Abstract
We have examined the kinetics and thermodynamics of the exchange of a fluorescent amphiphile derived from a phospholipid, NBD-DMPE, between serum albumin and the serum lipoproteins of high density (HDL2 and HDL3), LDL, and VLDL. Binding of the fluorescent lipid amphiphile to bovine serum albumin is characterized, at 35°C, by an equilibrium binding constant of ∼3 × 106 M−1 and a characteristic time ≤0.1 s. Association of NBD-DMPE with the lipoprotein particles, if considered as a partitioning of amphiphile monomers between the aqueous phase and the lipoprotein particles, is characterized by an equilibrium partition coefficient between 105 and 106, being highest for LDL and lowest for HDL. The association of NBD-DMPE monomers with lipoprotein particles can be described by insertion rate constants on the order of 105 M−1 s−1 for VLDL and LDL and 104 M−1 s−1 for HDL. The desorption rate constants are on the order of 10−5 s−1 for all particles. The study was performed as a function of temperature between 15 and 35°C. This permitted the calculation of the equilibrium thermodynamic parameters (ΔGo, ΔHo, and ΔSo) as well as the activation parameters (ΔG‡o, ΔH‡o, and ΔS‡o) for the insertion and desorption processes. The association equilibrium is dominated by the entropic contribution to the free energy in all cases. The results are discussed in relation to phospholipid and amphiphile exchange phenomena involving the lipoproteins.
INTRODUCTION
The kinetics and thermodynamics of the spontaneous exchange of amphiphiles (phospholipids, cholesterol, fatty acids, and amphiphilic xenobiotics) between organized lipid aggregates (cell membranes, lipoproteins) or between these and serum proteins have been the topic of a considerable amount of research over the past thirty years. Most of this work has been motivated by the necessity to understand the detailed mechanisms underlying processes of physiological, pathological, and/or pharmacological importance. A limitation in these studies has been the fact that amphiphiles have a tendency to aggregate in aqueous solution. Thus, obtaining molecular rate constants for the individual steps in the spontaneous exchange processes is hampered by the fact that complex amphiphile monomer—aggregate equilibria have to be included in the kinetic schemes, which, as a result, become practically unsolvable. It is, therefore, not surprising that the work in this field has been mostly limited to pseudo first-order processes involving transfer of amphiphiles between one lipid aggregate and another (Jonas, 1979; Jonas and Maine, 1979; Lund-Katz et al., 1982; McLean and Phillips, 1984; Ferrell et al., 1985; Nichols, 1985; Phillips et al., 1987; Jones and Thompson, 1990; Wimley and Thompson, 1990; Brown, 1992; Silvius and Leventis, 1993; and literature cited by these authors) or between a lipid aggregate and proteins (Zucker et al., 1995; Massey et al., 1997; Zucker, 2001; Abreu et al., 2003, 2004; and literature cited by these authors). One of the better studied amphiphile classes in this respect has been the nonesterified fatty acids (see Kleinfeld, 2000; Zakim, 2000; Pownall, 2001; and literature cited by these authors) but even in this case the literature shows a strong reliance upon generic partition coefficients (e.g., Haberland and Reynolds, 1975) to calculate the insertion rate constants from measured desorption rate constants. A further aspect of amphiphile exchange between organized lipid aggregates that has received much attention in the literature is the phenomenon of catalyzed transfer, generally involving lipid transfer proteins (Ihm et al., 1982; Lippiello and Waite, 1983; Barter et al., 2003; Albers and Cheung, 2004). In these cases, although it is not absolutely necessary for understanding the catalytic mechanism, a detailed knowledge of the basal noncatalyzed kinetics serves to evaluate the comparative efficiency if not the physiological relevance of the supposedly catalyzed process.
As stated in the preceding paragraph, study of the detailed kinetics of interaction of an amphiphile in aqueous solution with an organized lipid aggregate is hampered by the fact that the amphiphile tends to aggregate (form micelles, microcrystalline states, etc.) in aqueous solution and the aggregate—monomer equilibria can be complex processes that are usually poorly understood and very difficult, if not impossible, to resolve analytically. This problem becomes particularly relevant for those amphiphiles (phospholipids and their derivatives, long-chain fatty acids, and cholesterol in particular, but also xenobiotics) that form aggregates at very low concentrations in the aqueous phase. In recent work (Abreu et al., 2003) we have developed a method that partly overcomes this difficulty. If the amphiphile binds strongly to a protein (such as serum albumin), the presence of this protein in the aqueous solution of the amphiphile results in a reduction of the free amphiphile concentration in the aqueous solution that is dependent upon the KB and the concentration of the protein. Effectively, the concentration of free amphiphile in the aqueous solution can be reduced to values where the only two species of amphiphile in the system are the monomer in aqueous solution and the protein-bound amphiphile (Vaz and Melo, 2001). Removal of the monomer from the aqueous solution results in its being replenished from the protein-bound state and reasonably high concentrations of total amphiphile can be treated in this way. The detailed kinetics of this binding process can be studied to obtain the relevant molecular rate constants. If an organized lipid aggregate structure (bilayer vesicle, cell membrane fragment, or lipoprotein) is now added to the equilibrium solution of amphiphile and protein in which the amphiphile exists in aqueous solution exclusively as the monomer, the amphiphile monomers in the solution will associate with the lipid aggregate structure and the molecular rate constants for association with the lipid structure can be extracted from the kinetics. We have recently shown the applicability of this method in a detailed kinetic study of the association of the fluorescent derivative of a phospholipid, NBD-DMPE, with lipid bilayer membrane vesicles in the liquid-ordered and liquid-disordered phases (Abreu et al., 2004).
In this work, we report on the association of NBD-DMPE with the surface phospholipid–cholesterol monolayer of the lipoproteins (Chapman, 1986; Gotto et al., 1986) of VLDL (0.93 g mL−1 ≤ ρ ≤ 1.006 g mL−1), LDL (1.019 g mL−1 ≤ ρ ≤ 1.063 g mL−1), and HDL (HDL2, 0.063 g mL−1 ≤ ρ ≤ 1.125 g mL−1, and HDL3, 1.125 g mL−1 ≤ ρ ≤ 1.210 g mL−1). Equilibrium association constants for association of NBD-DMPE with lipoproteins, KL (M−1), and the respective rate constants for the amphiphile insertion and desorption processes, k+ (M−1 s−1) and k− (s−1), respectively, were obtained as a function of temperature between 15 and 35°C. This permitted the calculation of the equilibrium thermodynamic parameters (ΔGo, ΔHo, and ΔSo) as well as the activation parameters (ΔG‡o, ΔH‡o, and ΔS‡o) for the insertion and desorption processes. The results are discussed in comparison with similar results for the association, insertion and desorption of NBD-DMPE with/into/from lipid bilayer membranes (Abreu et al., 2004) under similar conditions.
