Abstract
Inositol 1,4,5-trisphosphate receptors (InsP3R) play a key role in intracellular calcium (Ca2+) signaling. Three mammalian InsP3R isoforms—InsP3R type 1 (InsP3R1), InsP3R type 2 (InsP3R2), and InsP3R type 3 (InsP3R3) are expressed in mammals, but the functional differences between the three mammalian InsP3R isoforms are poorly understood. Here we compared single-channel behavior of the recombinant rat InsP3R1, InsP3R2, and InsP3R3 expressed in Sf9 cells, reconstituted into planar lipid bilayers and recorded with 50 mM Ba2+ as a current carrier. We found that: 1), for all three mammalian InsP3R isoforms the size of the unitary current is 1.9 pA and single-channel conductance is 74–80 pS; 2), in optimal recording conditions the maximal single-channel open probability for all three mammalian InsP3R isoforms is in the range 30–40%; 3), in optimal recording conditions the mean open dwell time for all three mammalian InsP3R isoforms is 7–8 ms, the mean closed dwell time is ∼10 ms; 4), InsP3R2 has the highest apparent affinity for InsP3 (0.10 μM), followed by InsP3R1 (0.27 μM), and then by InsP3R3 (0.40 μM); 5), InsP3R1 has a high-affinity (0.13 mM) ATP modulatory site, InsP3R2 gating is ATP independent, and InsP3R3 has a low-affinity (2 mM) ATP modulatory site; 6), ATP modulates InsP3R1 gating in a noncooperative manner (nHill = 1.3); 7), ATP modulates InsP3R3 gating in a highly cooperative manner (nHill = 4.1). Obtained results provide novel information about functional properties of mammalian InsP3R isoforms.
INTRODUCTION
The inositol (1,4,5)-trisphosphate receptor (InsP3R) is an intracellular calcium (Ca2+) release channel that plays a key role in Ca2+ signaling in cells (Berridge, 1993). Three InsP3R isoforms—InsP3R type 1 (InsP3R1), InsP3R type 2 (InsP3R2), and InsP3R type 3 (InsP3R3) are expressed in mammals (Furuichi et al., 1994), each with a unique expression pattern. InsP3R1 is predominant in the central nervous system, but most other tissues express at least two and often all three InsP3R isoforms at different ratios (Taylor et al., 1999). The InsP3R is a large (∼1 MDa) tetrameric complex (Furuichi et al., 1994). The three mammalian InsP3R isoforms are 60–70% identical in sequence (Furuichi et al., 1994) and share a common domain structure (Mignery and Sudhof, 1990; Miyawaki et al., 1991) that consists of an amino-terminal InsP3-binding domain, a carboxy-terminal Ca2+ channel domain, and a middle coupling domain containing most of the putative regulatory sites. The InsP3-binding and Ca2+ channel-forming domains are highly conserved between InsP3R isoforms, whereas the middle coupling domain is the most divergent.
Functional properties of native and recombinant InsP3R1 have been extensively characterized by Ca2+ flux measurements, planar lipid bilayer or nuclear envelope patch-clamp recordings (reviewed in Bezprozvanny and Ehrlich, 1995; Thrower et al., 2001). In contrast with InsP3R1, functional properties of InsP3R2 and InsP3R3 are less known (Thrower et al., 2001). The functional properties of purified native cardiac InsP3R2 (Ramos-Franco et al., 1998), purified recombinant InsP3R2 expressed in COS cells (Ramos-Franco et al., 2000), and native InsP3R3 from RINm5F cells (Hagar et al., 1998; Hagar and Ehrlich, 2000) were characterized in planar lipid bilayers. The functional properties of recombinant InsP3R3 expressed in Xenopus oocytes were described in nuclear envelope patch-clamp experiments (Mak et al., 2001a,b, 2000). Some of these studies resulted in conflicting data, but because of differences in techniques, experimental conditions and expression systems used by various groups, systematic comparison of obtained results is difficult.
Ca2+ signals supported by different endogenous chicken InsP3R isoforms have been previously compared in a Ca2+ imaging study with genetically altered DT40 cells by systematically deleting two out of three isoforms (Miyakawa et al., 1999). In the latter approach, however, comparison of functional properties of the three InsP3R isoforms is complicated by the differences in the endogenous expression levels of each isoform in DT40 cells. To compare single-channel properties of mammalian InsP3R isoforms in identical conditions, here we expressed rat InsP3R1, InsP3R2, and InsP3R3 in Spodoptera frugiperda (Sf9) cells. The recombinant InsP3R isoforms were reconstituted into planar lipid bilayers and characterized in identical recording conditions using 50 mM Ba2+ as a current carrier. The Ca2+ imaging results obtained in DT40 cells (Miyakawa et al., 1999) formed a framework for interpretation of single-channel data with different InsP3R isoforms obtained in our experiments. Our results provide a first comprehensive description of single-channel properties of the three mammalian InsP3R isoforms in identical experimental conditions.
MATERIALS AND METHODS
Generation of recombinant baculoviruses
The baculoviruses expressing rat InsP3R1 (RT1) and rat InsP3R3 (RT3) have been previously described (Maes et al., 2000; Tu et al., 2002). To generate rat InsP3R2-encoding baculovirus (RT2) a coding sequence of rat InsP3R2 in pCMV5 vector (Ramos-Franco et al., 2000; Sudhof et al., 1991) was subcloned into pFastBac1 vector (Invitrogen, Carlsbad, CA) and 5′ untranslated region (UTR) was replaced with the Kozak sequence by PCR using the BssHII (5′ UTR) and SfiI (2143) restriction sites. The RT2 baculoviruses were generated and amplified using the Bac-to-Bac system according to the manufacturer's (Invitrogen) instructions.
Expression of InsP3R in Sf9 cells
S. frugiperda (Sf9) cells were obtained from American Type Culture Collection (Manassas, VA) and cultured in suspension culture in supplemented Grace's insect media (Invitrogen) with 10% fetal bovine serum at 27°C. The three isoforms of InsP3R (RT1, RT2, and RT3) were expressed in Sf9 cells as previously described for RT1 (Nosyreva et al., 2002; Tu et al., 2002). Briefly, 150 ml of Sf9 cell culture was infected by InsP3R-encoding baculoviruses at multiplicity of infection (MOI) of 5–10. Sf9 cells were collected 66–72 h postinfection by centrifugation at 4°C for 5 min at 800 rpm (GH 3.8 rotor, Beckman Instruments, Fullerton, CA). The cellular pellet was resuspended in 25 ml of homogenization buffer A (0.25 M sucrose, 5 mM Hepes, pH 7.4) supplemented with protease inhibitors cocktail (1 mM EDTA, aprotinin 2 μg/ml, leupeptin 10 μg/ml, benzamidine 1 mM, 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride 2.2 mM, pepstatin 10 μg/ml, phenylmethylsulfonyl fluoride 0.1 mg/ml). Cells were disrupted by sonication (Branson Ultrasonics, Danbury, CT) and manually homogenized on ice with a glass-Teflon homogenizer.
