Abstract
Once immortalized, human cells are susceptible to transformation by introduction of an oncogene such as ras. Several lines of evidence now suggest that the maintenance of telomere length is a major determinant of replicative lifespan in human cells and thus of the immortalized state. The majority of human tumor cells acquire immortality through expression of the catalytic subunit of telomerase (hTERT), whereas others activate an alternative mechanism of telomere maintenance (ALT) that does not depend on the actions of telomerase. We have examined whether ALT could substitute for telomerase in the processes of transformation in vitro and tumorigenesis in vivo. Expression of oncogenic H-Ras in the immortal ALT cell line GM847 did not result in their transformation. However, subsequent ectopic expression of hTERT in these cells imparted a tumorigenic phenotype. Indeed, this outcome was also observed after introduction of a mutant hTERT that retained catalytic activity but was incapable of maintaining telomere length. These studies indicate that hTERT confers an additional function that is required for tumorigenesis but does not depend on its ability to maintain telomeres.
Normal human cells possess a limited replicative lifespan when propagated in vitro (1). In these cells, telomeres—the specialized DNA–protein structures found at the ends of linear chromosomes—shorten with each cell division (2, 3). This observation has led to the suggestion that telomeres act as a molecular counting device that tallies the number of cell divisions and, upon shortening below a threshold length, limits further division (4). In contrast, the majority of cells derived from spontaneously arising human tumors proliferate indefinitely and maintain stable telomere lengths (5). This observation has led to the inference that maintenance of telomere length is a prerequisite for the acquisition of unlimited (i.e., immortalized) replicative capacity.
The great majority of human tumor-derived cell lines express hTERT, the catalytic subunit of the telomerase holoenzyme, and exhibit readily detectable telomerase activity (6–10). This observation, together with studies demonstrating that ectopic hTERT expression suffices to immortalize many normal human cells (11–13), suggests that telomere length stabilization is required for cellular immortality, and that tumor cells often acquire the telomere maintenance capability by derepressing expression of hTERT.
Telomerase activation is not the only mechanism by which cells can stabilize their telomeres. As many as 10% of human tumor-derived cell lines are telomerase-negative and rely on an alternative mechanism of telomere maintenance termed ALT (7, 14–16). ALT is defined operationally as a mechanism that allows stable telomere maintenance in the absence of telomerase, and this term may, in fact, subsume a number of distinct telomerase-independent mechanisms. The molecular basis of the ALT mechanism(s) operating in mammalian cells remains poorly defined. Telomere maintenance in yeast cells that are genetically deficient in telomerase activity depends in part on inter-chromosomal recombination mechanism(s) (17), and some evidence suggests that the ALT mechanism(s) operative in human cells may use a similar pathway (18).
Whether achieved by telomerase or ALT, the telomere maintenance capability of human tumor cells appears to be an intrinsic and essential component of their neoplastic growth (7, 11, 12, 19, 20). This capability can be conferred experimentally by introduction of an hTERT gene into normal human cells; the resulting telomerase activity is an essential component of the cell physiologic changes that in concert make it possible to experimentally transform normal human cells into tumor cells (21–23). In addition, inhibition of telomerase activity in telomerase-positive human tumor cells renders them nontumorigenic (24–26), further illustrating the importance of telomerase activity in tumor cell survival.
Cultured human cells can be transformed to a tumorigenic state by ectopic expression of an activated form of the ras oncogene, hTERT, and simian virus 40 (SV40) small and large T antigens (21–23, 27, 28). This observation has suggested that telomerase makes its essential contribution to transformation through its ability to continuously maintain telomeric DNA above a threshold length. This model predicts that any molecular mechanism that is capable of maintaining telomeres above this threshold length should be able to cooperate with concomitant expression of the SV40 early region (encoding both small and large T antigens) and the H-rasV12 oncogene to enable tumorigenic transformation. Here, we have examined whether ALT can substitute for hTERT in telomere maintenance and in this fashion contribute to the experimental creation of human tumor cells.
Materials and Methods
Cell Lines.
The GM847 cell line was the gift of Olivia Pereira-Smith, Baylor College of Medicine, Waco, TX. All cells were grown in DME plus 10% FCS (24).
Generation of Retroviruses.
Construction and production of the pBABE-puro, pBABE-puro-hTERT, pBABE-puro-hTERTHA, and pMIG retroviruses were as described (20, 21, 24, 29). pMIGHRas was created by cloning the V12 oncogenic H-Ras variant into pMIG. After infection with the pMIG constructs green fluorescent protein (GFP)-positive cells were sorted by fluorescence-activated cell sorting.
Telomerase Assay.
Cellular extracts were assayed for telomerase activity by using a PCR-based telomerase repeat amplification protocol (TRAP) assay as described (6).
Western Blot Analysis.