MATERIALS AND METHODS
BSA, essentially free of fatty acids, was obtained from Sigma-Aldrich Química (Sintra, Portugal); NBD-DMPE (>99% purity) was obtained from Avanti Polar Lipids (Alabaster, AL). All other chemicals were of the highest available purity. Bovine serum albumin concentrations in aqueous solution were determined by the absorbance of the solutions at 278 nm using an extinction coefficient of 0.66 mL mg−1 cm−1 (Peters, 1997), or by the method of Lowry et al. (1951). Concentrations of NBD-DMPE (in methanol) were determined by absorption spectrophotometry assuming a molar extinction coefficient of 21,000 M−1 cm−1 at 463 nm. Absorption spectrophotometry was done using a Unicam UV530 absorption spectrophotometer and fluorescence measurements were done using a Cary Eclipse spectrofluorimeter with a thermostated multisample holder. Samples were continuously agitated by a magnetic stirrer during measurements. The experimental kinetic curves were fitted by theoretical expressions (see Results section) using Microsoft Excel and Solver.
Lipoprotein fractions were obtained essentially as described by Vieira et al. (1996) from human blood drawn from a volunteer (healthy male aged 26 years), after a 12 h fasting period, by venous puncture into heparinized tubes. HDL2 and HDL3 fractions were prepared from the total HDL fraction (Kostner and Alaupovic, 1972; Schumaker and Puppione, 1986). All lipoprotein preparations were sterilized by filtration through a 0.22 μm membrane filter (Millipore-Amicon).
After isolation of the lipoprotein fractions, the protein content of the samples was estimated by the method of Lowry et al. (1951), and the lipoprotein concentration was estimated from this by assuming mean aggregate masses and protein contents (Gotto et al., 1986) for each of the lipoprotein fractions, as follows:
VLDL, mean aggregate mass of 45 × 106 Da with 7.7% protein (w/w).
LDL, mean aggregate mass of 2.3 × 106 Da with 20.9% protein (w/w).
HDL2, mean aggregate mass of 0.36 × 106 Da with 41% protein (w/w).
HDL3, mean aggregate mass of 0.175 × 106 Da with 55% protein (w/w).
The purity of the lipoprotein fractions was verified by 0.5% agarose gel electrophoresis (Vieira et al., 1996).
The kinetic and equilibrium characteristics of the association of NBD-DMPE to BSA have been described in detail elsewhere (Abreu et al., 2004). The rate constants for the association, kB and k−B, were obtained by stopped flow mixing using a HiTech model SF-61 thermostated stopped flow fluorimeter. The binding was characterized by an equilibrium binding constant, KB ≅ 3 × 106 M−1, and characteristic times, τB ≅ 0.1 s.
Equilibrium titration of NBD-DMPE with lipoproteins
The equilibrium titration of NBD-DMPE with lipoproteins makes use of the fact that the relative fluorescence quantum yield of the amphiphile is different in aqueous solution, associated with BSA, and associated with lipoprotein, being highest in the last case and lowest in the first (see Fig. 1 A). 8 × 10−7 M NBD-DMPE was first equilibrated with 2 × 10−4 M BSA for a period of 16 h. Under these conditions >99% of the NBD-DMPE is bound to BSA. An aliquot of a lipoprotein suspension was then added to this solution to give a desired final lipoprotein concentration and the mixture was allowed to reach equilibrium over a period of 24–48 h at a desired temperature. Typically the final concentrations of NBD-DMPE and BSA were 5 × 10−7 M and 1.3 × 10−4 M, respectively. Relative fluorescence quantum yield (emission intensity at 530 nm with excitation at 470 nm) was then measured at that temperature. Fig. 1, B and C show typical experimental titration curves. The value of KL was then obtained from these results from the best theoretical fit considering the simultaneous equilibria of NBD-DMPE with BSA and with the lipoproteins, KB being independently known as described above. It was possible to use this procedure with VLDL and LDL only. Incubation with HDL over long periods of time showed a slow process in which the relative fluorescence quantum yield of NBD-DMPE increased slowly in time. This process, for which we have no explanation, had a characteristic time ≫ 10 h but caused difficulties with reproducible equilibrium titration of NBD-DMPE with HDL. In this case, therefore, we obtained the values of KL from the analysis of kinetic curves of the transfer of NBD-DMPE from BSA to HDL making the assumption that the conversion factor, 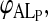 that relates the measured fluorescence intensity to probe concentration was the same for HDL and LDL.
that relates the measured fluorescence intensity to probe concentration was the same for HDL and LDL.
FIGURE 1.
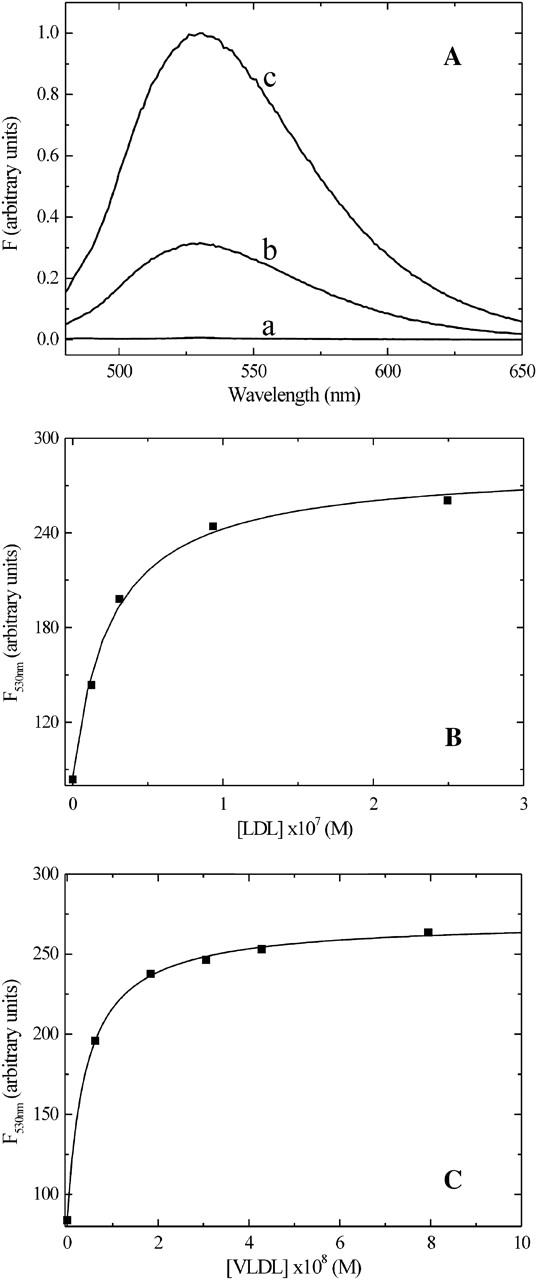
(A) Comparison of the fluorescence emission spectra of NBD-DMPE in aqueous solution at pH 7.4 (spectrum a); bound to BSA (spectrum b) and associated with HDL3 (spectrum c). (B) Equilibrium titration of a solution of 5 × 10−7 M NBD-DMPE preequilibrated with 1.3 × 10−4 M BSA in buffer at pH 7.4, with a suspension of LDL in the same buffer. (C) Equilibrium titration of a solution of 5 × 10−7 M NBD-DMPE preequilibrated with 1.3 × 10−4 M BSA in buffer at pH 7.4, with a suspension of VLDL in the same buffer.