The microsomes were isolated from the Sf9 cell homogenate by differential centrifugation as previously described (Kaznacheyeva et al., 1998). Briefly, 25 ml of Sf9 cell homogenate was centrifuged for 15 min at 4 k gmax (J 25.50 rotor, Beckman Instruments). The supernatant fluid was filtered through cheese cloth, and the filtrate was centrifuged for 30 min at 90 k gmax (Ti 50.2 rotor, Beckman Instruments). The pellet from the latter spin was resuspended in 25 ml of high-salt buffer B (0.6 M KCl, 5 mM NaN3, 20 mM Na4P2O7, 1 mM EDTA, 10 mM HEPES, pH 7.2) and manually homogenized on ice using Teflon/glass manual homogenizer and centrifuged for 15 min at 4 k gmax (J 25.50 rotor, Beckman Instruments). The resulting supernatant fluid was centrifuged for 30 min at 90 k gmax (Ti 50.2 rotor, Beckman Instruments). The pellet from the last spin was resuspended in 0.5 ml of the storage buffer (10% sucrose, 10 mM 3-morpholinopropanesulfonic acid, pH 7.0) to typically yield 6 mg/ml of protein (Bradford assay, Bio-Rad), aliquoted, quickly frozen in liquid nitrogen, and stored at −80°C.
Expression of InsP3R isoforms was confirmed by Western blotting with isoform-specific antibodies. Rabbit polyclonal anti-InsP3R1 antibody T443 was previously described (Kaznacheyeva et al., 1998). Rabbit polyclonal anti-InsP3R2 (IB7122) and anti-InsP3R3 (IB7124) antibodies were generated against keyhole limpet haemocyanin-conjugated InsP3R2 (RLGFLGSNTPHENHHMPPH) and InsP3R3 (RLGFVDVQNCMSR) carboxy-terminal peptides and affinity purified (AP) on antigenic peptides conjugated to N-hydroxysuccinimide-activated Sepharose (Amersham-Pharmacia Biotech).
Single-channel recordings of InsP3R activity
Planar lipid bilayers were formed from dioleoyl-phosphoethanolamine/dioleoyl-phosphoserine (3:1) synthetic lipid (Avanti Polar Lipids, Alabaster, AL) mixture in decane on the small (100–200 μm in diameter) hole in Teflon film separating two chambers 3 ml each (cis and trans). Before formation of the bilayer the hole was prepainted with phytanoyl-phosphocholine/dioleoyl-phosphoserine (3:1) synthetic lipid (Avanti Polar Lipids) mixture in decane. Recombinant InsP3R isoforms were incorporated into planar lipid bilayers by microsomal vesicle fusion as described previously for the wild-type and mutant InsP3R1 (Nosyreva et al., 2002; Tu et al., 2002). In these experiments endoplasmic reticulum (ER) microsomes were added to the cis chamber with stirring, and fusion of microsomes to the bilayer was induced by osmotic pressure resulting from an addition of 0.6–1 M KCl to the cis chamber. Fusion of ER microsomes to the bilayer leads to incorporation of the channels in such an orientation that the cis side is equivalent to cytosol and the trans side is equivalent to the lumen of ER (Miller, 1986). Fusion of ER vesicles with the bilayer was registered by the appearance of chloride currents. Once sufficient fusion was achieved (>100 pA of chloride currents), cis chamber (cytosolic) was perfused with 20 vol of cis recording solution (110 mM Tris dissolved in HEPES, pH 7.35) with stirring. The trans chamber (luminal) was filled with trans recording solution (50 mM Ba(OH)2 dissolved in HEPES, pH 7.35), leaving 50 mM Ba2+ as a main charge carrier (Bezprozvanny and Ehrlich, 1994). The cis chamber was held at virtual ground and the trans chamber was voltage clamped (Warner OC-725C bilayer clamp) in the range of membrane potentials (cis chamber potential relative to trans chamber potential) from +10 to −30 mV as indicated in the text. The liquid junction potential between cis and trans recording solutions was compensated before formation of the bilayer.
The InsP3R single-channel currents were amplified (Warner OC-725C), filtered at 1 kHz by low-pass eight-pole Bessel filter (model 900, Frequency Devices, Haverhill, MA), digitized at 5 kHz (Digidata 1200, Axon Instruments, Union City, CA), stored on a computer hard drive and recordable optical disks, and analyzed offline using pClamp 6 (Axon Instruments) and WinEDR V2.3 (Dempster, 2001). For single-channel analysis currents were filtered digitally at 500 Hz, and for presentation of the current traces data were filtered at 200 Hz. All-points amplitude histograms were generated from current records at least 100-s long and fit by a sum of two Gaussian functions (WinEDR V2.3). Channel openings were detected by half-threshold (t ≥ 2 ms) crossing criteria (Sakmann and Neher, 1983) using pClamp 6. We have not corrected for missed events in our analysis. Open and closed dwell-time distributions were fit by a single exponential fit (pClamp 6).
InsP3- and ATP-dependence of InsP3R was determined as described in (Bezprozvanny and Ehrlich, 1993; Lupu et al., 1998) by consecutive additions to the cis chamber from the concentrated stocks (1 mM InsP3 or 100 mM and 500 mM Na2ATP) with at least 30 s stirring of solutions in both chambers. Evidence for the presence of multiple channels in the bilayer (multiple open levels) was obtained in the majority of the experiments. The number of active channels in the bilayer was estimated as a maximal number of simultaneously open channels during the course of an experiment (Horn, 1991). The probability of the closed level, and first and second open levels was determined by using half-threshold crossing criteria (t ≥ 2 ms) from the records lasting at least 100 s at each InsP3 or ATP concentrations. The single-channel open probability (Po) was calculated using the binomial distribution for the levels 0, 1, and 2, and assuming that the channels in the bilayer were identical and independent (Colquhoun and Hawkes, 1983). Potential errors of absolute Po values associated with the possible underestimate of the number of active channels in the bilayer were minimized by normalizing the Po to the maximum Po observed in the same experiment. The normalized data from several experiments with each InsP3R isoform were averaged together for presentation and fitting. The fits were generated using least-squares routine (SigmaPlot 2001, Jandel Scientific, San Rafael, CA) and the quality of the fit was evaluated from the coefficient of determination (R2). The standard errors of resulting parameters were obtained as the estimates of the uncertainties in the values of regression coefficients obtained as a result of the fitting procedure (SigmaPlot 2001, Jandel Scientific).
To obtain the parameters of InsP3 dependence, the normalized and averaged data were fit by the equation
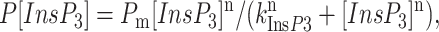 |
(1) |
modified from Lupu et al. (1998), where Pm is the maximal normalized open probability, n is the Hill coefficient, and kInsP3 is the apparent affinity for InsP3.
To obtain the parameters of ATP dependence, the normalized and averaged data were fit by the equation
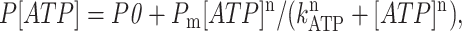 |
(2) |
modified from Bezprozvanny and Ehrlich (1993), where P0 is the normalized open probability in the absence of ATP, Pm is the maximal increase in normalized Po induced by ATP, n is the Hill coefficient, and kATP is the apparent affinity for ATP.
