Abstract
GUVs have been widely used for studies on lipid mobility, membrane dynamics and lipid domain (raft) formation, using single molecule techniques like fluorescence correlation spectroscopy. Reports on membrane protein dynamics in these types of model membranes are by far less advanced due to the difficulty of incorporating proteins into GUVs in a functional state. We have used sucrose to prevent four distinct membrane protein(s) (complexes) from inactivating during the dehydration step of the GUV-formation process. The amount of sucrose was optimized such that the proteins retained 100% biological activity, and many proteo-GUVs were obtained. Although GUVs could be formed by hydration of lipid mixtures composed of neutral and anionic lipids, an alternate current electric field was required for GUV formation from neutral lipids. Distribution, lateral mobility, and function of an ATP-binding cassette transport system, an ion-linked transporter, and a mechanosensitive channel in GUVs were determined by confocal imaging, fluorescence correlation spectroscopy, patch-clamp measurements, and biochemical techniques. In addition, we show that sucrose slows down the lateral mobility of fluorescent lipid analogs, possibly due to hydrogen-bonding with the lipid headgroups, leading to larger complexes with reduced mobility.
INTRODUCTION
Biological membranes are complex matrices mainly composed of lipids and proteins that separate the contents of cells or specialized compartments from the external surroundings. GUVs (diameter ∼5–100 μm) have proved to be useful model systems and have been widely used to study lipid dynamics (Korlach et al., 1999), lipid domain (raft) formation (Bagatolli and Gratton, 2000; Kahya et al., 2003), elastic properties of membranes (Angelova et al., 1992; Akashi et al., 1996), lipid-DNA interactions (Angelova and Tsoneva, 1999), vesicle shape changes (Tanaka et al., 2002), membrane tube formation (Roux et al., 2002), and membrane fusion (Tanaka and Yamazaki, 2004). In contrast, reports on membrane protein dynamics in GUVs are rare, since for a long time methods suitable for incorporating these proteins in a functional state into GUVs were not available (Kahya et al., 2001; Girard et al., 2004).
The mechanism of formation and properties of GUVs has been studied since the 1980s (Dimitrov and Angelova, 1986; Mueller and Chien, 1983). GUVs can be prepared by drying lipids dissolved in organic solvent (chloroform or chloroform/methanol mixtures) followed by addition of distilled water. Water penetrates the dried lamellar structures, and GUVs are formed spontaneously due to membrane fusion processes. Formation appears to be optimal if a fraction of anionic lipids is incorporated (phosphatidylglycerol or phosphatidylserine) or can be promoted by the addition of divalent cations (Ca2+ or Mg2+) when using only neutral lipids (Akashi et al., 1998). In addition, AC electric fields have been reported to facilitate or impede the formation process (Dimitrov and Angelova, 1986, 1987).
The major bottleneck for direct incorporation of membrane proteins into GUVs is the dehydration step that precedes the formation process. Kahya et al. (2001) have circumvented this problem by using peptide-induced fusion of LUVs (diameter ∼200 μm), containing the membrane protein of interest, with preformed GUVs. Although this method has been successfully applied to study the dynamics and aggregation state of bacteriorhodopsin in GUVs (Kahya et al., 2002), the method is laborious and required the presence of some highly unusual lipids and a fusogenic peptide in the model membranes. Recently, we (Folgering et al., 2004) and others (Girard et al., 2004) developed alternative methods for incorporating polytopic membrane proteins into GUVs by (partial) dehydration of LUVs, containing (purified) membrane proteins, followed by rehydration in the presence of an AC electrical field (Angelova et al., 1992). This method was applied for the Ca2+-ATPase from sarcoplasmic reticulum and two highly stable membrane proteins, the light-driven proton pump bacteriorhodopsin, and the mechanosensitive channel of large-conductance MscL. A drawback was that electroformation tolerates low ( <10 mM) ion concentrations during the GUV-formation process. Bacteriorhodopsin and MscL retained their activity during drying of the proteoliposomes, however, activity of the Ca2+-ATPase was reduced by ∼30% (Girard et al., 2004). Bacia et al. (2004) studied two single-span soluble N-ethylmaleimide-sensitive factor attachment protein receptors incorporated into GUVs by the same method, but the fraction of proteins that survived the GUV-formation process was not determined. For single-molecule techniques like FCS, fluorescence resonance energy transfer, and atomic force microscopy, it is essential that heterogeneities due to nonproductive protein conformations can be ruled out and that 100% protein activity can be recovered.
We have made use of the stabilizing properties of disaccharides on membranes and proteins to develop a direct and simple method by which 100% protein activity was retained during incorporation of four distinct membrane protein(s) (complexes) into GUVs. The proteins studied were the lipid-anchored oligopeptide-binding protein OppA and the translocator complex OppBCDF of the oligopeptide ABC transporter from Lactococcus lactis, the mechanosensitive channel of large conductance MscL from Escherichia coli, and the secondary lactose transport protein LacS from Streptococcus thermophilus. The distribution, mobility, and function of these membrane proteins from distinct protein families were studied in proteo-GUVs by confocal imaging, FCS, patch-clamp, and other biochemical techniques. In addition, the effect of sucrose on the lateral mobility of hydrated lipids was analyzed by FCS measurements on GUVs prepared in the presence of high concentrations of sugar.
MATERIALS AND METHODS
GUV formation
GUVs were prepared according to standard procedures (Mueller and Chien, 1983; Akashi et al., 1996) with some modifications. In short, 10 μl of 4 mg/ml lipids (w/w), dissolved in chloroform, was deposited on a glass cover slide. For detection of GUVs by fluorescence microscopy, a fluorescent lipid probe, DiO (excitation and emission wavelength maxima of DiO are 484 and 501 nm, respectively), or NBD C6-HPC (excitation and emission wavelength maxima of NBD C6-HPC are 460 and 534 nm, respectively), was added (1 μl of 0.5 mM probe dissolved in MeOH/mg lipid). A thin lipid film was formed by evaporating the chloroform under vacuum at 4°C. The lipids were rehydrated at room temperature by the addition of 0.5 ml distilled water or 10 mM KPi, pH 7.0, in a custom-built sample chamber. Optionally 10 mM MgCl2 was added after prehydration with distilled water (Akashi et al., 1998) or electroformation was performed (Angelova et al., 1992). For electroformation, lipids were dried onto ITO-coated cover slips (custom-coated by GeSim, Dresden, Germany). A platinum wire was assembled 1 mm above the ITO-coated slide in the sample chamber, after which an AC electric field was applied (10 Hz, 1.2 V). Formation of GUVs was followed by fluorescence microscopy.
