Abstract
Insects can rapidly clear microbial infections by producing a variety of immune-induced molecules including antibacterial and/or antifungal peptides/polypeptides. In this report, we present the isolation, structural characterization, and biological properties of two variants of a group of bioactive, slightly cationic peptides, referred to as alloferons. Two peptides were isolated from the blood of an experimentally infected insect, the blow fly Calliphora vicina (Diptera), with the following amino acid sequences: HGVSGHGQHGVHG (alloferon 1) and GVSGHGQHGVHG (alloferon 2). Although these peptides have no clear homologies with known immune response modifiers, protein database searches established some structural similarities with proteins containing amino acid stretches similar to alloferon. In vitro experiments reveal that the synthetic version of alloferon has stimulatory activities on natural killer lymphocytes, whereas in vivo trials indicate induction of IFN production in mice after treatments with synthetic alloferon. Additional in vivo experiments in mice indicate that alloferon has antiviral and antitumoral capabilities. Taken together, these results suggest that this peptide, which has immunomodulatory properties, may have therapeutic capacities. The fact that insects may produce cytokine-like materials modulating basic mechanisms for human immunity suggests a source of anti-infection and antitumoral biopharmaceuticals.
Keywords: insect innate immunity‖cytokine-like‖immunomodulatory peptide
Insects comprise about 55% of total biodiversity and approximately 85% of that from animals. It is believed that insects originated from a centipede-like ancestor as far back as the Devonian period, over 350 million years ago. Among the 3 million to 30 million living species estimated, less than 900,000 are described. Insects have colonized all of the terrestrial and freshwater niches under all climates, from polar to desert regions. The omnipresence of insects in any type of ecological system, from waterway to extremely septic environments, has stimulated scientists to look for new therapeutic agents in this class of arthropods. Among the bioactive peptides/polypeptides that have already been characterized from insects, antimicrobial peptides are fascinating scientists for their potential use as therapeutic agents (1). However, relatively little data are available on molecules from insects with antiparasitic, antiviral, and/or antitumoral activities. Most available antiviral and antitumor agents have been derived from plant, microbes, and, to a lesser extent, animal secondary metabolites (2, 3). Recent progress in biotechnology and immunology has contributed to the list cytokines and some other regulatory polypeptides of human origin. However, huge varieties of peptides/polypeptides from animal origin remain practically untapped in this area. To date, no peptide from an animal source has reached the level of commercial use as a pharmaceutical compound for anticancer or antiviral treatments.
Here, we describe the structure, biological activity, and therapeutic potential of a group of bioactive peptides originating from insects. The peptides, referred to as alloferons, were isolated from bacteria-challenged larvae of the blow fly Calliphora vicina. This species, along with other surgical maggots, has a long history of medical use in wound and ulcer healing (4). Calliphora is also known to produce, after an experimental challenge with bacteria, a series of potent antimicrobial substances with primary structures similar to those described for other insects, namely an insect defensin, diptericins, cecropins, and proline-rich peptides (5). Experiments preceding alloferon discovery noted that crude hemolymph of Calliphora contain a factor capable of stimulating antiviral and antitumor resistance when injected into mice (S. Chernysh, personal communication). This enhancement of resistance may reflect a marked increase of spleen lymphocyte natural killer (NK) activity. NK cells are known to be key elements of antiviral (6) and antitumor (7) innate immunity shared by vertebrates and some invertebrates (8). We have hypothesized that Calliphora hemolymph may contain cytokine-like material, cross-reactive with mouse NK cells, that may protect virus-infected or tumor-grafted recipients. To verify this hypothesis, we subjected an acidic extract of Calliphora hemolymph to HPLC purification and used mouse spleen lymphocyte cytotoxicity to monitor biological activity. After extensive purification, two peptides of 13 and 12 aa were isolated, and their amino acid sequences were identified. The largest 13-residue peptide, named alloferon 1, was synthesized and exposed to a panel of biological activity studies. Synthetic alloferon I exhibited an apparent capacity to stimulate NK cell activity and IFN synthesis in animal and human models, as well as to enhance antiviral and antitumoral activity in mice.