Cells were lysed in 50 mM Tris (pH 7.5), 150 mM NaCl, 0.5% Nonidet P-40, and protease inhibitor mixture (Roche, Indianapolis). One hundred micrograms of protein was separated on 7.5–15% gradient SDS/PAGE. Protein expression was confirmed by immunoblotting with the following antibodies: Ras (C20, Santa Cruz Biotechnology), large T antigen (Pab 101, Santa Cruz Biotechnology), small T antigen (Pab 108 and Pab 419, Santa Cruz Biotechnology), and hemagglutinin (HA) (HA-11, Convance, Richmond, CA).
Immunofluorescent Staining of ALT-Associated Promyelocytic Leukemia (AA-PML) Bodies in Cell Lines.
Cells were grown on 4-well Lab-Tek chamber slides, fixed with 4% paraformaldehyde, and permeabilized with 0.5% Triton X-100. Cells were stained with rabbit anti-PML (Medical and Biological Laboratories, Naka-Ku, Nagoya, Japan) and murine anti-TRF2 (Upstate Biotechnology, Lake Placid, NY). After antibody staining the cells were fixed with 4% paraformaldehyde, treated with RNase A (100 μg/ml in 2× SSC) at 37°C for 30 min, and hybridized with a FITC-conjugated (CCCTAA)3 peptide-nucleic acid probe [0.6 μg/ml in 70% formamide, 1% blocking reagent (Roche) and 10 mM Tris, pH 7.2]. Z-series images were collected on a Zeiss LSM 510 confocal microscope.
Immunofluorescent Staining of AA-PML Bodies in Tumor Sections.
Tissue sections were deparaffinized, hydrated as described (30), and stained with anti-PML and anti-TRF2 as described above.
Spectral Karyotyping Analysis.
Spectral karyotyping analysis was carried out as described with only minor modifications (31, 32).
Anchorage-Independent Growth and Tumor Formation.
Soft agar assays and growth of cells in immunodeficient animals were performed as described (21). Tumor volume was calculated by using the following formula: 4/3πr2, (where r is the radius of the tumor). BALB/c-ByJ-Hfh11nu mice were obtained from The Jackson Laboratory.
Results
GM847 constitute a line of immortal human fibroblasts that contain the SV40 early region (33). These cells lack telomerase activity, yet maintain long telomeres of heterogeneous size and are thus considered to exhibit the ALT phenotype (14). Because ALT can substitute for telomerase in telomere maintenance, we anticipated that the expression of an oncogenic ras allele would suffice to convert GM847 cells from a nontumorigenic to a tumorigenic growth state, doing so in a manner analogous to cells immortalized by ectopic expression of hTERT (21–23, 28).
To test this hypothesis, GM847 cells were transduced with a bicistronic vector encoding GFP alone or a vector encoding GFP plus the H-RasV12 oncoprotein (H-Ras). Cells expressing high levels of GFP were then isolated by fluorescence-activated cell sorting. H-Ras, large T antigen, and small T antigen expression in sorted cell populations was confirmed by Western blot analyses (Fig. 1A and data not shown).
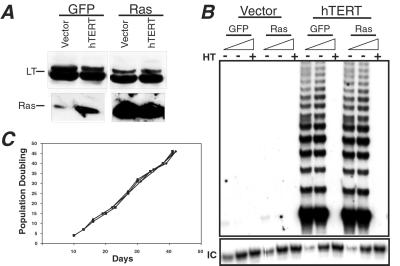
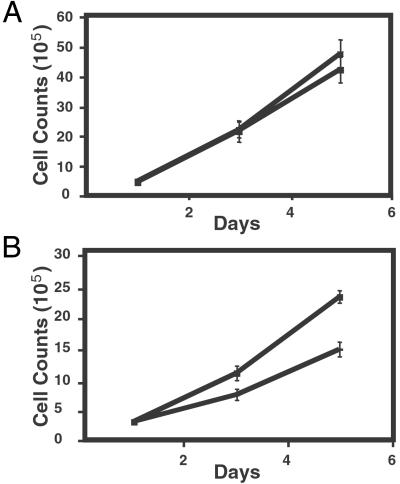
Figure 1.
Characterization of GM847 cells. (A) The expression of both the SV40 large T (LT) antigen and oncogenic H-Ras was confirmed by immunoblotting with 100 μg of total cell protein, separated on 7.5–15% gradient SDS/PAGE gels. (B) Telomerase expression in GM847 cells. Telomerase activity was analyzed by in vitro TRAP. Total cell lysates (2,000 and 200 ng) were analyzed. HT indicates heat-treated control, and IC indicates the location of the internal PCR control. (C) Growth kinetics of GM847 cells. After infection, selection, and fluorescence-activated cell sorting growth kinetics were assessed in each GM847 cell line. Population doubling zero was defined as the point when the first 10-cm2 plate reached confluence after fluorescence-activated cell sorting (this occurred approximately 10 days postsorting for all cultures). Cells were infected with a control vector expressing a drug-resistant marker alone or hTERT followed by infection with a second vector expressing either GFP alone or GFP plus H-ras. Cell lines are defined as follows: (⧫) Vector-GFP and (▴) hTERT-GFP, cell lines expressing a control vector and hTERT and GFP, respectively; (■) Vector-Ras and (●) hTERT-Ras, cell lines expressing a control vector or hTERT and H-Ras, respectively.
