate sensory hair cell mechanotransduction. Science. 301:96–99. [DOI] [PubMed] [Google Scholar]
- Siemens, J., C. Lillo, R. A. Dumont, A. Reynolds, D. S. Williams, P. G. Gillespie, and U. Mueller. 2004. Cadherin 23 is a component of the tip link in hair-cell stereocilia. Nature. 428:950–959. [DOI] [PubMed] [Google Scholar]
- Sollner, C., G.-J. Rauch, J. Simens, R. Geisler, S. Schuster, U. Muller, and T. Nicolson. 2004. Mutation in cadherin 23 affects tip link in zebrafish sensory hair cells. Nature. 428:955–959. [DOI] [PubMed] [Google Scholar]
- Tsuprun, V., and P. Santi. 2000. Helical structure of hair cell stereocilia tip links in the chinchilla cochlea. J. Assoc. Res. Otolaryngol. 1:224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuprun, V., and P. Santi. 2002. Structure of outer hair cell stereocilia side and attachment links in the chinchilla cochlea. J. Histochem. Cytochem. 50:493–502. [DOI] [PubMed] [Google Scholar]
- Tsuprun, V., P. Schachern, S. Cureoglu, and M. Paparella. 2003. Structure of the stereocilia side links and morphology of auditory hair bundle in relation to noise exposure in the chinchilla. J. Neurocytol. 32:1117–1128. [DOI] [PubMed] [Google Scholar]
- Walker, R. G., A. T. Willingham, and C. S. Zuker. 2000. A Drosophila mechansensory transduction channel. Science. 287:2132–2133. [DOI] [PubMed] [Google Scholar]
- Yagi, T., and M. Takeichi. 2000. Cadherin superfamily genes: function, genomic organization, and neurologic diversity. Genes Dev. 114:1169–1180. [PubMed] [Google Scholar]