Methods
Insect and Hemolymph Treatment.
Experiments were performed with a wild-type strain of the blow fly C. vicina (Diptera, Calliphoridae) as described (9). Adult flies were maintained in gauze-covered cages supplied with a diet of beet sugar, dry milk, and fresh beef under the temperature of 20 ± 1°C and short day conditions (12 h light/12 h dark), stimulating production of diapause-destined progeny. Pieces of meat containing eggs were placed in 500-ml glass jars on moistened sawdust covered by filter paper, and maintained under 12 h light/12 h dark at 12 ± 1°C. Fresh meat was added until the end of the feeding period. After feeding, third instar wandering larvae migrated into the sawdust and entered diapause. Two weeks later, approximately 3,000 diapausing larvae were experimentally infected by pricking the cuticle with a fine needle dipped into a mixture of heat-killed bacteria (Escherichia coli D31, Gram-negative, and Micrococcus luteus A270, Gram-positive) and kept at 20°C. Twenty-four hours postinfection, Calliphora larvae were sterilized with 70% ethanol, washed with distilled water, dried, and the hemolymph collected. After centrifugation, the supernatant was transferred into a solution of aqueous trifluoroacetic acid (0.05%) and extraction was performed in the presence of aprotinin (final concentration 20 μg/ml) and phenylthiourea (final concentration 1 μg/ml), which are inhibitors of proteases and of melanization, respectively.
Isolation and Structural Characterization of Alloferon.
A 30-ml aliquot of the hemolymph extract was prepurified by solid-phase extraction on reversed-phase Sep-Pak C18 cartridges (Waters) as described. Peptides and proteins were eluted with 50% acetonitrile (ACN), acidified with 0.05% trifluoroacetic acid, and lyophilized. The prepurified 50% Sep-Pak fraction (122 mg of lyophilized materials) was subjected to reversed-phase HPLC on a semipreparative Aquapore RP-300 column (250 × 7 mm, Brownlee Lab), with a linear gradient of 2–62% ACN in acidified water (0.05% trifluoroacetic acid), over 60 min, at a flow rate of 1.5 ml/min. The column effluent was monitored by absorbance at 225 nm, and fractions were hand collected, lyophilized, and resuspended in ultrapure water. Aliquots were assayed for NK cell-stimulating activity (see below). The active fraction, eluted by 5% ACN, was further purified on the same column as above, using a linear gradient of 0–15% ACN in acidified water over 60 min at a flow rate of 1.5 ml/min. The two active compounds were finally purified to homogeneity through three additional purification steps with appropriate gradients. First, the two active fractions were individually purified on an analytical Aquapore OD-300 column (220 × 4.6 mm, Brownlee Lab) with a linear gradient of 0–12% ACN in acidified water over 60 min, at a flow rate of 1 ml/min. The final purification was performed on a narrow bore Delta Pak HPIC18 column (150 × 2 mm, Waters) with a linear gradient of 2–10% ACN in acidified water over 40 min, at a flow rate of 0.5 ml/min. Purity of the fractions with NK cell-stimulating activity was assessed by capillary zone electrophoresis (model 270A-HT, Applied Biosystems) and by matrix-assisted laser desorption ionization–time-of-flight (MALDI-TOF) MS. Pure peptides were finally sequenced by Edman degradation.
Capillary zone electrophoresis.
The purity of the isolated peptides was ascertained by capillary zone electrophoresis on a model 270A-HT (Applied Biosystems) as described.
MALDI-TOF MS.
The mass spectrometry analyses were performed on a Bruker daltonique (Bremen, Germany) BIFLEX II mass spectrometer in a positive linear mode with an external calibration and α-cyano-4-hydroxycinnamic acid (Sigma) as matrix.
Peptide microsequencing.
The isolated peptides were sequenced by automated Edman degradation, and detection of the phenylthiohydantoin derivatives was carried out with a pulse liquid automatic sequencer (Applied Biosystems, model 473A).
Bioassays.
Cytotoxicity assay.
Tumor cell lines ([3H]uridine-labeled K562 and A431) routinely used in NK activity studies (10) were used at the effector/target ratio of 20:1. Mouse spleenocytes were isolated from the spleen of C3HA mice and immediately used for the cytotoxicity assay as described (11). Human peripheral blood lymphocytes were obtained from freshly donated blood, and the erythrocytes were purified with histopaque 1077 solution (Sigma). The fraction containing a lymphocyte and monocyte mixture was washed in phosphate buffer, centrifuged, resuspended in RPMI 1640 medium (Sigma), and immediately used for cytotoxicity analysis as for the mouse spleenocytes.
In vivo IFN induction.
Wild-type white mice (NMR1 strain) were subjected to an intranasal treatment with 25 μg (1.25 mg/kg) of synthetic alloferon. Blood samples from eight mice were collected at different time points (0, 2, 4, 6, 8, 16, 20, 24, 28, 48 h) and processed independently. IFN activity in the blood plasma was measured by using a cytopathogenicity bioassay (12), where the monolayer of L-929 cells and vesicular Stomatitis virus (Indiana strain) served as a test system. IFN activity was expressed as the reciprocal to the mouse serum dilution that protected 50% of the infected cells.
In vitro IFN induction.
Blood samples from three healthy humans were separated into aliquots, diluted with the inducer solution and the cell culture medium at the volume ratio of 1:1:8. The incubation was performed in the absence (control) or presence of 0.4 μg/ml alloferon during a period from 2- to 28-h intervals in different experimental groups, at 37°C. Each measurement was repeated twice, and the result was averaged. After centrifugation, serial dilutions of supernatant were deposited onto a monolayer of L-41 human lung carcinoma cells and incubated for 24 h at 37°C. The monolayer was then infected with 100 CPD50 (dose killing 50% of monolayer cells) of vesicular Stomatitis virus incubated 18 h at 37°C. Cells were stained with crystal violet solution (0.1% in 30% ethanol), washed, and dried; the stain was then extracted by 30% ethanol. The rate of monolayer destruction was estimated by measuring the absorbency of the extracted stain at 590 nm. The value obtained was compared with reference IFN-α preparation and expressed in experimental units as described (12). Newcastle disease virus (64 hemagglutination units per ml), staphylococcal endotoxin A (5 μg/ml), and cycloferon, a derivative of carboximetheneacridone (1.25 mg/ml), served as reference IFN-α inducers for comparison with alloferon. IFN activity was analyzed twice for each sample.
Antiviral activity assay.
Tests on mice infected by influenza virus were performed as recommended for preclinical studies of potential antiviral agents (13). NMR1 mice were subjected to an intranasal challenge with mouse virulent influenza virus A (Aichi/2/68 strain) or influenza virus B (Lee/1/40 strain). Mice were treated intranasally or s.c. with alloferon (25 μg) 1 day before virus inoculation, then 1, 2, 4, 6, and 8 days postvirus inoculation. Remantadin (1 mg) and ribavirin (200 μg), obtained from the Institute of Influenza (St. Petersburg, Russia), were used as positive antiviral preparations to viruses A and B, respectively.
Antitumor activity assay.
In vivo antitumoral activity was assayed by using as a test-system DBA/2 mice grafted with P388 murine leukemia cells as recommended for anticancer drug development (14). Briefly, mice with 20–22-g body mass were s.c. inoculated by 100, 1,000, or 10,000 cells per animal and were injected with alloferon (25 μg) dissolved in 0.1 ml of Hepes solution (Sigma) twice a week during 6 weeks starting from 1 day after tumor cell inoculation. Control animals were injected by Hepes solution. The tumor emergence and size were monitored for the 70-day period.
Results
Alloferon Purification and Characterization.