RESULTS
Expression and functional properties of mammalian InsP3R isoforms
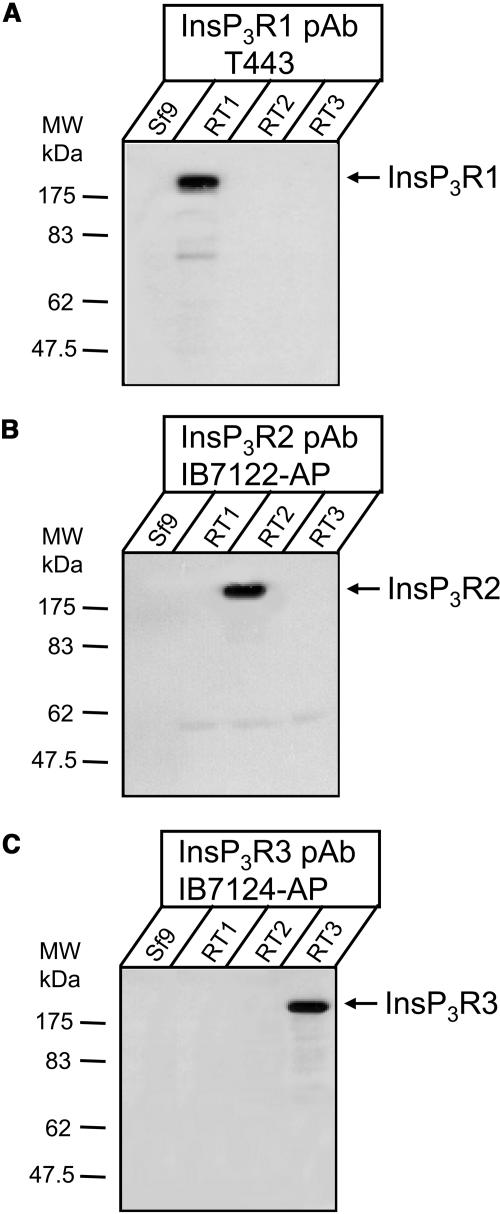
The baculoviruses encoding rat InsP3R1 (RT1) and rat InsP3R3 (RT3) have been previously described (Maes et al., 2000; Tu et al., 2002). The baculovirus encoding rat InsP3R2 (RT2) has been generated as described in Materials and Methods. The S. frugiperda (Sf9) cells infected with RT1, RT2, and RT3 baculoviruses were used to prepare microsomes 66–72 h postinfection as described in Materials and Methods. The microsomes prepared from noninfected Sf9 cells (Sf9) and from RT1-, RT2-, or RT3-infected Sf9 cells were analyzed by Western blotting with anti-InsP3R1 T443 antibodies (Fig. 1 A), anti-InsP3R2 affinity purified IB7122-AP antibodies (Fig. 1 B), and anti-InsP3R3 affinity purified IB7124-AP antibodies (Fig. 1 C). A prominent immunoreactive band of ∼260 kDa was detected in samples from baculovirus-infected cells, but not in noninfected control samples (Fig. 1, A–C). The specificity of the isoform-specific InsP3R antibodies used in these experiments is supported by the lack of cross-reactivity with microsomes from RT1, RT2, and RT3-infected Sf9 cells (Fig. 1, A–C). Our results support efficient expression of full-length rat InsP3R1, InsP3R2, and InsP3R3 in RT1-, RT2-, and RT3-infected Sf9 cells in our experimental conditions.
FIGURE 1.
Expression of mammalian InsP3R isoforms in Sf9 cells. Western blot of microsomal proteins. Microsomes isolated from noninfected Sf9 cells (Sf9) and from Sf9 cells infected with RT1, RT2, and RT3 baculoviruses were analyzed by Western blotting with polyclonal antibodies specific for InsP3R1 (A), InsP3R2 (B), and InsP3R3 (C) as indicated. For each microsomal preparation, 10 μg of total protein was loaded on the gel.
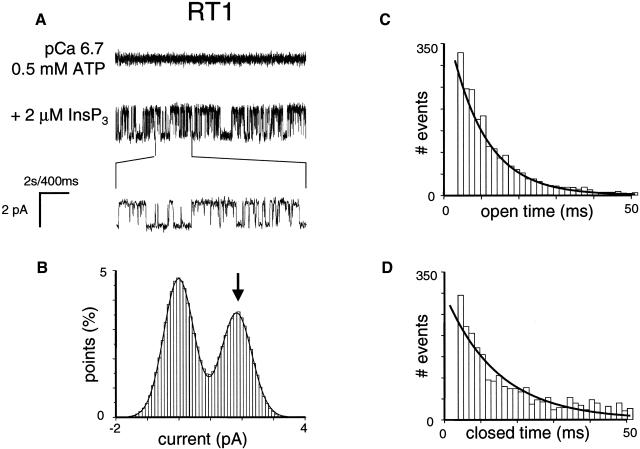
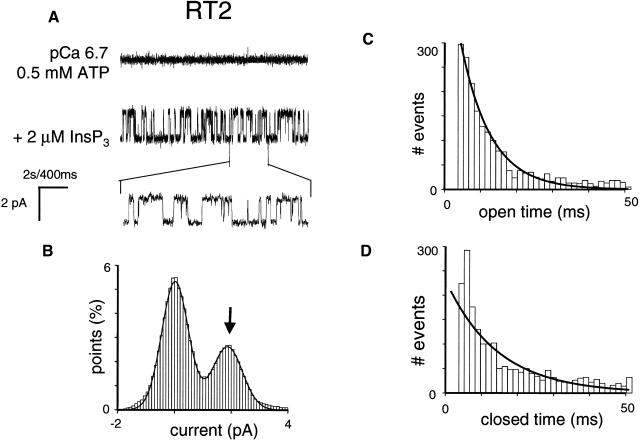
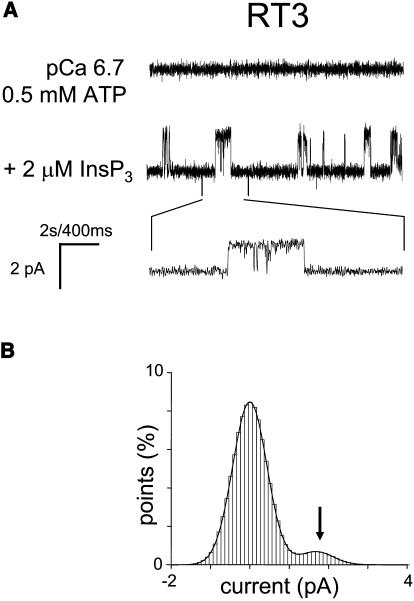
As we previously described (Nosyreva et al., 2002; Tu et al., 2002, 2003), when the microsomes isolated from RT1-infected Sf9 cells were fused with planar lipid bilayers, InsP3-gated channel activity was frequently (60/70) observed (Fig. 2 A). In contrast, the InsP3-gated channels were never (n = 10) observed in the experiments with microsomes isolated from uninfected Sf9 cells (Nosyreva et al., 2002; Tu et al., 2002, 2003). Similar to experiments with microsomes from RT1-infected Sf9 cells, InsP3-gated channels were frequently observed in experiments with microsomes from RT2-infected Sf9 cells (35/40) (Fig. 3 A) and with microsomes from RT3-infected Sf9 cells (40/46) (Fig. 4 A). Most of the experiments with microsomes from RT1-, RT2-, and RT3-infected Sf9 cells resulted in incorporation of multiple active InsP3R in the bilayer. To compare the basic channel properties of recombinant InsP3R1, InsP3R2, and InsP3R3 we performed single-channel analysis of currents recorded in a few experiments with only a single active InsP3R in the bilayer for each InsP3R isoform (as judged by the absence of multiple open levels). From this analysis we determined that in standard recording conditions (pCa 6.7, 0.5 mM ATP, 2 μM InsP3), InsP3R1 and InsP3R2 displayed similar levels of activity with Po values of ∼30% (Figs. 2 A and 3 A; Table 1). In contrast, InsP3R3 channels were less active in standard recording conditions, with Po values <5% (Fig. 4 A; Table 1).
