Abstract
We investigate miscibility transitions of two different ternary lipid mixtures, DOPC/DPPC/Chol and POPC/PSM/Chol. In vesicles, both of these mixtures of an unsaturated lipid, a saturated lipid, and cholesterol form micron-scale domains of immiscible liquid phases for only a limited range of compositions. In contrast, in monolayers, both of these mixtures produce two distinct regions of immiscible liquid phases that span all compositions studied, the α-region at low cholesterol and the β-region at high cholesterol. In other words, we find only limited overlap in miscibility phase behavior of monolayers and bilayers for the lipids studied. For vesicles at 25°C, the miscibility phase boundary spans portions of both the monolayer α-region and β-region. Within the monolayer β-region, domains persist to high pressures, yet within the α-region, miscibility phase transition pressures always fall below 15 mN/m, far below the bilayer equivalent pressure of 32 mN/m. Approximately equivalent phase behavior is observed for monolayers of DOPC/DPPC/Chol and for monolayers of POPC/PSM/Chol. As expected, pressure-area isotherms of our ternary lipid mixtures yield smaller molecular area and compressibility for monolayers containing more saturated acyl chains and cholesterol. All monolayer experiments were conducted under argon. We show that exposure of unsaturated lipids to air causes monolayer surface pressures to decrease rapidly and miscibility transition pressures to increase rapidly.
INTRODUCTION
When incorporated into giant unilamellar vesicles (GUVs), ternary mixtures of saturated phospholipids, unsaturated phospholipids, and cholesterol produce micron-scale liquid domains (Bagatolli, 2003; Dietrich et al., 2001; Veatch and Keller, 2002). The coexisting liquids have a rich phase behavior and the vesicles can undergo budding (Baumgart et al., 2003; Veatch and Keller, 2003). Aside from their physical properties, these systems are particularly interesting because the observed liquid domains may be good models for raft domains in cell membranes. Raft domains are thought to be important in cellular processes such as membrane trafficking and signaling (Simons and Ikonen, 1997). Within rafts, sphingomyelin lipids with long acyl chains and little unsaturation are thought to interact with cholesterol, packing tightly into a liquid-ordered (Lo) phase (London and Brown, 2000; Simons and Harder, 1997).
One method of assessing lateral packing is to study mixtures of lipids and cholesterol in a monolayer at an air-water interface. Monolayers containing sphingomyelin lipids have lower molecular area and compressibility than monolayers containing phosphatidylcholine lipids (Smaby et al., 1996). In some cases, a sharp minimum in molecular area is observed at a particular stoichiometry of lipid and cholesterol (Radhakrishnan and McConnell, 1999). Monolayers are also useful as units to be assembled into supported membranes containing domains (Dietrich et al., 2001). For example, supported membranes have been used to study protein binding to raft domains (Khan et al., 2003) and diffusion coefficients of lipids in liquid domains (Crane and Tamm, 2004; Dietrich et al., 2001). The assembly of lipid monolayers of different compositions to form each leaflet of a supported bilayer is particularly interesting because cell membranes are highly asymmetric. We have previously shown that each deposited monolayer in a supported bilayer retains the micron-scale domains from the original monolayer at the air-water interface (Stottrup et al., 2004).
To form a supported bilayer, monolayers are usually deposited at a surface pressure of 32 mN/m, at which lipids in a monolayer are thought to have a molecular area equivalent to lipids in a cell membrane. This value is derived from measurements of phospholipase activity in red blood cells and lipid monolayers (Demel et al., 1975), and is supported by calculations (Marsh, 1996). Other theoretical work implies that an equivalent pressure should be higher, near 50 mN/m (Feng, 1999; Nagel, 1986), which is higher than the collapse pressure of some monolayers. The appropriate value may change with lipids of the system, and a more relevant parameter to consider may be molecular density. Nevertheless, in studies of coexisting liquid phases, supported monolayers are nearly always deposited near 32 mN/m (e.g., Crane and Tamm, 2004; Dietrich et al., 2001). This leads us to the question of whether lipid phase behavior in vesicles and monolayers is equivalent under these conditions. The general question of how to quantitatively compare monolayer and bilayer results is a longstanding and venerable problem (de Kruyff et al., 1973; Phillips, 1972).
Here we present monolayer phase diagrams for two different ternary mixtures of lipids and cholesterol, and compare them to phase diagrams of the same lipids in giant unilamellar vesicles. We then evaluate the monolayer pressure-area isotherms, and comment on the effects of oxidation in monolayers. One of our ternary mixtures contains the saturated lipid di(16:0) phosphatidylcholine (DPPC), the unsaturated lipid di(18:1) phosphatidylcholine (DOPC), and cholesterol. We have extensively studied the miscibility phase behavior of this mixture in vesicles (Veatch and Keller, 2003; Veatch et al., 2004). The second mixture contains palmitoyl sphingomyelin (PSM), 1-palmitoyl-2-oleoyl-phosphatidylcholine (POPC), and cholesterol, and is thought to be more representative of lipids found in cell membranes (Smaby et al., 1996).
MATERIALS AND METHODS
Lipid monolayers
All phospholipids and cholesterol (Chol) were obtained from Avanti Polar Lipids (Alabaster, AL), which estimates purity at >99%, with the exception of palmitoyl sphingomyelin (PSM) from Sigma (St. Louis, MO), which estimates purity at 97–99%. The PSM used here is a racemic mixture of D-erythro and L-erythro forms. Racemic 16:0 sphingomyelin (SM) has a slightly lower gel transition, 39.9°C, than naturally occurring D-erytho-N-16:0-SM, 41.1°C (Ramstedt and Slotte, 1999). Giant vesicles of unpurified stock PSM exhibit an extended gel-liquid coexistence region, from 41°C to 51°C (Sarah Veatch, personal communication). Lipids were mixed at 10 mg/ml in chloroform and used without further purification; stock solutions were stored below −20°C. The fluorescent probe Texas red dipalmitoyl-phosphatidylethanolamine (TR-DPPE, Molecular Probes, Eugene, OR) was used at 0.5 mol % to provide contrast between immiscible lipid phases. Varying TR-DPPE concentration from 0.2% to 2% does not affect monolayer transition pressures. Monolayers were assembled on a subphase of purified water of resistivity >18 MΩ cm (Barnstead, Dubuque, IA).