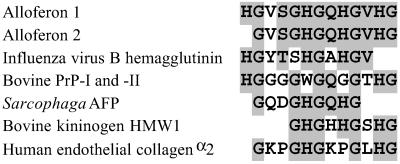
An acidic extract of hemolymph of C. vicina larvae, taken 24 h after a bacterial challenge, was prepurified by solid-phase extraction according to the procedure previously used for the isolation of C. vicina cationic antimicrobial peptides (5). Purification of the Sep-Pak fraction eluted with 50% acetonitrile was performed by reversed-phase HPLC, using acidified acetonitrile as eluent. Screening of the purified material revealed the presence of two fractions with cytotoxicity activity where mouse spleen lymphocytes were used as effector cells and [3H]uridine-labeled K562 tumor cells as target. Following extensive purification, two pure active molecules, with molecular masses measured by MALDI-TOF MS at monoisotopic values of 1265.15 MH+ and 1128.48 MH+, were sequenced by Edman degradation. For these two molecules, the 13- and 12-amino acid sequences HGVSGHGQHGVHG and GVSGHGQHGVHG, respectively, were obtained (Fig. 1). Deduced molecular masses at m/z 1265.59 and m/z 1128.63 were in good agreement with the molecular masses measured by MALDI-TOF MS, confirming the accuracy of the amino acid sequencing. As searches in FASTA.GENOME and SwissProt databases did not reveal clear sequence similarities with known antiviral, antitumor, or immunomodulatory peptides, these molecules were defined, respectively, as alloferon 1 and 2. The name of alloferon was selected to note the substance functional similarity with interferons, native regulators of cytotoxic lymphocytes in vertebrates (-feron), and origin from different, nonvertebrate species (allo-). However, similar amino acid stretches have been found in some functionally relevant proteins, such as influenza virus B hemagglutinin, several precursors of high molecular weight proteins, two bovine prion proteins, the human kininogen, and an antifungal protein isolated from the insect, Sarcophaga peregrina (see Fig. 1). To investigate the biological properties of these molecules, the 13-residue peptide (alloferon 1) was selected as a prototype molecule and synthesized by using fluorenylmethoxycarbonyl chemistry (15).
Figure 1.
Primary structure of alloferon 1 and 2 and sequence alignment with influenza virus B hemagglutinin precursor (fragment 382–392), bovine prion protein (PrP) I and II precursor (fragment 96–108 and 88–100, correspondingly), S. peregrina antifungal protein (Sarcophaga AFP) precursor (fragment 51–59), and bovine kininogen HMW I precursor (fragment 452–460). Gaps are introduced to maximize identities. Common and conservative amino acids are in shaded boxes.
Stimulation of NK Activity.
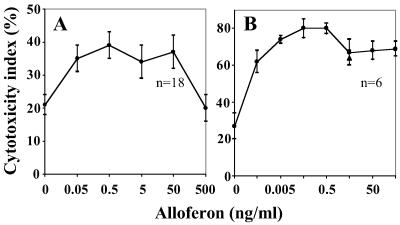
To characterize alloferon stimulatory activity quantitatively, naturally cytotoxic lymphocytes from mouse spleen or human peripheral blood were selected as effector cells and a K562 tumor cell line as a target. Averaged data of three experiments shows that alloferon added to the incubation medium stimulated mouse spleen lymphocyte cytotoxicity at the concentration range from 0.05 to 50 ng/ml (Fig. 2A). The dose-effect curve of the responsive healthy donor demonstrates an even broader range of pharmacologically effective concentration, with maximum efficacy at 0.05–0.5 ng/ml (Fig. 2B). Stimulatory activity of recombinant IFN-α 2b, specific inducer of human NK lymphocytes, was consistent with alloferon activity, whereas a few of the blood samples tested were not responsive to alloferon treatment; good correlation of responsiveness to alloferon and IFN treatment was noticeable in most cases.
Figure 2.