Kinetics of association of NBD-DMPE with lipoproteins
An equilibrated solution of NBD-DMPE and BSA (at concentrations high enough to bind 99% of the probe) was mixed with lipoprotein at the desired concentration and the fluorescence intensity at 530 nm (excitation at 450 nm) was followed in time. Typical concentrations in these experiments were 7 × 10−7 M NBD-DMPE, 2 × 10−4 M BSA, 3 × 10−7 M LDL, 6 × 10−8 M VLDL, 2 × 10−6 M HDL2 and 3 × 10−6 M HDL3. Transfer of NBD-DMPE from the BSA-bound state to the lipoprotein-bound state is accompanied by an increase in fluorescence intensity (Fig. 1 A). The experimental curves were fitted by theoretical curves described in the Results section using Microsoft Excel and Solver.
RESULTS
Equilibrium binding of NBD-DMPE to lipoproteins
NBD-DMPE in aqueous solution has a very low relative fluorescence quantum yield. Upon binding to BSA there is a significant increase in its relative fluorescence quantum yield and this fact has been used to study its association with the protein (Abreu et al., 2004). Upon association with lipoproteins there is also an increase in relative fluorescence quantum yield of the probe and this increase is even more than the increase seen upon its association with BSA. These results, shown in Fig. 1 A, permit following the equilibrium binding of NBD-DMPE to BSA and also its transfer from the BSA-associated state to lipoproteins. Results of the equilibrium titration of NBD-DMPE with VLDL and LDL in the presence of BSA are shown in Fig. 1, B and C. These results, measured in terms of relative fluorescence intensity of NBD-DMPE as a function of lipoprotein concentration, were fitted with a theoretical binding curve, which considers the existence of two simultaneous equilibria: NBD-DMPE with BSA and NBD-DMPE with lipoprotein. The asymptotic value of the theoretical curve at “infinite” lipoprotein concentration gives the fluorescence intensity of NBD-DMPE when all of the probe in the system is associated with lipoprotein and, therefore, the conversion factor, 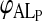 , that relates fluorescence intensity of lipoprotein-bound NBD-DMPE to its concentration. The equilibrium association constants for association of NBD-DMPE with lipoproteins at 35°C are tabulated in Table 1. In the case of HDL2 and HDL3 it was not possible to obtain the value of KL independently because of an unexplained slow process that was superimposed on the association equilibrium. The fluorescence intensity showed a tendency to rise continually at a rate that was very much slower than the association equilibrium. In this case we used the calculated
, that relates fluorescence intensity of lipoprotein-bound NBD-DMPE to its concentration. The equilibrium association constants for association of NBD-DMPE with lipoproteins at 35°C are tabulated in Table 1. In the case of HDL2 and HDL3 it was not possible to obtain the value of KL independently because of an unexplained slow process that was superimposed on the association equilibrium. The fluorescence intensity showed a tendency to rise continually at a rate that was very much slower than the association equilibrium. In this case we used the calculated 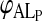 values obtained from an equilibrium titration of VLDL and LDL (identical within limits of error) and used the kinetic curves (see following section) to obtain the rate constants and, therefore, the corresponding association equilibrium constant.
values obtained from an equilibrium titration of VLDL and LDL (identical within limits of error) and used the kinetic curves (see following section) to obtain the rate constants and, therefore, the corresponding association equilibrium constant.
TABLE 1.
Kinetic and thermodynamic constants for insertion and desorption of NBD-DMPE into/from lipoproteins at 35°C
| VLDL | LDL | HDL2 | HDL3 | |
|---|---|---|---|---|
| k+ (M−1 s−1) | (8.0 ± 2.4) × 105 | (2.8 ± 0.3) × 105 | (7.7 ± 0.7) × 104 | (7.6 ± 1.9) × 104 |
| k− (s−1) | (4.0 ± 1.3) × 10−5 | (5.9 ± 2.7) × 10−5 | (2.7 ± 0.3) × 10−4 | (5.4 ± 0.9) × 10−4 |
| KL (M−1) | (2.2 ± 0.4) × 1010 | (5.6 ± 3.2) × 109 | (2.9 ± 0.5) × 108 | (1.4 ± 0.1) × 108 |
| KP | 1.8 × 106 | 3.9 × 106 | 1.0 × 106 | 1.4 × 106 |
| ΔGo (kJ mol−1) | −61 | −57 | −50 | −48 |
| ΔHo (kJ mol−1) | 52 | 38 | 33 | 15 |
| TΔSo (kJ mol−1) | 113 | 95 | 83 | 63 |
| Eact (insert) (kJ mol−1) | 87 | 84 | 93 | 90 |
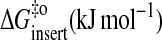 |
41 | 43 | 47 | 47 |
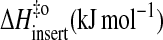 |
84 | 81 | 91 | 88 |
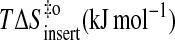 |
44 | 38 | 44 | 41 |
| Eact (desorb) (kJ mol−1) | 35 | 46 | 61 | 76 |
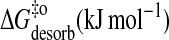 |
101 | 100 | 96 | 95 |
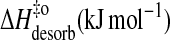 |
32 | 43 | 58 | 73 |
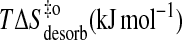 |
−69 | −57 | −38 | −22 |
Note: Values are reported as the mean ± SD of results obtained from three independent experiments. The values of KL were obtained for each individual kinetic experiment and are reported as the mean ± SD of three independent experiments. The mean values reported here are, within experimental error, identical to the mean values of KL obtained from equilibrium titrations.
