Abstract
Little is known about the influence of substrate-bound gradients on neuronal development, since it has been difficult to fabricate gradients over the distances typically required for biological studies (a few hundred micrometers). This article demonstrates a generally applicable technique for the fabrication of substrate-bound gradients of proteins with complex shapes, using laminar flows in microchannels. Gradients that range from pure laminin to pure BSA were formed in solution by using a network of microchannels, and these proteins were allowed to adsorb onto a homogeneous layer of poly-l-lysine. Rat hippocampal neurons were cultivated on these substrate-bound gradients. Analysis of optical images of these neurons showed that axon specification is oriented in the direction of increasing surface density of laminin. Linear gradients in laminin adsorbed from a gradient in solution having a slope of ∇[laminin] > about 0.06 μg (ml⋅μm)−1 (defined by dividing the change of concentration of laminin in solution over the distance of the gradient) orient axon specification, whereas those with ∇[laminin] < about 0.06 μg (ml⋅μm)−1 have no effect.
Keywords: microfluidics‖neuronal polarity‖hippocampal
Gradients of chemoattractant and chemorepellent substances play central roles in controlling the development of the brain (1). Although the influence of gradients of soluble substances on neuronal behavior has been studied extensively and has been used to unravel the molecular and cellular mechanisms of axon guidance (2), much less is known about gradients of substrate-bound substances (3). Generating consistent substrate-bound gradients over the distances required for biological studies (a few hundred micrometers) has been difficult, and this difficulty hindered the investigation of the role of immobilized gradients in neuronal development (e.g., establishment of cellular polarity and axon guidance) and cell migration. This article demonstrates a generally applicable technique for the fabrication of substrate-bound gradients that uses laminar flow of fluids in microchannels; we have described the microfluidic systems used to form these gradients (4). By generating gradients in the concentration of proteins in solution and allowing them to adsorb on a surface, we fabricated substrate-bound gradients that range from pure laminin to pure BSA immobilized on a uniform layer of poly-l-lysine (PLL) over distances of a few hundred micrometers. We demonstrate that axon specification of rat hippocampal neurons cultivated on these gradients is oriented in the direction of increasing concentration of laminin. We demonstrate that gradients of extracellular molecules adsorbed on the surface are capable of orienting the polarity of cultured hippocampal neurons.
Hippocampal neurons in culture develop their characteristic structural and functional polarity in a stereotyped sequence of developmental events (5). Cells first attach to the substrate and then develop processes. When new processes start to form, they cannot be distinguished as either axons or dendrites until one of them starts to elongate rapidly. This process quickly reaches a length at least 10–15 μm longer than any other process of the same neuron within 24 h; it invariably becomes the axon (6). In an isotropic environment, establishment of neuronal polarity of hippocampal neurons is random in orientation and occurs spontaneously within the first 2 days in culture (5).
Extracellular signals stimulating rapid outgrowth of one of the minor processes of an undifferentiated neuron can direct axon specification (7, 8). For example, axon specification of an undifferentiated neuron is triggered when one of the neurites of the cell crosses from a PLL substrate to laminin; this neurite starts elongating rapidly on laminin and becomes the axon (8). Generalization of this observation led us to the hypothesis that axon specification of an undifferentiated neuron can be oriented in an extracellular gradient that favors outgrowth of the neurites in one direction. A gradient that ranges from laminin to BSA adsorbed on a homogeneous layer of PLL represents such a gradient. Testing this hypothesis begins to answer the question of the influence of gradients of immobilized, extracellular proteins on neuronal development. Our method is also a general one that enables the studies of haptotaxis, directed cellular movement in a gradient of adhesion molecule on the surface.
Materials and Methods
Design of Microfluidic Networks.
Our approach to the fabrication of substrate-bound gradients has two components: (i) the generation of gradients in the concentration of proteins in solution by using a microfluidic device designed for this purpose (4), and (ii) the transfer of this gradient in the concentration of proteins from solution onto a surface by adsorption. The design of the networks for the generation of linear gradients followed procedures similar to those described (4, 9). In brief, linear gradients in concentration spanning 250 μm were approximated with five streams forming a step-profile (Fig. 1A); a network with two inlets and five outputs was used (two-input/five-output). Analogously, linear gradients spanning 200 μm were approximated with four laminar streams by using a network with two inlets and four outputs.