Sorted cells were injected s.c. into sublethally irradiated, immunodeficient nude mice. Unexpectedly, no tumor formation was observed in mice injected with GM847 cells expressing the oncogenic H-Ras protein (data not shown), despite expression levels of H-Ras similar to those seen in transformed cells that had been previously immortalized by the expression of hTERT (21, 23, 27). Because similar experiments with hTERT-immortalized cells have repeatedly led to tumorigenic cells in our hands, this response suggested that the contribution of the ALT mechanism to transformation was not equivalent to that of hTERT.
To address what additional role(s) telomerase plays in tumorigenesis, we created polyclonal populations of GFP- and H-Ras-expressing GM847 cells that also expressed hTERT. Introduction of hTERT resulted in telomerase activity as gauged by the TRAP assay (Fig. 1B), confirming that the level of the hTERT protein is the rate-limiting determinant of telomerase activity in GM847 cells (24, 33). Introduction of the hTERT gene had no effect on either H-Ras levels (Fig. 1A) or the proliferation rate of these cells in vitro (Fig. 1C).
The ability of cells to grow in an anchorage-independent manner in vitro is often used as a surrogate test for tumorigenicity (34). Therefore, we examined whether these cells formed colonies in a soft agar assay. GM847 cells expressing oncogenic H-Ras grew efficiently in an anchorage-independent fashion; derivatives of these cells expressing additionally hTERT also grew well under these conditions. As expected, in the absence of an H-ras oncogene, none of the GM847 cell lines formed colonies in soft agar (Table 1). Because the GM847 cells expressing H-Ras could grow in an anchorage-independent fashion but were nontumorigenic (Table 1), we concluded that, at least in this instance, anchorage-independent growth did not provide a good predictor of the ability of these cells to form tumors.
Table 1.
Anchorage-independent growth and tumor formation
| Cell type | Soft agar
|
Tumors | ||
|---|---|---|---|---|
| 103 | 104 | 105 | ||
| Vector-GFP | 4 | 10 | 39 | 0/9 |
| TERT-GFP | 11 | 20 | 20 | 0/9 |
| Vector-Ras | 10 | 91 | 1,564 | 1*/12 |
| TERT-Ras | 5 | 62 | 1,558 | 12/12 |
1 × 103, 1 × 104, and 1 × 105 cells were plated in soft agar and colony formation was assessed 4 weeks later. Sublethally irradiated mice were injected s.c. with 2 × 106 cells in three independent sites and tumor formation was monitored weekly. Numbers in the tumor column represent the number of tumors/number of injection sites that formed after 10 weeks.
Tumor formed after an extended latency and remained less than 3 mm in diameter.
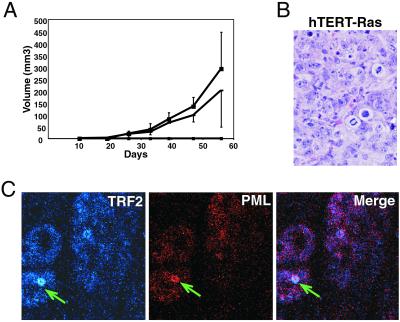
We next tested whether introduction of hTERT allowed the previously nontumorigenic, ras-bearing GM847 cells to form tumors in nude mice. We found that the addition of catalytically active telomerase did indeed allow Ras-expressing GM847 cells to form tumors (Fig. 2A and Table 1). Analysis of tissue sections indicated that the tumor cells had a high mitotic index, were undifferentiated, and were highly malignant (Fig. 2B).
Figure 2.
Tumorigenicity of cells expressing telomerase and H-Ras: 2 × 106 cells were injected s.c. into sublethally irradiated nude mice (400 rad) and tumor formation was monitored weekly. (A) Each point on the graph represents the average volume of three tumors per mouse and the corresponding standard deviations. A single tumor did arise in GM847 cells expressing H-Ras alone but it did so only after an extended latency and it was limited in size, suggesting that it arose only after the cells had sustained a secondary alteration. Only (dash, square) hTERT-Ras cells appear on the graph. (B) Tumors were removed, fixed in formalin, sectioned, and stained for hematoxylin and eosin. A representative tissue section from a tumor formed by cells expressing hTERT and H-Ras is shown. Tumors have a defined border, appear round and epithelium-like, and have a high mitotic index. Results are representative of five independent sets of experiments. (Magnification: ×400.) (C) Tumors formed by injection of GM847 cells expressing hTERT and H-Ras were processed, deparaffinized, and stained with anti-TRF2 (blue) and anti-PML (red). The arrow indicates the presence of an AA-PML body. (Magnification: ×100.)