Kinetics of NBD-DMPE association with lipoproteins
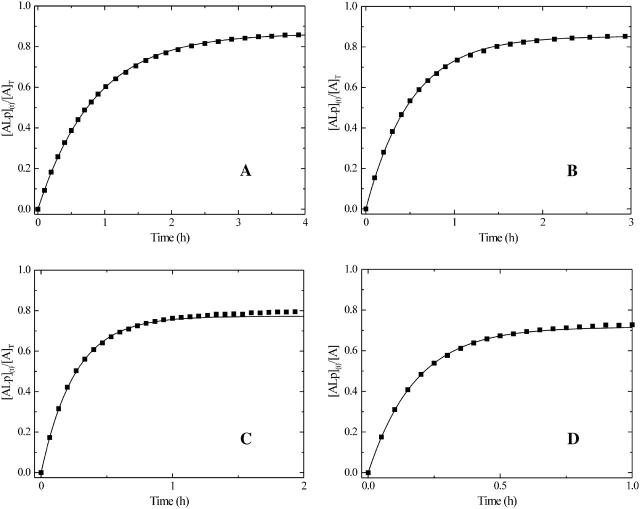
When lipoprotein is added to a solution of NBD-DMPE in equilibrium with BSA, there is a transfer of some of the amphiphile from its BSA-bound state via monomers in the aqueous phase to the lipoprotein. We have shown in previous work (Abreu et al., 2003) that this transfer occurs via monomers of the amphiphile in the aqueous phase in equilibrium with the protein-bound amphiphile and not via a direct interaction of BSA with the lipid aggregate surface. The transfer is accompanied by an increase in the fluorescence intensity since the relative quantum yield of NBD-DMPE is higher when associated with lipoprotein than it is when associated with BSA (see Fig. 1 A). Fig. 2, A–D shows the time course of this increase in fluorescence. These experimental time courses can be compared to theoretical expectations based upon a kinetic scheme that attempts to describe the process. In the case under consideration, the following kinetic scheme may be considered:
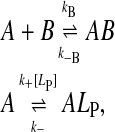 |
(1) |
where A is the amphiphile, NBD-DMPE, in the aqueous phase, ALP is the amphiphile associated with the lipoprotein particles, B is the BSA, LP is the lipoprotein aggregate, kB and k−B are the forward and reverse rate constants for the association with BSA, and k+[LP] and k− are the forward and reverse rate constants for interaction with the lipoprotein, respectively. (The representation of chemical equations that describe the interaction of small molecules with lipid aggregates (lipid vesicles or lipoproteins) presents problems since it is impossible to represent the lipid particle stoichiometry in these reactions unless a clearly defined “binding site” in these particles can be described (for a discussion see Gennis, 1989). In analogy to our previous work (Abreu et al., 2003, 2004) we should have described the association of NBD-DMPE with lipoprotein particles as 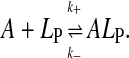 A reviewer has correctly pointed out that this equation implies that [A]T = [A] + [ALP] and [LP]T = [LP] + [ALP] where [A]T and [LP]T are total concentrations of A and LP species, respectively. This representation would imply that LP is consumed in the course of the reaction, which is not the case. We have, therefore, modified the equation that describes the association equilibrium of NBD-DMPE with the lipoproteins as shown in scheme 1. It is intended here that k+[LP] is a pseudo first-order rate constant that includes the concentration of lipoprotein. As described in the text, [LP] does not change in the course of the reaction.) LP is not consumed in the reaction and its concentration in the reaction mixture is effectively constant since labeled LP particles are just as able to react with fresh A as unlabeled ones. This condition is satisfied as long as [ALP]/[LP] is not high enough to significantly affect the properties of the lipoprotein particles (a probe to surface-polar-lipid ratio of ∼0.01). As discussed elsewhere (Abreu et al., 2004), the binding of NBD-DMPE to BSA is more complex than represented in the kinetic scheme 1 above. However, since the characteristic time for association with the protein (∼0.1 s) is very much shorter than the characteristic time for association with the lipoprotein particles (typically hours), the fast equilibrium approximation is valid and the complex kinetics of NBD-DMPE binding to BSA become irrelevant. The association equilibria are defined by the equilibrium association constants,
A reviewer has correctly pointed out that this equation implies that [A]T = [A] + [ALP] and [LP]T = [LP] + [ALP] where [A]T and [LP]T are total concentrations of A and LP species, respectively. This representation would imply that LP is consumed in the course of the reaction, which is not the case. We have, therefore, modified the equation that describes the association equilibrium of NBD-DMPE with the lipoproteins as shown in scheme 1. It is intended here that k+[LP] is a pseudo first-order rate constant that includes the concentration of lipoprotein. As described in the text, [LP] does not change in the course of the reaction.) LP is not consumed in the reaction and its concentration in the reaction mixture is effectively constant since labeled LP particles are just as able to react with fresh A as unlabeled ones. This condition is satisfied as long as [ALP]/[LP] is not high enough to significantly affect the properties of the lipoprotein particles (a probe to surface-polar-lipid ratio of ∼0.01). As discussed elsewhere (Abreu et al., 2004), the binding of NBD-DMPE to BSA is more complex than represented in the kinetic scheme 1 above. However, since the characteristic time for association with the protein (∼0.1 s) is very much shorter than the characteristic time for association with the lipoprotein particles (typically hours), the fast equilibrium approximation is valid and the complex kinetics of NBD-DMPE binding to BSA become irrelevant. The association equilibria are defined by the equilibrium association constants,
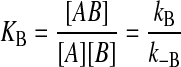 |
(2) |
and
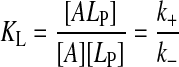 |
(3) |
for association to the BSA and to the lipoprotein, respectively. Integration of the differential equations that describe kinetic scheme 1 in consideration of the rapid equilibrium condition for binding to BSA, and also considering the fact that [B] ≈ [B]T where [B]T is the total concentration of BSA, results in the following expressions for the time dependence of the concentration of each of the species of interest:
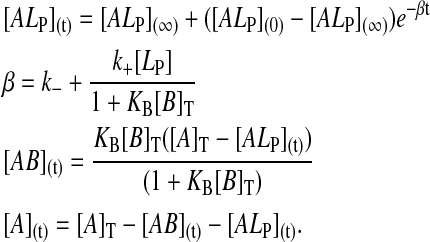 |
(4) |
FIGURE 2.
Time course of the association of a solution of NBD-DMPE preequilibrated with BSA in buffer at pH 7.4, with a suspension of lipoprotein particles in the same buffer at 35°C. Concentrations at the start of the reaction were: (A) 5.6 × 10−7 M NBD-DMPE, 1.4 × 10−4 M BSA, and 5.4 × 10−8 M VLDL; (B) 7.0 × 10−7 M NBD-DMPE, 1.7 × 10−4 M BSA, and 2.8 × 10−7 M LDL; (C) 5.6 × 10−7 M NBD-DMPE, 1.7 × 10−4 M BSA, and 1.4 × 10−6 M HDL2; and (D) 5.6 × 10−7 M NBD-DMPE, 1.7 × 10−4 M BSA, and 2.9 × 10−6 M HDL3.