Surface pressures of lipid monolayers at an air-water interface were measured using a home-built Langmuir trough with a Wilhelmy plate (Riegler & Kirstein, Berlin, Germany). Lipid monolayers were imaged using a Nikon Y-FL microscope (Melville, NY) and a Photometrics Coolsnap FX charge-coupled device camera (Roper, Princeton, NJ). Pressure-area isotherms for monolayers were obtained using a home-built Langmuir trough and Wilhelmy plate (Baneyx and Vogel, 1999; Halter et al., 2004) calibrated using arachidic acid. Isotherm data was taken 4–10 min after deposition of lipids at the air-water interface.
To prevent oxidation of monolayer lipids, Langmuir troughs were used inside an argon-filled glove bag. Argon was flushed through the bag before lipid deposition and a positive pressure was maintained throughout the experiment. For isotherms, new ampules of the unsaturated lipid, DOPC, or POPC, were opened daily and lipids were mixed in a glove bag filled with nitrogen. Samples were prepared immediately before (∼2 h) use for monolayer experiments. Experiments were conducted at room temperature (25 ± 2°C).
Identifying two-phase regions
At low surface pressures, monolayers containing 1:1 DOPC/DPPC and cholesterol separate into two distinct regions of liquid phases, called the α-region at low cholesterol and the β-region at high cholesterol (Veatch and Keller, 2002). Using fluorescence microscopy, the α-region is easy to identify. As the surface pressure is lowered, a uniform monolayer abruptly demixes into two coexisting liquid phases. The error in determining transition pressures for the α-region is typically ±0.5 mN/m.
Miscibility transition pressures in the β-region and the transition from the α-region to the β-region are not as easily identified. Monolayers in the β-region usually fulfill more than one of the following criteria:
Domains in the β-region persist at surface pressures much greater than in the α-region. At high pressures these domains are sparse.
At high pressures, the contrast often inverts from bright domains on a dark background to the opposite (Okonogi and McConnell, 2004).
As the surface pressure is lowered below ∼5–15 mN/m, the sparse, bright domains are suddenly joined by uniformly dispersed small bright domains.
Monolayers were investigated from 15 to 70 mol % cholesterol. At both low and high cholesterol concentrations, domains become small and difficult to image. At very low cholesterol fractions, coexisting solid and liquid phases are expected (Crane and Tamm, 2004). At high cholesterol fractions in vesicle suspensions, cholesterol crystals form in solution (Huang et al., 1999).
Isotherm analysis
Isotherm data from 1 to 35 mN/m were fit to polynomials. This fit was used to calculate both molecular area and compressibility. Errors contain a component due to the systematic instrument precision, and a component due to volume measurement of lipid solutions. The Langmuir trough itself, with no lipids, measures molecular area with a standard deviation of 0.6 Å2. At least once daily we calibrated our isotherms against arachidic acid, for which the liftoff area varied by ±∼1.5 Å2.
Monolayer compressibility, C, for each lipid composition was calculated from isotherms using C = (−1/A)(dA/dΠ) where A is the average area per lipid and Π is the monolayer surface pressure. C−1 is the inverse compressibility.
RESULTS
Miscibility phase diagrams
We searched for immiscible liquid phases in monolayers of two different ternary lipid mixtures. Both mixtures contain a saturated lipid (DPPC or PSM), an unsaturated lipid (DOPC or POPC), and cholesterol. Both ternary mixtures produce two distinct regions of immiscible liquid phases, the α-region at low cholesterol and the β-region at high cholesterol. Typical fluorescence micrographs are shown in Fig. 1. Within the α-region, more dark phase is observed as the fraction of cholesterol increases, implying that our dye partitions away from the cholesterol-rich phase. Within the β-region, bright domains are sparsely distributed throughout in the monolayer and often persist to surface pressures well above 15 mN/m.
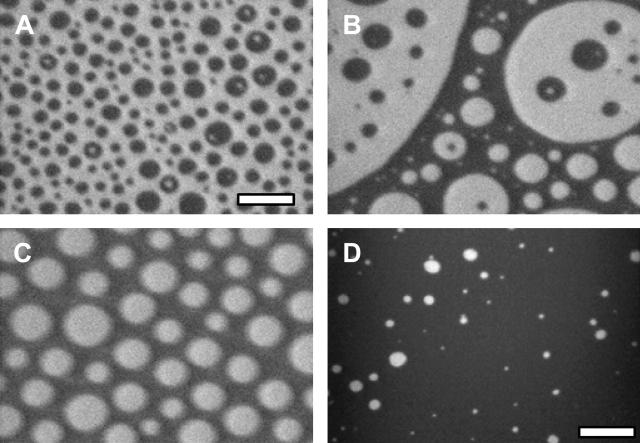
FIGURE 1.
Coexisting liquid phases in lipid monolayers of 1:2 POPC/PSM + X% Chol with increasing cholesterol fraction. (A) 25% Chol at 9 ± 0.5 mN/m, the α-region with small dark domains on a bright background. (B) 30% Chol at 9 ± 0.5 mN/m, the α-region with approximately equal area fractions of bright and dark domains. (C) 35% Chol at 9 ± 0.5 mN/m, the α-region with small bright domains on a dark background. (D) 45% Chol at 22 ± 0.5 mN/m, the β-region showing sparse, large bright domains on a dark background. Similar micrographs are found with increasing cholesterol throughout the phase diagram. The scale bar is 10 μm in images A, B, and C, and 20 μm in image D.