To control for the possibility that tumor formation by cells expressing H-Ras and hTERT was not caused by the isolation of rare clonal variants, we created a second set of cell lines. As was initially observed, only cells that expressed both hTERT and H-Ras repeatedly produced tumors in mice, indicating that our observations were not the result of rare clonal variants (data not shown).
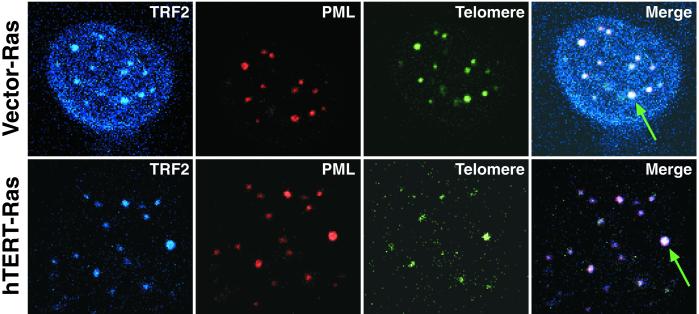
ALT and telomerase can coexist in some cell lines (33, 35–37). However, it has been shown that the ALT phenotype can be lost in a minority of clones upon expression of hTERT (35). To determine whether ALT remained active in our hTERT-expressing GM847 cells, we analyzed these cells for the presence of AA-PML bodies whose presence has been correlated with the long telomeres of ALT cells and is now frequently used as a marker of the ALT phenotype (33, 35–38). AA-PML bodies contain a number of proteins including Rad51, TRF1, TRF2, and PML and telomeric DNA (38). Accordingly, we stained these cells with antibodies specific for TRF2 and PML and carried out peptide-nucleic acid fluorescence in situ hybridization to detect telomeric DNA. In agreement with previous results, we did not observe AA-PML bodies in a telomerase-positive human ovarian cancer cell line 36M (data not shown) (33). In contrast, we clearly observed AA-PML bodies in all of our ALT cell lines, indicating that ALT remained active in the presence of ectopically expressed hTERT and H-Ras (Fig. 3).
Figure 3.
AA-PML bodies in GM847 cells. AA-PML bodies were identified in GM847 cells expressing a vector or hTERT and H-Ras. Column one represents staining with anti-TRF2 (blue), column two represents staining with anti-PML (red), column three represents telomere fluorescence in situ hybridization (green), and column four is the merged image of all three where white indicates colocalization. The arrows indicate the presence of an AA-PML body. (Magnification: ×100.)
We also analyzed tissue sections from tumor masses to determine whether the ALT mechanism remained active in vivo. Tumor sections from mice injected with GM847 cells expressing hTERT and H-Ras were stained for the presence of PML and TRF2. AA-PML bodies were found in the tissue sections (Fig. 2C), indicating that ALT persisted in these cells in vivo. Furthermore, isolation of the cells from the tumor mass confirmed that they remained telomerase-positive, indicating that telomerase activity and ALT coexisted in vivo (data not shown).
In previous reports, telomere fluorescence in situ hybridization was used to demonstrate that telomerase expression in GM847 cells led to lengthening of the shortest telomeres (33). This result is compatible with a mechanism in which telomerase is able to confer a survival advantage on these ALT cells by elongating the shortest of their telomeres. Despite heterogeneity in the lengths of the telomeres in these cells, these short telomeres might well represent rate-limiting determinants for survival in vivo. Analysis of the telomeres in GM847 cells expressing a vector alone or hTERT by pulse-field electrophoresis did not reveal any notable differences in telomere length (data not shown).
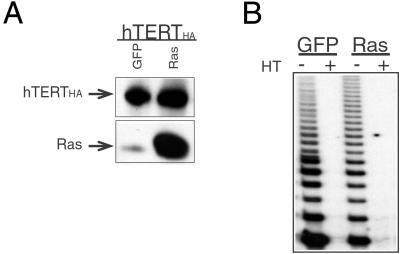
We wanted to determine whether the ability of telomerase to elongate telomeres was required for it to contribute to transformation. To do so, we introduced a telomerase enzyme that carries the HA tag at its carboxyl terminus (hTERTHA) into GM847 cells. Previously, it was shown that this hTERTHA enzyme retains robust enzymatic activity when assayed in vitro but is unable to elongate telomeres in vivo (20, 39). Use of this mutant therefore allowed us to dissect the contribution of hTERT catalytic activity from its telomere-lengthening functions.
GM847 cells were transduced with a retroviral vector expressing hTERTHA. After selection, cells were infected with the bicistronic vector expressing GFP alone or GFP plus H-Ras, and cells were sorted for GFP expression. H-Ras expression was confirmed by Western blot analysis (Fig. 4A), and telomerase activity was confirmed by the TRAP assay (Fig. 4B). Introduction of hTERTHA and H-Ras had no effect on either the in vitro growth rate of these cells or the presence of AA-PML bodies (data not shown).