The fluorescence at any given time during the reaction, F(t), is given by
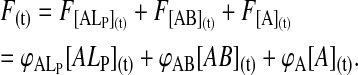 |
(5) |
The values of 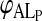 were obtained as described earlier from the fits to the equilibrium titration curves. The experimental kinetic curves were fitted with theoretical curves described by scheme 5 using a least squares fitting procedure and the values of k+ and k− were extracted from the best fits. We note that the only adjusting parameter in the case of the kinetic curves with LDL and VLDL was k+ since KL was independently known in this case. In the case of HDL2 and HDL3 it was not possible to obtain the values of KL independently so that in these cases both k+ and k− had to be variable parameters in the fits to the kinetic curves. The results are listed, for 35°C in Table 1.
were obtained as described earlier from the fits to the equilibrium titration curves. The experimental kinetic curves were fitted with theoretical curves described by scheme 5 using a least squares fitting procedure and the values of k+ and k− were extracted from the best fits. We note that the only adjusting parameter in the case of the kinetic curves with LDL and VLDL was k+ since KL was independently known in this case. In the case of HDL2 and HDL3 it was not possible to obtain the values of KL independently so that in these cases both k+ and k− had to be variable parameters in the fits to the kinetic curves. The results are listed, for 35°C in Table 1.
Temperature-dependence and energetics
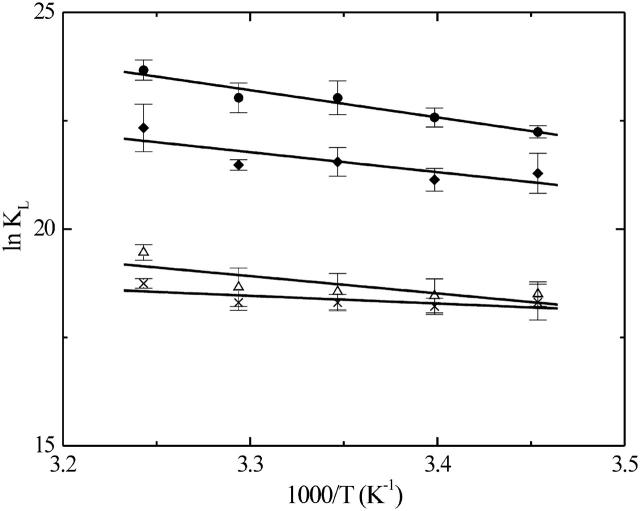
The entire study (equilibrium titrations, kinetics) was performed as a function of temperature between 15 and 35°C. The temperature-dependence of KL for association of NBD-DMPE with VLDL, LDL, HDL2, and HDL3 are plotted as van't Hoff plots in Fig. 3. From these results we obtained the thermodynamic description in terms of ΔGo, ΔHo, and TΔSo for the respective association processes. These are listed in Table 1. The kinetics of association was also studied as a function of temperature between 15 and 35°C and the respective activation energies and thermodynamic parameters of activation (ΔG‡o, ΔH‡o, and TΔS‡o) were calculated on the basis of transition state theory (Steinfeld et al., 1999) from the respective Arrhenius plots shown in Fig. 4. The results for the activation energetics are also listed in Table 1.
FIGURE 3.
Van't Hoff plots for the association of NBD-DMPE with VLDL (•), LDL (♦), HDL2 (Δ), and HDL3 (×). The results shown represent the average values ± SD of three independent experiments.
FIGURE 4.
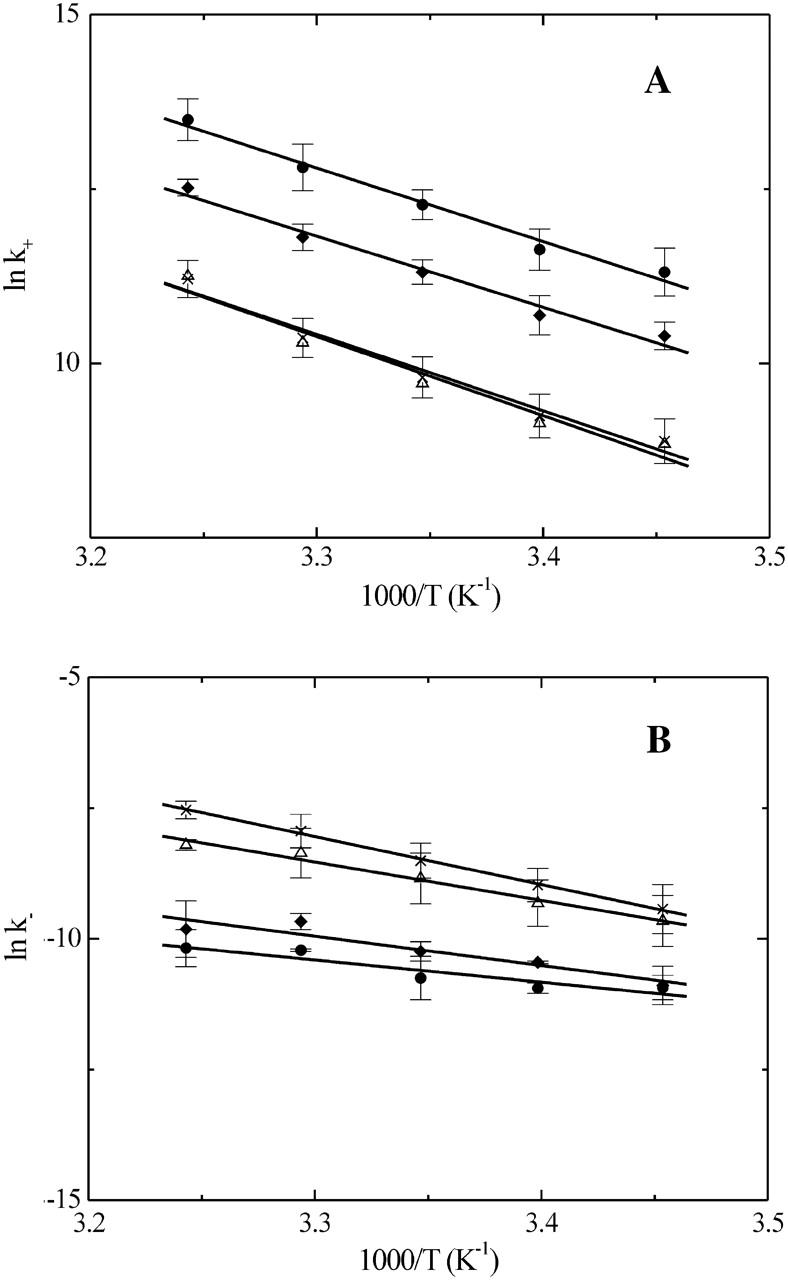
Arrhenius plots for the insertion (panel A) and desorption (panel B) of NBD-DMPE into/from lipoprotein particles. The symbols are the same as in Fig. 3 and the results shown are average values ± SD of three independent experiments.