FIGURE 2.
Single-channel properties of recombinant rat InsP3R1 (RT1). (A) Single-channel records of recombinant InsP3R1 in planar lipid bilayers. The experiments were performed at pCa 6.7 in the presence of 0.5 mM ATP (top trace). Addition of 2 μM InsP3 to the cis (cytoplasmic) side activated InsP3R1 (middle trace). Current trace at the expanded timescale is also shown (bottom trace). (B–D) Analysis of the InsP3R1 single-channel currents. All-points current amplitude histogram (B), open dwell time distribution (C), and closed dwell time distribution (D) are shown. The amplitude histogram (B) was fitted with a sum of two Gaussian functions corresponding to closed and open states of the InsP3R1. The Gaussian peak corresponding to an open state of InsP3R1 (arrow) was centered at 1.82 pA, had σ = 0.49 pA, and area 41%. Open and closed time distributions (C and D; number of events = 5000) were fitted with a single exponential function (curves) that yielded mean open time τ0 = 6.9 ms and mean closed time τc = 10.1 ms. The data from the same experiment were used for panels A–D. Similar analysis of at least three independent experiments with a single active InsP3R1 in the bilayer was performed to generate the data for Table 1.
FIGURE 3.
Single-channel properties of recombinant rat InsP3R2 (RT2). (B) Single-channel records of recombinant InsP3R2 in planar lipid bilayers. The experiments were performed at pCa 6.7 in the presence of 0.5 mM ATP (top trace). Addition of 2 μM InsP3 to the cis (cytoplasmic) side activated InsP3R2 (middle trace). Current trace at the expanded timescale is also shown (bottom trace). (B–D) Analysis of the single-channel records of InsP3R2 was performed and analyzed as described for InsP3R1 in the Fig. 2 legend. The Gaussian peak corresponding to an open state of InsP3R2 (arrow) was centered at 1.89 pA, had σ = 0.53 pA, and area 35%. Open and closed time distributions (C and D; number of events = 5000) were fitted with a single exponential function (curves) that yielded mean open time τ0 = 7.6 ms and mean closed time τC = 10.8 ms. The data from the same experiment were used for panels A–D. Similar analysis of at least three independent experiments with a single active InsP3R2 in the bilayer was performed to generate the data for Table 1.
FIGURE 4.
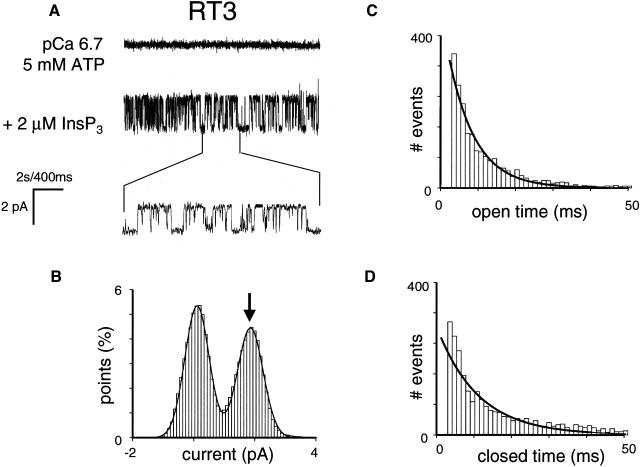
Single-channel properties of recombinant rat InsP3R3 (RT3). (A) Single-channel records of recombinant InsP3R3 in planar lipid bilayers. The experiments were performed at pCa 6.7 in the presence of 0.5 mM ATP (top trace). Addition of 2 μM InsP3 to the cis (cytoplasmic) side activated InsP3R3 (middle trace). Current trace at the expanded timescale is also shown (bottom trace). (B) Amplitude histogram of InsP3R3 was generated as described for InsP3R1 in the Fig. 2 B legend. The Gaussian peak corresponding to an open state of InsP3R3 (arrow) was centered at 1.76 pA, had σ = 0.43 pA, and area 7%. The data from the same experiment were used for panels A and B. Similar analysis of at least three independent experiments with a single active InsP3R3 in the bilayer was performed to generate the data for Table 1.
TABLE 1.
Single-channel properties of mammalian InsP3R isoforms
| InsP3R | ATP (mM) | Po (%) | i (pA) | τo (mS) | τc (mS) | γ (pS) |
|---|---|---|---|---|---|---|
| RT1 | 0.5 | 33 ± 5 | 1.9 ± 0.1 | 7.0 ± 0.3 | 9.9 ± 1.3 | 80.0 ± 0.3 |
| RT2 | 0.5 | 27 ± 6 | 1.9 ± 0.1 | 7.3 ± 0.4 | 10.6 ± 1.6 | 78.0 ± 0.5 |
| RT3 | 0.5 | 4 ± 1 | 1.91 ± 0.04 | ND | ND | 74.0 ± 0.4 |
| 5 | 33 ± 9 | 1.8 ± 0.2 | 7.4 ± 1.2 | 9.8 ± 1.1 | ND |
ND, not determined.
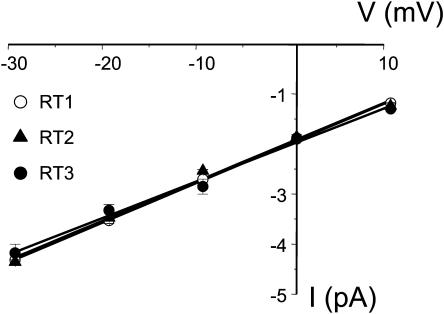
All-points current amplitude histograms for each InsP3R isoform were fit by a sum of two Gaussian functions corresponding to the closed and open states of the channels (panel B in Figs. 2–4). Thus, all three InsP3R isoforms open primarily to the main conductance state and subconductance states (Watras et al., 1991) are infrequent. At 0 mV holding potential, the size of the unitary current for the main conductance state of all 3 InsP3R isoforms in our experimental conditions was close to 1.9 pA (Figs. 2 B, 3 B, and 4 B; Table 1). The open dwell time distribution was fit by a single exponential function (Figs. 2 C and 3 C) with the mean open time in the range 7–8 ms for InsP3R1 and InsP3R2 isoforms (Table 1). The dwell closed time distribution was also fit by a single exponential function (Figs. 2 D and 3 D) with the mean closed time close to 10 ms for InsP3R1 and InsP3R2 (Table 1). Because of the low frequency of openings (Fig. 4), we were not able to collect enough events to perform kinetic analysis of InsP3R3 channels in standard recording conditions. To further characterize the conductance properties of three mammalian InsP3R isoforms, we measured the unitary currents supported by these channels at various transmembrane potentials between +10 and −30 mV (Fig. 5). The slope of the resulting current-voltage relationship provided us with the values of single-channel conductance equal to 80.0 ± 0.3 pS (n = 6) for RT1, 78.0 ± 0.5 pS (n = 4) for RT2, and 74 ± 0.4 pS (n = 3) for RT3 (Fig. 5; Table 1).
FIGURE 5.
Current-voltage relationship of mammalian InsP3R isoforms. InsP3R currents were recorded in the planar lipid bilayers in the range of transmembrane potentials from −30 mV to +10 mV (cis versus trans). Single-channel current amplitude at each voltage was determined from a Gaussian fit as shown in Figs. 2 B, 3 B, and 4 B. The data for RT1 (○), RT2 (▴), and RT3 (•) are shown as means ± SE (n ≥ 3). The slope of the linear fit to the data (lines; r = 0.99) yielded a single-channel conductance of 80 pS for RT1, 78 pS for RT2, and 74 pS for RT3.
InsP3 sensitivity of mammalian InsP3R isoforms
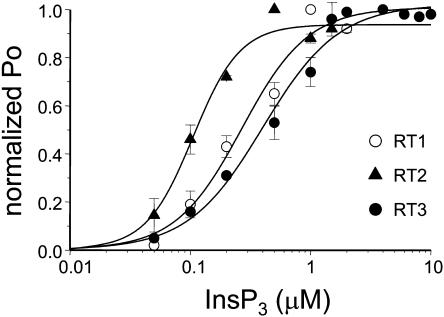
In the next series of experiments we studied the sensitivity of mammalian InsP3R isoforms to InsP3. The channel activity was recorded at pCa 6.7 in the presence of 0.5 mM ATP and in the range from 50 nM to 2 μM of InsP3 concentrations for InsP3R1 and InsP3R2 and in the range from 50 nM to 10 μM range of InsP3 concentrations for InsP3R3. Because most of the experiments resulted in multichannel bilayers, the Po values in each experiment were normalized to the maximal Po in the same experiment as described in Materials and Methods and the normalized data from different experiments with each InsP3R isoform were averaged together for presentation and analysis. Consistent with the previous findings (Lupu et al., 1998; Tang et al., 2003; Watras et al., 1991) we found that the open probability of InsP3R1 was elevated with increase in InsP3 concentration (Fig. 6, open circles). Fit to the RT1 data using Eq. 1 (Fig. 6, curve, R2 = 0.99) yielded an apparent affinity kInsP3 = 0.27 ± 0.07 μM InsP3 (Table 2). When compared to InsP3R1, InsP3R2 were more sensitive to activation by InsP3 (Fig. 6, solid triangles). Fit to the RT2 data using Eq. 1 (Fig. 6, smooth curve, R2 = 0.98) yielded an apparent affinity kInsP3 = 0.10 ± 0.01 μM InsP3 (Table 2). In contrast, InsP3R3 were least sensitive to activation by InsP3 (Fig. 6, solid circles). Fit to the RT3 data using Eq. 1 (Fig. 6, curve, R2 = 0.98) yielded an apparent affinity kInsP3 = 0.40 ± 0.05 μM InsP3 (Table 2).
FIGURE 6.
InsP3 sensitivity of mammalian InsP3R isoforms. The single-channel open probability (Po) for each InsP3R isoform was measured as a function of InsP3 concentrations on the cis (cytoplasmic) side of the membrane at pCa 6.7 in the presence of 0.5 mM ATP. The normalized and averaged data (see Materials and Methods) are shown as means ± SE (n ≥ 3) for RT1 (○), RT2 (▴), and RT3 (•). The data were fitted using Eq. 1 (see Materials and Methods). The parameters of the best fit (curves) are in Table 2.
TABLE 2.
InsP3 and ATP sensitivity of mammalian InsP3R isoforms
| InsP3 dependence
|
ATP dependence
|
||||
|---|---|---|---|---|---|
| InsP3R | kInsP3 (μM) | nHill | kATP (mM) | nHill | (Pm + P0)/P0 |
| RT1 | 0.27 ± 0.07 | 1.6 | 0.13 ± 0.04 | 1.3 | 5.26 |
| RT2 | 0.10 ± 0.01 | 2.2 | N/A | N/A | N/A |
| RT3 | 0.40 ± 0.05 | 1.4 | 2.0 ± 0.1 | 4.1 | 7.14 |
Modulation of mammalian InsP3R isoforms by ATP
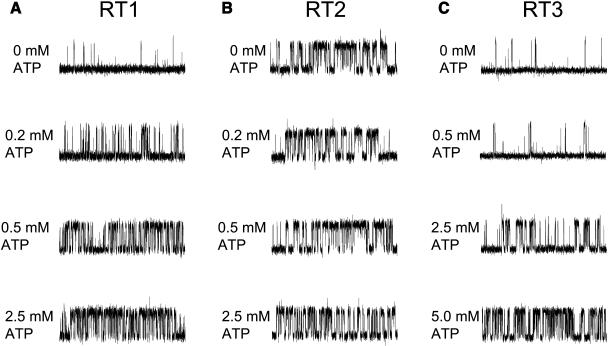
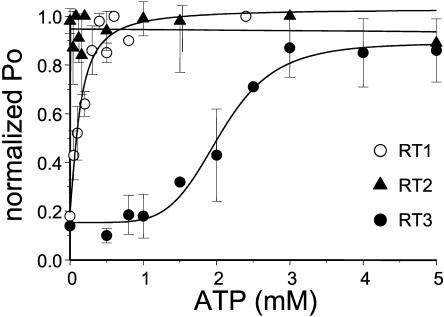
InsP3R1 is allosterically activated by ATP (Bezprozvanny and Ehrlich, 1993; Ferris et al., 1990; Iino, 1991; Maes et al., 2000; Tu et al., 2002). Does ATP affect gating of other InsP3R isoforms? To investigate isoform-specific modulation of InsP3R by ATP, we measured ATP sensitivity of channel gating for the three mammalian InsP3R isoforms in the presence of 2 μM InsP3 at pCa 6.7 and in the range of ATP concentrations from 0 to 2.5 mM for InsP3R1 and from 0 to 5 mM for InsP3R2 and InsP3R3. In agreement with the previous findings (Bezprozvanny and Ehrlich, 1993; Ferris et al., 1990; Iino 1991; Maes et al., 2000; Tu et al., 2002) we found that activity of RT1 channels was potentiated by submillimolar ATP (Fig. 7 A). In contrast, the gating of RT2 channels was ATP independent (Fig. 7 B). Activity of RT3 channels was elevated in the presence of ATP, but ATP concentrations in excess of 2 mM were required (Fig. 7 C). Most of the experiments resulted in multichannel bilayers. Thus, to analyze obtained results quantitatively, the Po values in each experiment were normalized to the maximal Po in the same experiment as described in Materials and Methods and the normalized data from different experiments with each InsP3R isoform were averaged together at each ATP concentration. Consistent with previous findings, we found that millimolar ATP increases RT1 activity approximately fivefold (Fig. 8, open circles). Fit to the RT1 data using Eq. 2 (see Materials and Methods) (Fig. 8, curve, R2 = 0.95) indicated that an apparent affinity of InsP3R1 for ATP (kATP) is equal to 0.13 ± 0.04 mM and the effects of ATP on InsP3R1 are not cooperative (nHill = 1.3) (Table 2). The parameter (Pm + P0)/P0 in Table 2 reflects a degree of ATP-dependent potentiation of InsP3R gating. Similar to RT1, RT3 activity was increased approximately sevenfold in the presence of ATP (Fig. 8, solid circles). Fit to the RT3 data using Eq. 2 (Fig. 8, curve, R2 = 0.98) indicated that an apparent affinity of InsP3R3 for ATP is equal to 2.0 ± 0.1 mM and the effects of ATP are highly cooperative (nHill = 4.1) (Table 2). In contrast with RT1 and RT3, gating of RT2 was ATP independent (Fig. 8, solid triangles).