Effect of synthetic alloferon 1 on the natural killer activity of mouse spleenocytes (A) and human lymphocytes (B). The human lymphocytes, the mouse spleenocytes, and the [3H]uridine labeled K562 cells were coincubated in the absence or presence of alloferon 1 at various concentrations. The cytotoxicity indices (M ± SE) were calculated for each concentration as described by Trinchieri (10) based on 18 or 6 assays of mouse splenocyte or human lymphocyte activity, respectively. Human lymphocyte responsiveness was also compared with response to IFN-α 2b (▴).
Induction of IFN Synthesis.
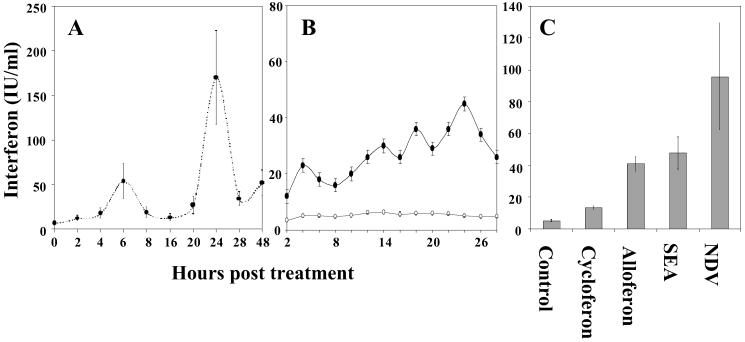
The rate of IFN synthesis was monitored in mice receiving s.c. or intranasally 25 μg of alloferon. Both s.c. injection and intranasal application induced IFN synthesis in mice, although intranasal administration was particularly effective. Two clear peaks of IFN concentration were registered in the blood plasma 6 and 24 h posttreatment (Fig. 3A). Significant IFN induction was also noted when human blood cells were incubated in the presence of alloferon (Fig. 3B). A comparison with known IFN inducers demonstrated that alloferon induction capacity was less acute compared with the Newcastle disease virus, almost identical with staphylococcal enterotoxin A, and exceeded those of cycloferon, the only reference preparation approved for medical use (Fig. 3C).
Figure 3.
Induction of IFN production by alloferon in mouse in vivo and human in vitro models. IFN concentration is expressed in IFN-α equivalents as mean ± SE value. (A) In vivo efficacy of alloferon after intranasal administration to mice. Each value summarizes data of eight independent IFN determinations. (B) In vitro effect of alloferon (black cycles) compared with the absence of alloferon (open cycles) on human leucocytes. Three blood samples were tested twice for each time point. (C) Comparative efficacy of synthetic alloferon, cycloferon, staphylococcal endotoxin A (SEA), and Newcastle disease virus (NDV) as IFN inducers after a 24-h treatment of human leucocytes.
Antiviral Activity.
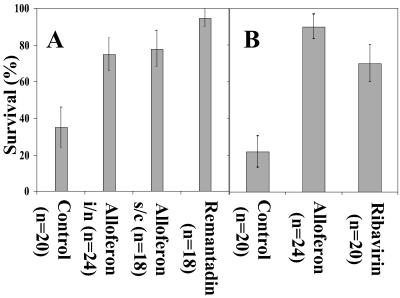
Alloferon antiviral activity was evaluated by using the model of mice lethal pulmonary infection with human influenza viruses A and B. Alloferon intranasal or s.c. administration (25 μg) prevented mortality of most animals challenged by influenza virus A during the period of 10 days postinfection (Fig. 4A). Remantadin, a reference preparation selectively active against this strain, seems to create better protection, although it must be applied in a dosage approximately 40 times higher (1 mg) compared with alloferon. An injection of 25 μg of alloferon stimulated resistance to influenza virus B at the level similar to 200 μg of ribavirin, a broad-spectrum antiviral agent (Fig. 4B), whereas intranasal administration was less effective (data not shown).
Figure 4.
Effect of alloferon as antiviral agent. Mice were intranasally challenged with (A) influenza virus A or (B) influenza virus B and treated either intranasally or s.c. by alloferon (25 μg). In positive control experiments, infected mice received, instead of alloferon, perorally remantadin (1 mg) or ribavirin (200 μg) as reference preparation against influenza virus A and B, respectively. Variance bar indicates the percentage standard deviation.
Antitumor Activity.
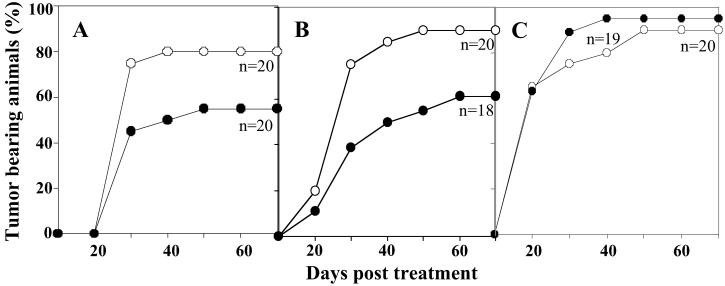
Initial attempts to evaluate alloferon anticancer activity by using a model designed for cytostatic drugs assay (DBA/2 mice inoculated with 1 million P388 leukemia cells) demonstrated a failure to suppress tumor growth. Further experiments, however, demonstrated that lower doses of cancer cells could be eliminated after administration of 25 μg of alloferon. A significant proportion of animals grafted s.c. with 102–103 tumor cells remained tumor free within the 70-day study (Fig. 5 A and B). When primary tumors appeared, their growth was significantly slower compared with the control group (solvent injection). However, at an inoculation dose of 104 tumor cells, no significant differences were observed in the size and rate of tumor emergence between animals treated with alloferon or solvent as control (Fig. 5C).
Figure 5.
In vivo antitumoral activity was tested on mice challenged with an increasing number of P388 lymphoma cells: 100 (A), 1,000 (B), or 10,000 (C). After injection of 25 μg of alloferon twice a week during a 6-day period (black cycles) or solvent (open cycles), tumor emergence and size were estimated over a period of 70 days.
Discussion
The present study reports the identification of alloferon, a peptide family from the insect C. vicina, with the capability to stimulate natural cytotoxicity of mouse spleen lymphocytes. Interestingly, these molecules have no clear homology with known peptides. Two variants of alloferons (13 and 12 residues) were found in the hemolymph of Calliphora after a bacterial challenge. Alloferon 2 corresponds to an N-terminal truncated form of alloferon 1 (H, see Fig. 1). However, it is not known whether the presence of the two peptides is the result of natural degradation of alloferon 1 or if the alloferon molecules are coded by different genes. Even if alloferon structure is unique among known immunomodulatory peptides, data bank searches revealed very little identities with known large functionally relevant proteins (see Fig. 1). One of these proteins was an 85-residue antifungal protein isolated from the flesh fly S. peregrina that presumably damages fungal cell membrane (16). Alloferon also contained some structural elements common to the 583-residue influenza virus B hemagglutinin precursor. This precursor has an 11-aa stretch closely related to alloferon (8 residues over 11 residues). The hemagglutinin is a critical part of the viral envelope, responsible for attaching the virus to the cell surface. Interestingly, alloferon also showed some slight similarities to the two bovine prion proteins I and II over a 13-residue stretch. Prion proteins are abundantly expressed in tissues of the central nervous system and many extraneuronal tissues, particularly lymphocytes. Although little is known about the normal function of prion proteins, recent findings suggest that such proteins, besides other activities, may participate in T cell activation (17). Transformation of the secondary structure of the prion proteins may lead to the appearance of pathological scrapies isoforms. The acquisition of neurotoxicity and proteinase resistance for these scrapies isoforms have been attributed to the peptide fragment corresponding to residues 106–126 of prion protein II and homologous fragment 114–134 of prion protein I, particularly to the amyloidogenic and hydrophobic core AGAAAAGA of the fragment (18, 19). The alloferon-related part of the prion protein precursors (fragments 96–108 of PrP I and 88–100 of PrP II) is totally different from the fragment 106–126. Based on the structural similarity, one may speculate that the alloferon-related fragment mediates immunomodulatory function of the prion protein molecule in a manner similar to alloferon. Finally, some similarities were also observed between alloferon and two short domains (see Fig. 1) of the high molecular weight bovine kininogen 1 (amino acids 452–460) and the human endothelial collagen α2 (amino acids 33–44). The high molecular weight kininogens are polyfunctional blood-circulating glycoproteins that can act as antiadhesive molecules when they are bound to multinuclear leucocytes. Endothelial collagen α2 is a cell-adhesive molecule forming the extracellular matrix for connective tissue cells.