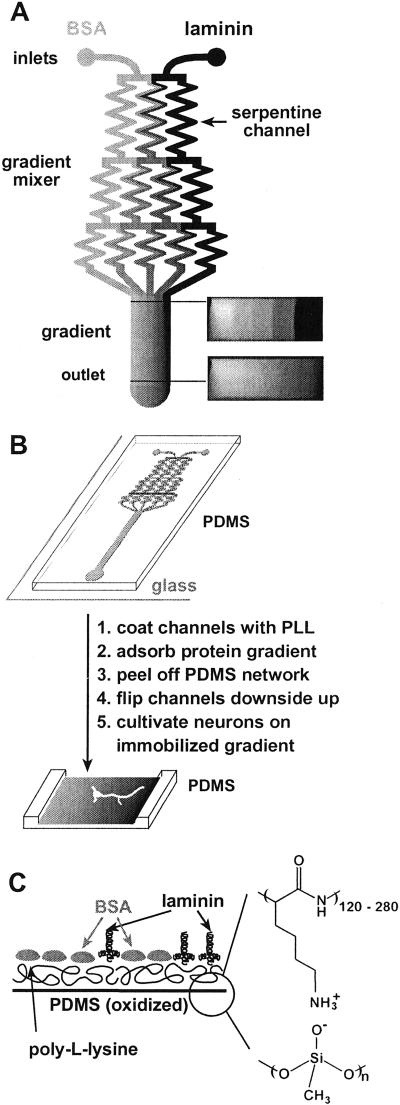
Figure 1.
(A) Schematic drawing of the design of a typical microfluidic network in PDMS that we used for the fabrication of immobilized gradients. Solutions of laminin and BSA were injected into the microfluidic network (inlets). Several streams, each carrying different concentrations of laminin and BSA, were generated in the gradient mixer and combined in a single channel to form a gradient perpendicular to the direction of the flow. The initial step profile created at the junction blurs slightly due to diffusion as the fluid travels downstream. The right-hand side of the diagram shows cross-sectional images of the concentration profile (using pure buffer and fluorescein, D = 5.0 × 10−6 cm2⋅s−1) of the flow visualized by using a confocal microscope. The shadows in the corners of the confocal images are due to the distortion of the light at the channel walls. (B) The diagram summarizes the steps from the fabrication of the substrate-bound gradient to the cultivation of neurons on the gradient immobilized on the substrate. A gradient was deposited on the floor of the channels by adsorption from the flowing stream. The network of microfluidic channels was removed, inverted, and placed in a culture dish. Neurons were plated on the floor of the channel in the region where the gradient had formed. (C) An idealized schematic diagram of the cross section of the surface composition of a substrate-bound gradient composed of laminin and BSA on PLL.
The proper performance of the gradient mixer relies on complete diffusive mixing of all streams in the long serpentine channels of the network (9). To ensure complete mixing, we used channels 55 mm in length (45 μm wide, 50 μm high) and adjusted the flow rates to <1 mm/s. Thus, streams with different concentrations of proteins were allowed to mix by diffusion for ≈1 min in the serpentine channels of the network; this interval is sufficient to ensure complete diffusive mixing of extracellular matrix (ECM) proteins.
Fabrication of Networks and Protein Gradients.
Networks of microchannels in poly(dimethylsiloxane) (PDMS) were fabricated by using soft lithography and rapid prototyping (10–14). The surface of the PDMS replica and a clean glass substrate were treated in an air plasma (2 Torr, 60 sec, 100 W, Harrick Scientific, Ossining, NY) and brought together immediately after activation. An irreversible seal was formed between the PDMS and the glass substrate (10); this assembly produced the required systems of microfluidic channels. To protect the lower part of the network (in which the gradient forms) from sealing irreversibly to the glass coverslip, this part was covered with a glass coverslip during the exposure to the plasma. Prevention of an irreversible seal was necessary to be able to separate the PDMS channel network from the glass substrate after gradient fabrication and to cultivate neurons in the channel. Immediately after sealing the PDMS network to the glass coverslip, the microchannels were filled with PLL solution (1 mg/ml, Sigma, molecular mass 30–70 kDa). The positively charged PLL was allowed to adsorb overnight on the negatively charged surface of plasma-oxidized PDMS to coat the inner surface of the channels. Gradients of laminin vs. BSA in solutions were established for 6 h; a total volume of 200 μl was displaced per network in generating a linear gradient. Each inlet was connected to a 100-μl syringe; one syringe contained the solution of laminin (50 μg/ml, Sigma, L2020) and the other solution of BSA. After adsorption of the gradient, the network enclosing the gradient in the wide channel was removed by cutting and peeling it from the substrate (since this part of the PDMS was not irreversibly sealed against the glass coverslip), rinsed in PBS (Dulbecco's PBS, GIBCO, 14190-136) and stored in 3% BSA (Sigma, A2153) until neurons were plated (typically overnight).