Cysteine mutagenesis
Cysteine mutants of the lipid-anchored oligopeptide-binding protein from L. lactis, OppA I602C, the mechanosensitive channel protein from E. coli, MscL K55C, and the secondary lactose transporter from S. thermophilus, LacS C320A/A635C, were prepared by standard molecular biology techniques. Each protein contains a C-terminal His6-tag that was used for purification by Ni2+-affinity chromatography. OppA I602C was made by replacing the 368 basepair BamHI-XbaI fragment of plasmid pAMP42 (Doeven et al., 2004) with a synthetic double-stranded oligonucleotide linker (5′-GATCCTGTATTGAGGGTCGTCATCATCACCACCATCACTGACGCGTCTGCAGT-3′ annealed to 5′-CTAGACTGCAGACGCGTCAGTGATGGTGGTGATGATGACGACCCTCAATACAG-3′) containing three extra basepairs coding for cysteine (letters in bold). The linker also contained a PstI site (underlined) outside the coding region to facilitate restriction analysis after ligation. Next, the insert of pAMP42 (oppDFBC) was replaced with a 1802 basepair NcoI-BamHI fragment of pAMP31 (Picon et al., 2000) containing the oppA gene. The resulting plasmid was named pNZOppA (I602C). LacS C320A/A635C was made by changing the codon specifying alanine 635 in pSKE8his(C320A) (Veenhoff et al., 2000) into a cysteine codon, using the QuikChange mutagenesis kit (Stratagene, La Jolla, CA). The plasmid coding for MscL K55C was a gift from L. Dijkink from the BioMaDe Technology Foundation, Groningen, The Netherlands.
Protein production and purification
OppA was produced in L. lactis NZ9000, using the nisin expression system (Kuipers et al., 1993; Kunji et al., 2003). Production of MscL K55C was done in E. coli PB104 (Blount et al., 1996), using the pB10b expression vector (Sukharev et al., 1994). LacS C320A/A635C was produced by E. coli HB101, using the pSKE8his expression vector (Veenhoff et al., 2000). Membrane vesicles were prepared by rupturing the cells with a high-pressure homogenizer (Kindler Maschinen AG, Zurich, Switzerland) and solubilized by 0.5% DDM (OppA and LacS) or 3% octyl-β-glucoside (MscL), and the proteins were purified by nickel affinity chromatography essentially as described previously (Knol et al., 1996; Detmers et al., 2000; Folgering et al., 2004). Solubilization buffers were 50 mM KPi, pH 8.0, 200 mM KCl, 10% (w/v) glycerol, 10 mM imidazole (OppA), 50 mM KPi, pH 8.0, 100 mM NaCl, 10% (w/v) glycerol, 15 mM imidazole (LacS), and 50 mM KPi, pH 7.0, 300 mM NaCl, 35 mM imidazole (MscL). OppBCDF was produced and purified as described previously (Doeven et al., 2004).
Protein labeling
After 12′ centrifugation at 280,000 × g, the solubilized material was incubated with Ni2+- nitrilotriacetic acid resin for 1.5 h at 4°C while rotating. Subsequently, the resin was drained and washed with 10 column volumes of solubilization buffer containing 0.05% DDM (OppA and LacS) or 0.2% Triton X-100 (MscL). The columns with OppA and LacS were washed with another 10 column volumes of the same buffer with 25 mM imidazole. For FCS experiments, the proteins were labeled with Alexa Fluor 488 C5 maleimide (Molecular Probes, Eugene, OR) by incubating the proteins after the initial washing steps, while bound to the Ni2+- nitrilotriacetic acid resin, with a ∼30 times excess of label for 2 h up to overnight at 4°C. Labeling was done in solubilization buffer without imidazole supplemented with 0.05% DDM (OppA and LacS) or 0.2% Triton X-100 (MscL). After labeling, the column was washed with 20 column volumes of the same buffer, and the proteins were eluted in the same buffer, pH 7.0, supplemented with 300, 400, or 200 mM imidazole for OppA, MscL, and LacS, respectively. The degree of labeling was estimated by measuring the absorbance of Alexa Fluor 488 (extinction coefficient is 71,000 M−1cm−1 at 495 nm) and protein concentration and was found to be 80–100% for each protein preparation.
Functional reconstitution of membrane proteins into LUVs
Purified proteins were inserted into Triton X-100 destabilized lipsomes as described (Knol et al., 1996). Activity of the proteins was determined by measuring peptide-binding (OppA; Detmers et al., 2000), peptide transport (OppBCDF plus OppA; Doeven et al., 2004), lactose transport (LacS; Knol et al., 1996), and channel activity (MscL; Folgering et al., 2004) as described previously.
Formation of proteo-GUVs
LUVs (10 μl of 20 mg/ml lipids), containing Alexa Fluor 488-labeled membrane protein at given protein/lipid ratio in 50 mM NH4HCO3, pH 8.0, were dried overnight under vacuum at 4°C on ultraviolet-ozone cleaned glass or ITO-coated cover slips. Ultraviolet-ozone cleaning was not essential for GUV formation but increased the wetting properties of the cover slip surface, making it easier to dry liposomes from aqueous solution. Sucrose was added at given amounts to stabilize the proteins during dehydration. Rehydration was done by adding 0.5 ml 10 mM KPi, pH 7.0, at room temperature. Optionally, 10 mM MgCl2 was added or electroformation was performed (as described above) when using neutral lipids only. GUV formation was monitored by fluorescence microscopy.
Confocal imaging and FCS measurements
FCS measurements were carried out on a laser scanning confocal microscope. The laser scanning confocal microscope is based on an inverted microscope Axiovert S 100 TV (Zeiss, Jena, Germany) in combination with a galvanometer optical scanner (model 6860, Cambridge Technology, Watertown, MA). The laser beam (488 nm, argon ion laser, Innova 99, Coherent, Louisville, CO) was focused by a Zeiss C-Apochromat infinity-corrected 1.2 NA 63× water immersion objective for excitation of the Alexa Fluor 488 fluorophore. The fluorescence was collected through the same objective, separated by a dichroic beam splitter (61003bs, Chroma Technology, Rockingham, VT) and directed through an emission filter (HQ 535/50, Chroma Technology) and a pinhole (diameter of 30 μm) onto an avalanche photodiode (SPCM-AQR-14, EG&G). The fluorescence signal was digitized, and autocorrelation curves were calculated on a PC using a multiple τ algorithm. The setup was calibrated by measuring the known diffusion coefficient of Alexa Fluor 488 in water (Molecular Probes; D = 300 μm2/s). Autocorrelation curves were fitted with a one-component two-dimensional diffusion model (Schwille, 2001) using Origin software (OriginLab, Northampton, MA).
Miscellaneous
Mutations were confirmed by restriction analysis and DNA sequencing. Protein concentrations were determined according to the method of Lowry et al. (1951) using bovine serum albumin as a standard. The concentrations of purified OppA, OppBCDF, and LacS were determined spectrophotometrically by measuring the absorption at 280 nm and using extinction coefficients of 1.605, 0.990, and 0.926 (mg/ml)−1cm−1, respectively.
RESULTS
Investigation of GUV formation from different lipid mixtures
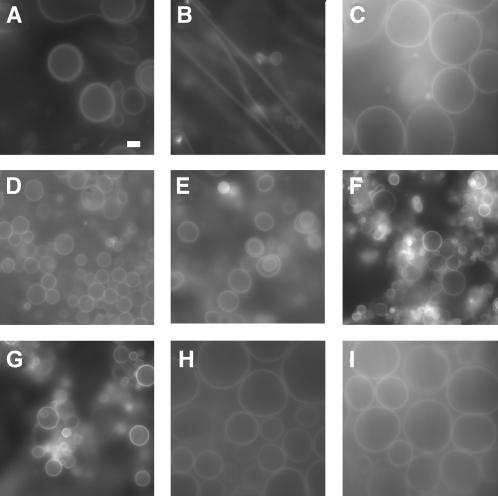
The formation of GUVs from lipid mixtures commonly used for reconstitution of membrane proteins in LUVs was evaluated. GUVs could be formed from all lipid compositions tested, although with different efficiency. When using neutral lipids (DOPC), formation was slow >1 h) and GUV yield was relatively low (Fig. 1 A). With DOPC/DOPE 1:1 (w/w), mixture threadlike structures were observed and GUVs were rarely formed (Fig. 1 B). However, when 25% (w/w) anionic lipids (DOPG or DOPS) was included, formation of GUVs was fast (within 15 min) and the yield was high (Fig. 1, C–E). Formation of GUVs from neutral lipids (DOPC and DOPC/DOPE) could be enhanced by adding 10 mM MgCl2, in accordance with observations made previously (Akashi et al., 1998). Addition of Mg2+ collapsed existing structures, after which many GUVs started to grow (Fig. 1, F and G). Electroformation (Angelova et al., 1992) also dramatically increased the yield of GUVs formed from neutral lipids (Fig. 1, H and I). For formation of proteo-GUVs (see below), a lipid mixture of DOPC/DOPS 3:1 (w/w) was used, because most membrane proteins studied to date require anionic lipids for (optimal) activity.
FIGURE 1.
Formation of GUVs from lipid mixtures used for proteo-LUV preparation. Membranes were visualized with a fluorescence microscope equipped with a Zeiss C-Apochromat infinity-corrected 1.2 NA 63× water immersion objective and a charge-coupled device camera. The fluorescent probe DiO was present at a mole ratio of 0.35 μmol DiO/mol total lipid. Scale bar (A) is 20 μm and is the same for all pictures shown. Formation of GUVs from neutral lipids: DOPC (A) and DOPC/DOPE 1:1 (w/w) (B). High yields of GUV formation were obtained when anionic lipids were incorporated: DOPC/DOPG 3:1 (w/w) (C), DOPC/DOPS 3:1 (w/w) (D), and DOPC/DOPE/DOPS 2:1:1 (w/w) (E). Addition of 10 mM MgCl2 after 30 min of prehydration in distilled water increased the yield of DOPC (F) and DOPC/DOPE (G) GUVs. Application of an electric field (10 Hz, 1.2 V, distance between electrodes was 1 mm) also dramatically improved the formation of GUVs from neutral lipids: DOPC (H) and DOPC/DOPE 1:1 (w/w) (I).
Production, purification, and fluorescent labeling of membrane proteins
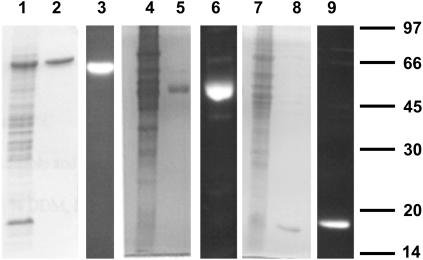
The four model membrane protein(s) (complexes) used in this study are the oligopeptide-binding protein OppA, and the translocator complex OppBCDF of the oligopeptide ABC transporter from L. lactis, the mechanosensitive channel of large conductance MscL from E. coli, and the secondary lactose transport protein LacS from S. thermophilus. To selectively label the proteins with fluorescent probes for detection by confocal imaging and FCS measurements, single-cysteine mutants were constructed. The position of the cysteine was located either near the C-terminus of the protein (OppA and LacS), or in an extracellular loop (MscL). Wild-type OppA contains an N-terminal cysteine residue that is lipid-modified, and this residue could not be used for labeling; OppA is anchored to the membrane via this lipid modification. The native cysteine of LacS at position 320 is located in the middle of a transmembrane helix and is not accessible or unreactive for labeling. Therefore, this cysteine was replaced by an alanine, and a cysteine for labeling was introduced near the C-terminus of the protein. The cysteines in the different proteins were labeled with Alexa Fluor 488 C5 maleimide, after which excess label was removed by nickel affinity chromatography. Fig. 2 shows SDS-PAGE analysis of membrane vesicles containing the overexpressed cysteine mutants, the purified proteins, and the proteins labeled with Alexa Fluor 488.
FIGURE 2.
SDS-PAGE analysis of overexpressed, purified, and labeled cysteine mutants. Membrane vesicles containing overexpressed cysteine mutants are shown in lanes 1, 4, and 7. Purified proteins labeled with Alexa Fluor 488 C5 maleimide (Molecular Probes) stained with Coomassie brilliant blue (lanes 2, 5, and 8) and visualized with an ultraviolet lamp (lanes 3, 6, and 9) are also shown. Mutants used were OppA I602C (lanes 1–3), LacS C320A/A635C (lanes 4–6), and MscL K55C (lanes 7–9).
Membrane reconstitution and activity of the labeled mutants
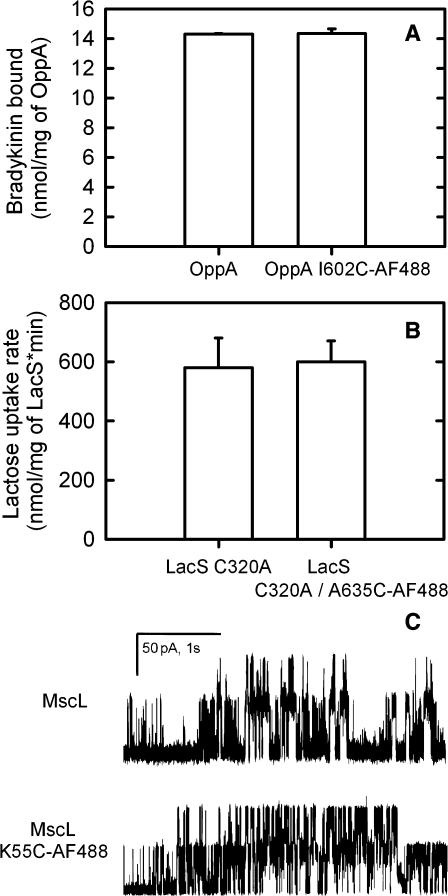
The Alexa Fluor 488 labeled proteins were inserted into Triton X-100 destabilized LUVs (Knol et al., 1996), and protein activity was assayed as described previously (Knol et al., 1996; Detmers et al., 2000; Folgering et al., 2004). Peptide binding by OppA, channel activity by MscL, and lactose transport by LacS were not affected by the mutations and the labeling of the proteins, as is shown in Fig. 3. In addition, bradykinin uptake via Opp (= OppA + OppBCDF) was also not influenced by labeling of OppA (not shown).
FIGURE 3.
Activity of labeled cysteine mutants. (A) Peptide binding by wild-type OppA and Alexa Fluor 488-labeled cysteine mutant OppA I602C AF488. The concentration of [3H]-bradykinin was 3 μM. (B) Lactose counterflow via LacS C320A and LacS C320A/A635C labeled with Alexa Fluor 488. The internal lactose and external [14C]-lactose concentrations were 10 mM and 100 μM, respectively. (C) Channel activity of wild-type MscL (upper trace) and MscL K55C labeled with Alexa Fluor 488 (lower trace). Patch-clamp measurements were done in 5mM Hepes, pH 7.2, 200 mM KCl, 40 mM MgCl2, and at +20 mV pipette voltage. The unit conductance of a single MscL channel is ∼2.5 nS as indicated by the 50 pA scale bar. Protein/lipid ratios were 1:50 (w/w). Proteins were inserted into DOPC/DOPS 3:1 (w/w) lipid mixtures.
Stabilization of membrane proteins by sucrose during dehydration
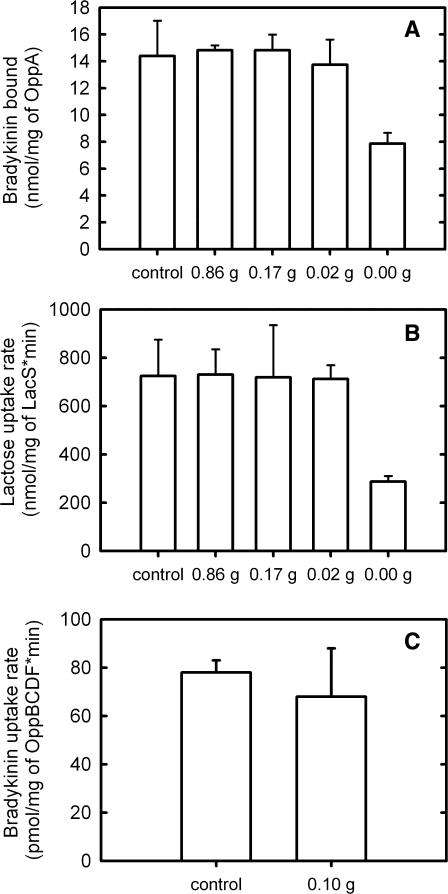
The major difficulty with incorporating membrane proteins in a functional state into GUVs is the dehydration step of the GUV-formation process. During drying, labile (membrane) proteins are prone to lose their biological activity (Crowe et al., 1984, 1988, 1996b). In our case, only the highly stable channel protein MscL was able to survive completely when water was removed from proteo-LUVs containing this protein (Folgering et al., 2004). OppA and LacS, on the other hand, were severely affected by the removal of water, resulting in loss of protein activity after de- and rehydration. Protein activity could be preserved by adding sucrose during drying of OppA- and LacS-containing LUVs (Fig. 4, A and B), and as little as 20 mg sucrose/g of lipid was sufficient for the recovery of full activity. Notice that during drying, the sucrose concentration increases from 1 to an estimated several hundreds of mM. The multi-subunit oligopeptide ABC transporter OppABCDF could be stabilized during dehydration by the addition of 100 mg sucrose/g of lipid (Fig. 4 C).
FIGURE 4.
Sucrose stabilizes membrane proteins during dehydration. Peptide binding by OppA (A), lactose counterflow via LacS C320A (B), and peptide uptake by OppABCDF (C; [3H]-bradykinin concentration was 0.7 μM) were measured after dehydration of the membranes in the presence of 0–0.86 g sucrose/g lipids and rehydration in 10 mM KPi, pH 7.0. “Control” samples were not dried and rehydrated. For further details, see Fig. 2 legend.
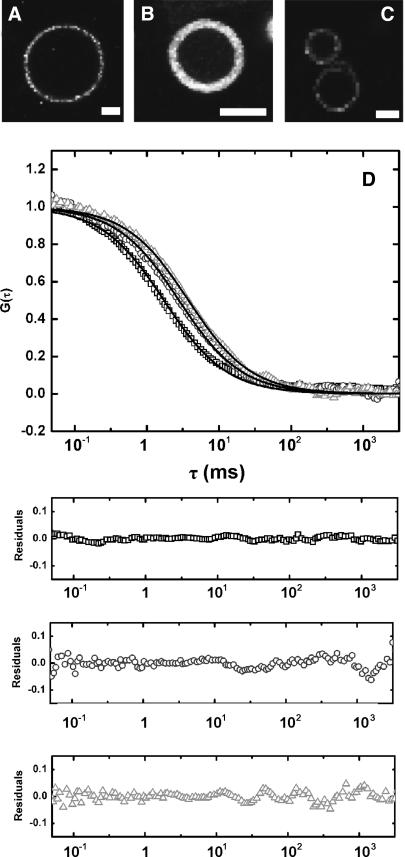
Confocal imaging of functional membrane proteins in GUVs
After optimization of the amount of sucrose needed to retain 100% biological activity for each protein (Fig. 4), proteo-GUVs were prepared for confocal imaging. Proteo-LUVs were dried in the presence of stabilizing amounts of sucrose and rehydrated in aqueous buffer. GUV formation was followed by fluorescence microscopy. The addition of Mg2+ or application of an electric field was not required for the formation of GUVs, since 25% (w/w) DOPS was included in the LUVs (see above). Confocal imaging confirmed that the proteins were incorporated homogeneously into the GUVs (Fig. 5, A–C). Differences in brightness of the GUVs are a result of different amounts of labels attached to the proteins. Pentameric MscL (Fig. 5 B), for example, has five labels attached whereas OppA (Fig. 5 A) has only one. The presence of sucrose during the drying of the proteo-LUVs inhibited the GUV-formation process. When sucrose was used at 0.86 g/g lipid or higher concentration, GUV formation was no longer observed. Addition of Mg2+ or application of an electric field also could not induce GUV formation under these conditions. However, the minimal amount of sucrose needed for the protection of the proteins during dehydration was much lower (∼0.02 g sucrose/g lipid; Fig. 4), making it possible to form GUVs containing fully functional membrane proteins.
FIGURE 5.
Confocal imaging and diffusion measurements of membrane proteins in GUVs. Confocal images of proteo-GUVs containing functional Alexa Fluor 488-labeled OppA I602C (A), MscL K55C (B), or LacS C320A/A635C (C). Scale bars are 10 μm. GUVs were prepared in the presence of 0.02 (A), 0 (B), or 0.17 (C) g sucrose/g lipids. The protein/lipid ratio was 1:500 (w/w) and the lipid composition was DOPC/DOPS 3:1 (w/w). (D) Autocorrelation curves for OppA I602C (□), MscL K55C (○), and LacS C320A/A635C (Δ) in GUVs. Curves were fit with a one-component two-dimensional diffusion model (solid lines) using Origin software (OriginLab); the residuals of the fits are shown in the panels below the figure.
Lateral mobility of membrane proteins in GUVs
The mobility of OppA, MscL, and LacS in GUVs was determined by FCS measurements. Confocal images were made of proteo-GUVs containing fluorescent labeled protein, and the focal volume was focused on the pole of the GUVs. Representative autocorrelation curves for each of the three proteins studied are shown in Fig. 5 D; the experimental data could be fitted reasonably well with a one-component two-dimensional diffusion model. Occasionally the membranes appeared to move or fluctuate during the autocorrelation measurements, which could be observed as a decay or systematic deviation in the count rate. Autocorrelation curves affected by these membrane undulations were not taken into account when analyzing the data. Undulations seemingly appeared more frequently when measuring autocorrelation curves with very large GUVs (30–100 μm) and less when the GUVs were somewhat smaller (<30 μm). The measured diffusion coefficients are summarized in Table 1. Lipid-anchored oligopeptide-binding protein OppA diffused with the same speed as the fluorescent lipid DiO. The mobility of the integral membrane proteins MscL and LacS was lower, with LacS being the slowest. The lateral mobility of the integral membrane proteins was ∼2–3-fold lower than the mobility of DiO.
TABLE 1.
Diffusion coefficients for lipid and membrane proteins in GUVs
| DiO* | OppA I602C AF488 | MscL K55C AF488 | LacS C320A/A635C AF488 | |
|---|---|---|---|---|
| Diffusion coefficient† (10−8 cm2/s) | 7.7 ± 0.8 | 7.5 ± 0.9 | 3.9 ± 0.3 | 3.0 ± 0.3 |
Diffusion of DiO was measured in GUVs, that is, in the absence of protein.
Diffusion coefficients represent the average of at least five measurements, and the SD values are given. FCS setup was calibrated with Alexa Fluor 488.
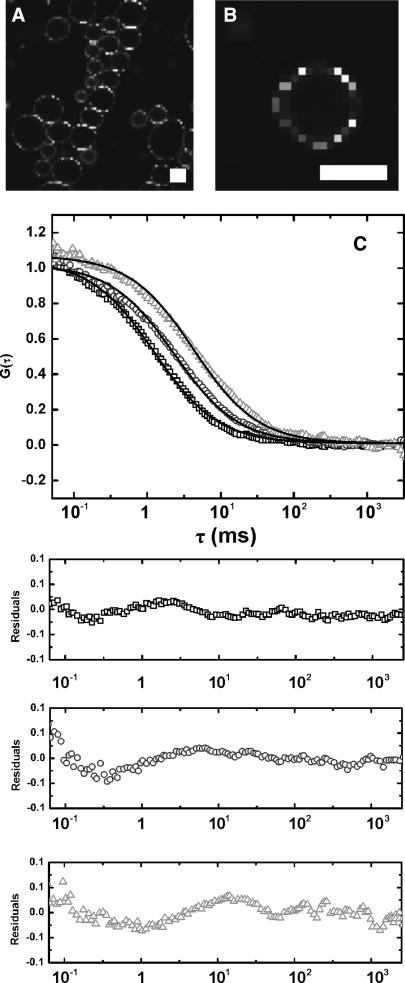
Interaction of sucrose with hydrated lipids
To investigate the effect of high concentrations of sucrose on the lateral mobility of lipids, we prepared GUVs in the absence and presence of high concentrations of sugar. Although the efficiency of GUV formation was lowered when sugar was present in the rehydration liquid, GUVs could still be formed with up to 1.5 M sucrose (or glucose) in the rehydration medium (Fig. 6, A and B). Representative autocorrelation curves for the fluorescent lipid probe DiO in GUVs prepared in water or 1.5 M sugar are shown in Fig. 6 C. It can be seen that at a high sucrose concentration, the lateral diffusion of DiO was slowed by a factor of ∼3. This suggests that the sugar is binding to the lipids, and cluster formation may be taking place. When glucose was used instead of sucrose, the effect on the lateral mobility of DiO was much smaller, but still a reduction of ∼2-fold was observed. In Table 2, a summary of the measured diffusion coefficients is given. Control experiments with another fluorescent lipid analog (NBD C6-HPC; Molecular Probes) gave similar results (not shown), indicating that the results were not probe-specific.
FIGURE 6.
Effect of sucrose on lipid lateral mobility in GUVs. Confocal images of GUVs prepared in the absence (A) or presence (B) of 1.5 M sucrose. Scale bar is 10 μm. The fluorescent lipid probe DiO was used to visualize the membranes. (C) Autocorrelation curves of DiO in GUVs prepared in the absence of sugar (□) or in the presence of 1.5 M glucose (○) or sucrose (Δ). Curves were fit with a one-component two-dimensional diffusion model (solid lines) using Origin software (OriginLab); the residuals of the fits are shown in the panels below the figure. With high concentrations of sucrose (and glucose), the data points deviated significantly from the one-component two-dimensional diffusion model, which may reflect the clustering of the lipids. Excellent fits were obtained when anomalous or two-component diffusion models were used to analyze the data (not shown). The lipid composition was DOPC/DOPS 3:1 (w/w).
TABLE 2.
Effect of sugars on lipid lateral mobility in GUVs
| Water | 1.5 M sucrose | 1.5 M glucose | |
|---|---|---|---|
| Diffusion coefficient* (10−8 cm2/s) | 7.7 ± 0.8 | 2.5 ± 0.6 | 4.1 ± 0.7 |
Diffusion coefficients represent the average of at least five measurements, and the SD values are given. FCS setup was calibrated with Alexa Fluor 488. Fluorescent lipid probe was DiO.
DISCUSSION
A method for direct incorporation of membrane proteins into GUVs without losing protein activity is described. Protein activity was retained by drying proteo-LUVs in the presence of sucrose. The distribution, lateral mobility, and activity of representative membrane proteins from distinct families in GUVs was analyzed by confocal imaging, FCS, patch-clamp, and other biochemical techniques. Furthermore, the effect of high concentrations of sucrose on lateral mobility of lipids in GUVs was studied by FCS.
First, we evaluated the formation of GUVs from lipid mixtures commonly used to reconstitute membrane proteins into LUVs (Fig. 1). Formation was optimal when a fraction of anionic lipids (DOPC or DOPS) was incorporated or could be promoted by the addition of Mg2+ or by performing electroformation when using only neutral lipids. These results are in accordance with previously made observations (Akashi et al., 1998; Angelova et al., 1992). Importantly, to the best of our knowledge, a systematic comparison of GUV formation in the presence or absence of an AC electric field using a wide range of lipid compositions has not been reported before.
For the formation of proteo-GUVs, we used proteo-LUVs containing purified membrane proteins as starting material. However, after drying and rehydration to form GUVs, three out of the four protein(s) (complexes) had lost biological activity. The only protein that survived completely was the mechanosensitive channel of large conductance MscL, which is not entirely surprising since MscL is a highly stable membrane protein. The other three proteins studied—the lipid-anchored oligopeptide binding protein OppA, the oligopeptide translocator complex OppBCDF, and the secondary lactose transporter LacS—were found to be less stable. Rigaud and colleagues attempted to circumvent the problem of losing protein activity during the drying step by performing partial dehydration of the proteo-LUVs under controlled humidity (Girard et al., 2004). However, of the two proteins studied, the light-driven proton pump bacteriorhodopsin and the Ca2+-ATPase, only the former completely survived the GUV-formation process. The Ca2+-ATPase had lost 30% of its biological activity. We prevented OppA, OppBCDF, and LacS from losing activity during dehydration by adding stabilizing amounts of sucrose. Only low amounts were needed to retain 100% activity (the minimal amount required was 0.02 g sucrose/g lipid) (Fig. 4).
Disaccharides (e.g., sucrose) are known to stabilize the folded state of proteins in solution via a mechanism termed preferential exclusion (Lee and Timasheff, 1981; Arakawa and Timasheff, 1985). At a high solute concentration, the sugar is excluded from the protein surface and the native state is thermodynamically favored over the unfolded state of the protein. The stabilization of soluble proteins during freezing is thought to occur via a similar mechanism. However, stabilization of soluble proteins by disaccharides during air drying is thought to occur via direct interaction (hydrogen bonding) of the sugar with polar groups of the protein (Crowe et al., 1988). In addition, disaccharides have been known for a long time to stabilize membranes during freezing or drying (Crowe et al., 1984, 1988, 1996a,b). The transitions from liquid-crystalline to gel phase (during dehydration) and gel to liquid crystalline phase (during subsequent rehydration) in the absence of sucrose may cause aggregation of integral membrane proteins and loss of activity. Upon drying, the sugar molecules replace water by hydrogen bonding to the lipid headgroups, thereby maintaining the spacing between the headgroups and preventing the membrane from going from the liquid-crystalline to the gel phase. The interactions of sucrose with the proteins and the maintenance of the liquid-crystalline phase of the membrane are most probably the determining factors for the stabilization of the OppA, OppBCDF, and LacS proteins. A side effect of high concentrations of sugars during drying is that they inhibit membrane fusion (Hincha et al., 2003). This may explain why at high amounts of sucrose during drying (≥0.86 g/g lipid), no GUVs were formed. Addition of Mg2+ or application of an electric field also could not induce GUV formation under these conditions. However, there appears to be an optimum in sucrose concentration at which membrane protein activity is retained (minimal amount required is 0.02 g sucrose/g lipid) and membrane fusion is still possible (<0.86 g sucrose/g lipid), which enables proteo-GUV formation.
The distribution of the proteins in the GUVs was assessed by confocal imaging and found to be homogeneous. FCS experiments showed that the diffusion of lipid-anchored OppA was as fast as that of the lipid analog DiO. This suggests that OppA does not have interactions with the membrane other than through its lipid anchor. The integral membrane proteins MscL and LacS diffused ∼2–3 times slower compared to DiO. The values found for diffusion of DiO and that of integral membrane proteins in GUVs are in the same range as determined previously (Kahya et al., 2001, 2003). The difference in mobility of DiO and the integral membrane proteins is in accordance with the Saffman and Delbrűck model for diffusion in biological membranes (Saffman and Delbrűck, 1975, Eq. 1):
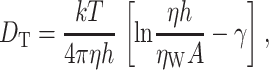 |
(1) |
where k is the Boltzmann constant, η and ηW are the viscosity of the membrane and of the surrounding aqueous medium, respectively, h is the membrane thickness, A the CSSA of the particle, and γ is Euler's constant. The difference in mobility of LacS (dimer with a molecular mass of ∼140 kDa and CSSA of ∼32 nm2; Friesen et al., 2000; Veenhoff et al., 2001) and that of MscL (pentamer with a molecular mass of ∼70 kDa and CSSA of ∼20 nm2 for the “closed” state; Chang et al., 1998) was ∼1.3 times. This difference is expected to be this small due to the logarithmic dependence of the lateral mobility of membrane proteins on the cross-sectional area of the aggregate (Saffman and Delbrűck, 1975; Kucik et al., 1999; Lee and Petersen, 2003).
Surprisingly, GUVs could still be formed when high amounts of sugar (up to 1.5 M) were present during rehydration only. It has been suggested by molecular dynamics simulations that the lateral diffusion of lipids in membranes is reduced in the presence of high concentrations of disaccharides (Sum et al., 2003; Pereira et al., 2004). Clusters of a single sucrose or trehalose molecule bound to 2–3 (Sum et al., 2003) or even more than 5 lipids (Pereira et al., 2004) were observed, with the phosphate group of the lipids as the principal interaction site with the sugars. Consistent with this prediction, we observed a reduction of the lateral mobility of lipids in the presence of 1.5 M sucrose, and the data could no longer be fitted with a one-component two-dimensional diffusion model. However, formation of clusters of 2–3 or even 5 lipids cannot explain a ∼3-fold reduction in diffusion coefficient on the basis of the increased cross-sectional surface area only (see Eq. 1). Based on our results, and in accordance with the molecular dynamics simulations, we conclude that high concentrations of sucrose (or trehalose) may indeed cluster lipids, and hydrogen bonding of sugars to the lipids increases the overall viscosity of the membrane, thereby slowing down the lipid mobility even further.
In conclusion, we developed a generic method to insert membrane proteins into GUVs without losing protein activity. Sucrose inhibited GUV formation when present during drying, but the amount needed for protein stabilization was lower than the amount that completely blocked GUV formation.
Acknowledgments
We thank L. Dijkink from the BioMaDe Technology Foundation for providing us with the plasmid encoding MscL K55C.
This work was supported by “Top-subsidie” grant 700-50-302 from the Netherlands Organization for Scientific Research-Chemische Wetenschappen (to B.P.) and funding from the Material Science Centre.
Abbreviations used: GUV, giant unilamellar vesicle; LUV, large unilamellar vesicle; ABC, ATP-binding cassette; AC, alternating current; DDM, n-dodecyl-β-d-maltoside; DiO, 3,3′-dioctadecyloxacarbocyanine perchlorate (DiOC18(3)); DOPC, 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine; DOPE, L-α-dioleoyl phosphatidylethanolamine; DOPG, 1,2-dioleolyl-sn-glycerol-3-[phospho-rac-(1-glycerol)]; DOPS, 1,2-dioleoyl-sn-glycero-3-phosphatidylserine; FCS, fluorescence correlation spectroscopy; NBD C6-HPC, 2-(6-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)hexanoyl-1-hexadecanoyl phosphocholine; ITO, indium tin oxide; and CSSA, cross-sectional surface area.
References
- Akashi, K., H. Miyata, H. Itoh, and K. Kinosita Jr. 1996. Preparation of giant liposomes in physiological conditions and their characterization under an optical microscope. Biophys. J. 71:3242–3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akashi, K., H. Miyata, H. Itoh, and K. Kinosita Jr. 1998. Formation of giant liposomes promoted by divalent cations: critical role of electrostatic repulsion. Biophys. J. 74:2973–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelova, M. I., S. Soléau, Ph. Méléard, J. F. Faucon, and P. Bothorel. 1992. Preparation of giant vesicles by external AC electric fields. Kinetics and applications. Progr. Colloid Polym. Sci. 89:127–131. [Google Scholar]
- Angelova, M. I., and I. Tsoneva. 1999. Interactions of DNA with giant liposomes. Chem. Phys. Lip. 101:123–137. [DOI] [PubMed] [Google Scholar]
- Arakawa, T., and S. N. Timasheff. 1985. The stabilization of proteins by osmolytes. Biophys. J. 47:411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacia, K., C. G. Schuette, N. Kahya, R. Jahn, and P. Schwille. 2004. SNAREs prefer liquid-disordered over “raft” (liquid-ordered) domains when reconstituted into giant unilamellar vesicles. J. Biol. Chem. 279:37951–37955. [DOI] [PubMed] [Google Scholar]
- Bagatolli, L. A., and E. Gratton. 2000. Two photon fluorescence microscopy of coexisting lipid domains in giant unilamellar vesicles of binary phospholipid mixtures. Biophys. J. 78:290–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount, P., S. I. Sukharev, M. J. Schroeder, S. K. Nagle, and C. Kung. 1996. Single residue substitutions that change the gating properties of a mechanosensitive channel in Escherichia coli. Proc. Natl. Acad. Sci. USA. 93:11652–11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, G., R. H. Spencer, A. T. Lee, M. T. Barclay, and D. C. Rees. 1998. Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science. 282:2220–2226. [DOI] [PubMed] [Google Scholar]
- Crowe, J., L. M. Crowe, J. F. Carpenter, A. S. Rudolph, C. A. Wistrom, B. J. Spargo, and T. J. Anchordoguy. 1988. Interactions of sugars with membranes. Biochim. Biophys. Acta. 947:367–384. [DOI] [PubMed] [Google Scholar]
- Crowe, J., F. A. Hoekstra, K. H. N. Nguyen, and L. M. Crowe. 1996a. Is vitrification involved in depression of the phase transition temperature in dry phospholipids? Biochim. Biophys. Acta. 1280:187–196. [DOI] [PubMed] [Google Scholar]
- Crowe, L. M., R. Mouradian, J. Crowe, S. A. Jackson, and C. Womersley. 1984. Effects of carbohydrates on membrane stability at low water activities. Biochim. Biophys. Acta. 769:141–150. [DOI] [PubMed] [Google Scholar]
- Crowe, L. M., D. S. Reid, and J. H. Crowe. 1996b. Is trehalose special for preserving dry biomaterials? Biophys. J. 71:2087–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detmers, F. J. M., F. C. Lanfermeijer, R. Abele, R. W. Jack, R. Tampé, W. N. Konings, and B. Poolman. 2000. Combinatorial peptide libraries reveal the ligand-binding mechanism of the oligopeptide receptor OppA of Lactococcus lactis. Proc. Natl. Acad. Sci. USA. 97:12487–12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov, D. S., and M. I. Angelova. 1986. Swelling and electroswelling of lipids: theory and experiment. Stud. Biophys. 113:15–20. [Google Scholar]
- Dimitrov, D. S., and M. I. Angelova. 1987. Electric field mediated lipid swelling and liposome formation. Stud. Biophys. 119:61–65. [Google Scholar]
- Doeven, M. K., R. Abele, R. Tampé, and B. Poolman. 2004. The binding specificity of OppA determines the transport selectivity of the oligopeptide ATP-binding cassette transporter. J. Biol. Chem. 279:32301–32307. [DOI] [PubMed] [Google Scholar]
- Folgering, J. H. A., J. M. Kuiper, A. H. de Vries, J. B. F. N. Engberts, and B. Poolman. 2004. Lipid-mediated light activation of a mechanosensitive channel of large conductance. Langmuir. 20:6985–6987. [DOI] [PubMed] [Google Scholar]
- Friesen, R. H. E., J. Knol, and B. Poolman. 2000. Quaternary structure of the lactose transport protein of Streptococcus thermophilus in the detergent-solubilized and membrane-reconstituted state. J. Biol. Chem. 275:33527–33535. [DOI] [PubMed] [Google Scholar]
- Girard, P., J. Pécréaux, G. Lenoir, P. Falson, J. Rigaud, and P. Bassereau. 2004. A new method for the reconstitution of membrane proteins into giant unilamellar vesicles. Biophys. J. 87:419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hincha, D. K., E. Zuther, and A. G. Heyer. 2003. The preservation of liposomes by raffinose family oligosaccharides during drying is mediated by effects on fusion and lipid phase transitions. Biochim. Biophys. Acta. 1612:172–177. [DOI] [PubMed] [Google Scholar]
- Kahya, N., E. Pécheur, W. P. de Boeij, D. A. Wiersma, and D. Hoekstra. 2001. Reconstitution of membrane proteins into giant unilamellar vesicles via peptide-induced fusion. Biophys. J. 81:1464–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahya, N., D. A. Wiersma, B. Poolman, and D. Hoekstra. 2002. Spatial organization of bacteriorhodopsin in model membranes. Light-induced mobility changes. J. Biol. Chem. 277:39304–39311. [DOI] [PubMed] [Google Scholar]
- Kahya, N., D. Scherfeld, K. Bacia, B. Poolman, and P. Schwille. 2003. Probing lipid mobility of raft-exhibiting model membranes by fluorescence correlation spectroscopy. J. Biol. Chem. 278:28109–28115. [DOI] [PubMed] [Google Scholar]
- Knol, J., L. Veenhoff, W. J. Liang, P. J. Henderson, G. Leblanc, and B. Poolman. 1996. Unidirectional reconstitution into detergent-destabilized liposomes of the purified lactose transport system of Streptococcus thermophilus. J. Biol. Chem. 271:15358–15366. [DOI] [PubMed] [Google Scholar]
- Korlach, J., P. Schwille, W. W. Webb, and G. W. Feigenson. 1999. Characterization of lipid bilayer phases by confocal microscopy and fluorescence correlation spectroscopy. Proc. Natl. Acad. Sci. USA. 96:8461–8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucik, D. F., E. L. Elson, and M. Sheetz. 1999. Weak dependence of mobility of membrane protein aggregates on aggregate size supports a viscous model of retardation of diffusion. Biophys. J. 76:314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers, O. P., M. M. Beerthuyzen, R. J. Siezen, and W. M. de Vos. 1993. Characterization of the nisin gene cluster nisABTCIPR of Lactococcus lactis. Requirement of expression of the nisA and nisI genes for development of immunity. Eur. J. Biochem. 216:281–291. [DOI] [PubMed] [Google Scholar]
- Kunji, E. R. S., D. Slotboom, and B. Poolman. 2003. Lactococcus lactis as host for overproduction of functional membrane proteins. Biochim. Biophys. Acta. 1610:97–108. [DOI] [PubMed] [Google Scholar]
- Lee, C. C., and N. O. Petersen. 2003. The lateral diffusion of selectively aggregated peptides in giant unilamellar vesicles. Biophys. J. 84:1756–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. C., and S. N. Timasheff. 1981. The stabilization of proteins by sucrose. J. Biol. Chem. 256:7193–7201. [PubMed] [Google Scholar]
- Lowry, O. N., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275. [PubMed] [Google Scholar]
- Mueller, P., and T. F. Chien. 1983. Formation and properties of cell-size lipid bilayer vesicles. Biophys. J. 44:375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira, C. S., R. D. Lins, I. Chandrasekhar, L. C. G. Freitas, and P. H. Hűnenberger. 2004. Interaction of the disaccharide trehalose with a phospholipid bilayer: a molecular dynamics study. Biophys. J. 86:2273–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picon, A., E. R. S. Kunji, F. C. Lanfermeijer, W. N. Konings, and B. Poolman. 2000. Specificity mutants of the binding protein of the oligopeptide transport system of Lactococcus lactis. J. Bacteriol. 182:1600–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux, A., G. Cappello, J. Cartaud, J. Prost, B. Goud, and P. Bassereau. 2002. A minimal system allowing tubulation with molecular motors pulling on giant liposomes. Proc. Natl. Acad. Sci. USA. 99:5394–5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffman, P. G., and M. Delbrűck. 1975. Brownian motion in biological membranes. Proc. Natl. Acad. Sci. USA. 72:3111–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwille, P. 2001. Fluorescence correlation spectroscopy and its potential for intracellular applications. Cell Biochem. Biophys. 34:383–408. [DOI] [PubMed] [Google Scholar]
- Sukharev, S. I., P. Blount, B. Martinac, F. R. Blattner, and C. Kung. 1994. A large-conductance mechanosensitive channel in E. coli encoded by MscL alone. Nature. 368:265–268. [DOI] [PubMed] [Google Scholar]
- Sum, A. K., R. Faller, and J. J. de Pablo. 2003. Molecular simulation study of phospholipid bilayers and insights of the interactions with disaccharides. Biophys. J. 85:2830–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, T., Y. Tamba, S. M. Masum, Y. Yamashita, and M. Yamazaki. 2002. La3+ and Gd3+ induce shape change of giant unilamellar vesicles of phosphatidylcholine. Biochim. Biophys. Acta. 1564:173–182. [DOI] [PubMed] [Google Scholar]
- Tanaka, T., and M. Yamazaki. 2004. Membrane fusion of giant unilamellar vesicles of neutral phospholipid membranes induced by La3+. Langmuir. 20:5160–5164. [DOI] [PubMed] [Google Scholar]
- