Abstract
The origin of DNA axis curvature in complexes of the catabolite activator protein with DNA is studied using multiple molecular dynamics (MD) simulations of the free and protein-bound forms of the DNA. The results are compared to available solution and crystal structure data. The MD simulations reproduce the experimentally observed bend in DNA and indicate that ∼40% of the bending observed in the complex is intrinsic to the DNA sequence, whereas ∼60% is induced on protein binding. The MD provides a model for the dynamical structure of the DNA free in solution and for ligand-induced bending.
DNA sequence-dependent structural deformation, intrinsic axis curvature, and ligand-induced bending are all significant elements in obtaining a basic understanding of protein DNA recognition in genomic regulatory processes (1, 2). One of the most extreme cases of DNA bending was discovered in the crystal structure of the complex with catabolite activator protein (CAP) (3), in which the DNA “makes a right turn”. In this case, the ∼90° bend occurs primarily as a consequence of two ∼45° kinks at TpG steps, one in each of the two consensus half-sites. To determine how much of this is intrinsic curvature and how much is ligand-induced bending requires structures of the complexed and uncomplexed forms of the DNA. In the case of CAP, the structure of the uncomplexed DNA in solution is currently beyond the reach of crystallography or NMR spectroscopy. However, recent advances in compute power and methods developments make it possible to obtain all atom models of the dynamical structures of protein-DNA complexes and corresponding uncomplexed protein and DNA using molecular dynamics (MD) simulation (4, 5). The models can be validated independently with respect to experimentally determined structures (6) and serve as a basis for comparing intrinsic and induced DNA structure and studies of the structural adaptation process. A set of six new nanosecond MD simulations have been performed to investigate the nature and extent of structural adaptation of the DNA on complex formation, how much of any DNA deformation is ligand-induced, and how much is intrinsic to the uncomplexed structure, and to address the question of whether the structure of DNA in the complex is a metastable substate or simply a strained form. Jen-Jacobsen et al. (7) observed that the formation of protein DNA complexes with significant DNA bending are entropy controlled, whereas the formation of complexes with little DNA bending are enthalpy controlled. Thus the extent of DNA bending in a complex has clear implications with respect to thermodynamics and structural energetics.
The crystal structure of CAP bound to a palindromic 30 basepair DNA sequence with the conserved 5′-TGTGA sequence in both the half-sites was first solved by Steitz and co-workers (3) (Protein Data Bank ID: 1CGP). However, the DNA in the complex was not fully intact but nicked at one position. Subsequent structures of the CAP DNA complex and variants thereof have been obtained (8–10), and a range of DNA bending angles has been reported. The CAP-DNA complexes, such as 1CGP and 1J59, obtained in an orthorhombic unit cell, tend toward a net DNA bending of ∼90° effected by large positive roll and twist at TpG steps in the conserved region. The structures solved with a 46-basepair DNA sequence, of which 26 basepairs are resolved in the crystal structure in a trigonal space group (e.g., 2CGP), in general exhibit a smaller bend of ∼70° on the average. The specific values of the DNA bending angle from crystallography range all the way from 51° to 107°.
Topological measurements by Lutter et al. (11) indicate that the DNA bend in complex has an intermediate value of ∼69° in solution. Fluorescence resonance energy transfer studies by Ebright and co-workers have indicated that the bend in DNA is ∼77° degrees (12). Crothers and co-workers (13) estimate a preexisting bend of 15° in the uncomplexed DNA based on cyclization experiments and Monte Carlo simulation, whereas Maher and co-workers (14) report a 26° bend based on phosphate charge neutralization studies. Although all the results are unanimous in pointing the bend in DNA, the degree of bending has been variable.
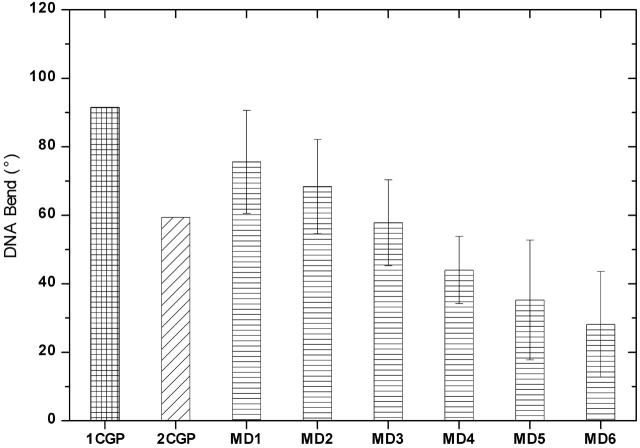
MD on DNA including explicit solvent and ions were performed using the AMBER suite of programs and the parm94 force field (15). Details about the simulation procedure are available in the Supplementary Material. The DNA bend angles in the simulations in the bound and unbound forms, in comparison to the crystal structure values, are shown in Fig. 1. In the timescale of the simulations, the DNA bending angle is reasonably well stabilized in all the systems. The complex structure simulated in a minimal solution environment maintains 75° bend with an uncertainity of ±17° due to thermal motions in the DNA structure over the 5 ns simulation length, ∼15° less than the value observed in the co-crystal structure. MD on the CAP-DNA complex with excess salt concentration of 120 mM shows curvature of ∼70°. Thus, the simulations indicate that salt concentration may be a factor in the DNA bending. MD simulation of the 2CGP structure tends to maintain a structural form close to ∼50° bend.
FIGURE 1 .
MD1 and MD2 present the average curvature after 5 ns simulation of the CAP DNA complex with minimal and excess salt, respectively. The error bars represent the statistical uncertainty of one standard deviation in the calculated curvature. MD3 denotes the average curvature in simulation with intact DNA, and MD4 is the average observed in the simulation of 2CGP structure. MD5 and MD6 are the curvature of free DNA in simulations starting from the protein-bound and canonical B-form, respectively.
The time course of the structural adaptation process can be followed in detail by examining in reverse order the structures obtained from an MD on the DNA free in solution, beginning with the DNA structure in the complex (Fig. 2). The results indicate that MD starting from the ∼90° bent form relaxes in the course of 5 ns of MD to a value of ∼40°. A corresponding simulation of the free DNA starting from canonical B-DNA structure spontaneously develops a curvature of ∼30° over the course of 5 ns simulation. This is close enough to indicate that in longer simulations, the two structures would converge into the same structural form with ∼30°–40° curvature. The MD simulations support the results from crystallography that primary origin of axis deformation in 1CGP and 1J59 structures of CAP DNA is a kink of ∼45°at the TpG steps in each of the two half-sites of the DNA. The importance of pyrimidine-purine (YpR) steps in general in DNA curvature and protein-induced bending has been emphasized in a recent review (4). In the 2CGP crystal structure, curvature is distributed across the basepair steps interacting with the recognition helix of the protein. The MD results indicate that although the TpG step exhibits a significantly large basepair roll (∼12°) that contributes to the net curvature, it is not as large as that observed in the 1CGP crystal structure, and the curvature in the MD model of the complexed DNA resembles more closely that observed in the 2CGP structure.
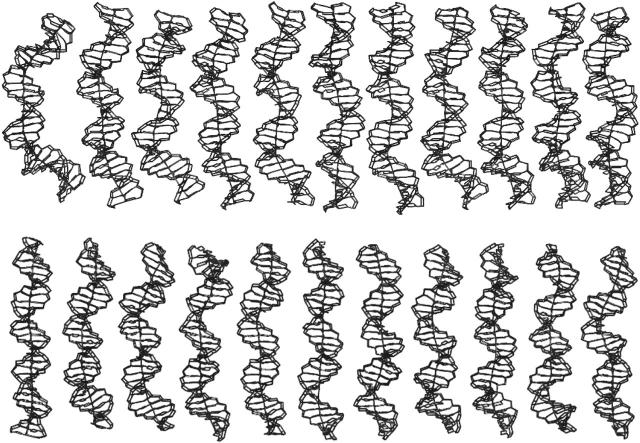
FIGURE 2 .
The time evolution of DNA structure viewed left to right during 5 ns MD simulation in unbound form. The structures in simulations starting from the 90° bent DNA and the straight canonical B-form are shown in the top and lower panels, respectively.
In conclusion, a series of six new nanosecond MD simulations of the CAP DNA complexes and corresponding unbound DNA have produced plausible calculations of DNA curvature and bending when compared with corresponding experimental data. The MD simulations predict that the DNA bending on complexation with CAP is 65°–80° in the solution state, and depends slightly but significantly on salt concentration. Considering the structure of the CAP-bound DNA relative to a canonical B reference state with an essentially straight helix axis, the protein-bound form is ∼40% due to intrinsic curvature in the cognate DNA and ∼60% due to protein-induced bending. The MD modeling indicates that the sequence-dependent structure of the free DNA is preorganized for complex formation, i.e., the intrinsic curvature of the DNA is in the general direction of the protein-bound form with incipient kinks evident at TpG steps. The MD indicates that the structure of the DNA in the complex is an intrinsically strained structure and not a stable or metastable substate.