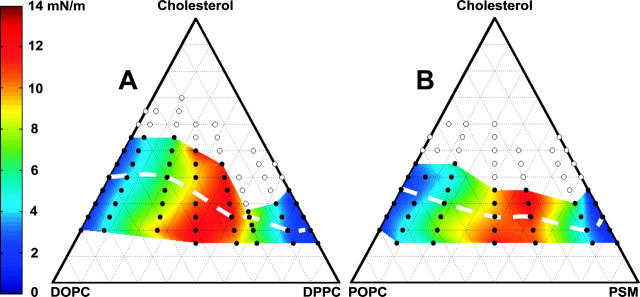
Monolayer miscibility phase boundaries for the two ternary lipid mixtures are shown in Fig. 2. For the cholesterol compositions explored (∼15–70 mol %), two coexisting liquid phases are always observed, in both the α-region (solid circles), and the β-region (open circles). The colored surface is an interpolated fit of the miscibility transition pressures measured for the filled points in the α-region. Transition pressures are highest near equal ratios of the two phospholipids, consistent with observations in other ternary lipid systems (Keller et al., 2000). Within the α-region, there is little systematic dependence of surface pressure on cholesterol fraction. For our ternary mixtures, miscibility transition pressures in the α-region are always below 15 mN/m, far below 32 mN/m.
FIGURE 2.
Monolayer miscibility phase diagrams for ternary mixtures of (A) DOPC/DPPC/Chol and (B) POPC/PSM/Chol. In both cases, two different regions of immiscible liquid phases are observed at low surface pressure, the α-region at low cholesterol (solid circles) and the β-region at high cholesterol (open circles). Transition pressures in the α-region are recorded in color with a polynomial fit between data points, and are highest near equimolar ratios of DOPC to PSM. Transition pressures in the β-region are greater than in the α-region, often well above 15 mN/m.
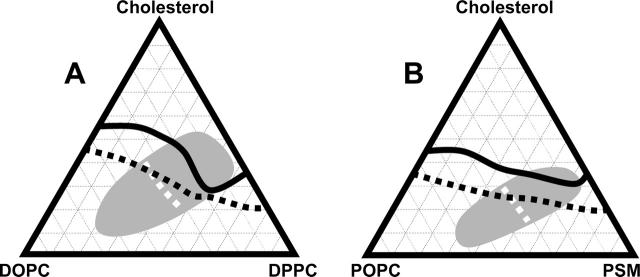
Within the α-region, dark domains on a bright background are observed in our ternary mixtures at cholesterol fractions below the dotted black line in Fig. 3. Along the dotted line, domain striping occurs near the miscibility transition, which implies that the system is near a critical point (Keller and McConnell, 1999). At cholesterol fractions above the dotted line, contrast reverses to bright domains on a dark background. The dotted line tilts such that more dark phase is observed as the fraction of DPPC increases. In other words with more DPPC, dark backgrounds are observed at smaller cholesterol fractions. The transition from the α-region to the β-region is recorded as a solid black line in Fig. 3. Using the results above, we can compare phase diagrams of monolayers and bilayers of the same ternary lipid mixtures. Phase diagrams of giant unilamellar vesicles are taken from Veatch and Keller (2002, 2003) for DOPC/DPPC/Chol, and from a manuscript in preparation for POPC/PSM/Chol.
FIGURE 3.
Comparison of miscibility phase behavior in monolayers and bilayers of ternary mixtures of (A) DOPC/DPPC/Chol and (B) POPC/PSM/Chol. Change of contrast from bright domains on a dark background to the opposite occurs in monolayers at an air-water interface (dotted black line) and in giant unilamellar vesicle bilayers (dotted white line). In monolayers, the transition from the α-region to the β-region is denoted by a solid black line. In giant unilamellar vesicles, the region in which coexisting liquid phases are observed is denoted by shading. Although these boundaries coincide for some compositions (where the two dotted lines cross and where the black line intersects the shaded region), the agreement is not general for all compositions.
A large difference between the bilayer and monolayer systems is the boundary of the region of two liquid phases. For vesicles, coexisting micron-scale liquid domains are seen only within the shaded region in Fig. 3. In contrast, for monolayers, micron-scale liquid phases are seen at all compositions studied, including along the binary axis of unsaturated lipid (e.g., DOPC) and cholesterol. The vesicle phase coexistence boundary at 25°C spans both the α-region and the β-region in the monolayer phase diagram, yet within the α-region domains are observed only at low pressures, far below 32 mN/m. As a specific example, the composition 1:1:1 DOPC/DPPC/Chol lies directly in the center of Fig. 3 A. In vesicles, this mixture produces micron-scale liquid domains at room temperature (Veatch and Keller, 2003). In monolayers this particular mixture produces liquid domains only up to ∼11 mN/m.
A second distinction between the vesicle and monolayer systems lies in domain ripening. In vesicles, domains typically collide and coalesce into one bright domain and one dark domain over the course of minutes. In contrast, in monolayers there is a distribution of domain sizes, and domains rarely coalesce unless the monolayer is close to a critical point, where the difference in dipole density between phases becomes small (Keller and McConnell, 1999). Monolayer domains remain dispersed even if the monolayers are assembled into a supported bilayer (Stottrup et al., 2004). For the domains shown in Fig. 1 to grow considerably, domains must be gathered under electric field gradients (Lee et al., 1994), or maintained for extremely long periods (Hu et al., 2003).
A third point to discuss is the ratio of bright to dark domains seen in fluorescence micrographs. In our previous work using GUVs, we made a qualitative argument that if only two liquid phases are present, then the two ends of a tie-line fall at the compositions of vesicles with the most bright or dark phases, respectively (Veatch and Keller, 2003). We concluded that the tie lines in the DOPC/DPPC/Chol system run across the phase diagram at a shallow slope, and we supported this argument with subsequent quantitative measurements (Veatch et al., 2004).