As shown by a series of biological assays, synthetic alloferon (i) stimulates natural cytotoxicity of human peripheral blood lymphocytes, (ii) induces IFN synthesis in mouse and human models, and (iii) enhances antiviral and antitumor resistance in mice. Simultaneous activation of natural killing and IFN production is not surprising because both mechanisms work in close cooperation: interferons stimulate NK cell cytotoxicity whereas NK cells produce IFNs on stimulation (6, 20). Even though the crucial role of NK cell/IFN network in the control of viral infections and cancer is well documented, the data presented here suggest an interplay of alloferon antiviral and anticancer activity with its immunomodulatory properties. Nevertheless, direct action on virus-infected or malignant cells can also contribute to an overall efficacy.
The immunomodulatory properties of alloferon remain to be clarified in detail. As for interferonogenic activity, alloferon peculiarity is a wave-like shape of IFN concentration, which may be seen from in vitro study of human leukocytes response (see Fig. 3B) and seems most evident when alloferon is administered to mice in vivo (see Fig. 3A). In this last case, two clear peaks have been found 6 and 24 h postinjection, although a trend to IFN activity growth was also seen 48 h later. This probably reflects a cascade-like reaction where early cytokine synthesis induced by alloferon administration may stimulate secondary response in same or other IFN-producing cells. Assuming that many cell populations, especially lymphoid cells, fibroblasts, and epithelial and endothelial cells, can be induced to express type I IFNs via a cytokine network, it is not surprising that a secondary response may exceed the primary one.
We now have evidence that the activity spectrum of alloferon as an antiviral agent is not restricted to influenza viruses. In fact, alloferon-based therapy of herpes simplex and hepatitis B and C infections is now under extensive preclinical and clinical study. However, it has been shown that preventive and/or therapeutic administration of alloferon essentially increased survival rate and suppressed virus reproduction in mice intracerebrally infected with type 2 herpes simplex virus.
Summarizing preclinical and preliminary clinical data, we suggest that alloferon may serve to design a group of biopharmaceutical compounds with the capability to improve important effector mechanisms of innate immunity, with emphasis on cell-mediated natural cytotoxicity and IFN synthesis.
Acknowledgments
We thank Dr. Phil Irving (Strasbourg, France) for critical reading of the manuscript, Prof. David L. Denlinger of Ohio State University (Columbus), and Dr. Ruslan Medzhitov of Yale University Medical School (New Haven, CT) for helpful discussion and critical review of the manuscript. We thank Dr. Jean-Paul Brian of the Institute of Molecular and Cell Biology (Strasbourg, France) and Dr. Michael V. Ovchinnikov of the Cardiology Center (Moscow) for alloferon synthesis. We are also grateful to Nina Simonenko, Irina Artsibasheva, Natalia Chernysh, and Irina Kozhucharova for their expert technical assistance. This work was financially supported by Entopharm Co. Ltd. (Seoul) and was supported in part by Alloferon Corp. (Moscow) and Centre National de la Recherche Scientifique (France).
Abbreviations
- ACN
acetonitrile
- MALDI-TOF
matrix-assisted laser desorption ionization–time-of-flight
- NK
natural killer
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. P83412).
