Abstract
The initial arrest and subsequent rolling of a leukocyte on the vascular endothelium is believed to be facilitated by the extraction of tethers, which are narrow membranous tubes drawn from the leukocyte. Although single tether extraction from neutrophils has been studied thoroughly, the relationship between the tether force (F) and tether-growth velocity (Ut) is still unknown for double tethers drawn from neutrophils. In this study, we have determined this relationship with the micropipette-aspiration technique. As a comparison, tether extraction from CD4+ T-lymphocytes was also studied. The threshold force and effective viscosity for single tether extraction from passive CD4+ T-lymphocytes were found to be 46 pN and 1.55 pN · s/μm, respectively. These values were modulated by stimulation with phorbol myristate acetate (PMA), but not interleukin-8 (IL-8). More importantly, for both types of leukocyte, the threshold force and effective viscosity for double tether extraction are about twice as large as those corresponding to single tether extraction. Neither IL-8 nor PMA stimulation had any effect on this correlation. These results indicate that double tethers are highly localized on cellular surfaces and independent of each other during the rolling process.
INTRODUCTION
Rolling on the vascular endothelium is critical for trafficking, extravasation, and retention of certain leukocytes such as neutrophils and lymphocytes. Rolling allows for the ensuing firm adhesion of these immune cells on the endothelium and their subsequent migration into tissues to fulfill their various immunological functions. The traffic signals for neutrophil emigration to inflammatory sites and for lymphocyte recirculation are strikingly similar at the molecular level (Springer, 1995). The initial attachment and rolling are mediated by the selectin family of adhesion molecules on both leukocytes and endothelial cells and their carbohydrate ligands on opposing cells. The firm adhesion of leukocytes on the endothelium is mediated by β2 integrins on the leukocyte surface and their ligands of the immunoglobulin family on the endothelial cells. Furthermore, rolling is a highly-complex dynamic process regulated cooperatively not only by these specialized adhesion molecules and locally-released chemokines, but also by fluid shear stress and the mechanical properties of leukocytes. In particular, membrane tether extraction from leukocytes is believed to play an important role in regulating the rolling process (Shao and Hochmuth, 1996; Shao et al., 1998; Schmidtke and Diamond, 2000; Marcus and Hochmuth, 2002; Park et al., 2002; Yago et al., 2002).
Studies on membrane tether extraction in general can help us understand the association between the leukocyte membrane and its underlying cytoskeleton, which is particularly relevant to the processes of secretion and signaling (Hochmuth et al., 1996; Sheetz and Dai, 1996; Hochmuth and Marcus, 2002). Tethers have been extracted from lipid vesicles (Waugh, 1982) and from various living cells, including neutrophils (Shao and Hochmuth, 1996; Shao and Xu, 2002), erythrocytes (Hochmuth et al., 1973, 1982), neuronal growth cones (Dai and Sheetz, 1995; Hochmuth et al., 1996), and outer hair cells (Li et al., 2002). For single tether extraction from all these cell types, it has been shown that (Evans and Yeung, 1994; Yeung, 1994; Dai and Sheetz, 1995; Hochmuth et al., 1996; Shao and Hochmuth, 1996)
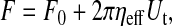 |
(1) |
where F is the tether force (the tensile force imposed at the tether head), F0 is the threshold (minimum) force required for extracting a single tether, ηeff is the effective viscosity, and Ut is the tether-growth velocity. The threshold force F0 is determined by the intrinsic membrane surface tension, the membrane bending rigidity, and the membrane-cytoskeleton adhesion energy. In addition, ηeff is determined by the intrinsic membrane surface viscosity, the relative interbilayer slip, and the membrane slip relative to the cytoskeleton (Evans and Yeung, 1994; Hochmuth et al., 1996).
For single tether extraction from passive neutrophils, F0 = 45 pN and ηeff = 1.8 pN · s/μm (Shao and Hochmuth, 1996). Stimulation of the cells with chemokines such as interleukin-8 (IL-8) or phorbol myristate acetate (PMA) resulted in a threshold force twice as large as that for passive neutrophils and dramatically decreased the effective viscosity (Shao and Xu, 2002). Because of the high surface density of leukocyte microvilli, more than one microvillus could be in contact with the endothelium at the onset of rolling and double or multiple tethers could be extracted. In fact, double or multiple tethers have to be present at least intermittently during the rolling process because a stable rolling cannot be achieved otherwise. Although double tethers drawn from neutrophils have been observed in some flow chamber studies (Schmidtke and Diamond, 2000; Park et al., 2002) and in our previous micropipette experiments (Shao and Hochmuth, 1996; Shao and Xu, 2002), to date, the relationship between the pulling force and tether-growth velocity is still unknown for double tethers extracted from passive or stimulated neutrophils. It is important to determine this relationship so that the detailed force history of each adhesive bond can be calculated during the rolling process. Equally important, the same relationship for single and double tether extraction from CD4+ T-lymphocytes (chosen to avoid variation among different subpopulations of lymphocytes) is also unknown. This study will provide us some valuable information on the mechanical properties of these cells as well as the basis for their rolling and recirculation. Using the micropipette-aspiration technique (MAT), for the first time we determined these previously-unknown properties of double tether extraction from neutrophils as well as single and double tether extraction from CD4+ T-lymphocytes.
MATERIALS AND METHODS
Cell and bead preparation
Neutrophils were collected from healthy donors by finger prick as described previously (Shao and Hochmuth, 1996). CD4+ T-lymphocytes were isolated as follows. A few drops of blood were collected by finger prick into a heparinized capillary glass tube (Fisher Scientific, Pittsburgh, PA). About the same amount of Ficoll-Hypaque gradient (Histopaque 1077; Sigma, St. Louis, MO) was then collected into the same tube from the blood end. The blood end of the tube was sealed with tube-sealing compound (Fisher Scientific, Pittsburgh, PA), and the tube was centrifuged at 13,450 × g for a few seconds at room temperature (∼20°C). Thereafter, the tube was cut at a point in the gradient region that was near but free of erythrocytes and neutrophils (a white layer sandwiched between the gradient solution and erythrocytes). The erythrocytes and neutrophils were discarded. The remaining cells were mixed with 0.1-ml 50% autologous plasma-HBSS solution (modified endotoxin-free Hanks' balanced salt solution; no Ca2+ or Mg2+; buffered with 25 mM Hepes; pH = 7.4; Sigma) and incubated with FITC-conjugated monoclonal mouse anti-human CD4 (Sigma) at 37°C for 15 min in order for them to be differentiated from other subpopulations of lymphocytes. Finally, the cells were washed twice with 1.1-ml HBSS solution at 2,700 × g for 5 min at room temperature (∼20°C), resuspended in a 0.1-ml 50% autologous plasma-HBSS solution, and transferred to the experimental chamber. During the experiments, CD4+ T-lymphocytes were identified by using fluorescence microscopy. Without any other treatment, the cells were spherical under the microscope and not appear to be activated by the labeling. For stimulation experiments, cells were treated with 25 ng/ml IL-8 (R&D Systems, Minneapolis, MN) or 50 ng/ml PMA (Sigma) in the experimental chamber as described previously (Shao and Xu, 2002).