FIGURE 7.
ATP sensitivity of mammalian InsP3R isoforms. Representative current records of RT1 (A), RT2 (B), and RT3 (C) channels in the bilayers in the presence of 2 μM InsP3 and pCa 6.7 at concentrations of Na2ATP as indicated on the cis (cytoplasmic) side of the membrane. The recordings from the same experiment are shown on each panel. Similar results were obtained in at least three experiments with each InsP3R isoform.
FIGURE 8.
ATP modulation of mammalian InsP3R isoforms. The single-channel open probability (Po) for each InsP3R isoform was measured as a function of Na2ATP concentrations on the cis (cytoplasmic) side of the membrane at pCa 6.7 in the presence of 2 μM InsP3. The normalized and averaged data (see Materials and Methods) are shown as means ± SE (n ≥ 3) for RT1 (○), RT2 (▴), and RT3 (•). The data for RT1 and RT3 were fit by Eq. 2 (see Materials and Methods). The parameters of the best fit (curves) are in Table 2. The data for RT2 were fit by a linear regression (line).
Relatively low sensitivity of RT3 channels to ATP (Fig. 8, solid circles) indicated that 0.5 mM ATP used in standard recording conditions corresponds to suboptimal ATP concentration for InsP3R3 activation. Thus, we repeated single-channel analysis of RT3-supported currents at pCa 6.7 in the presence of 2 μM InsP3 and 5 mM ATP (Fig. 9 A). In these conditions, Po of InsP3R3 was equal to 33 ± 9% (n = 3) (Fig. 9 B; Table 1), the size of the unitary current at 0 mV was equal to 1.8 ± 0.1 pA (n = 3) (Fig. 9 B; Table 1), the mean open dwell time was equal to 7 ± 1 ms (n = 3) (Fig. 9 C; Table 1), and the mean closed time was equal to 10 ± 1 ms (n = 3) (Fig. 9 D; Table 1). Thus, gating properties and open probability of RT3 channels in the presence of 5 mM ATP are similar to gating properties and open probability of RT1 and RT2 channels recorded in the presence of 0.5 mM ATP (Figs. 2, 3, and 9; Table 1).
FIGURE 9.
Single-channel properties of recombinant rat InsP3R3 (RT3) at 5 mM ATP. Single-channel records of recombinant InsP3R3 in planar lipid bilayers. The experiments were performed at pCa 6.7 in the presence of 5 mM ATP (top trace). Addition of 2 μM InsP3 to the cis (cytoplasmic) side activated InsP3R3 (middle trace). Current trace at the expanded timescale is also shown (bottom trace). (B–D) Analysis of the single-channel records of InsP3R3 was performed and analyzed as described for InsP3R1 in the Fig. 2 legend. The Gaussian peak corresponding to an open state of InsP3R3 (arrow) was centered at 1.84 pA, had σ = 0.34 pA, and area 47%. Open and closed time distributions (C and D; number of events = 5000) were fitted with a single exponential function (curves) that yielded mean open time τ0 = 7.8 ms and mean closed time τC = 10.2 ms. The data from the same experiment were used for panels A–D. Similar analysis of at least three independent experiments with a single active InsP3R3 in bilayer was performed to generate the data for Table 1.
DISCUSSION
Functional properties of mammalian InsP3R isoforms
All three mammalian InsP3R isoforms analyzed in our study expressed efficiently in Sf9 cells using baculoviral infection (Fig. 1) and formed InsP3-gated channels in planar lipid bilayers (Figs. 2 A, 3 A, and 4 A). With 50 mM Ba2+ as a current carrier the size of the unitary current for all three mammalian InsP3R isoforms was equal to 1.9 pA at 0 mV transmembrane potential (Figs. 2 B, 3 B, and 4 B; Table 1). The single-channel conductance for three mammalian InsP3R isoforms was in the range 74–80 pS (Fig. 5; Table 1). In standard recording conditions (pCa 6.7, 2 μM InsP3, and 0.5 mM ATP on the cytosolic side of the membrane) the single-channel open probability (Po) of the InsP3R1 and InsP3R2 channels was ∼30% (Figs. 2 B and 3 B; Table 1), whereas Po of InsP3R3 was <5% (Fig. 4 B; Table 1). In the same recording conditions the mean open dwell time of InsP3R1 and InsP3R2 was in the range 7–8 ms (Figs. 2 C and 3 C; Table 1) and the mean closed time was ∼10 ms (Figs. 2 D and 3 D; Table 1). When ATP dependence of InsP3R isoforms was compared (Figs. 7 and 8; Table 2), we found that 0.5 mM ATP maximally activated InsP3R1, but was not sufficient for maximal activation of InsP3R3. We also found that gating of InsP3R2 was ATP independent. In the presence of pCa 6.7, 2 μM InsP3 and 5 mM ATP on the cytosolic side of the membrane Po of InsP3R3 was elevated to 30% (Fig. 9 B; Table 1), the mean open time was equal to 8 ms (Fig. 9 C; Table 1) and the mean closed time was equal to 10 ms (Fig. 9 D; Table 1). Thus, all three mammalian InsP3R isoforms display similar maximal open probability in optimal recording conditions (∼30%) and share common gating and conductance properties (Table 1). The similarity in conductance and gating properties is consistent with the high degree of sequence conservation in channel-forming carboxy-terminal domain of InsP3R (Furuichi et al., 1994) and with the previous single-channel studies (Hagar et al., 1998; Mak et al., 2000; Ramos-Franco et al., 2000, 1998). In a recent study we described functional properties of recombinant Drosophila melanogaster InsP3R (DmInsP3R) expressed in Sf9 cells by baculoviral infection and reconstituted into planar lipid bilayers (Srikanth et al., 2004). We found that DmInsP3R displayed conductance and gating properties remarkably similar to mammalian InsP3R isoforms (Srikanth et al., 2004), indicating that these major functional properties of InsP3R are conserved in evolution.
The three mammalian InsP3R isoforms differ in sensitivity to activation by InsP3 (Fig. 6; Table 2). The InsP3R2 isoform is most sensitive to activation by InsP3 (kInsP3 = 0.10 μM), followed by InsP3R1 (kInsP3 = 0.27 μM InsP3), and then by InsP3R3 (kInsP3 = 0.40 μM InsP3) (Fig. 6; Table 2). The differences in apparent affinities of mammalian InsP3R isoforms to activation by InsP3 observed in our experiments are consistent with the functional analysis of InsP3R isoforms in DT40 cells (Miyakawa et al., 1999), and with the [3H]InsP3 binding (Maranto, 1994; Ramos-Franco et al., 2000; Sudhof et al., 1991) (but see Nerou et al., 2001) and single-channel (Hagar and Ehrlich, 2000; Ramos-Franco et al., 2000) studies.
The three mammalian InsP3R isoforms also differ in sensitivity to allosteric modulation by ATP (Figs. 7 and 8; Table 2). Search for potential ATP-binding sites with the query GXGXXG (Wierenga and Hol, 1983) reveals a presence of two potential ATP-binding sites in the InsP3R1 sequence 1773GGGGGGPG1780 (ATPA) and 2015GGLGLLG2021 (ATPB); two potential ATP-binding sites in the InsP3R2 sequence 1727GGGFTG1732 (ATPA) and 1968GGLGLLG1974 (ATPB); and a single potential ATP-binding site in the InsP3R3 sequence 1919GGLGLLG1925 (ATPB). Previous biochemical experiments indicated that the ATPA site in the InsP3R1 sequence binds ATP with high affinity (Maes et al., 1999) and ATPB sites in InsP3R1 and InsP3R3 bind ATP with low affinity (Maes et al., 2001, 1999). ATP-binding properties of ATPA and ATPB sites in InsP3R2 have not been examined in biochemical experiments. We found that both InsP3R1 and InsP3R3 are activated approximately fivefold by ATP, whereas InsP3R2 does not depend on ATP for maximal activation (Figs. 7 and 8). In agreement with the previous functional studies of InsP3R1 (Bezprozvanny and Ehrlich, 1993; Ferris et al., 1990; Iino, 1991; Maes et al., 2000; Tu et al., 2002), we found that the InsP3R1 apparent affinity for ATP is high (kATP = 0.13 mM) and effects of ATP are not cooperative (nHill = 1.3) (Fig. 8; Table 2). Also in agreement with the previous functional studies of InsP3R3 (Hagar and Ehrlich, 2000; Maes et al., 2000; Mak et al., 2001a; Missiaen et al., 1998), we found that the InsP3R3 apparent affinity for ATP is low (kATP = 2 mM) (Fig. 8; Table 2). We also discovered that effects of ATP on InsP3R3 are highly cooperative (nHill = 4.1) (Fig. 8; Table 2). High sensitivity of InsP3R1 to modulation by ATP most likely results from the unique high-affinity ATPA site (Bezprozvanny and Ehrlich, 1993; Ferris et al., 1990; Iino, 1991; Maes et al., 2000; Tu et al., 2002). Indeed, InsP3R1 sensitivity to modulation by ATP was greatly reduced in the InsP3R1-opt mutant that lacks ATPA site (Tu et al., 2002). Low sensitivity of InsP3R3 to modulation by ATP is consistent with low affinity of the ATPB binding site, which is likely to account for modulation of InsP3R3 by ATP. We would like to suggest that due to low affinity and high cooperativity, InsP3R3 regulation by ATP may be important in a physiological range of intracellular ATP concentrations (∼2 mM). This idea is consistent with predominant InsP3R3 expression in pancreatic β-cells (Taylor et al., 1999). We would like to propose that ATP modulation of InsP3R3 in pancreatic β-cells may play a role in control of glucose-dependent insulin secretion. ATP modulation of InsP3R1 is more likely to play a role in pathological conditions such as ischemia, when ATP concentrations can fall below 0.1 mM (Abe et al., 1987). Despite the presence of both ATPA and ATPB putative ATP-binding sites in the InsP3R2 sequence, InsP3R2 gating is ATP independent (Figs. 7 and 8). The ATP sensitivity of InsP3R2 has not been previously examined in electrophysiological experiments. However, in agreement with our findings, InsP3R2 was not modulated by ATP in Ca2+ flux experiments with DT40 cells (Miyakawa et al., 1999).
Acknowledgments
We are grateful to Gregory Mignery and Thomas C. Südhof for the kind gift of the rat InsP3R1 and InsP3R2 clones, to Dr. Graeme Bell for the kind gift of the rat InsP3R3 clone. We thank Phyllis Foley for the administrative assistance.
This work was supported by the Welch Foundation and National Institutes of Health (R01 NS38082 to I.B.) and the Fund for Scientific Research, Flanders, Belgium (G.0210.03 to H.D.S.).
References
- Abe, K., K. Kogure, H. Yamamoto, M. Imazawa, and K. Miyamoto. 1987. Mechanism of arachidonic acid liberation during ischemia in gerbil cerebral cortex. J. Neurochem. 48:503–509. [DOI] [PubMed] [Google Scholar]
- Berridge, M. J. 1993. Inositol trisphosphate and calcium signalling. Nature. 361:315–325. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny, I., and B. E. Ehrlich. 1993. ATP modulates the function of inositol 1,4,5-trisphosphate-gated channels at two sites. Neuron. 10:1175–1184. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny, I., and B. E. Ehrlich. 1994. Inositol (1,4,5)-trisphosphate (InsP3)-gated Ca channels from cerebellum: conduction properties for divalent cations and regulation by intraluminal calcium. J. Gen. Physiol. 104:821–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny, I., and B. E. Ehrlich. 1995. The inositol 1,4,5-trisphosphate (InsP3) receptor. J. Membr. Biol. 145:205–216. [DOI] [PubMed] [Google Scholar]
- Colquhoun, D., and A. G. Hawkes. 1983. The principles of stochastic interpretation of ion-channel mechanisms. In Single-Channel Recording. B. Sakmann and E. Neher, editors. Plenum Press, New York. 135–174.
- Dempster, J. 2001. The Laboratory Computer: A Practical Guide for Neuroscientists and Physiologists. Academic Press, New York.