DISCUSSION
Understanding how amphiphilic molecules interact with lipoproteins is important from a physiological perspective because it provides a baseline for understanding the exchange of chemical components of these organized lipid aggregates among themselves and between them and cell membranes and serum proteins. From the pharmacological perspective it provides an important model for understanding the processes underlying the transport and distribution of amphiphilic drugs and their pharmacokinetics. From the physical/chemical perspective it is an important tool in describing the physical properties of the polar lipid surface that acts as the interface between the lipoproteins and their serum environment. In particular, systematic kinetic and thermodynamic studies on amphiphile interaction with lipoproteins have an important predictive value. It is, therefore, not surprising that this field has been the subject of intensive investigation since several decades.
A complete description of the association of amphiphiles with lipoproteins requires that the association and dissociation rate constants, the equilibrium constants, and the thermodynamic properties of the interaction be quantitatively defined. Such a definition requires that the temperature-dependence of at least two of the three parameters, namely, forward and reverse reaction rate constants and equilibrium association constant, be directly measured. Amphiphile aggregation in the aqueous phase complicates these measurements due to the complex kinetics of amphiphile monomer—aggregate equilibria. Thus, experimental conditions have to be found in which the only free amphiphile species in aqueous solution that can interact with the lipoprotein surface in the reaction mixture is the monomer. This is particularly a problem for amphiphiles with a very low CAC (≤10−8 M) such as phospholipids and their derivatives as well as cholesterol. Consequently, the number of reports in which the required two out of three variables have been directly measured for the interaction of phospholipids and their derivatives with lipoproteins or other lipid aggregates such as membranes is very limited (Nichols, 1985; Abreu et al, 2003, 2004).
The use of simultaneous equilibria of an amphiphile in the aqueous phase between a lipoprotein and a protein that does not have a catalytic amphiphile-exchange function is one way to circumvent the problems involved (Abreu et al., 2003). If the binding and dissociation rate constants and the equilibrium association constant(s) for amphiphile interaction with the protein are independently known, the protein concentration can be adjusted so that the only free amphiphile species in aqueous solution is the monomer. The direct measurement of the rate constants for transfer of the amphiphile to lipoproteins added to the mixture is then rather straightforward. An important aspect in this approach is that amphiphile transfer between the protein and the lipoproteins should not be a second order process involving a lipoprotein-protein complex as intermediate. BSA binds the amphiphilic phospholipid derivative, NBD-DMPE, rather strongly (KB ≈ 106 M−1) so that 2 × 10−4 M BSA (∼13 mg/mL) in the solution can reduce the free amphiphile concentration to <0.2% of its total concentration in the system. This reduces the free NBD-DMPE concentration in the aqueous phase under our experimental conditions to <2 × 10−9 M which is well below its CAC of ∼5 × 10−9 M (Abreu et al, 2004). The methodology described makes it possible to use amphiphiles with a CAC in the 10−10 M range. In previous work we had shown that the transfer of BSA-bound amphiphiles to membranes occurs via monomers in the aqueous phase and not through a collisional interaction between the amphiphile-loaded protein and the membrane (Abreu et al., 2003). Since the lipoprotein surface is, in principle, not very different from that of a lipid bilayer, direct lipoprotein-BSA association may be excluded as a mechanism of bound amphiphile transfer from the protein to the lipoprotein or vice-versa.
Three factors have to be borne in mind when comparing the association of monomeric NBD-DMPE from the aqueous phase with the different lipoproteins, or its association with lipoproteins and phospholipid bilayer membranes. First, the different lipoprotein particles have very different diameters, from a maximum of ∼55 nm for VLDL to a minimum of ∼7 nm for HDL3. A typical large unilamellar vesicle has a diameter of ∼100 nm. Thus, the curvature of the polar lipid surfaces that these structures present to the aqueous phase are quite distinct from each other. Second, the polar surfaces of lipoproteins have associated proteins that are different and characteristic for each class of lipoproteins, and the density of surface-coverage by the proteins is likely to be quite different from one class of lipoproteins to another whereas the surface of an artificial lipid bilayer vesicle has no associated protein. This will certainly affect the kinetics of association but is not likely to affect the equilibrium state if it is assumed that NBD-DMPE behaves like a normal phospholipid in the surface layer and has no preferred interaction with a specific apolipoprotein. Third, the polar lipid composition of the surface layer is different for each class of the lipoproteins and the behavior of this layer will depend upon its phase characteristics. From the available lipid composition of the different lipoprotein classes (Chapman, 1986), and assuming that all of the phospholipid and cholesterol is in the surface layers of the lipoproteins, it may be concluded that the surface layers of VLDL and LDL show a coexistence of a liquid-ordered phase (rich in sphingomyelin and cholesterol) and a liquid-disordered phase (rich in glycerophospholipids) (Simons and Vaz, 2004). Cholesterol, in particular, possibly partition between the surface layers and the hydrophobic core of the lipoprotein particles although it is not known to what extent. This partitioning will reduce the equilibrium molar fraction of cholesterol in the surface layers. The consequences for VLDL and LDL surface layers will be to reduce the mass fraction of sphingomyelin- and cholesterol-rich liquid-ordered phase and in the HDL will probably result in a single liquid-disordered phase surface layer.
With the above precautions we may proceed to an analysis of the data presented in Table 1. Due to size differences between the lipoproteins, the kinetic rate constants, k+ and k−, as well as the equilibrium association constants, KL, are not directly comparable with each other or with the values we have previously presented (Abreu et al., 2004) for lipid bilayer vesicles. The use of the equilibrium partition coefficient, KP, eliminates this difficulty and the values of KP for the different lipoproteins and lipid bilayer vesicles (regardless of size) can be directly compared. As seen in Table 1, and intuitively expected, the equilibrium partition coefficients are roughly comparable for all the lipoproteins examined and are also comparable to the equilibrium partition coefficients between the lipid phase and the aqueous phase in suspensions of large unilamellar lipid vesicles in the liquid-disordered phase (Abreu et al., 2004).