Applying the same qualitative reasoning to our monolayer phase diagram of the same DOPC/DPPC/Chol system, we notice the most-bright phase appears in monolayers rich in DOPC, at the lower left of the phase diagram in Fig. 3 B. The amount of dark phase increases mildly by traveling to the right, toward more DPPC, and strongly by traveling upward, toward more cholesterol. The change of contrast is recorded as a dotted black line in Fig. 3. The most-dark phase is found when both DPPC and cholesterol fractions are high. Given the phase diagrams in Fig. 3 B, tie-lines in this monolayer system likely run toward higher cholesterol.
Monolayer pressure-area isotherms
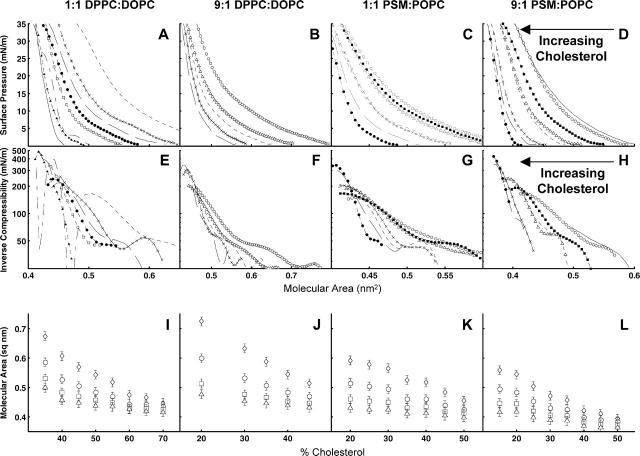
An advantage of studying lipid monolayers is the ability to access a wide range of molecular areas. Pressure-area isotherms for monolayers of our ternary lipid compositions appear in Fig. 4. Monolayers containing more saturated acyl chains have smaller molecular area and compressibility (Fig. 4, B and D, versus Fig. 4, A and C), as expected from other systems (Smaby et al., 1996). Similarly, monolayers containing more cholesterol have smaller molecular areas and compressibility, as expected (Smaby et al., 1997).
FIGURE 4.
(A–D) Pressure-area isotherms of monolayers of 1:1 DOPC/DPPC, 1:9 DOPC/DPPC, 1:1 POPC/PSM, and 1:9 POPC/PSM with varying amounts of cholesterol. Monolayers contain 15% Chol (—), 20% Chol (-○-), 25% Chol (-▪-), 30% Chol (-▵-), 35% Chol (- - - -), 40% Chol (-×-), 45% Chol (— —), 50% Chol (-•-), 55% Chol (-□-), 60% Chol (— -), 65% Chol (-▴-), and 70% Chol (—). (E–H) Monolayer compressibility calculated from the isotherms in A–D. (I–L) Molecular areas versus mole fraction cholesterol at 3 mN/m (⋄), 10 mN/m (○), 20 mN/m (□), and 30 mN/m (Δ).
In general, we find smaller molecular areas in monolayers containing PSM and POPC than in monolayers containing DPPC and DOPC. This is expected for two reasons. Monolayers of PSM and cholesterol have lower molecular areas than monolayers of DPPC and cholesterol (Lund-Katz et al., 1988). In addition, the saturated and unsaturated chains of POPC require less area than the two unsaturated chains of DOPC (Smaby et al., 1996).
Deviations from ideal mixing have long been observed in molecular areas of monolayers containing phospholipids and cholesterol (Smaby et al., 1994). A similar area condensation is observed in our ternary mixtures of lipids and cholesterol (Fig. 4, I–L). Molecular area decreases monotonically with increasing cholesterol, within our measurement errors.
Effects of oxidation on lipid monolayers
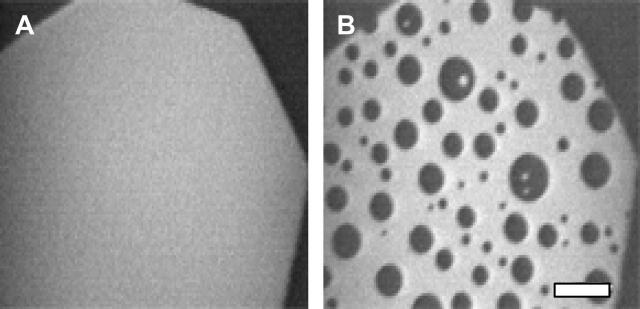
It is well known that exposure of unsaturated lipids to air and ozone can result in changes in surface pressure and miscibility transition pressures in lipid monolayers (Benvegnu and McConnell, 1993; Lai et al., 1994). In previous experiments, we mitigated these changes by completing experiments quickly (Veatch and Keller, 2002). A superior technique is to conduct experiments under argon (Abousalham et al., 2000; Hagen and McConnell, 1997). Here we use a raft-forming lipid composition containing unsaturated lipids used by Crane and Tamm (2004) to compare lipid miscibility of a monolayer formed and maintained under argon to the same monolayer exposed to air. The results in Fig. 5 are striking. Under argon, the monolayer of 1:1 brain PC/brain SM and 25% cholesterol has a low miscibility transition pressure at 5 mN/m. At 32 mN/m, the monolayer is in one uniform phase. In contrast, when the monolayer is exposed to air for less than an hour, the miscibility transition rises so that the same monolayer composition exhibits coexisting liquid phases at 32 mN/m. We have observed increases in the miscibility transition for a variety of monolayers containing unsaturated lipids (Stottrup et al., 2004).
FIGURE 5.
Effects of oxidation. (A) A monolayer of 1:1 brain PC/brain SM and 25 mol % cholesterol deposited under argon and viewed at ∼32 mN/m. (B) A monolayer of the same mixture at 32 mN/m after 40 min of observation and exposure to air.
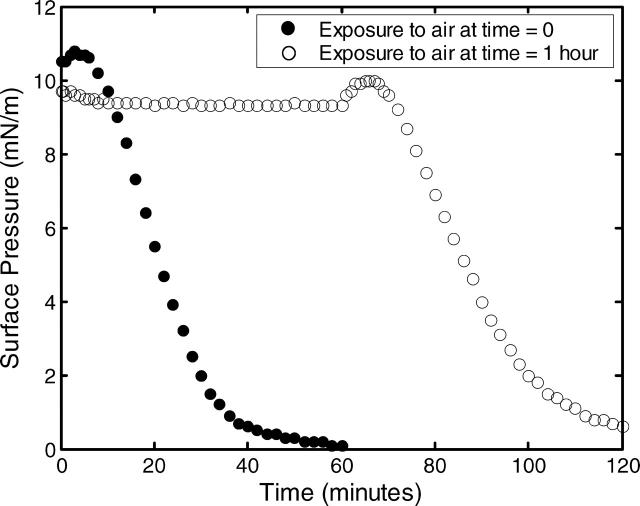
Exposure to air can drastically change not only miscibility transitions, but also surface pressures. Fig. 6 shows surface pressures for monolayers of pure DOPC either exposed to air immediately, or maintained under argon for 1 h before exposure to air. The monolayer was not illuminated, thus eliminating photo-oxidation. Upon exposure to air, surface pressure decreases rapidly. This change in monolayer pressure may be a result of cleaved tails submerging into the subphase (Abousalham et al., 2000; Lai et al., 1994). In contrast, the surface pressure of monolayers maintained under argon is stable to ±0.5 mN/m over 1 h.
FIGURE 6.
Decrease in surface pressure of a DOPC monolayer of constant area upon exposure to air at time = 0 (•), or maintained under argon for 60 min before exposure to air (○).
DISCUSSION
Sphingomyelin
In the results above, we have mapped miscibility transitions in monolayers of ternary lipid mixtures. Each of the lipids of our first mixture, DOPC/DPPC/Chol, is well studied and can easily be acquired at high purity. The lipids of our second mixture, POPC/PSM/Chol, are more commonly found in biological membranes. In plasma membranes, sphingomyelin is a major lipid (Barenholz and Thompson, 1980), and phosphatidylcholines (PCs) with asymmetric chains appear more frequently than with symmetric chains (Smaby et al., 1996). Sphingomyelin and cholesterol are colocalized in cell plasma membranes (Lange et al., 1989).
Sphingomyelin is thought to interact more strongly with cholesterol than phosphatidylcholine lipids, based on measurements of high monolayer area condensation, slow cholesterol desorption from bilayers (Lund-Katz et al., 1988), and low bilayer permeability (Barenholz and Thompson, 1980). In many cases, properties attributed to sphingomyelins may simply be due to chain length and unsaturation. Sphingomyelins extracted from natural sources have more saturation and higher melting temperatures than their phosphatidylcholine counterparts (Barenholz and Thompson, 1980). For example, recent work has concluded that sphingomyelin confers a high area condensation on monolayers because its acyl chains tend to be long and saturated (McIntosh et al., 1992; Smaby et al., 1994). Compressibility moduli for mixtures of sphingomyelin and cholesterol are comparably high for mixtures of saturated phosphatidylcholines and cholesterol (McIntosh et al., 1992). When comparing sphingomyelin and phosphatidylcholine lipids, important physical parameters to consider are the gel-melting temperature in bilayers and the liquid-expanded to liquid-condensed transition in monolayers (Smaby et al., 1994). In monolayers, PSM has a liquid-expanded to liquid-condensed transition at 19 mN/m, whereas DPPC has a lower transition, at only 9 mN/m (Lund-Katz et al., 1988).
Cholesterol may interact more strongly with sphingolipids than phosphatidylcholine lipids through hydrogen bonding. It has long been known that sphingomyelin has the capability to hydrogen-bond with the 3-OH group of cholesterol (Barenholz and Thompson, 1980). Experiments probing this hydrogen bond through the exchange of cholesterol between vesicles and the condensation of monolayers have shown that the sphingomyelin-cholesterol interaction does not depend on sphingomyelin's hydroxyl group at C-3 (Grönberg et al., 1991; Kan et al., 1991). However, sphingomyelin's amine group may be important. Without the amine, oxidation of monolayer cholesterol proceeds faster, although monolayer condensation is not significantly altered (Bittman et al., 1994), and vesicle chemical shifts give no evidence of strong hydrogen bonding between cholesterol and the amide group of sphingomyelin (Guo et al., 2002). The amine group's influence on the sphingomyelin molecule may also be through increasing the rotational barrier of the acyl chain (Bittman et al., 1994).
In our work above, we find only subtle differences in the monolayer phase behavior between the systems of DOPC/DPPC/Chol and POPC/PSM/Chol. Both systems exhibit two different regions of coexisting liquid phases at low surface pressures. The miscibility behavior gives no compelling evidence for a strong sphingomyelin-cholesterol interaction.
The molecular areas we measure are reasonable given areas previously measured for binary mixtures of cholesterol and lipids (Lund-Katz et al., 1988; Smaby et al., 1994). At 30 mN/m, mixtures of 50 mol % cholesterol with DOPC, DPPC, POPC, and PSM show an area condensation and yield molecular areas of 40–49 Å2, 40–45 Å2, 42 Å2, and 40.5 Å2, respectively. If these cholesterol-condensed monolayers added ideally, we would expect the molecular area of 1:1 DPPC/DOPC + 50% Chol to be 40–47 Å2, and we measure 44 Å2. Similarly, we would expect the molecular area of 1:1 POPC/PSM + 50% Chol to be 41 Å2, and we measure 40 Å2.
Monolayers versus bilayers
We have mapped monolayer phase diagrams to search for correlations in miscibility behavior between monolayers and bilayers. In our monolayers containing a saturated lipid (DPPC or PSM), an unsaturated lipid (DOPC or POPC), and cholesterol, two distinct regions of immiscible liquid phases are observed—the α-region and the β-region. In the α-region, coexisting domains are observed at surface pressures below 15 mN/m (Fig. 2), far below the equivalent bilayer pressure of 32 mN/m. A more appropriate unit of comparison between monolayer and bilayer systems may be molecular area. The average lipid area in an erythrocyte membrane or in a phospholipid bilayer is ∼40 Å2 or ∼60 Å2, respectively (Marsh, 1990; van Deenen and De Gier, 1974). For the isotherms in Fig. 4, all of the miscibility transitions from Fig. 2 indeed fall at molecular areas below 60 Å2, with the exception of 1:9 DOPC/DPPC + 20 mol % cholesterol. However, we do not expect such good agreement for compositions containing low concentrations of cholesterol and high concentrations of unsaturated lipid, which should have high molecular areas and low miscibility transition pressures.
Is there any general method of comparing phase diagrams of monolayers and bilayers? A proposal of how to do so was proposed by Harden McConnell (personal communication). In the monolayer phase diagram of DMPS/GM1/DChol, a solid phase appears at low cholesterol and high pressure. With increasing cholesterol, the solid phase is replaced by two liquid phases and then one uniform liquid phase (Radhakrishnan and McConnell, 2002). The high pressure boundary of this two-phase region occurs at the same cholesterol composition as the low pressure transition between the α-region and the β-region.
Monolayer phase diagrams are plotted as lipid composition versus surface pressure whereas bilayer diagrams are plotted versus temperature. Solid phases appear high on the pressure axis for monolayers and low on the temperature axis for bilayers, so it may be possible to translate from a monolayer phase diagram to a bilayer by inverting the pressure axis into temperature. Inverting the phase diagram of Radhakrishnan and McConnell, we would expect that bilayers at low temperature would contain a solid phase at low cholesterol. With increasing cholesterol, we would expect the solid phase to be replaced by first a solid-liquid coexistence, followed by two liquid phases, and then one uniform phase. This is exactly what we have observed in GUVs containing ternary mixtures of lipids and cholesterol (Veatch and Keller, 2002).
If the bilayer phase diagram is produced by a simple inversion of the monolayer phase diagram then we expect to find the same lipid composition at both the miscibility phase boundary in the bilayer and the transition between the α-region and β-region in the monolayer. We found rough preliminary evidence for this correlation in mixtures of 1:1 DOPC/DPPC with varying cholesterol (Veatch and Keller, 2002). However, the full phase diagrams presented here show that there is no general correlation between the miscibility phase boundary in the vesicle bilayer and the transition between the α- and β-regions in the monolayer (Fig. 3, solid lines). Therefore, studies which determine the contrast inversion or percolation threshold in supported monolayers (e.g., Crane and Tamm, 2004) may not be relevant to bilayer systems of the same lipids.
In monolayers, even binary mixtures of lipids and cholesterol can produce two distinct regions of coexisting liquid phases (α-region and β-region). This phase behavior has been explained by hypothesizing a condensed complex of lipid and cholesterol at a stoichiometry near the transition between the α- and β-regions. Previously, a minimum or a sharp change in slope has been observed in the monolayer area per molecule at the stoichiometry of the complex (Radhakrishnan and McConnell, 1999). However, we find no striking change of slope for our monolayer areas. No abrupt decreases are discernible from a smooth area condensation curve, given the measurement uncertainties in Fig. 4.
An obvious difference between monolayer and bilayer systems lies in the interactions of the lipid tails. Other researchers have attempted to mitigate this difference by placing monolayers at an oil-water interface rather than an air-water interface (Mingins et al., 1982). Electrostatics also contribute to differences in phase behavior between monolayers and bilayers. A competition between lipid dipole density and domain line tension has been utilized to derive monolayer domain sizes (McConnell and De Koker, 1992; McConnell and Moy, 1988). Charged dye can undergo contrast inversion with increasing monolayer surface pressure (Okonogi and McConnell, 2004). When the charged lipid PS is incorporated into monolayers of the ganglioside GM1 and cholesterol, novel liquid phases are observed at high surface pressure (Radhakrishnan and McConnell, 2002).
Finally, given the frequent use of supported monolayers, it is important to comment on oxidation. For monolayers containing unsaturated lipids, brief exposure to air can easily increase the miscibility transition pressure above 32 mN/m. For example, we find that a monolayer of 1:1 brain PC/brain sphingomyelin (SM) and 25% cholesterol has a low miscibility transition pressure when maintained under argon, which rises above 32 mN/m in less than an hour upon exposure to air. Our results suggest that the supported monolayers studied by Crane and Tamm (2004) may have harbored some oxidized lipids. Comparing fluorescence micrographs, it is likely that their brain PC/brain SM mixtures containing 5–35 mol % cholesterol were in the α-region in monolayers before deposition.
Oxidation of unsaturated lipids has been documented in a Langmuir monolayer exposed to 0.3 ppm ozone (Lai et al., 1994). Ozone levels measured in our lab range from 0.01 to 0.04 ppm, which compare well with other local measurements (Puget Sound Clean Air Agency, 2004; http://www.pscleanair.org/airq/datareq.aspx). Ozone is likely partially responsible for the alteration of our lipid monolayer upon exposure to air. For experiments utilizing unsaturated lipids, we recommend maintaining the lipids and Langmuir trough under argon or nitrogen.
CONCLUSION
Monolayers containing ternary mixtures of DOPC/DPPC/Chol and POPC/PSM/Chol have similar miscibility phase behavior. Results presented here are consistent with previous work showing monolayers containing more cholesterol and saturated lipid have smaller molecular areas and compressibility. Finally, the exposure of monolayers containing unsaturated lipids to air has dramatic effects on miscibility transition pressures; one explanation for this is a change in monolayer composition. Although lipid monolayers provide an excellent system for the study of lipid packing, our results show large differences in the miscibility phase behavior, particularly in the composition of the phase boundary, between monolayer and bilayer systems. Understanding these differences is important in comparing monolayer, supported bilayer, and vesicle systems.
Acknowledgments
We are grateful to Sarah Veatch for generous use of her unpublished data, Ryan Rule for preliminary monolayer experiments, and Harden McConnell for insightful discussions. We thank Viola Vogel and Michael Halter for assistance with their mechanized Langmuir trough. We also thank Jane Koenig and Karen Jansen for their help with ozone measurements.
B.L.S. was supported in part by a National Science Foundation Integrated Graduate Education and Research Training Program Fellowship from the University of Washington Center for Nanotechnology. S.L.K. acknowledges laboratory support from a National Science Foundation CAREER award (MCB-0133484), from the Research Corporation Innovation Award and Cottrell Scholar Award, and from the Petroleum Research Fund, administered by the American Chemical Society.
References
- Abousalham, A., F. Fotiadu, G. Buono, and R. Verger. 2000. Surface properties of unsaturated non-oxidized and oxidized free fatty acids spread as monomolecular films at an argon/water interface. Chem. Phys. Lipids. 104:93–99. [DOI] [PubMed] [Google Scholar]
- Bagatolli, L. A. 2003. Direct observation of lipid domains in free standing bilayers: from simple to complex lipid mixtures. Chem. Phys. Lipids. 122:137–145. [DOI] [PubMed] [Google Scholar]
- Baneyx, G., and V. Vogel. 1999. Self-assembly of fibronectin into fibrillar networks underneath dipalmitoyl phosphatidylcholine monolayers: role of lipid matrix and tensile forces. Proc. Natl. Acad. Sci. USA. 96:12518–12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenholz, Y., and T. E. Thompson. 1980. Sphingomyelins in bilayers and biological membranes. Biochim. Biophys. Acta. 604:129–158. [DOI] [PubMed] [Google Scholar]
- Baumgart, T., S. T. Hess, and W. W. Webb. 2003. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature. 425:821–824. [DOI] [PubMed] [Google Scholar]
- Benvegnu, D. J., and H. M. McConnell. 1993. Surface dipole densities in lipid monolayers. J. Phys. Chem. 97:6686–6691. [Google Scholar]
- Bittman, R., C. R. Kasireddy, P. Mattjus, and J. P. Slotte. 1994. Interaction of cholesterol with sphingomyelin in monolayers and vesicles. Biochemistry. 33:11776–11781. [DOI] [PubMed] [Google Scholar]
- Crane, J. M., and L. K. Tamm. 2004. Role of cholesterol in the formation and nature of lipid rafts in planar and spherical model membranes. Biophys. J. 86:2965–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kruyff, B., R. A. Demel, A. J. Slotboom, L. L. M. van Deenen, and A. F. Rosenthal. 1973. The effect of the polar headgroup on the lipid-cholesterol interaction: a monolayer and differential scanning calorimetry study. Biochim. Biophys. Acta. 307:1–19. [DOI] [PubMed] [Google Scholar]
- Demel, R. A., W. S. M. Geurts van Kessel, R. F. Zwaal, B. Roelofsen, and L. L. van Deenen. 1975. Relation between various phospholipase actions on human red cell membranes and interfacial phospholipid pressure in monolayers. Biochim. Biophys. Acta. 406:97–107. [DOI] [PubMed] [Google Scholar]
- Dietrich, C., L. A. Bagatolli, Z. N. Volovyk, N. L. Thompson, M. Levi, K. Jacobson, and E. Gratton. 2001. Lipid rafts reconstituted in model membranes. Biophys. J. 80:1417–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, S.-S. 1999. Interpretation of mechanochemical properties of lipid bilayer vesicles from the equation of state or pressure-area measurement of the monolayer at the air-water or oil-water interface. Langmuir. 15:998–1010. [DOI] [PubMed] [Google Scholar]
- Grönberg, L., Z. Ruan, R. Bittman, and J. P. Slotte. 1991. Interaction of cholesterol with synthetic sphingomyelin derivatives in mixed monolayers. Biochemistry. 30:10746–10754. [DOI] [PubMed] [Google Scholar]
- Guo, W., V. Kurze, T. Huber, N. H. Afdhal, K. Beyer, and J. A. Hamilton. 2002. A solid-state NMR study of phospholipid-cholesterol interactions: sphingomyelin-cholesterol binary systems. Biophys. J. 83:1465–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen, J. P., and H. M. McConnell. 1997. Liquid-liquid immiscibility in lipid monolayers. Biochim. Biophys. Acta. 1329:7–11. [DOI] [PubMed] [Google Scholar]
- Halter, M., Y. Nogata, O. Dannenberger, T. Sasaki, and V. Vogel. 2004. Engineered lipids that cross-link the inner and outer leaflets of lipid bilayers. Langmuir. 20:2416–2423. [DOI] [PubMed] [Google Scholar]
- Hu, Y., K. Y. C. Lee, and J. N. Israelachvili. 2003. Sealed minitrough for microscopy and long-term stability studies of Langmuir monolayers. Langmuir. 19:100–104. [Google Scholar]
- Huang, J., J. T. Buboltz, and G. W. Feigenson. 1999. Maximum solubility of cholesterol in phosphatidylcholine and phosphatidylethanolamine bilayers. Biochim. Biophys. Acta. 1417:89–100. [DOI] [PubMed] [Google Scholar]
- Kan, C. C., Z. Ruan, and R. Bittman. 1991. Interaction of cholesterol with sphingomyelin in bilayer membranes: evidence that the hydroxy group of sphingomyelin does not modulate the rate of cholesterol exchange between vesicles. Biochemistry. 30:7759–7766. [DOI] [PubMed] [Google Scholar]
- Keller, S. L., T. G. Anderson, and H. M. McConnell. 2000. Miscibility critical pressures in monolayers of ternary lipid mixtures. Biophys. J. 79:2033–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, S. L., and H. M. McConnell. 1999. Stripe phases in lipid monolayers near a miscibility critical point. Phys. Rev. Lett. 82:1602–1605. [Google Scholar]
- Khan, T. K., B. Yang, N. L. Thompson, S. Maekawa, R. M. Epand, and K. Jacobson. 2003. Binding of NAP-22, a calmodulin-binding neuronal protein, to raft-like domains in model membranes. Biochemistry. 42:4780–4786. [DOI] [PubMed] [Google Scholar]
- Lai, C. C., S. H. Tang, and B. J. Finlayson-Pitts. 1994. Interactions of monolayers of unsaturated phosphatidylcholines with ozone at the air-water interface. Langmuir. 10:4637–4644. [Google Scholar]
- Lange, Y., M. H. Swaisgood, B. V. Ramos, and T. L. Steck. 1989. Plasma membranes contain half the phospholipid and 90% of the cholesterol and sphingomyelin in cultured human fibroblasts. J. Biol. Chem. 264:3786–3793. [PubMed] [Google Scholar]
- Lee, K. Y. C., J. F. Klingler, and H. M. McConnell. 1994. Electric field-induced concentration gradients in lipid monolayers. Science. 263:655–658. [DOI] [PubMed] [Google Scholar]
- London, E., and D. A. Brown. 2000. Insolubility of lipids in Triton X-100: physical origin and relationship to sphingolipid/cholesterol membrane and domains (rafts). Biochim. Biophys. Acta. 1508:182–195. [DOI] [PubMed] [Google Scholar]
- Lund-Katz, S., H. M. Laboda, L. R. McLean, and M. C. Phillips. 1988. Influence of molecular packing and phospholipid type rates of cholesterol exchange. Biochemistry. 27:3416–3423. [DOI] [PubMed] [Google Scholar]
- Marsh, D. 1990. CRC Handbook of Lipid Bilayers. CRC Press, Boca Raton, FL.