Latex beads coated with goat anti-mouse IgG antibodies (∼8.3 μm in diameter; Sigma) were washed twice in PBS and incubated with mouse anti-human CD162 or CD18 (PharMingen, San Diego, CA) for tether-extraction experiments from passive or stimulated cells, respectively. The beads were then stored at 4°C and washed twice with PBS before use. During the incubation, the concentration of the primary antibody (∼10 μg/ml) was large enough to saturate all the binding sites of the secondary antibody on the bead surface. The beads used in this study were the same as the ones used in two previous studies (Shao and Hochmuth, 1999; Levin et al., 2001), where the site density of the primary antibody was determined to be ∼220/μm2 with two independent methods: the micropipette technique and flow cytometry.
Tether extraction with the MAT
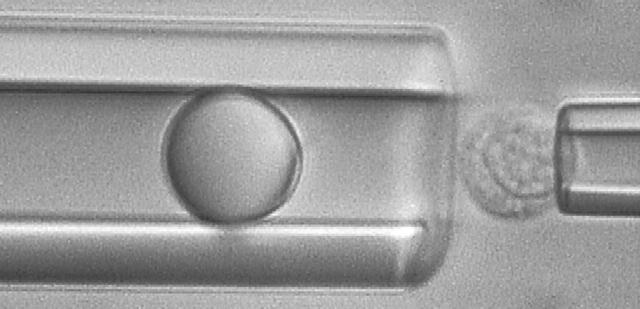
Tether-extraction experiments from neutrophils and CD4+ T-lymphocytes were conducted with the MAT (Shao and Xu, 2002). A video micrograph from one of the lymphocyte experiments is shown in Fig. 1. The micropipette on the left is shown containing an antibody-coated bead, which is the force transducer of the MAT. The pipette on the right is shown holding a CD4+ T-lymphocyte at the mouth of the left pipette. At the beginning of an experiment, a precisely-controlled aspiration pressure (Δp < 0) is applied inside the left pipette, which causes the bead to move initially to the left. Then a positive internal pressure is superimposed, and the bead is driven to the right until it contacts the cell. After the bead is in contact with the cell for a certain period of time, the positive pressure is removed and the negative aspiration pressure (Δp) is imposed to pull the bead again to the left. If the contact does not result in adhesion between the bead and cell, the bead translates freely to the left at its “free motion velocity (Uf)”. If, on the contrary, adhesion occurs during the contact, tether extraction and extension ensues under the action of the aspiration pressure, which is often of sufficient magnitude for double tether extraction. One or two tethers may occur. The leftward velocity of withdrawal of the bead-transducer (tether-growth velocity, Ut) is a function of Δp and the number of tethers formed. Several typical modes of bead displacement versus time are shown in Fig. 2, a–c, indicating zero, one, and two tethers formed in this process, respectively. The corresponding pulling force (F) can be calculated from (Shao and Hochmuth, 1996)
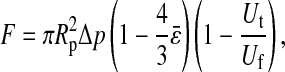 |
(2) |
where Rp is the radius of the micropipette, and
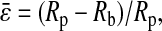 |
(3) |
where Rb is the radius of the bead. When the bead does not adhere or after the tether breaks, the pressure inside the left micropipette is returned to a positive value and the whole process is repeated. For each cell-bead pair, this process is repeated for ∼3 min. Since the pressure in the experimental chamber changes slowly over time, the zero pressure has to be verified frequently, usually before a new cell-bead pair is used. The negative aspiration pressures (Δp) used in our experiments ranged from 2 pN/μm2 to 12 pN/μm2. All experiments were recorded on an S-VHS videotape and analyzed as described below.
FIGURE 1.
A video micrograph showing how to extract tethers from a passive CD4+ T-lymphocyte with the MAT where the bead was coated with mouse anti-human CD162 antibodies.
FIGURE 2.
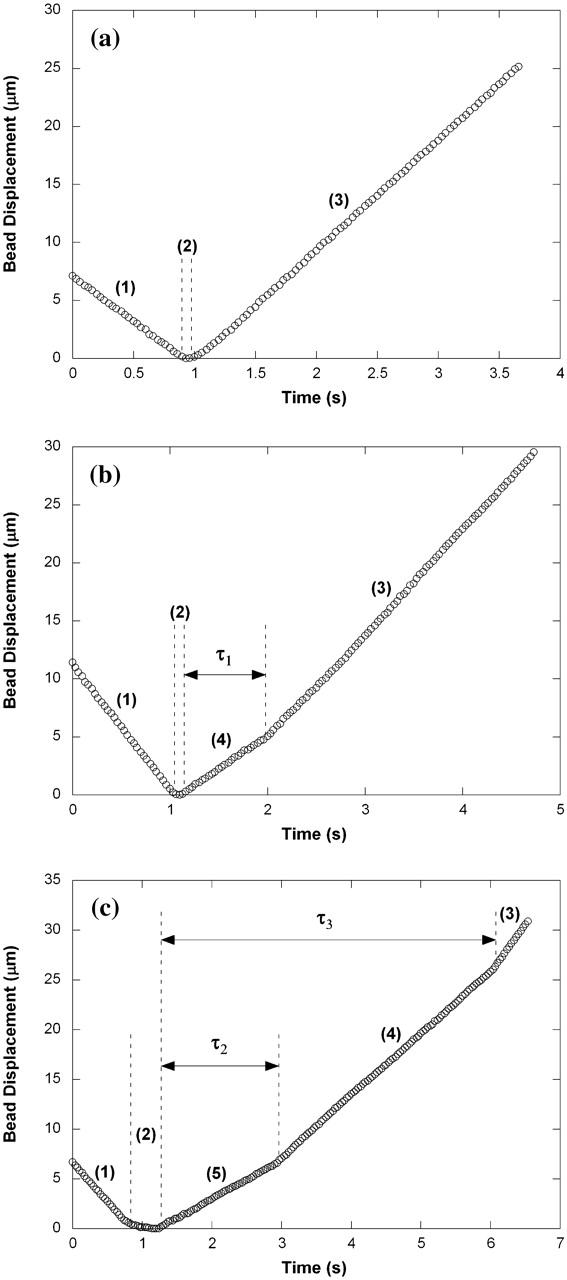
Bead displacements when (a) no tethers, (b) single tethers, or (c) double tethers were extracted from a PMA-treated lymphocyte under the aspiration pressure (Δp) of 5 pN/μm2. Different bead translation velocities are separated by dashed lines and marked as (1) approaching the cell, (2) making contact with the cell, (3) moving away freely from the cell, (4) extracting a single tether, and (5) extracting double tethers. Here, the free motion velocity of the bead (Uf) is ∼9.5 μm/s (a–c), the growth velocity of the single tether is ∼5.7 μm/s (b and c), and the growth velocity of the double tether is ∼3.9 μm/s (c). In c, after one tether was broken, the other grew at a velocity that was almost the same as the velocity when only one tether was initially extracted as in b. The adhesion lifetime is defined as follows: τ1 is the adhesion lifetime of single tethers, τ2 is the adhesion lifetime of double tethers when both tethers are present, and τ3 is the adhesion lifetime of double tethers when at least one tether is present.
Data analysis of the MAT
The videotape recorded during an experiment was played on an S-VHS video cassette recorder and the signal was transmitted to a CCTV monitor and to a Windows PC computer through a monochrome frame grabber (Scion, Frederick, MD). The action occurring within the region of interest during one adhesion event (i.e., the left micropipette with the moving bead inside) was stored in the computer as a movie at a speed of 30 frames/s. Then the displacement of the bead was tracked with Scion Image macros written in our laboratory, yielding an ASCII file that contained the displacement-time data of the bead such as those shown in Fig. 2. The data were then imported into KaleidaGraph (Synergy Software, Reading, PA), plotted, and fitted with a straight line for both single and double tether elongation or free bead motion that occurred in the absence of an adhesion event. The statistical comparison between the slopes or intercepts of two intersecting regression lines, or between the elevations of two parallel lines was carried out by using the methods described by Zar (1999). For all the statistical analyses, 0.05 was chosen as the significance level.
RESULTS
F0 and ηeff are not different for single tether extraction from CD4+ T-lymphocytes and neutrophils
For passive CD4+ T-lymphocytes and neutrophils, we chose anti-CD162-coated beads as the force transducer of the MAT because CD162, also called P-selectin glycoprotein ligand-1 or PSGL-1, mediates the rolling of leukocytes on the endothelium through its interaction with P-selectin on the endothelial cell surface (Moore et al., 1995; Bruehl et al., 1997). With these beads, a total of 738 and 465 single tethers were extracted from 54 passive CD4+ T-lymphocytes and 43 passive neutrophils, respectively, at various pressures. Membrane tethers are usually only tens of nanometers in diameter, so it is very difficult to actually see a tether under the microscope. However, if a tether happens to be in the focal plane, a very faint shadow becomes visible on the video monitor of our microscopic system. In our experiments, we did observe these faint shadows occasionally.
Almost all tethers grew at constant velocity under a constant aspiration pressure. With the MAT, single tether extraction was typically inferred from a slow bead translation followed by a free faster bead translation (Uf) indicating rupture of the adhesive bond between the bead and cell (Shao and Hochmuth, 1996; Shao and Xu, 2002). Furthermore, an adhesion frequency, defined as the percentage of adhesive events in the total bead-cell contact trials, <20%, was also taken to be an indicator of single receptor-ligand interactions and hence single tether extractions. Fig. 3, a and b, compare the correlations between the tether force and tether-growth velocity for single and double tethers drawn from passive CD4+ T-lymphocytes and passive neutrophils, respectively. For CD4+ T-lymphocytes, the threshold force (intercept) necessary to extract a single tether was found to be 46 pN, and the effective membrane surface viscosity (slope divided by 2π) was found to be 1.55 pN · s/μm. These values are essentially the same as those derived from the correlation for single tethers from passive neutrophils (Fig. 3 b). The results for single tether extraction from passive neutrophils are in good agreement with those in the literature (Shao and Hochmuth, 1996; Shao and Xu, 2002).
FIGURE 3.
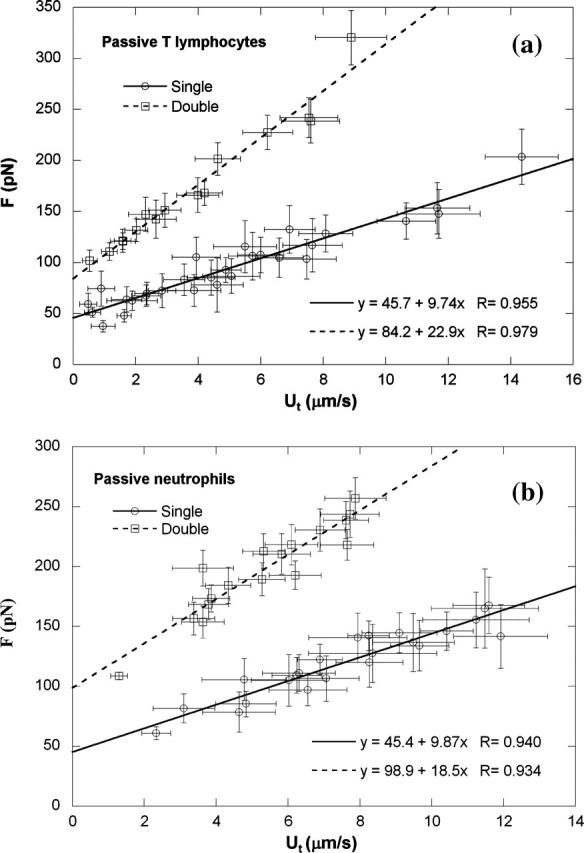
Single and double tether extraction from (a) passive CD4+ T-lymphocytes and (b) passive neutrophils. Each point represents an average of 10–50 tethers from two to six cells at the same aspiration pressure. The error bars in both directions stand for the standard deviations of F and Ut, respectively. Also shown is the linear regression equation that yields the threshold force (intercept) and effective viscosity (slope/2π), as well as the correlation coefficient.
F0 and ηeff double for double tether extraction from CD4+ T-lymphocytes and neutrophils
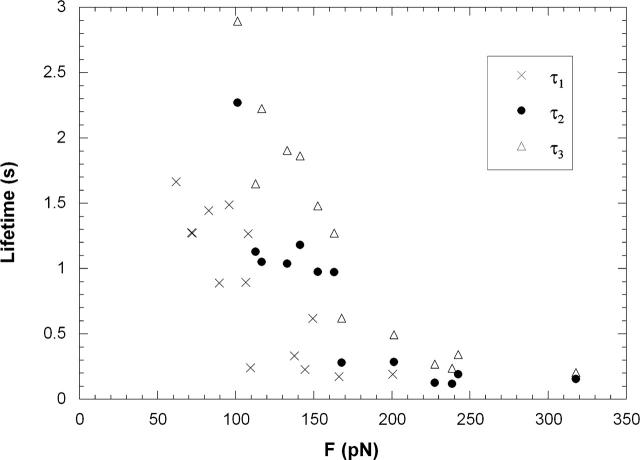
To extract double tethers, we increased the bead-cell adhesion frequency (conducive to double or multiple bond formation) by increasing the contact time between the bead and cell. Double bonds can be inferred from the fact that, under similar pulling forces, double bonds had longer lifetimes than single ones. This is shown in Fig. 4, where τ1, τ2, and τ3 (defined in Fig. 2) are plotted against their corresponding pulling forces for passive CD4+ T-lymphocytes. It should be noted that, during double tether extraction, the pulling force was not a constant. When double tethers were present, the force was larger than the force after one of the tethers was broken. Had the other tether also been broken by this larger force, τ3 would have been smaller. Consequently, the adhesion lifetime under this larger force should lie somewhere between τ2 and τ3. As shown in Fig. 4, even τ2 is larger than τ1 under similar pulling forces. Therefore, we can conclude that double bonds were indeed formed in our double tether experiment with passive CD4+ T-lymphocytes. For stimulated lymphocytes and passive or stimulated neutrophils, the same conclusion can be drawn (data not shown).
FIGURE 4.
Average adhesion lifetime (τ1, τ2, and τ3) under various pulling forces obtained in the experiments with passive CD4+ T-lymphocytes.
A total of 271 and 307 double tethers were obtained from 39 passive CD4+ T-lymphocytes and 43 passive neutrophils, respectively. In some cases, after the adhesion was ruptured between the bead and cell, we could see clearly that the shadows of two tethers retracted to the cell body, one after the other. Triple tethers, where the bead velocity changed twice before it reached its free motion velocity, were only occasionally seen in our experiments. For double tethers, it is highly unlikely that two tethers would break at the same instant because of the stochastic nature of receptor-ligand or antibody-antigen binding. Hence, some of our single tether data shown in Fig. 3 correspond to single tethers that were initially one of a double tether pair, measured after the other was ruptured (as shown in Fig. 2 c). When two tethers were simultaneously extracted from passive CD4+ T-lymphocytes, the effective viscosity became 3.65 pN · s/μm and the threshold force increased to 84 pN (Fig. 3 a). For double tether extraction from passive neutrophils, the effective viscosity was 2.95 pN · s/μm and the threshold force was 99 pN (Fig. 3 b). Obviously, double tethers drawn from these passive leukocytes behave dynamically almost as a linear summation of two single tethers in parallel: both threshold force and effective viscosity for double tethers are about twice as large as those properties in single tethers, respectively (see Table 1). This indicates that double tethers are highly localized and independent of each other, which suggests that there is no significant “competition” for membrane material between the two tethers owing to an abundant membrane reservoir in these microvillus-rich leukocytes.
TABLE 1.
A summary of all the threshold force and effective viscosity (value ± 68% confidence interval) for single and double tether extraction from passive or treated neutrophils and CD4+ T-lymphocytes
| Single tether |
Double tether |
Double/single ratio |
|||||
|---|---|---|---|---|---|---|---|
| Cell type | Condition | F0 (pN) | ηeff (pN · s/μm) | F0 (pN) | ηeff (pN · s/μm) | F0 | ηeff |
| Neutrophils | Passive | 45 ± 6.5 | 1.57 ± 0.127 | 99 ± 10.6 | 2.95 ± 0.301 | 2.2 ± 0.6 | 1.9 ± 0.4 |
| IL8 | 91 ± 5.0* | 0.40 ± 0.054* | 176 ± 8.9* | 0.83 ± 0.103* | 1.9 ± 0.2 | 2.1 ± 0.6 | |
| PMA | 77 ± 4.4* | 0.56 ± 0.078* | 150 ± 7.5* | 1.18 ± 0.196* | 1.9 ± 0.2 | 2.1 ± 0.7 | |
| Lymphocytes | Passive | 46 ± 3.7 | 1.55 ± 0.093 | 84 ± 6.3 | 3.65 ± 0.217 | 1.8 ± 0.3 | 2.4 ± 0.3 |
| IL8 | 52 ± 8.6 | 1.34 ± 0.199 | 95 ± 13.3 | 3.15 ± 0.541 | 1.8 ± 0.6 | 2.4 ± 0.8 | |
| PMA | 69 ± 9.9* | 1.03 ± 0.217* | 113 ± 7.0* | 2.44 ± 0.240* | 1.6 ± 0.4 | 2.4 ± 0.8 | |
Denotes a significant difference (p < 0.05) versus passive cells (Zar, 1999).
PMA affects tether extraction from CD4+ T-lymphocytes and neutrophils
Neutrophils stimulated with PMA would down-regulate their CD162 expression (Davenpeck et al., 2000). On the contrary, the expression of β2 integrins would be up-regulated upon leukocyte activation (Diamond and Springer, 1993; Erlandsen et al., 1993). Therefore, for stimulated lymphocytes and neutrophils, anti-CD18-coated beads were chosen as the force transducer of the MAT. With these beads, a total of 515 and 624 single tethers were extracted from 30 CD4+ T-lymphocytes and 18 neutrophils, respectively, after being treated with PMA (50 ng/ml). For single tether extraction from CD4+ T-lymphocytes treated with PMA, the threshold force was 69 pN and the effective viscosity was 1.03 pN × s/μm (Fig. 5 a; p < 0.02 for both parameters versus passive T-cells). For single tether extraction from neutrophils treated with PMA, the threshold force became 77 pN and the effective viscosity became 0.56 pN · s/μm (Fig. 5 b; p < 0.001 for both parameters versus passive neutrophils), values which are again consistent with published results (Shao and Xu, 2002). A total of 277 and 238 double tethers were also extracted from 27 CD4+ T-lymphocytes and 18 neutrophils, respectively, after being treated with PMA. Plotted in Fig. 5 is the correlation between F and Ut for double tether extraction from these PMA-treated leukocytes. The experimental values of threshold force and effective viscosity for double tether extraction from both types of cell treated with PMA were, respectively, modulated by PMA but remained about twice the corresponding magnitudes for single tether extraction from these treated cells (Table 1).
FIGURE 5.
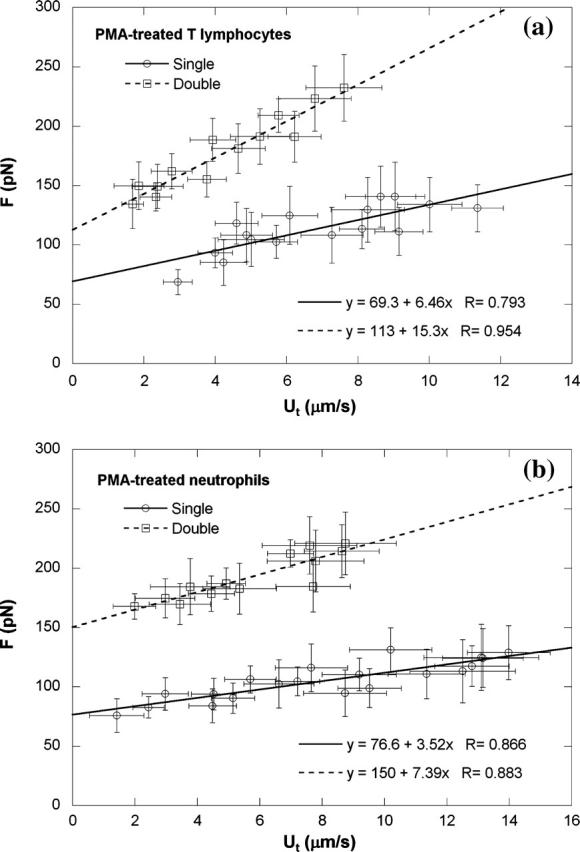
Single and double tether extraction from (a) CD4+ T-lymphocytes and (b) neutrophils when treated with 50 ng/ml PMA. Each point represents an average of 10–60 tethers from two to six cells at the same aspiration pressure. The error bars in both directions stand for the standard deviations of F and Ut, respectively. Also shown is the linear regression equation that yields the threshold force and effective viscosity, as well as the correlation coefficient.
IL-8 affects tether extraction from neutrophils, but not CD4+ T-lymphocytes
It has been shown that single tether extraction from neutrophils is modulated by IL-8 and PMA (Shao and Xu, 2002). As found previously (Shao and Xu, 2002), neutrophils treated with 25 ng/ml IL-8 or 50 ng/ml PMA became very active, protruding pseudopodia in various directions. However, CD4+ T-lymphocytes treated with the same concentration of IL-8 are relatively “passive”, maintaining a spherical shape. Although T-cells treated with PMA developed some protrusions, these cells were not as active as neutrophils treated with PMA. Nevertheless, an observed increase in adhesion frequency indicates that both neutrophils and T-lymphocytes up-regulated the expression of integrins in response to IL-8 or PMA. With anti-CD18-coated beads as the force transducer, a total of 453 and 898 single tethers were extracted from 19 and 33 IL-8-treated T-lymphocytes and neutrophils, respectively. For single tether extraction from T-lymphocytes after IL-8 treatment, the threshold force was 52 pN and the effective viscosity was 1.34 pN · s/μm (Fig. 6 a), with no significant difference compared with passive T-cells (p > 0.2 for both parameters). This finding indicates that IL-8 has no effect on tether extraction from T-lymphocytes. For single tether extraction from neutrophils after IL-8 treatment, the threshold force became 91 pN and the effective viscosity became 0.40 pN · s/μm (Fig. 6 b; p < 0.001 for both parameters versus passive neutrophils). These results agree well with our previous findings (Shao and Xu, 2002). Furthermore, a total of 208 and 344 double tethers were extracted from 19 T-lymphocytes and 33 neutrophils, respectively, after being treated with IL-8. Shown in Fig. 6 are the corresponding data for double tether extraction from these leukocytes after being treated with IL-8. It is clear that the values of threshold force and effective viscosity for double tether extraction from both types of cell treated with IL-8 were, respectively, about twice the magnitude of those for single tether extraction from treated cells (Table 1).
FIGURE 6.
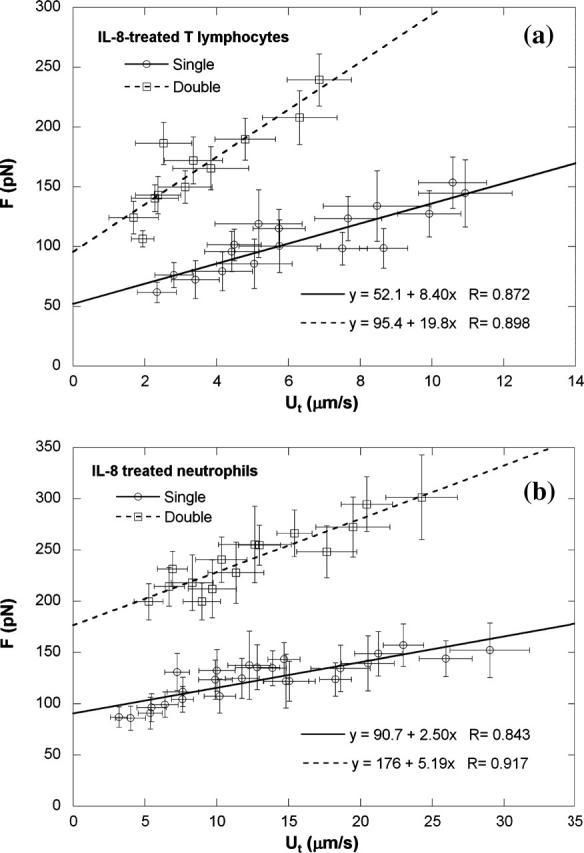
Single and double tether extraction from (a) CD4+ T-lymphocytes and (b) neutrophils after they were treated with 25 ng/ml IL-8. Each point represents an average of 10–50 tethers from two to six cells at the same aspiration pressure. The error bars in both directions stand for the standard deviations of F and Ut, respectively. Also shown is the linear regression equation that yields the threshold force and effective viscosity, as well as the correlation coefficient.
DISCUSSION
With the MAT, we extracted single and double tethers from CD4+ T-lymphocytes and neutrophils, which were either unstimulated or stimulated with PMA or IL-8. In these experiments, the most convincing evidence for double tether extraction is our direct, albeit occasional, observation of double tethers and their retraction to the cell body after being broken. In most cases, double tethers could only be inferred from the change in the bead velocity before its free motion (the slope change as shown in Fig. 2 c). Although the tether length shown in Fig. 2 c is long, this slope change is not caused by the growth of the tether length. On average, the double tethers extracted from the PMA-stimulated lymphocytes were only ∼8 μm in total length (from the instant of adhesion to the instant when the second tether was broken). This value is comparable to the average tether length of the single tethers extracted from these cells at low adhesion frequency, where no slope change was observed (data not shown; this is also true for other cells). Likewise, the slope change shown in Fig. 2 c is not caused by two different receptor pools or one receptor population with two different linkages to the cytoskeleton (one results in single tether extraction and the other results in double tether extraction) either. If these two different receptor pools or linkages existed, we would have observed the same slope change when single tethers were extracted at low adhesion frequency.
Membrane tethers extracted from fibroblasts do not have F-actin inside (Raucher et al., 2000), which indicates that the cytoskeleton is not deformed or dislocated during tether extraction. This is likely also the case for the lymphocytes and neutrophils studied in this article. If tether extraction involves deforming the cytoskeleton (a network of rodlike cytoskeletal components), we should expect that the tether-growth velocity would become smaller and smaller under a constant pulling force. The fact that we obtained a constant tether-growth velocity under a constant pulling force indicates that no cytoskeletal component was dislocated, so even a receptor with two different linkages to the cytoskeleton would not cause the slope change in Fig. 2 c. Therefore, the slope change in Fig. 2 c is very likely due to double tether extraction.
For single tether extraction from passive neutrophils at large forces (>100 pN), it is possible that the local structure at the junction of the tether and cell (mainly the separation radius) will cause a decrease in the effective viscosity (Marcus and Hochmuth, 2002). According to Marcus and Hochmuth (2002), the smaller the separation radius (corresponding to smaller pulling forces), the larger the effective viscosity. If this were the reason for the slope change shown in Fig. 2 c, it would imply that, after the slope change, the separation radius became smaller and the effective viscosity became larger because a smaller force was present (corresponding to the faster bead motion as shown in Fig. 2 c and Eq. 2). However, what we found was that the effective viscosity became smaller after the slope change (Figs. 3, 5, and 6). This contradiction, in addition to the fact that the slope change has nearly never been observed in our low adhesion-frequency (single tether extraction) experiments, indicates that the slope change in Fig. 2 c is not caused by the change in the local structure of the cell.
Lymphocytes (∼7.5 μm in diameter) are smaller than neutrophils (∼8.3 μm in diameter) and have a relatively larger nucleus (44% of cell volume) than neutrophils (21% of cell volume) (Waugh and Hochmuth, 1995). We did not know whether lymphocytes and neutrophils would exhibit different mechanical properties governing tether extraction because of their different cellular and nuclear sizes. In this study, we found virtually no difference in the mechanics of tether extraction (single or double) from these passive cells. Both passive CD4+ T-lymphocytes and neutrophils have the same effective viscosity (∼1.55 pN · s/μm). They also have the same total adhesion energy (∼130 pN/μm) as determined from the threshold force (Hochmuth and Marcus, 2002). The cortical tension of lymphocytes, determined to be ∼35 pN/μm (Waugh and Hochmuth, 1995), is very close to that of neutrophils (24–35 pN/μm) (Evans and Yeung, 1989; Needham and Hochmuth, 1992; Tsai et al., 1993, 1994). These results demonstrate that these two types of leukocyte possess the same overall intrinsic mechanical properties as manifested by membrane tension, bending, membrane viscosity, and dynamic membrane-cytoskeleton association.
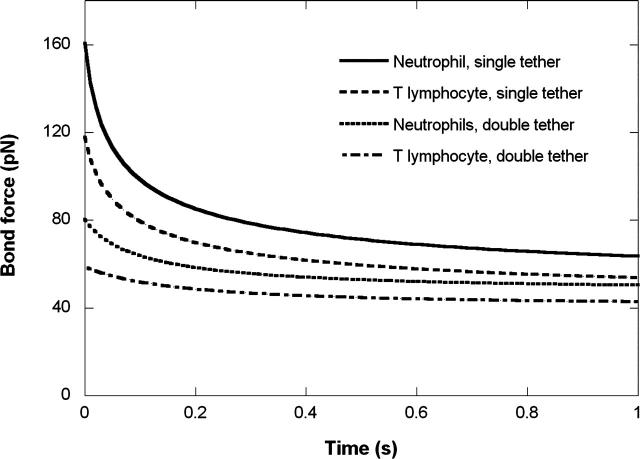
Because lymphocytes are smaller than neutrophils, they would be expected to experience different tether forces under the same blood flow conditions when rolling on the vascular endothelium. By using a simple biophysical model of leukocyte rolling (Shao et al., 1998), we were able to estimate the variation of the bond force in a single tether being extracted from either a lymphocyte or a neutrophil rolling under the action of the same shear stress. As shown in Fig. 7, at a wall shear stress of 0.08 pN/μm2, the tensile force on a single tether pulled from a lymphocyte (with an initial microvillus length of 0.35 μm) decreases from ∼120 pN to ∼50 pN in 1 s. Under the same shear stress, the tensile force on a single tether from a neutrophil (with the same initial microvillus length) decreases from ∼160 pN to ∼60 pN in 1 s (Shao et al., 1998).