SUPPLEMENTARY MATERIAL
An online supplement to this article can be found by visiting BJ Online at http://www.biophysj.org.
Acknowledgments
Discussions with Dr. Kelly Thayer are gratefully acknowledged.
This work was supported by grant No. GM 37909 from the National Institutes of Health and an allocation of supercomputer time at the National Center for Supercomputing Applications of the University of Illinois and the Texas Advanced Computing Center at the University of Texas under the auspices of the Partnership for Advanced Computational Infrastructure program.
References
- (1).Steitz, T. A. 1990. Structural studies of protein-nucleic acid interaction: the sources of sequence-specific binding. Q. Rev. Biophys. 23:205–280. [DOI] [PubMed] [Google Scholar]
- (2).Travers, A. 1993. DNA-Protein Interactions. Chapman and Hall, London.
- (3).Schultz, S. C., G. C. Shields, and T. A. Steitz. 1991. Crystal structure of a CAP-DNA complex: the DNA is bent by 90 degrees. Science. 253:1001–1007. [DOI] [PubMed] [Google Scholar]
- (4).Beveridge, D. L., S. B. Dixit, G. Barreiro, and K. M. Thayer. 2004. Molecular dynamics simulations of DNA curvature and flexibility: dynamical aspects of helix phasing and premelting phenomena. Biopolymers. 73:380–403. [DOI] [PubMed] [Google Scholar]
- (5).Cheatham 3rd, T. E. 2004. Simulation and modeling of nucleic acid structure, dynamics and interactions. Curr. Opin. Struct. Biol. 14:360–367. [DOI] [PubMed] [Google Scholar]
- (6).Arthanari, H., K. J. McConnell, R. Beger, M. A. Young, D. L. Beveridge, and P. H. Bolton. 2003. Assessment of the molecular dynamics structure of DNA in solution based on calculated and observed NMR NOESY volumes and dihedral angles from scalar coupling constants. Biopolymers. 68:3–15. [DOI] [PubMed] [Google Scholar]
- (7).Jen-Jacobson, L., L. E. Engler, and L. A. Jacobson. 2000. Structural and thermodynamic strategies for site-specific DNA binding proteins. Structure. 8:1015–1023. [DOI] [PubMed] [Google Scholar]
- (8).Parkinson, G., C. Wilson, A. Gunasekera, Y. W. Ebright, R. E. Ebright, and H. M. Berman. 1996. Structure of the CAP-DNA complex at 2.5 angstroms resolution: a complete picture of the protein-DNA interface. J. Mol. Biol. 260:395–408. [DOI] [PubMed] [Google Scholar]
- (9).Passner, J. M., and T. A. Steitz. 1997. The structure of a CAP-DNA complex having two cAMP molecules bound to each monomer. Proc. Natl. Acad. Sci. USA. 94:2843–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Lawson, C. L., D. Swigon, K. S. Murakami, S. A. Darst, H. M. Berman, and R. H. Ebright. 2004. Catabolite activator protein: DNA binding and transcription activation. Curr. Opin. Struct. Biol. 14:10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Lutter, L. C., H. R. Halvorson, and C. R. Calladine. 1996. Topological measurement of protein-induced DNA bend angles. J. Mol. Biol. 261:620–633. [DOI] [PubMed] [Google Scholar]
- (12).Kapanidis, A. N., Y. W. Ebright, R. D. Ludescher, S. Chan, and R. H. Ebright. 2001. Mean DNA bend angle and distribution of DNA bend angles in the CAP-DNA complex in solution. J. Mol. Biol. 312:453–468. [DOI] [PubMed] [Google Scholar]
- (13).Kahn, J. D., and D. M. Crothers. 1998. Measurement of the DNA bend angle induced by the catabolite activator protein using Monte Carlo simulation of cyclization kinetics. J. Mol. Biol. 276:287–309. [DOI] [PubMed] [Google Scholar]
- (14).Hardwidge, P. R., J. M. Zimmerman, and L. J. Maher 3rd. 2002. Charge neutralization and DNA bending by the Escherichia coli catabolite activator protein. Nucleic Acids Res. 30:1879–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Cornell, W. D., P. Cieplak, C. I. Bayly, I. R. Gould, K. M. Merz Jr., D. M. Ferguson, D. C. Spellmeyer, T. Fox, J. W. Cladwell, and P. A. Kollman. 1995. A second generation force field for the simulation of proteins, nucleic acids and organic molecules. J. Am. Chem. Soc. 117:5179–5197. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.