References
- 1.Bulet P, Hetru M, Dimarcq J-L, Hoffmann D. Dev Comp Immunol. 1999;23:329–344. doi: 10.1016/s0145-305x(99)00015-4. [DOI] [PubMed] [Google Scholar]
- 2.Cragg G M, Newman D J, Weiss R B. Semin Oncol. 1997;24:156–163. [PubMed] [Google Scholar]
- 3.McConnell O J, Longley R E, Koehn F E. Biotechnology. 1994;26:109–174. [PubMed] [Google Scholar]
- 4.Sherman R A, Hall M J, Thomas S. Annu Rev Entomol. 2000;45:55–81. doi: 10.1146/annurev.ento.45.1.55. [DOI] [PubMed] [Google Scholar]
- 5.Chernysh S I, Gordja N A, Simonenko N P. Entomol Sci. 2000;3:139–144. [Google Scholar]
- 6.Biron C A. Curr Opin Immunol. 1997;9:24–34. doi: 10.1016/s0952-7915(97)80155-0. [DOI] [PubMed] [Google Scholar]
- 7.Brittenden J, Heys S D, Ross J, Eremin O. Cancer. 1996;77:1226–1243. doi: 10.1002/(sici)1097-0142(19960401)77:7<1226::aid-cncr2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 8.Savary C A, Lotzova E. In: Immunobiology of Natural Killer Cells. Lotzova E, Herberman R B, editors. I. Boca Raton, FL: CRC; 1986. pp. 45–61. [Google Scholar]
- 9.Nesin A P, Simonenko N P, Numata H, Chernysh S I. Appl Entomol Zool. 1995;30:351–356. [Google Scholar]
- 10.Trinchieri G. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filatova N A, Malygin A M, Goriunova L B, Fel V I, Khavinson V K. Tsitologiia. 1990;32:652–658. [PubMed] [Google Scholar]
- 12.Fernandez R C, Lee S H, Fernandez L A, Pope B L, Rozee K R. J Interferon Res. 1986;6:573–580. doi: 10.1089/jir.1986.6.573. [DOI] [PubMed] [Google Scholar]
- 13.Guskova T A, Nikolaeva I S, Peters V V. In: Guide to Experimental (Preclinical) Study of Novel Pharmacological Materials. Fisenko V P, Arzamascev E V, Babayan E A, Bulaev V M, Gerasimov V B, Glushkov R G, Guskova T A, Drozzin A P, Ignatov Y D, Kukes V G, et al., editors. Moscow: RF Ministry of Health; 2000. pp. 274–280. [Google Scholar]
- 14.Burger A M, Fiebig H H. In: Anticancer Drug Development. Baguley B C, Kerr D J, editors. San Diego: Academic; 2002. pp. 285–299. [Google Scholar]
- 15.Neimark J, Briand J-P. Peptide Res. 1993;6:219–228. [PubMed] [Google Scholar]
- 16.Iijima R, Kurata S, Natori S. J Biol Chem. 1993;268:12055–12061. [PubMed] [Google Scholar]
- 17.Mabbott N A, Brown K L, Manson J, Bruce M E. Immunology. 1997;92:161–165. doi: 10.1046/j.1365-2567.1997.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kourie J I. Chem Biol Interact. 2001;138:1–26. doi: 10.1016/s0009-2797(01)00228-9. [DOI] [PubMed] [Google Scholar]
- 19.Taylor S C, Green K N, Smith I F, Peers C. Am J Physiol Cell Physiol. 2001;281:1850–1857. doi: 10.1152/ajpcell.2001.281.6.C1850. [DOI] [PubMed] [Google Scholar]
- 20.Vilcek J. In: Cytokine Reference. Oppenheim J J, Feldmann M, Durum S K, Hirano T, Vilcek J, Nicola N A, editors. San Diego: Academic; 2001. pp. 615–626. [Google Scholar]