Cultivation of Neurons on Gradients.
Hippocampal neurons from neonatal rats (P0-P1) were cultured by using methods as described (15). Typically, we plated 12,000 neurons in the microchannel of one sample over a channel length of 2 cm.
Characterization of Substrate-Bound Gradients and Visualization of Neurons.
Cells were routinely fixed and stained following standard protocols (8). In brief, neurons were fixed with 4% p-formaldehyde, permeabilized with 0.3% Triton-X, and blocked with 5% BSA for 3 h. Antibodies against laminin (rabbit, 1:40, Sigma, L-9393) and tubulin (mouse, 1:200, Covance, Richmond, CA, MMS-435p) or tau-1 (mouse, 1:100, Chemicon, MAB3420) were applied in 3% BSA at 4°C overnight. Secondary antibodies labeled with FITC (anti-rabbit Ig, 1:40, Amersham Pharmacia, N1034) and Texas red (anti-mouse Ig, 1:40, Amersham Pharmacia, N2031) were applied for 3 h at room temperature. After rinsing with PBS the samples were covered with mounting media (Vectashield) and fluorescent images were taken with a confocal microscope (Leica, TCS 4D).
Results
Generation of Gradients in Solution.
Fig. 1A shows a network of microchannels that we used to generate gradients in the concentration of proteins present in a stream of buffer (4). We started with a small number of fluid streams and allowed them to divide and mix by diffusion in channels with appropriate length (here compacted linearly into a serpentine profile to save area on the chip) into multiple streams differing in the concentrations of proteins. Recombination of these multiple streams into a single stream in a long channel, under conditions in which fluid flow is laminar, formed the gradient and allowed it to propagate along the length of this channel. Diffusion of proteins with high molecular masses (e.g., laminin with molecular mass ≈900 kDa, and BSA molecular mass ≈66 kDa, their diffusion coefficients are on the order of 10−7 cm2/s) over distances of microns was slow relative to the time required for the fluid to move from one end of the channel to the other (≈30 s, in this time, the proteins diffuse 10 ≈ μm). As a result, the concentration gradient of proteins perpendicular to the direction of the flow was maintained along the length of the larger channel. The shape of the gradient was at steady state at any point in the channel, since the solution was constantly renewed.
Formation of Substrate-Bound Gradients.
We fabricated immobilized gradients that range from pure laminin to pure BSA, both on PLL, in two steps. First, we formed a homogeneous layer of PLL on the inner surface of the microchannel—plasma-oxidized PDMS—by adsorption. Second, a binary gradient running from pure laminin to pure BSA was established in solution. The proteins adsorbed from solution and formed a gradient on the uniform PLL layer of the coated walls of the microchannel. Fig. 1B shows in cartoon form the composition of the surface after formation of the substrate-bound gradient of BSA and laminin on PLL. We quantified the profile of the adsorbed gradient of laminin by measuring the fluorescence intensity of immunostained laminin. Absolute concentrations of adsorbed laminin cannot be measured with this technique.
To form linear gradients of laminin adsorbed on the substrates, we allowed a mixture of two noninteracting proteins—laminin and BSA—to adsorb. (One cannot use a single protein in these experiments, because protein adsorption is irreversible, and even low concentrations of a single protein will saturate all of the surface area and form a uniform layer of protein over time.) An increasing gradient in the concentration of laminin in solution was always matched by a decreasing gradient in the concentration of BSA. At the maximum concentration of each protein in solution, the concentration of the complementary protein was zero; the individual maximum concentration of laminin was 25 μg/ml, and that of BSA was 3% (wt/vol; 30 mg/ml). These empirically developed conditions gave approximately linear gradients on the surface. We used BSA as the competing molecule in adsorption to adjust the shape of the gradient in laminin, because adsorbed BSA does not affect the growth of hippocampal neurons (8). BSA also does not change the ability of substrate-bound PLL and laminin to support attachment and outgrowth of hippocampal neurons (8); we confirmed these observations in separate experiments.