- Ferris, C. D., R. L. Huganir, and S. H. Snyder. 1990. Calcium flux mediated by purified inositol 1,4,5-trisphosphate receptor in reconstituted lipid vesicles is allosterically regulated by adenine nucleotides. Proc. Natl. Acad. Sci. USA. 87:2147–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi, T., K. Kohda, A. Miyawaki, and K. Mikoshiba. 1994. Intracellular channels. Curr. Opin. Neurobiol. 4:294–303. [DOI] [PubMed] [Google Scholar]
- Hagar, R. E., A. D. Burgstahler, M. H. Nathanson, and B. E. Ehrlich. 1998. Type III InsP3 receptor channel stays open in the presence of increased calcium. Nature. 396:81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagar, R. E., and B. E. Ehrlich. 2000. Regulation of the type III InsP(3) receptor by InsP(3) and ATP. Biophys. J. 79:271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn, R. 1991. Estimating the number of channels in patch recordings. Biophys. J. 60:433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino, M. 1991. Effects of adenine nucleotides on inositol 1,4,5-trisphosphate-induced calcium release in vascular smooth muscle cells. J. Gen. Physiol. 98:681–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaznacheyeva, E., V. D. Lupu, and I. Bezprozvanny. 1998. Single-channel properties of inositol (1,4,5)-trisphosphate receptor heterologously expressed in HEK-293 cells. J. Gen. Physiol. 111:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupu, V. D., E. Kaznacheyeva, U. M. Krishna, J. R. Falck, and I. Bezprozvanny. 1998. Functional coupling of phosphatidylinositol 4,5-bisphosphate to inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 273:14067–14070. [DOI] [PubMed] [Google Scholar]
- Maes, K., L. Missiaen, P. De Smet, S. Vanlingen, G. Callewaert, J. B. Parys, and H. De Smedt. 2000. Differential modulation of inositol 1,4,5-trisphosphate receptor type 1 and type 3 by ATP. Cell Calcium. 27:257–267. [DOI] [PubMed] [Google Scholar]
- Maes, K., L. Missiaen, J. B. Parys, P. De Smet, I. Sienaert, E. Waelkens, G. Callewaert, and H. De Smedt. 2001. Mapping of the ATP-binding sites on inositol 1,4,5-trisphosphate receptor type 1 and type 3 homotetramers by controlled proteolysis and photoaffinity labeling. J. Biol. Chem. 276:3492–3497. [DOI] [PubMed] [Google Scholar]
- Maes, K., L. Missiaen, J. B. Parys, I. Sienaert, G. Bultynck, M. Zizi, P. De Smet, R. Casteels, and H. De Smedt. 1999. Adenine-nucleotide binding sites on the inositol 1,4,5-trisphosphate receptor bind caffeine, but not adenophostin A or cyclic ADP-ribose. Cell Calcium. 25:143–152. [DOI] [PubMed] [Google Scholar]
- Mak, D. O., S. McBride, and J. K. Foskett. 2001a. ATP regulation of recombinant type 3 inositol 1,4,5-trisphosphate receptor gating. J. Gen. Physiol. 117:447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak, D. O., S. McBride, and J. K. Foskett. 2001b. Regulation by Ca2+ and inositol 1,4,5-trisphosphate (InsP3) of single recombinant type 3 InsP3 receptor channels. Ca2+ activation uniquely distinguishes types 1 and 3 InsP3 receptors. J. Gen. Physiol. 117:435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak, D. O., S. McBride, V. Raghuram, Y. Yue, S. K. Joseph, and J. K. Foskett. 2000. Single-channel properties in endoplasmic reticulum membrane of recombinant type 3 inositol trisphosphate receptor. J. Gen. Physiol. 115:241–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maranto, A. R. 1994. Primary structure, ligand binding, and localization of the human type 3 inositol 1,4,5-trisphosphate receptor expressed in intestinal epithelium. J. Biol. Chem. 269:1222–1230. [PubMed] [Google Scholar]
- Mignery, G. A., and T. C. Sudhof. 1990. The ligand binding site and transduction mechanism in the inositol-1,4,5-triphosphate receptor. EMBO J. 9:3893–3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, C. 1986. Ion Channel Reconstitution. C. Miller, editor. Plenum Press, New York.
- Missiaen, L., J. B. Parys, I. Sienaert, K. Maes, K. Kunzelmann, M. Takahashi, K. Tanzawa, and H. De Smedt. 1998. Functional properties of the type-3 InsP3 receptor in 16HBE14o- bronchial mucosal cells. J. Biol. Chem. 273:8983–8986. [DOI] [PubMed] [Google Scholar]
- Miyakawa, T., A. Maeda, T. Yamazawa, K. Hirose, T. Kurosaki, and M. Iino. 1999. Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. EMBO J. 18:1303–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki, A., T. Furuichi, Y. Ryou, S. Yoshikawa, T. Nakagawa, T. Saitoh, and K. Mikoshiba. 1991. Structure-function relationships of the mouse inositol 1,4,5- trisphosphate receptor. Proc. Natl. Acad. Sci. USA. 88:4911–4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerou, E. P., A. M. Riley, B. V. Potter, and C. W. Taylor. 2001. Selective recognition of inositol phosphates by subtypes of the inositol trisphosphate receptor. Biochem. J. 355:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyreva, E., T. Miyakawa, Z. Wang, L. Glouchankova, A. Mizushima, M. Iino, and I. Bezprozvanny. 2002. The high affinity calcium-calmodulin-binding site does not play a role in modulation of type 1 inositol (1,4,5)-trisphosphate receptor function by calcium and calmodulin. Biochem. J. 365:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Franco, J., D. Bare, S. Caenepeel, A. Nani, M. Fill, and G. Mignery. 2000. Single-channel function of recombinant type 2 inositol 1,4, 5-trisphosphate receptor. Biophys. J. 79:1388–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Franco, J., M. Fill, and G. A. Mignery. 1998. Isoform-specific function of single inositol 1,4,5-trisphosphate receptor channels. Biophys. J. 75:834–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann, B., and E. Neher. 1983. Single-Channel Recording. B. Sakmann and E. Neher, editors. Plenum Press, New York.
- Srikanth, S., Z. Wang, H. Tu, S. Nair, M. K. Mathew, G. Hasan, and I. Bezprozvanny. 2004. Functional properties of the Drosophila melanogaster inositol 1,4,5-trisphosphate receptor mutants. Biophys. J. 86:3634–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof, T. C., C. L. Newton, B. T. Archer, Y. A. Ushkaryov, and G. A. Mignery. 1991. Structure of a novel InsP3 receptor. EMBO J. 10:3199–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, T. S., H. Tu, Z. Wang, and I. Bezprozvanny. 2003. Modulation of type 1 inositol (1,4,5)-trisphosphate receptor function by protein kinase A and protein phosphatase 1alpha. J. Neurosci. 23:403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, C. W., A. A. Genazzani, and S. A. Morris. 1999. Expression of inositol trisphosphate receptors. Cell Calcium. 26:237–251. [DOI] [PubMed] [Google Scholar]
- Thrower, E. C., R. E. Hagar, and B. E. Ehrlich. 2001. Regulation of Ins(1,4,5)P3 receptor isoforms by endogenous modulators. Trends Pharmacol. Sci. 22:580–586. [DOI] [PubMed] [Google Scholar]
- Tu, H., T. Miyakawa, Z. Wang, L. Glouchankova, M. Iino, and I. Bezprozvanny. 2002. Functional characterization of the type 1 inositol 1,4,5-trisphosphate receptor coupling domain SII(+/−) splice variants and the opisthotonos mutant form. Biophys. J. 82:1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu, H., E. Nosyreva, T. Miyakawa, Z. Wang, A. Mizushima, M. Iino, and I. Bezprozvanny. 2003. Functional and biochemical analysis of the type 1 inositol (1,4,5)-trisphosphate receptor calcium sensor. Biophys. J. 85:290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watras, J., I. Bezprozvanny, and B. E. Ehrlich. 1991. Inositol 1,4,5-trisphosphate-gated channels in cerebellum: presence of multiple conductance states. J. Neurosci. 11:3239–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga, R. K., and W. G. Hol. 1983. Predicted nucleotide-binding properties of p21 protein and its cancer-associated variant. Nature. 302:842–844. [DOI] [PubMed] [Google Scholar]