Figure 4.
Characterization of GM847 cells expressing the C-terminally tagged telomerase enzyme (hTERTHA) and H-Ras. (A) hTERTHA and H-Ras protein expression. Protein expression was confirmed by Western blot analysis of 100 μg total cell protein, separated on 7.5–15% a gradient SDS/PAGE gel. (B) The catalytic activity of the hTERTHA protein was assessed by the TRAP assay. The TRAP assay was performed on 100 ng total cell lysate. HT indicates the heat-treated control.
The hTERTHA-GFP and hTERTHA-Ras cells were injected into immunodeficient mice, and the ability of these cells to form tumors was assessed. As expected, the hTERTHA-GFP expressing cells did not form tumors. In contrast, the hTERTHA-Ras expressing cells formed tumors at the same efficiency as the hTERT-Ras-expressing cells (Table 2), indicating that telomere elongation was not critical for tumor formation.
Table 2.
Tumor formation
| Cell type | Tumors |
|---|---|
| TERTHA-GFP | 0/9 |
| TERTHA-Ras | 9/12 |
| TERT-Ras | 4/6 |
Sublethally irradiated mice were injected s.c. with 2 × 106 cells in three independent sites and tumor formation was monitored weekly. Numbers represent the number of tumors/number of injection sites that formed after 10 weeks.
In light of the striking effect of telomerase on the tumorigenicity of GM847 cells that were already immortalized through ALT, we wanted to identify potential mechanisms by which hTERT might contribute to tumorigenicity in addition to its established role in telomere length maintenance. Recent reports have indicated that hTERT expression is associated with resistance to apoptosis in vitro (40–42), suggesting that cells expressing hTERT may demonstrate enhanced growth or survival when compared with those that do not express hTERT. However, the in vitro growth characteristics of our GM847 derivatives, regardless of the expression of H-Ras, GFP, or hTERT were indistinguishable when cells were grown under typical tissue culture conditions (10% serum and 20% oxygen). However, these conditions only poorly approximate those experienced by cells implanted in a host animal. For this reason, we wanted to test whether the ectopic expression of hTERT in GM847 cells facilitated growth under conditions of limiting nutrient concentrations and oxygen tensions.
In seven independent experiments, we reproducibly found that when GM847 cells were shifted to low serum, low oxygen tension, and low nutrient concentrations (from 10% serum, 5 mM glucose, and 20% oxygen to 0.1% serum, 2.5 mM glucose, and 0.4% oxygen), the cells expressing hTERT had an increased growth rate compared with cells expressing a control vector (Fig. 5). Further analysis of these cells grown under both culture conditions by spectral karyotypic analysis did not reveal any detectable recurrent changes in their complex karyotypes (data not shown). Because GM847 cells are already immortalized, we concluded that the ectopic expression of hTERT might contribute to the tumorigenic phenotype by facilitating the growth of cells under conditions of physiologic stress. Moreover, this facilitation is not conferred by the ALT process despite the fact that it results in far longer telomeres than those possessed by hTERT-positive cells.
Figure 5.
Growth curves of cells grown in limiting growth factors and reduced glucose and oxygen levels. (A) The graph represents the growth curve of cells grown in 10% serum, 5 mM glucose, and 20% oxygen. Cell lines are defined as follows: (⧫) Vector-Ras and (■) hTERT-Ras. These data are representative of seven independent experiments. (B) The graph represents the growth kinetics of cells grown in 0.1% serum, 2.5 mM glucose, and 0.4% oxygen. Cells grown in 0.4% oxygen were maintained in a vacuum-sealed container that was flushed with 0.4% oxygen, 5% carbon dioxide, and 94.9% nitrogen every 2 days. Cell lines are defined as follows: (dash) Vector-Ras and (■) hTERT-Ras. These data are representative of seven independent experiments.
Discussion
The expression of the SV40 early region, hTERT, and H-Ras suffices to transform a variety of human cells (21–23, 27, 28). In each case, expression of hTERT, the catalytic subunit of telomerase, permitted telomere elongation and maintenance and correlated with the acquisition of the immortal phenotype. These observations suggested that the maintenance of telomere length should be sufficient to allow cell transformation by certain oncogenes. Because a significant minority of human tumors and immortal human cell lines appear to use a telomerase-independent mechanism to maintain telomere length, we tested whether the maintenance of telomere length by these mechanisms was able to substitute for telomerase expression. Surprisingly, we found that ALT could not substitute for telomerase expression in this cell transformation assay and that an additional function of hTERT that goes beyond its ability to elongate telomeres was required for cell transformation.