Since we have not measured the absolute surface densities of adsorbed proteins, we specified immobilized gradients by the slope of the concentration of laminin in solution. In solution, the slope was defined as the change in the concentration of laminin per micrometer. For instance, in a channel 250 μm wide, supporting a gradient in laminin in solution that runs from 0 μg/ml to 25 μg/ml and a gradient in BSA of opposite sign that runs from 3% to 0%, the slope of the gradient of laminin in solution was ∇[laminin] = 0.1 μg(ml⋅μm)−1 (∇[laminin] = (∂/∂x + ∂/∂y + ∂/∂z)[laminin] ≈ d[laminin]/dx).
Gradients of Laminin and BSA on PLL Orient Axonogenesis.
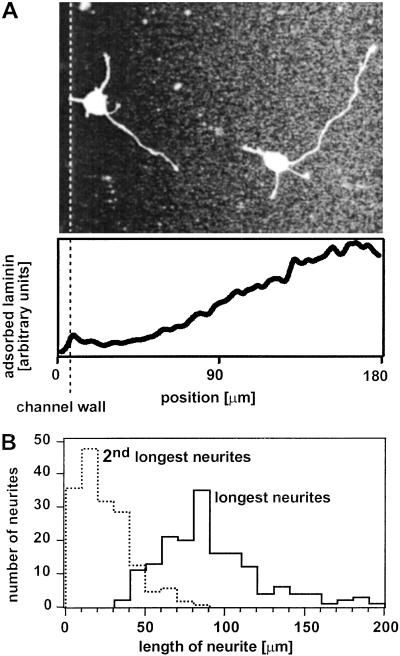
Fig. 2A shows a representative micrograph of two neurons on a gradient of laminin and BSA on PLL after 24 h in culture. Both cells display the characteristic morphology of polarized neurons; both have several short processes (which become dendrites when the neuron matures) and one long process (which becomes the axon) (16). As this micrograph demonstrates, there is a preference for the longest process of a neuron—the nascent axon (5, 6)—to extend in the direction of increasing concentration of laminin. By analyzing the relative lengths of the processes of individual cells, we found that the longest process after 24 h in culture is on average four times longer than the second longest of the same cell. Fig. 2B shows a histogram of the length of the longest and second longest process; in this analysis, we included all neurons from the experiments that form the basis for the analysis presented in this article. Earlier work from other groups demonstrated that this ratio is a clear indicator: the longest process invariably becomes the axon of the neuron (5, 6). Immunostaining for tau-1—the diagnostic tool normally used to identify axons—did not work reliably with neurons cultivated for less than 3 days (8).
Figure 2.
Rat hippocampal neurons preferentially extend their presumptive axon (their longest process) in the direction of increasing surface density of laminin. (A) A fluorescence micrograph of neurons that were fixed after 24 h in culture and immunostained for laminin (to visualize the substrate-bound gradient in laminin) and tubulin (to picture the microtubules of the neurons); staining for tubulin is commonly used to visualize the morphology of a neuron. Fluorescent images of tubulin and laminin have been superimposed. The shape of the immobilized gradient in laminin is shown in the graph below the micrograph. The fluorescent signal of the immunostained laminin has been used to measure the shape of the gradient. The slope of the laminin gradient in solution used to fabricate the substrate-bound gradient was ∇[laminin] = 0.125 μg (ml⋅μm)−1. The dotted line indicates the left wall of the channel. The walls of the channel and local bright spots distort the uniformity of the fluorescent intensities (e.g., on the left and right edges of the micrograph). The right wall of the channel is at 250 μm (not shown). (B) A histogram of the length of the longest and second longest process of all neurons that were included in the statistical analysis of axon orientation. After 24 h in culture, the longest process of a neuron is on average four times longer than the second longest process.