FIGURE 7.
Evolution of the bond force when single or double tethers were extracted from a rolling lymphocyte or neutrophil under the shear stress of 0.08 pN/μm2. In the case of double tether extraction, the force on one bond is shown.
During the rolling of leukocytes on the endothelium, double tether extraction could occur in at least two scenarios: a), two or more microvilli are in contact with the endothelium or substrate and both adhere, hence two parallel tethers will be extracted; and b), one microvillus adheres to the endothelium or substrate and one tether is extracted first, then due to the cell rolling, another microvillus adheres to the endothelium or substrate and the second tether is extracted. Case a is definitely beneficial for a stable rolling because of its longer adhesion lifetime, whereas case b is essential for the rolling to occur. In both cases, our results have shown that these two tethers will be independent of each other when their force and growth velocity are considered. Shown in Fig. 7 is the bond force history of case a when double tethers are extracted, calculated with the same approach for single tethers. At a wall shear stress of 0.08 pN/μm2, the tensile force on one of the two tethers extracted from a lymphocyte decreases from ∼60 pN to ∼43 pN in 1 s. Under the same shear stress, the tensile force on one of the two tethers from a neutrophil decreases from ∼80 pN to ∼54 pN in 1 s. Obviously, the presence of double tethers can decrease the force on each bond much more effectively than single tethers under the same shear stress. Hence, for both lymphocytes and neutrophils, the extraction and subsequent extension of single or double tethers can result in a dramatic decrease in the stress tending to break the bond between the leukocyte and endothelium. This stress relief prolongs the bond lifetime and lowers the chance of extraction of selectins from the rolling cell surface and hence helps keep the cell linked to the endothelium until firm arrest and diapedesis occur.
The transition of rolling to firm adhesion of a leukocyte on the endothelium is regulated by specific chemokines released locally by endothelial cells (Springer, 1995). These chemokines bind to their G-protein-coupled receptors on circulating leukocyte surfaces and hence induce adhesion and migration in a manner analogous to adhesion molecules (Olson and Ley, 2002). IL-8 and PMA are commonly-used in the study of leukocyte functions. IL-8 is a potent neutrophil chemoattractant that can bind the receptors on neutrophils. IL-8 has various effects on neutrophils such as pseudopodium extraction, degranulation, and respiratory burst. Our results demonstrate further that IL-8 affects the mechanics of tether extraction (single and double) from neutrophils. Some controversy exists about whether IL-8 has an impact on the migration of T-lymphocytes. Recent findings suggest that IL-8 activates neutrophils to release some prestored chemotactic products from their granules that can mediate the migration of human T-lymphocytes (Taub et al., 1996; Wang et al., 1996). Nevertheless, our results indicate that IL-8 has no direct effect on tether extraction from T-lymphocytes since our procedure for isolating lymphocytes ensured that there were no neutrophils in the experimental chamber.
On the other hand, PMA can activate protein kinase C and induce respiratory burst and actin polymerization in both neutrophils and lymphocytes. Our results show that PMA decreases the effective viscosity and increases the total adhesion energy (as determined from the increased threshold force) of both types of leukocyte. It has also been postulated that IL-8 and PMA both cause a decrease in the membrane-cytoskeletal slip viscosity of neutrophils, but not the membrane viscosity nor interbilayer slip viscosity (Shao and Xu, 2002). The decrease in the membrane-cytoskeletal slip viscosity will thus result in dramatic decrease of the effective viscosity when tethers are extracted from neutrophils. Our results show that PMA, but not IL-8, has similar effects on CD4+ T-lymphocytes.
When double tethers were extracted in our experiment, there were probably just two antibody-receptor bonds between the bead and cell. If there were more than two bonds, it would likely result in either no tether extraction or more tethers (≥3) extracted. The initial bond formation in the contact area was a random process, so the locations of these two bonds were likely not too close to each other within an average contact area of 1–2 μm2. This indicates that they would likely be located on two different microvilli on passive lymphocytes and neutrophils or at an average distance of a few hundred nanometers on treated cells. Therefore, it is not surprising that the correlation between F and Ut for double tether extraction from these leukocytes (passive or treated) is shifted upward, relative to that of single tether extraction (Table 1), in accordance with an approximate doubling of threshold force and effective viscosity. This indicates that double tethers generally do not compete for the lipid membrane source even though the tethers could be 20- or 30-μm long. This seems reasonable considering that there is so much excess membrane material in these microvillus-rich leukocytes and that individual tethers are only tens of nanometers in diameter. During the rolling of leukocytes on the endothelium, double tethers occur occasionally as demonstrated in flow chamber studies (Schmidtke and Diamond, 2000; Park et al., 2002). Our experimentally-determined F versus Ut relationship indicates that these two tethers are very likely independent of each other while being extracted.
Acknowledgments
We thank Dr. Salvatore P. Sutera for his critical reading of the manuscript.
This work was supported by the National Institutes of Health (R01 HL069947).