Because the rates of insertion of the amphiphile in the different lipid aggregates depend on the size of the aggregates, the values of k+ obtained for the different lipoproteins (this work) or lipid vesicles (Abreu et al., 2004) are not directly comparable. The effect of size may, however, be taken into account if one considers the process to proceed via the formation of an encounter complex between both reactants as an intermediate between the aqueous and inserted amphiphile (Steinfeld et al., 1999):
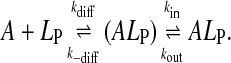 |
(6) |
The encounter complex, (ALP), is formed from A and LP with a diffusion-controlled rate constant, kdiff, and the two entities are held in close proximity by the solvent cage formed around them and by eventual interactions between their surfaces. This encounter complex is broken into the two free entities by diffusion with the rate constant k−diff. The rate constants kdiff and k−diff are given, respectively, as
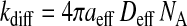 |
(7a) |
and
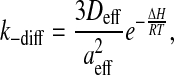 |
(7b) |
where
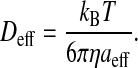 |
(7c) |
Here, aeff is the sum of the radii of the amphiphile and the lipid aggregate, Deff is the effective translational diffusion coefficient, NA is the Avogadro constant, ΔH is energy of interaction between the two entities in the encounter complex, R is the ideal gas constant, T is the temperature, kB is the Boltzmann constant, and η is the viscosity of the medium (water in our case). During the lifetime of the encounter complex a reaction between the two species, such as insertion of the amphiphile into the lipid aggregate, may occur with a rate constant kin. The encounter complex is also formed when the amphiphile exits the lipid aggregate with the rate constant kout. As the aggregate size increases, the encounter complex lives longer and therefore the probability of insertion increases with the apparent increase of the bimolecular rate of insertion as given by scheme 6. The unimolecular rate of insertion, kin, is related to the experimentally measured bimolecular insertion rate constant, k+, for insertion of the amphiphile into the lipid surface from the aqueous phase, obtained assuming a fast equilibrium between the two entities and the encounter complex, by:
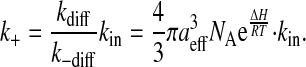 |
(7d) |
The unimolecular insertion rate, kin, calculated from scheme 7 d and assuming ΔH = 0, is presented in Table 2 for all the lipoproteins examined in this work and for large unilamellar vesicles (LUV) previously studied by us (Abreu et al., 2004). Its value for VLDL is very similar to that for LUV of POPC and an equimolar mixture of POPC and cholesterol (14 vs. 7 and 8, respectively) reflecting the similarities of the surface properties of the lipid particles. As the size of the lipoprotein decreases, the curvature of the monolayer becomes significant. For LDL the ratio of the outer and inner surface of the lipid monolayer is 0.66 and it is <0.5 for HDL. As a consequence of this high curvature the free spaces at the monolayer increase and the insertion of the amphiphile is facilitated. The same effect is observed on the exit rate constant (easy in, easy out) resulting in a partition coefficient insensitive to the size of the lipid aggregate.
TABLE 2.
Kinetic and thermodynamic constants for insertion and desorption of NBD-DMPE into/from lipoproteins at 35°C
| VLDL | LDL | HDL2 | HDL3 | POPC−LUV* | POPC:Chol (1:1)−LUV * | SpM:Chol (6:4)−LUV* | |
|---|---|---|---|---|---|---|---|
| radius (nm) | 28 | 11 | 5 | 4 | 50 | ||
| Sout/Sin | 0.86 | 0.66 | 0.39 | 0.18 | 0.92† | ||
| kin (s−1) | 1.4 × 101 | 7.5 × 101 | 1.5 × 102 | 4.4 × 102 | 0.7 × 101 | 0.8 × 101 | 6.0 × 10−1 |
| kout (10−5 s−1) | 4.0 × 10−5 | 5.9 × 10−5 | 2.7 × 10−4 | 5.4 × 10−4 | 2.8 × 10−5 | 4.6 × 10−5 | 1.4 × 10−5 |
Calculated from the results of Abreu et al. (2004).
Refers to the ratio of the outer and inner surface areas of the outer monolayer of the vesicles.
The Gibbs' molar free energy of transfer is clearly dominated in all cases by entropy. This is what one might expect from the hydrophobic effect. The relative contributions of enthalpic and entropic effects to the free energy of transfer are about the same in all the cases examined. The activation processes can be considered in terms of transition state theory (Steinfeld et al., 1999) using a model that has been widely used in the literature to describe the activated state in amphiphile transfer between lipid bilayers or between surfactant micelles and the aqueous phase. We have recently used this model to understand the association of NBD-DMPE with liquid-ordered and liquid-disordered phase lipid bilayer membranes (Abreu et al., 2004). The conclusions are, perhaps not surprisingly, very similar for association of this probe with the lipoprotein surfaces examined in this work and need not be further discussed here.
In conclusion, we have examined the detailed kinetics and thermodynamics of the association of an amphiphilic phospholipid derivative, NBD-DMPE, with various lipoproteins. The process is very similar in almost all its characteristics with the association of the same amphiphile with lipid bilayer membranes prepared from 1-palmitoyl-2-oleoylphosphatidylcholine in the liquid-disordered and liquid-ordered phases. It must be noted here, and has been discussed in greater detail in recent work from our laboratory (Abreu et al., 2004), that the liquid-disordered and liquid-ordered phase layers of 1-palmitoyl-2-oleoylphosphatidylcholine show only small differences in what concerns the kinetics and thermodynamics of NBD-DMPE association with them. The results reported here present, we believe for the first time, a complete description of the kinetics of the association of a lipid-derived amphiphile with lipoproteins that can serve as a generally predictive base for the association of other amphiphiles with these particles.
Acknowledgments
This work was supported through research grants from the POCTI program of the Fundacao para a Ciencia e a Tecnologia (FCT) of the Portuguese Ministry for Higher Education and Scientific Research. L.M.B.B.E. was supported by doctoral stipend No. SFRH/BD/6746/2001 by the FCT.
Abbreviations used: KB, equilibrium binding constant for the binding of an amphiphile to a protein (serum albumin); ρ, density (g mL−1); BSA, fatty acid-free bovine serum albumin; CAC, critical aggregation (micelle) concentration; HDL, high density lipoprotein; KL, equilibrium association constant for the association of NBD-DMPE with lipoproteins particles; LDL, low density lipoprotein; NBD-DMPE, N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-amino-1,2-dimyristoylphosphatidyl ethanolamine; VLDL, very low density lipoprotein.
References
- Abreu, M. S. C., M. J. Moreno, L. M. B. B. Estronca, and W. L. C. Vaz. 2003. Binding of a fluorescent lipid amphiphile to albumin and its transfer to lipid bilayer membranes. Biophys. J. 84:386–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu, M. S. C., M. J. Moreno, and W. L. C. Vaz. 2004. Kinetics and thermodynamics of association of a phospholipid derivative with lipid bilayers in liquid-disordered and liquid-ordered phases. Biophys. J. 87:353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers, J. J., and M. C. Cheung. 2004. Emerging roles for phospholipid transfer protein in lipid and lipoprotein metabolism. Curr. Opin. Lipidol. 15:255–260. [DOI] [PubMed] [Google Scholar]
- Barter, P. J., H. B. Brewer, M. J. Chapman, C. H. Hennekens, D. J. Rader, and A. R. Tall. 2003. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 23:160–167. [DOI] [PubMed] [Google Scholar]
- Brown, R. E. 1992. Spontaneous lipid transfer between organized lipid assemblies. Biochim. Biophys. Acta. 1113:375–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman, M. J. 1986. Comparative analysis of mammalian plasma lipoproteins. Methods Enzymol. 128:70–143. [DOI] [PubMed] [Google Scholar]
- Ferrell, J. E., K. J. Lee, and W. H. Huestis. 1985. Lipid transfer between phosphatidylcholine vesicles and human erythrocytes: exponential decrease in rate with increasing chain length. Biochemistry. 24:2857–2864. [DOI] [PubMed] [Google Scholar]
- Gennis, R. B. 1989. Biomembranes—Molecular Structure and Function. Springer-Verlag, New York.