- Marsh, D. 1996. Lateral pressure in membranes. Biochim. Biophys. Acta. 1286:183–223. [DOI] [PubMed] [Google Scholar]
- McConnell, H. M., and R. J. De Koker. 1992. Note on the theory of the sizes and shapes of lipid domains in monolayers. J. Phys. Chem. 96:7101. [Google Scholar]
- McConnell, H. M., and V. T. Moy. 1988. Shapes of finite two-dimensional lipid domains. J. Phys. Chem. 92:4520. [Google Scholar]
- McIntosh, T. J., S. A. Simon, D. Needham, and C.-H. Huang. 1992. Structure and cohesive properties of sphingomyelin/cholesterol bilayers. Biochemistry. 1992:2012–2020. [DOI] [PubMed] [Google Scholar]
- Mingins, J., J. A. G. Taylor, B. Pethica, C. M. Jackson, and B. Y. T. Yue. 1982. Phospholipid monolayers at nonpolar oil-water interfaces. III. Effect of chain-length on phase-transitions in saturated di-acyl lecithins at the normal-heptane aqueous sodium-chloride interface. J. Chem. Soc. Faraday Trans. 78:323–339. [Google Scholar]
- Nagel, J. F. 1986. Theory of lipid monolayer and bilayer chain-melting phase transitions. Faraday Disc. Chem. Soc. 81:151–162. [DOI] [PubMed] [Google Scholar]
- Okonogi, T. M., and H. M. McConnell. 2004. Contrast inversion in the epifluorescence of cholesterol-phospholipid monolayers. Biophys. J. 86:880–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, M. C. 1972. The physical state of phospholipids and cholesterol in monolayers, bilayers, and membranes. In Progress in Surface and Membrane Science. J.F. Danielli, M.D. Rosenberg, and D.A. Cadenhead, editors. Academic Press, New York. 139–221.
- Radhakrishnan, A., and H. M. McConnell. 1999. Condensed complexes of cholesterol and phospholipids. Biophys. J. 77:1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan, A., and H. M. McConnell. 2002. Critical points in charged membranes containing cholesterol. Proc. Natl. Acad. Sci. USA. 99:13391–13396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramstedt, B., and J. P. Slotte. 1999. Comparison of the biophysical properties of racemic and d-Erythro-N-acyl sphingomyelins. Biophys. J. 77:1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons, K., and T. Harder. 1997. Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Curr. Opin. Cell Biol. 9:534–542. [DOI] [PubMed] [Google Scholar]
- Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature. 387:569–572. [DOI] [PubMed] [Google Scholar]
- Smaby, J. M., H. L. Brockman, and R. E. Brown. 1994. Cholesterol's interfacial interactions with sphingomyelins and phosphatidylcholines: hydrocarbon chain structure determines the magnitude of condensation. Biochemistry. 33:9135–9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaby, J. M., V. S. Kulkarni, M. Momsen, and R. E. Brown. 1996. The interfacial elastic packing interactions of galactosylceramides, sphingomyelins, and phosphatidylcholines. Biophys. J. 70:868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaby, J. M., M. M. Momsen, H. L. Brockman, and R. E. Brown. 1997. Phosphatidylcholine acyl unsaturation modulates the decrease in interfacial elasticity induced by cholesterol. Biophys. J. 73:1492–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stottrup, B. L., S. L. Veatch, and S. L. Keller. 2004. Nonequilibrium behavior in supported lipid membranes containing cholesterol. Biophys. J. 86:2942–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deenen, L. L. M., and J. De Gier. 1974. Lipids of the red cell membrane. In The Red Blood Cell. D.M. Surgenor, editor. Academic Press, New York. 147–211.
- Veatch, S. L., and S. L. Keller. 2002. Organization in lipid membranes containing cholesterol. Phys. Rev. Lett. 89:268101. [DOI] [PubMed] [Google Scholar]
- Veatch, S. L., and S. L. Keller. 2003. Separation of liquid phases in giant unilamellar vesicles of ternary mixtures of phospholipids and cholesterol. Biophys. J. 85:3074–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch, S. L., I. V. Polozov, K. Gawrisch, and S. L. Keller. 2004. Liquid domains in vesicles investigated by NMR and fluorescence microscopy. Biophys. J. 86:2910–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]