References
- Bruehl, R. E., K. L. Moore, D. E. Lorant, N. Borregaard, G. A. Zimmerman, R. P. McEver, and D. F. Bainton. 1997. Leukocyte activation induces surface redistribution of P-selectin glycoprotein ligand-1. J. Leukoc. Biol. 61:489–499. [DOI] [PubMed] [Google Scholar]
- Dai, J., and M. P. Sheetz. 1995. Mechanical properties of neuronal growth cone membrane studied by tether formation with laser optical tweezers. Biophys. J. 68:988–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenpeck, K. L., M. E. Brummet, S. A. Hudson, R. J. Mayer, and B. S. Bochner. 2000. Activation of human leukocytes reduces surface P-selectin glycoprotein ligand-1 (PSGL-1, CD162) and adhesion to P-selectin in vitro. J. Immunol. 165:2764–2772. [DOI] [PubMed] [Google Scholar]
- Diamond, M. S., and T. A. Springer. 1993. A subpopulation of mac-1(CD11b/CD18) molecules mediates neutrophil adhesion to ICAM-1 and fibrinogen. J. Cell Biol. 120:545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandsen, S. L., S. R. Hasslen, and R. D. Nelson. 1993. Detection and spatial distribution of the β2 integrin (Mac-1) and L-selectin (LECAM-1) adherence receptors on human neutrophils by high-resolution field emission SEM. J. Histochem. Cytochem. 41:327–333. [DOI] [PubMed] [Google Scholar]
- Evans, E., and A. Yeung. 1989. Apparent viscosity and cortical tension of blood granulocytes determined by micropipette aspiration. Biophys. J. 56:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, E., and A. Yeung. 1994. Hidden dynamics in rapid changes of bilayer shape. Chem. Phys. Lipids. 73:39–56. [Google Scholar]
- Hochmuth, R. M., and W. D. Marcus. 2002. Membrane tethers formed from blood cells with available area and determination of their adhesion energy. Biophys. J. 82:2964–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochmuth, R. M., N. Mohandas, and P. L. J. Blackshear. 1973. Measurement of the elastic modulus for red cell membrane using a fluid mechanical technique. Biophys. J. 30:747–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochmuth, R. M., J.-Y. Shao, J. Dai, and M. P. Sheetz. 1996. Deformation and flow of membrane into tethers extracted from neuronal growth cones. Biophys. J. 70:358–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochmuth, R. M., H. C. Wiles, E. A. Evans, and J. T. McCown. 1982. Extensional flow of erythrocyte membrane from cell body to elastic tether: II. Experiment. Biophys. J. 39:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin, J. D., H. P. Ting-Beall, and R. M. Hochmuth. 2001. Correlating the kinetics of cytokine-induced E-selectin adhesion and expression on endothelial cells. Biophys. J. 80:656–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z., B. Anvari, M. Takashima, P. Brecht, J. H. Torres, and W. E. Brownell. 2002. Membrane tether formation from outer hair cells with optical tweezers. Biophys. J. 82:1386–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus, W. D., and R. M. Hochmuth. 2002. Experimental studies of membrane tethers formed from human neutrophils. Ann. Biomed. Eng. 30:1273–1280. [DOI] [PubMed] [Google Scholar]
- Moore, K. L., K. D. Patel, R. E. Bruehl, F. Li, D. A. Johnson, H. S. Lichenstein, R. D. Cummings, D. F. Bainton, and R. P. McEver. 1995. P-selectin Glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J. Cell Biol. 128:661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham, D., and R. M. Hochmuth. 1992. A sensitive measure of surface stress in the resting neutrophil. Biophys. J. 61:1664–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson, T. S., and K. Ley. 2002. Chemokines and chemokine receptors in leukocyte trafficking. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283:R7–28. [DOI] [PubMed] [Google Scholar]
- Park, E. Y., M. J. Smith, E. S. Stropp, K. R. Snapp, J. A. DiVietro, W. F. Walker, D. W. Schmidtke, S. L. Diamond, and M. B. Lawrence. 2002. Comparison of PSGL-1 microbead and neutrophil rolling: microvillus elongation stabilizes P-selectin bond clusters. Biophys. J. 82:1835–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raucher, D., T. Stauffer, W. Chen, K. Shen, S. Guo, J. D. York, M. P. Sheetz, and T. Meyer. 2000. Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton-plasma membrane adhesion. Cell. 100:221–228. [DOI] [PubMed] [Google Scholar]
- Schmidtke, D. W., and S. L. Diamond. 2000. Direct observation of membrane tethers formed during neutrophil attachment to platelets or P-selectin under physiological flow. J. Cell Biol. 149:719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao, J.-Y., and R. M. Hochmuth. 1996. Micropipette suction for measuring piconewton forces of adhesion and tether formation from neutrophil membranes. Biophys. J. 71:2892–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao, J.-Y., and R. M. Hochmuth. 1999. Mechanical anchoring strength of L-selectin, β2 integrins and CD45 to neutrophil cytoskeleton and membrane. Biophys. J. 77:587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao, J.-Y., H. P. Ting-Beall, and R. M. Hochmuth. 1998. Static and dynamic lengths of neutrophil microvilli. Proc. Natl. Acad. Sci. USA. 95:6797–6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao, J.-Y., and J. Xu. 2002. A modified micropipette aspiration technique and its application to tether formation from human neutrophils. J. Biomech. Eng. 124:388–396. [DOI] [PubMed] [Google Scholar]
- Sheetz, M. P., and J. Dai. 1996. Modulation of membrane dynamics and cell motility by membrane tension. Trends Cell Biol. 6:85–89. [DOI] [PubMed] [Google Scholar]
- Springer, T. A. 1995. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu. Rev. Physiol. 57:827–872. [DOI] [PubMed] [Google Scholar]
- Taub, D. D., M. Anver, J. J. Oppenheim, D. L. Longo, and W. J. Murphy. 1996. T-lymphocyte recruitment by interleukin-8 (IL-8). IL-8-induced degranulation of neutrophils releases potent chemoattractants for human T-lymphocytes both in vitro and in vivo. J. Clin. Invest. 97:1931–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, M. A., R. S. Frank, and R. E. Waugh. 1993. Passive mechanical behavior of human neutrophils: power-law fluid. Biophys. J. 65:2078–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, M. A., R. S. Frank, and R. E. Waugh. 1994. Passive mechanical behavior of human neutrophils: effect of cytochalasin B. Biophys. J. 66:2166–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. M., L. Xu, W. J. Murphy, D. D. Taub, and O. Chertov. 1996. IL-8-induced T-lymphocyte migration: direct as well as indirect mechanisms. Methods. 10:135–144. [DOI] [PubMed] [Google Scholar]
- Waugh, R. E. 1982. Surface viscosity measurements from large bilayer vesicle tether formation: II. Experiments. Biophys. J. 38:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh, R. E., and R. M. Hochmuth. 1995. Mechanics and deformability of hematocytes. In The Biomedical Engineering Handbook. J. D. Bronzino, editor. CRC Press, Boca Raton, FL. 474–486.
- Yago, T., A. Leppanen, H. Qiu, W. D. Marcus, M. U. Nollert, C. Zhu, R. D. Cummings, and R. P. McEver. 2002. Distinct molecular and cellular contributions to stabilizing selectin-mediated rolling under flow. J. Cell Biol. 158:787–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung, A. K. C. 1994. Mechanics of intermonolayer coupling in fluid surfactant bilayers. PhD dissertation. Dept. of Physics, University of British Columbia, Vancouver, Canada.
- Zar, J. H. 1999. Biostatistical Analysis. Prentice Hall, Upper Saddle River, N.J.