- Gotto, A. M., H. J. Pownall, and R. J. Havel. 1986. Introduction to the plasma lipoproteins. Methods Enzymol. 128:3–41. [DOI] [PubMed] [Google Scholar]
- Haberland, M. E., and J. A. Reynolds. 1975. Interaction of L-alpha-palmitoyl lysophosphatidylcholine with the AI polypeptide of high density lipoprotein. J. Biol. Chem. 250:6636–6639. [PubMed] [Google Scholar]
- Ihm, J., D. M. Quinn, S. J. Busch, B. Chataing, and J. A. K. Harmony. 1982. Kinetics of plasma protein catalyzed exchange of phosphatidylcholine and cholesteryl ester between plasma lipoproteins. J. Lipid Res. 23:1328–1340. [PubMed] [Google Scholar]
- Jonas, A. 1979. Interaction of bovine serum high density lipoprotein with mixed vesicles of phosphatidylcholine and cholesterol. J. Lipid Res. 20:817–824. [PubMed] [Google Scholar]
- Jonas, A., and G. T. Maine. 1979. Kinetics and mechanism of phosphatidylcholine and cholesterol exchange between single bilayer vesicles and bovine serum high-density lipoprotein. Biochemistry. 18:1722–1728. [DOI] [PubMed] [Google Scholar]
- Jones, J. D., and T. E. Thompson. 1990. Mechanism of spontaneous, concentration-dependent phospholipid transfer between bilayers. Biochemistry. 29:1593–1600. [DOI] [PubMed] [Google Scholar]
- Kleinfeld, A. M. 2000. Topical Review: Lipid phase fatty acid flip-flop, Is it fast enough for cellular transport? J. Membr. Biol. 175:79–86. [DOI] [PubMed] [Google Scholar]
- Kostner, D. M., and P. Alaupovic. 1972. Composition and structure of plasma lipoproteins. Separation and quantification of the lipoprotein families occurring in the high density lipoproteins of human plasma. Biochemistry. 11:3419–3428. [DOI] [PubMed] [Google Scholar]
- Lippiello, P. M., and M. Waite. 1983. Kinetics and mechanism of phosphatidylcholine and cholesterol exchange between chylomicrons and high-density lipoproteins. Biochem. J. 215:279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin reagent. J. Biol. Chem. 193:265–275. [PubMed] [Google Scholar]
- Lund-Katz, S., B. Hammerschlag, and M. C. Phillips. 1982. Kinetics and mechanism of free cholesterol exchange between human serum high and low-density lipoproteins. Biochemistry. 21:2964–2969. [DOI] [PubMed] [Google Scholar]
- Massey, J. B., D. H. Bick, and H. J. Pownall. 1997. Spontaneous transfer of monoacyl amphiphiles between lipid and protein surfaces. Biophys. J. 72:1732–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean, L. R., and M. C. Phillips. 1984. Kinetics of phosphatidylcholine and lysophosphatidylcholine exchange between unilamellar vesicles. Biochemistry. 23:4624–4630. [DOI] [PubMed] [Google Scholar]
- Nichols, J. W. 1985. Thermodynamics and kinetics of phospholipid monomer-vesicle interaction. Biochemistry. 24:6390–6398. [DOI] [PubMed] [Google Scholar]
- Peters, T., Jr. 1997. All About Albumin—Biochemistry, Genetics, and Medical Applications. Academic Press, San Diego, CA.
- Phillips, M. C., W. J. Johnson, and G. H. Rothblat. 1987. Mechanisms and consequences of cellular cholesterol exchange and transfer. Biochim. Biophys. Acta. 906:223–276. [DOI] [PubMed] [Google Scholar]
- Pownall, H. J. 2001. Cellular transport of nonesterified fatty acids. J. Mol. Neurosci. 16:109–115. [DOI] [PubMed] [Google Scholar]
- Schumaker, V. N., and D. L. Puppione. 1986. Sequential floatation ultracentrifugation. Methods Enzymol. 128:155–170. [DOI] [PubMed] [Google Scholar]
- Simons, K., and W. L. C. Vaz. 2004. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. Struct. 33:269–295. [DOI] [PubMed] [Google Scholar]
- Silvius, J. R., and R. Leventis. 1993. Spontaneous interbilayer transfer of phospholipids: dependence on acyl chain composition. Biochemistry. 32:13318–13326. [DOI] [PubMed] [Google Scholar]
- Steinfeld, J. I., J. S. Francisco, and W. L. Hase. 1999. Chemical Kinetics and Dynamics, 2nd ed. Prentice Hall, Upper Saddle River, NJ.
- Vaz, W. L. C., and E. Melo. 2001. Fluorescence studies on phase heterogeneity in lipid bilayer membranes. J. Fluorescence. 11:255–271. [Google Scholar]
- Vieira, O. V., J. A. N. Laranjinha, V. M. C. Madeira, and L. M. Almeida. 1996. Rapid isolation of low-density lipoproteins in a concentrated fraction free from water-soluble plasma antioxidants. J. Lipid Res. 37:2715–2721. [PubMed] [Google Scholar]
- Wimley, W. C., and T. E. Thompson. 1990. Exchange and flip-flop of dimyristoylphosphatidylcholine in liquid-crystalline, gel, and two-component, two-phase large unilamellar vesicles. Biochemistry. 29:1296–1303. [DOI] [PubMed] [Google Scholar]
- Zakim, D. 2000. Topical review: Thermodynamics of fatty acid transfer. J. Membr. Biol. 176:101–109. [DOI] [PubMed] [Google Scholar]
- Zucker, S. D., W. Goessling, and J. L. Gollan. 1995. Kinetics of bilirubin transfer between serum albumin and membrane vesicles. J. Biol. Chem. 270:1074–1081. [DOI] [PubMed] [Google Scholar]
- Zucker, S. D. 2001. Kinetic model of protein-mediated ligand transport: influence of soluble binding proteins on the intermembrane diffusion of a fluorescent fatty acid. Biochemistry. 40:977–986. [DOI] [PubMed] [Google Scholar]