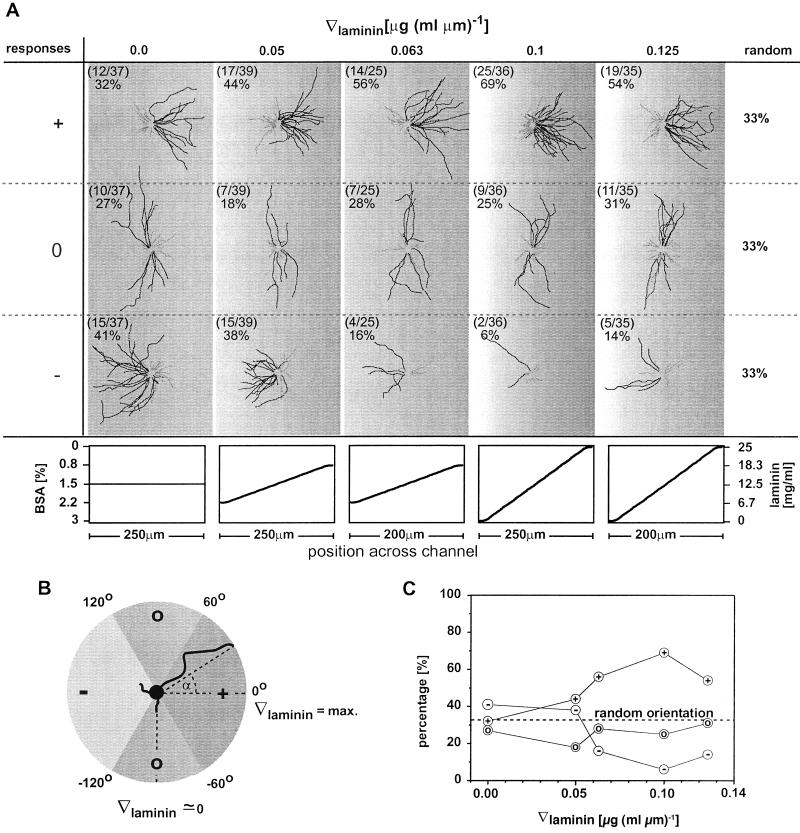
To quantify the effect of the gradient on axonal specification and find the threshold that orients formation of axons, we cultivated neurons on gradients with different slopes in laminin. Fig. 3 shows drawings of all neurons grown on five different gradients with slopes that range from ∇[laminin] = 0.0 μg(ml⋅μm)−1 to ∇[laminin] = 0.125 μg(ml⋅μm)−1; in these drawings, the neurons were grouped and superimposed according to the orientation of the tip of their longest process after 24 h in culture. Only neurons located in the center of a gradient (the center of the gradient was always identical with the center of the channel) were included in our analysis. The composition of the substrate in the center of the channels was the same in all experiments, since the substrate in the center was always exposed to a stream carrying 12.5 μg⋅ml−1 laminin and 1.5% BSA.
Figure 3.
(A) Superimposed drawings of all processes of neurons grown on gradients of laminin on PLL. Neurons were grouped and superimposed according to the orientation of the tip of the axon (the longest process) after 24 h in culture. This analysis was done for each gradient slope separately (columns). The single axon of a neuron is drawn in black; the remaining short processes (dendrites) are drawn in gray. Neurons with axons extending in the direction of increasing laminin concentration are shown in the first row. Neurons with axons extending parallel to the gradient or in the direction of decreasing laminin concentration are shown in the second and third row, respectively. The shapes of the gradients in laminin and BSA in solution that were used to fabricate the substrate-bound gradient are given in the graphs below. The gradients in laminin and BSA were always formed on a homogeneous layer of PLL. In each drawing, the number of neurons in each classification (+, 0, or −) is listed with the total number of neurons grown on a particular gradient on the upper left corner. (B) Definition of the angle α of axonal process grown on a gradient in laminin. The sectors that formed the basis for the classification in (+)-, (0)-, and (−)-responding neurons are indicated. (C) Summary of the percentage of (+)-, (0)-, and (−)-responding neurons cultivated on gradients with different slopes in laminin.