The precise mechanism by which telomerase facilitates tumorigenicity of ras-transformed, already-immortalized GM847 cells remains unclear. However, our results clearly indicate that the ability of hTERT to elongate telomeres is not essential for this function. Telomerase expression is also implicated in a number of other functions that would appear to be unrelated to telomere elongation. For example, telomerase expression has been linked to chromosomal healing and increased cell survival (40–44). In addition, the transformation of murine cells correlates with increased mTERT expression, and transgenic mice expressing high levels of mTERT are more susceptible to tumors than are littermate controls (45, 46), suggesting that increased telomerase activity in cells with already-long telomeres increases transformation in vivo.
To date, the majority of biochemical properties ascribed to hTERT have been limited to its ability to elongate telomeres. However, this activity does not preclude other actions of hTERT carried out at the telomere ends, which may affect, for example, the single-strand overhangs or the specialized T loop structures that may be important for chromosomal integrity (47, 48). One report that has suggested a function for hTERT beyond telomere elongation demonstrated that introduction of hTERT into telomerase-negative, precrisis fibroblasts resulted in immortalization whereas control cells experienced telomere dysfunction and cell death (49). The telomeres in these immortalized cells continued to shorten beyond those in the control cells that entered crisis, suggesting that telomerase allowed cells to survive with telomeres that were shorter than those normally required to protect chromosomal ends. This observation has led to the hypothesis that telomerase forms a protective cap at the end of the telomere that protects the chromosome from end-to-end fusions or from participating in illegitimate recombination events.
Although the ALT mechanism operating in GM847 cells cannot substitute for hTERT in tumorigenesis, it would seem that ALT operating in other cells can contribute to tumorigenicity even in the absence of telomerase expression. Approximately 10% of human tumors are telomerase-negative, and some telomerase-negative ALT cell lines form tumors in nude mice in the absence of telomerase (data not shown and ref. 50). These observations suggest that such cells use an ALT mechanism that is distinct from the one operating in GM847 cells. Alternatively, these cells may use the same ALT mechanism but have acquired an additional, unknown alteration(s) that enables them to survive in vivo.
ALT is present in only 7–10% of human tumors but is found in as many as 35% of in vitro immortalized cell lines (15), raising the question as to why ALT is not seen at comparable frequency in spontaneously arising human tumors. The present results suggest that spontaneously arising ALT-positive human tumors are less frequent because the ALT mechanism, or some versions of the ALT mechanism, cannot on its own fully replace telomerase function during the process of tumor progression. Indeed cytogenetic studies have suggested that telomere maintenance via an ALT mechanism or telomerase activity is not equivalent (51). Thus, some ALT-positive cells may require an additional alteration before they reach a physiologic state equivalent to that conferred on cells by telomerase expression. Of consequence here is the still-unresolved question of whether the ALT phenotype consists of a single mechanism or several distinct mechanisms that may not be equivalent in tumorigenesis. It is clear that telomerase plays at least two roles in tumorigenesis and a better understanding of each distinct role will provide insight into the basic mechanisms of tumorigenesis and will contribute to our ability to develop novel anticancer therapeutics.
Acknowledgments
We thank Nicki Watson and the Keck Foundation for assistance with confocal microscopy, Dr. Ittai Ben-Porath and other members of the Weinberg laboratory for helpful discussions, and Luk van Parijs and David Baltimore for the gift of the pMIG vector. This work was supported in part by Merck/MIT and Co. Inc. (R.A.W.), National Institutes of Health/National Cancer Institute Grant R01 CA78461 (to R.A.W.), G. Harold and Leila Y. Mathers Charitable Fund, a Charles E. Culpeper Biomedical Pilot Initiative grant (to R.A.W.), an American Cancer Society and American Association for Cancer Research Postdoctoral Fellowship (to S.A.S.), a Doris Duke Charitable Fund Clinical Scientist Development Scholar Award (to W.C.H.), Howard Temin Award KO1 CA94223 (to W.C.H.), and a Department of Defense New Investigator Award (to W.C.H.). S.A.S. is a Herman and Margaret Sokol Postdoctoral Fellow. R.A.W. is an American Cancer Society Research Professor and a Daniel K. Ludwig Cancer Research Professor.
Abbreviations
- SV40
simian virus 40
- GFP
green fluorescent protein
- HA
hemagglutinin
- PML
promyelocytic leukemia
- AA-PML
ALT-associated PML
- TRAP
telomerase repeat amplification protocol
Footnotes
See commentary on page 12520.
References
- 1.Hayflick L, Moorhead P S. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 2.Harley C B, Futcher A B, Greider C W. Nature (London) 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 3.Hastie N D, Dempster M, Dunlop M G, Thompson A M, Green D K, Allshire R C. Nature (London) 1990;346:866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- 4.Harley C B, Vaziri H, Counter C M, Allsopp R C. Exp Gerontol. 1992;27:375–382. doi: 10.1016/0531-5565(92)90068-b. [DOI] [PubMed] [Google Scholar]
- 5.Harley C B, Kim N W, Prowse K R, Weinrich S L, Hirsch K S, West M D, Bacchetti S, Hirte H W, Counter C M, Greider C W, et al. Cold Spring Harbor Symp Quant Biol. 1994;59:307–315. doi: 10.1101/sqb.1994.059.01.035. [DOI] [PubMed] [Google Scholar]
- 6.Kim N W, Piatyszek M A, Prowse K R, Harley C B, West M D, Ho P L, Coviello G M, Wright W E, Weinrich S L, Shay J W. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 7.Shay J W. J Cell Physiol. 1997;173:266–270. doi: 10.1002/(SICI)1097-4652(199711)173:2<266::AID-JCP33>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura T M, Morin G B, Chapman K B, Weinrich S L, Andrews W H, Lingner J, Harley C B, Cech T R. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- 9.Meyerson M, Counter C M, Eaton E N, Ellisen L W, Steiner P, Caddle S D, Ziaugra L, Beijersbergen R L, Davidoff M J, Liu Q, et al. Cell. 1997;90:785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 10.Counter C M, Avilion A A, Le Feuvre C E, Stewart N G, Greider C W, Harley C B, Bacchetti S. EMBO J. 1992;11:1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodnar A G, Ouellette M, Frolkis M, Holt S E, Chiu C P, Morin G B, Harley C B, Shay J W, Lichtsteiner S, Wright W E. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 12.Vaziri H, Benchimol S. Curr Biol. 1998;8:279–282. doi: 10.1016/s0960-9822(98)70109-5. [DOI] [PubMed] [Google Scholar]
- 13.Harley C B. Oncogene. 2002;21:494–502. doi: 10.1038/sj.onc.1205076. [DOI] [PubMed] [Google Scholar]
- 14.Bryan T M, Englezou A, Gupta J, Bacchetti S, Reddel R R. EMBO J. 1995;14:4240–4248. doi: 10.1002/j.1460-2075.1995.tb00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bryan T M, Englezou A, Dalla-Poazz L, Dunham M A, Reddel R R. Nat Med. 1997;3:1271–1274. doi: 10.1038/nm1197-1271. [DOI] [PubMed] [Google Scholar]
- 16.Henson J D, Neumann A A, Yeager T R, Reddel R R. Oncogene. 2002;21:598–610. doi: 10.1038/sj.onc.1205058. [DOI] [PubMed] [Google Scholar]
- 17.Lundblad V, Blackburn E H. Cell. 1993;73:347–360. doi: 10.1016/0092-8674(93)90234-h. [DOI] [PubMed] [Google Scholar]
- 18.Dunham M A, Neumann A A, Fasching C L, Reddel R R. Nat Genet. 2000;26:447–450. doi: 10.1038/82586. [DOI] [PubMed] [Google Scholar]
- 19.Granger M P, Wright W E, Shay J W. Crit Rev Oncol Hematol. 2002;41:29–40. doi: 10.1016/s1040-8428(01)00188-3. [DOI] [PubMed] [Google Scholar]
- 20.Counter C M, Hahn W C, Wei W, Caddle S D, Beijersbergen R L, Lansdorp P M, Sedivy J M, Weinberg R A. Proc Natl Acad Sci USA. 1998;95:14723–14728. doi: 10.1073/pnas.95.25.14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hahn W C, Counter C M, Lundberg A S, Beijersbergen R L, Brooks M W, Weinberg R A. Nature (London) 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 22.Yu J, Boyapati A, Rundell K. Virology. 2001;290:192–198. doi: 10.1006/viro.2001.1204. [DOI] [PubMed] [Google Scholar]
- 23.Elenbaas B, Spirio L, Koerner F, Fleming M D, Zimonjic D B, Donaher J L, Popescu N C, Hahn W C, Weinberg R A. Genes Dev. 2001;15:50–65. doi: 10.1101/gad.828901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hahn W C, Stewart S A, Brooks M W, York S G, Eaton E, Kurachi A, Beijersbergen R L, Knoll J H, Meyerson M, Weinberg R A. Nat Med. 1999;5:1164–1170. doi: 10.1038/13495. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Mar V, Zhou W, Harrington L, Robinson M O. Genes Dev. 1999;13:2388–2399. doi: 10.1101/gad.13.18.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herbert B, Pitts A E, Baker S I, Hamilton S E, Wright W E, Shay J W, Corey D R. Proc Natl Acad Sci USA. 1999;96:14276–14281. doi: 10.1073/pnas.96.25.14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hahn W C, Dessain S K, Brooks M W, King J E, Elenbaas B, Sabatini D M, DeCaprio J A, Weinberg R A. Mol Cell Biol. 2002;22:2111–2123. doi: 10.1128/MCB.22.7.2111-2123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rich J N, Guo C, McLendon R E, Bigner D D, Wang X F, Counter C M. Cancer Res. 2001;61:3556–3560. [PubMed] [Google Scholar]
- 29.Van Parijs L, Refaeli Y, Abbas A K, Baltimore D. Immunity. 1999;11:763–770. doi: 10.1016/s1074-7613(00)80150-8. [DOI] [PubMed] [Google Scholar]
- 30.Kuperwasser C, Pinkas J, Hurlbut G D, Naber S P, Jerry D J. Cancer Res. 2000;60:2723–2729. [PubMed] [Google Scholar]
- 31.Zimonjic D, Brooks M W, Popescu N, Weinberg R A, Hahn W C. Cancer Res. 2001;61:8838–8844. [PubMed] [Google Scholar]
- 32.Schrock E, du Manoir S, Veldman T, Schoell B, Wienberg J, Ferguson-Smith M A, Ning Y, Ledbetter D H, Bar-Am I, Soenksen D, et al. Science. 1996;273:494–497. doi: 10.1126/science.273.5274.494. [DOI] [PubMed] [Google Scholar]
- 33.Perrem K, Colgin L M, Neumann A A, Yeager T R, Reddel R R. Mol Cell Biol. 2001;21:3862–3875. doi: 10.1128/MCB.21.12.3862-3875.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freedman V H, Shin S I. Cell. 1974;3:355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- 35.Ford L P, Zou Y, Pongracz K, Gryaznov S M, Shay J W, Wright W E. J Biol Chem. 2001;276:32198–32203. doi: 10.1074/jbc.M104469200. [DOI] [PubMed] [Google Scholar]
- 36.Grobelny J V, Kulp-McEliece M, Broccoli D. Hum Mol Genet. 2001;10:1953–1961. doi: 10.1093/hmg/10.18.1953. [DOI] [PubMed] [Google Scholar]
- 37.Cerone M A, Londono-Vallejo J A, Bacchetti S. Hum Mol Genet. 2001;10:1945–1952. doi: 10.1093/hmg/10.18.1945. [DOI] [PubMed] [Google Scholar]
- 38.Yeager T R, Neumann A A, Englezou A, Huschtscha L I, Noble J R, Reddel R R. Cancer Res. 1999;59:4175–4179. [PubMed] [Google Scholar]
- 39.Ouellette M M, Aisner D L, Savre-Train I, Wright W E, Shay J W. Biochem Biophys Res Commun. 1999;254:795–803. doi: 10.1006/bbrc.1998.0114. [DOI] [PubMed] [Google Scholar]
- 40.Zhu H, Fu W, Mattson M P. J Neurochem. 2000;75:117–124. doi: 10.1046/j.1471-4159.2000.0750117.x. [DOI] [PubMed] [Google Scholar]
- 41.Holt S E, Glinsky V V, Ivanova A B, Glinsky G V. Mol Carcinog. 1999;25:241–248. [PubMed] [Google Scholar]
- 42.Fu W, Begley J G, Killen M W, Mattson M P. J Biol Chem. 1999;274:7264–7271. doi: 10.1074/jbc.274.11.7264. [DOI] [PubMed] [Google Scholar]
- 43.Vermeesch J R, Falzetti D, Van Buggenhout G, Fryns J P, Marynen P. Cytogenet Cell Genet. 1998;81:68–72. doi: 10.1159/000014991. [DOI] [PubMed] [Google Scholar]
- 44.Sprung C N, Reynolds G E, Jasin M, Murnane J P. Proc Natl Acad Sci USA. 1999;96:6781–6786. doi: 10.1073/pnas.96.12.6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gonzalez-Suarez E, Samper E, Ramirez A, Flores J M, Martin-Caballero J, Jorcano J L, Blasco M A. EMBO J. 2001;20:2619–2630. doi: 10.1093/emboj/20.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bednarek A K, Chu Y, Slaga T J, Aldaz C M. Mol Carcinog. 1997;20:329–331. doi: 10.1002/(sici)1098-2744(199712)20:4<329::aid-mc1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 47.von Zglinicki T. Cancer Lett. 2001;168:111–116. doi: 10.1016/s0304-3835(01)00546-8. [DOI] [PubMed] [Google Scholar]
- 48.Griffith J D, Comeau L, Rosenfield S, Stansel R M, Bianchi A, Moss H, de Lange T. Cell. 1999;97:503–514. doi: 10.1016/s0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]
- 49.Zhu J, Wang H, Bishop J M, Blackburn E H. Proc Natl Acad Sci USA. 1999;96:3723–3728. doi: 10.1073/pnas.96.7.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gollahon L S, Kraus E, Wu T A, Yim S O, Strong L C, Shay J W, Tainsky M A. Oncogene. 1998;17:709–717. doi: 10.1038/sj.onc.1201987. [DOI] [PubMed] [Google Scholar]
- 51.Scheel C, Schaefer K L, Jauch A, Keller M, Wai D, Brinkschmidt C, van Valen F, Boecker W, Dockhorn-Dworniczak B, Poremba C. Oncogene. 2001;20:3835–3844. doi: 10.1038/sj.onc.1204493. [DOI] [PubMed] [Google Scholar]