Neurites were counted as (+)-responding to the gradient when the angle between the line connecting the cell body and the tip of the longest process (the incipient axon) and the line following the steepest increase in the surface density of laminin (∇[laminin] = maximum) lay between −60° < α < 60° (Fig. 3B). This area corresponded to the two sextants (2 × 60°) comprising the increasing gradient in laminin. Neurites that grew within ±60° from the maximum slopes of a gradient experienced (averaging over the sextants) 50% of the maximum slope of ∇[laminin] of that gradient. We counted neurites that grew perpendicular to the gradient (60° ≤ α < 120° and −120° < α ≤ − 60°) as (0)-responding; we counted axons extending in the direction of decreasing concentration of laminin (120° ≤ α < 180° and −180° < α ≤ −120°) as (−)-responding. Fig. 3B shows a diagram summarizing these definitions.
We analyzed only isolated neurons to eliminate intercellular factors having the capability of influencing the outgrowth of neurons and distorting the effect of the laminin gradient. Neurons whose polarities are not established were excluded. Cells having contact with neighboring neurons, glial cells, or channel walls were excluded from the analysis. Fig. 3C shows a graph summarizing the response of neurons on gradients with a different slope of laminin. On linear gradients having ∇[laminin] > about 0.06 μg (ml⋅μm)−1 in laminin, on average 60% of the neurons extended their axon in the direction of increasing laminin concentration; based on chance, the number would be 33%. On gradients ∇[laminin] < about 0.06 μg (ml⋅μm)−1 the axons were randomly oriented. A χ2 test confirmed that the effect of the gradient with ∇[laminin] > about 0.06 μg (ml⋅μm)−1 on the orientation of the axons deviates statistically significantly from a random orientation (P < 0.005; χ2 test). For ∇[laminin] < about 0.06 μg (ml⋅μm)−1, the orientation of the axons was randomly distributed (P < 0.005; χ2 test).
We have chosen to evaluate the orientation of the axons by using the angle between the cell body and the tip of the longest process after 24 h in culture. At that time most of the neurons (>90%) were already in the process of axon specification (17) and a usefully large number of neurons (≈1% of the entire population in the channel) have not yet made contact with other neurons, glial cells, or the walls of the channels. We did not use the estimated angle at the onset of axon specification to measure the orientation of the axons, because such an analysis requires interpolating the position of the tip at the onset of axon specification (see Fig. 4, which is published as supporting information on the PNAS web site, www.pnas.org, for this analysis.) The rapidly changing morphology of neurons at this stage in development (caused by cell migration and erratic extension and retraction of neurites) makes an accurate estimation difficult.
Once the axon is specified, the gradient does not seem to be critical for axon guidance, the subsequent step in development. The axonal trajectories in Fig. 3A show that the gradient in laminin does not further guide newly formed axons: Neither do (+)-responding axons show a preference to follow the steepest slope in laminin [the orientation of these axons is evenly distributed in the (+)-sector], nor do axons preferentially bend in the direction of increasing laminin concentration (bending in the direction of increasing and decreasing concentration of laminin occurred with equal probability). Since most neurites follow an approximately straight line in the first 2 days in culture, the initial axonal orientation induced during axon specification is preserved during further outgrowth.
If the neurons are grouped according to the orientation of axons, the dendrites of the neurons also show a low degree of orientation that is opposite to the one of the axons. This orientation is a consequence of the orientation of the axons and can be rationalized on the basis of excluded area: The processes of a neuron are evenly distributed over the entire cell perimeter (8); once the orientation of the axon is determined, the dendrites are evenly distributed over the remaining perimeter of the cell body.
Discussion
This article demonstrates that gradients in laminin immobilized on PLL are capable of orienting axonal specification of rat hippocampal neurons. Our experimental findings represent direct evidence that gradients of immobilized ECM proteins such as laminin have the potential to influence an important step in early neuronal development.
Most previous investigations have examined the effect of sharp boundaries between different proteins on axon specification (for example, using substrates with stripes of alternating proteins) (8, 18). The extracellular stimulus that a neurite experiences when crossing from PLL to laminin is capable of triggering an undifferentiated neuron to enter the stage of axon specification and to stimulate the neurite crossing the boundary to become the axon (8). Our investigations indicated that the gradual transition from PLL to laminin has a similar orienting effect on axon specification. On gradient substrates, however, there is no abrupt transition between two proteins at which axon specification can be triggered. Our finding indicates that neurons are able to sense gradual changes in laminin presented on the surface. This finding may be of greater physiological relevance than findings involving step gradients, since, in vivo, the changes of laminin concentration on the surface are much more likely to be gradual rather than sharp.
The composition of the substrate-bound gradients used in this work differs significantly from the composition of the gradients in laminin that have been investigated in previous investigations of rat hippocampal neurons (19, 20). In these studies, laminin gradients were immobilized on glass or plastic. The viability of neurons on such gradients decreases dramatically when the concentration of adsorbed proteins falls below a critical level, because many types of neuronal cells cannot grow on unmodified glass or plastic and require substrates coated with ECM proteins or PLL to attach and grow. As a result, neurons cannot attach and grow in regions of the gradient substrates with low levels of adsorbed proteins. The different abilities of these substrates to support cell growth obscures the investigation of subtle effects such as axon guidance and establishment of polarity. Our strategy of depositing a gradient in laminin onto a homogenous layer of PLL that supports adhesion and long-term outgrowth of neurons provides uniform cell viability over the entire gradient. This approach allows us to assess the biological activity of immobilized gradients of laminin and to avoid experimental artifacts such as varying viability of neurons on these gradients.
In agreement with previous reports, our data indicate that linear gradients in laminin do not guide axons (19, 20). In our experimental system, axonal orientation can be explained solely on the basis of preferential axon specification in the direction of increasing concentration of laminin and of the growth-promoting effect of laminin on axons (17). We have investigated only linear gradients, and we cannot rule out the possibility that a different shape of gradient (e.g., an exponentially decreasing gradient) might have the ability to guide axons after they have been specified. In this context, an interesting parallel to our study can be found in a recent demonstration that the diffusible factor semaphorin III (a molecule known to guide axonal outgrowth) could specify the initial trajectory of axons in cortical slices (21).
An interesting inference from Fig. 3C is that the proportion of (0)-responding neurons stays roughly the same on all gradients: i.e., that a gain of (+)-responding neurons comes at the expense of a loss in (−)-responding neurons. The simplest explanation for these observations is a general and coincidental shift of axons in the direction of axonal specification as the gradient increase in the concentration of laminin. Further experiments using time-lapse recordings of the outgrowing neurons on substrate-bound gradients, combined with genetic and pharmacological approaches, are necessary to devise a mechanism to rationalize these observations.
Substrate-bound molecules have known to mediate cellular haptotaxis. Rogers et al. (22) have studied how neurons behave on substrates coated with different concentrations of ECM molecules. These gradients are not continuous and it is difficult to assess how a single neuron would behave on these gradients. Brandley and Schnaar (23) have studied tumor cell haptotaxis on gradients of cell adhesion peptides (23). The steepness of the gradients in this type of study is difficult to control. We believe that these studies will benefit from our technique for making substrate-bound gradients of ECM molecules. For example, it would become much easier to dissect the molecular pathways underlying haptotaxis of tumor cells by fibronectin, laminin, and collagen, using overlapping gradients of these proteins (24). It is also possible to obtain quantitative information about cell haptotaxis by varying the steepness of the gradients.
The microfluidic technique described here offers a general route to many types of substrate-bound gradients. In contrast to techniques that use diffusion of proteins in gels, controlled doping of porous membranes with solutions of proteins (3), or electric field-induced formation of gradients in supported lipid bilayers (25), this approach allows the formation and immobilization of gradients, with different steepness, extending over dimensions ranging from tens of micrometers to millimeters. This technique has the potential to form the basis for screening of substances that are involved in cell differentiation, cell migration, axon guidance, and other processes involved in biological pattern formation.
Supplementary Material
Acknowledgments
This work was supported by the Defense Advanced Research Planning Agency, National Science Foundation Grants ECS-9729405 and ECS-0004030, and National Institutes of Health Grants GM 30367 and NS039059. S.K.W.D. thanks the Deutsche Forschungsgemeinschaft for a research fellowship. V.N.M. is an Alfred P. Sloan Foundation Fellow, a Pew and EJLB Scholar, and a National Alliance for Research on Schizophrenia and Depression Young Investigator.
Abbreviations
- PLL
poly-l-lysine
- ECM
extracellular matrix
- PDMS
poly(dimethylsiloxane)
References
- 1.Tessier-Lavigne M, Goodman C S. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 2.Song H J, Poo M M. Curr Opin Neurobiol. 1999;9:355–363. doi: 10.1016/s0959-4388(99)80052-x. [DOI] [PubMed] [Google Scholar]
- 3.Baier H, Bonhoeffer F. Science. 1992;255:472–475. doi: 10.1126/science.1734526. [DOI] [PubMed] [Google Scholar]
- 4.Dertinger S K W, Chiu D, Jeon N L, Whitesides G M. Anal Chem. 2001;73:1240–1246. [Google Scholar]
- 5.Dotti C G, Sullivan C A, Banker G A. J Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goslin K, Banker G. J Cell Biol. 1989;108:1507–1516. doi: 10.1083/jcb.108.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradke F, Dotti C G. Science. 1999;283:1931–1934. doi: 10.1126/science.283.5409.1931. [DOI] [PubMed] [Google Scholar]
- 8.Esch T, Lemmon V, Banker G. J Neurosci. 1999;19:6417–6426. doi: 10.1523/JNEUROSCI.19-15-06417.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeon N L, Dertinger S K W, Chiu D T, Choi I S, Stroock A D, Whitesides G M. Langmuir. 2000;16:8311–8316. [Google Scholar]
- 10.Duffy D C, McDonald J C, Schueller O J A, Whitesides G M. Anal Chem. 1998;70:4974–4984. doi: 10.1021/ac980656z. [DOI] [PubMed] [Google Scholar]
- 11.McDonald J C, Duffy D C, Anderson J R, Chiu D T, Wu H K, Schueller O J A, Whitesides G M. Electrophoresis. 2000;21:27–40. doi: 10.1002/(SICI)1522-2683(20000101)21:1<27::AID-ELPS27>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 12.Xia Y, Whitesides G M. Angew Chem Int Ed Engl. 1998;37:550–575. doi: 10.1002/(SICI)1521-3773(19980316)37:5<550::AID-ANIE550>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 13.Anderson J R, Chiu D T, Jackman R J, Cherniavskaya O, McDonald J C, Wu H K, Whitesides S H, Whitesides G M. Anal Chem. 2000;72:3158–3164. doi: 10.1021/ac9912294. [DOI] [PubMed] [Google Scholar]
- 14.Chiu D T, Jeon N L, Huang S, Kane R S, Wargo C J, Choi I S, Ingber D E, Whitesides G M. Proc Natl Acad Sci USA. 2000;97:2408–2413. doi: 10.1073/pnas.040562297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murthy V N, Sejnowski T J, Stevens C F. Neuron. 1997;18:599–612. doi: 10.1016/s0896-6273(00)80301-3. [DOI] [PubMed] [Google Scholar]
- 16.Craig A M, Banker G. Annu Rev Neurosci. 1994;17:267–310. doi: 10.1146/annurev.ne.17.030194.001411. [DOI] [PubMed] [Google Scholar]
- 17.Lein P J, Banker G A, Higgins D. Dev Brain Res. 1992;69:191–197. doi: 10.1016/0165-3806(92)90159-t. [DOI] [PubMed] [Google Scholar]
- 18.Kuhn T B, Williams C V, Dou P, Kater S B. J Neurosci. 1998;18:184–194. doi: 10.1523/JNEUROSCI.18-01-00184.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halfter W. J Neurosci. 1996;16:4389–4401. doi: 10.1523/JNEUROSCI.16-14-04389.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKenna M P, Raper J A. Dev Biol. 1988;130:232–236. doi: 10.1016/0012-1606(88)90429-0. [DOI] [PubMed] [Google Scholar]
- 21.Polleux F, Giger R J, Ginty D D, Kolodkin A L, Ghosh A. Science. 1998;282:1904–1906. doi: 10.1126/science.282.5395.1904. [DOI] [PubMed] [Google Scholar]
- 22.Rogers S L, Palm S L, Letourneau P C, Hanlon K, McCarthy J B, Furcht L T. J Neurosci Res. 1988;21:315–322. doi: 10.1002/jnr.490210224. [DOI] [PubMed] [Google Scholar]
- 23.Brandley B K, Schnaar R L. Dev Biol. 1989;135:74–86. doi: 10.1016/0012-1606(89)90159-0. [DOI] [PubMed] [Google Scholar]
- 24.Aznavoorian S, Stracke M L, Krutzsch H, Schiffmann E, Liotta L A. J Cell Biol. 1990;110:1427–1438. doi: 10.1083/jcb.110.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kam L, Boxer S G. J Am Chem Soc. 2000;122:12901–12902. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.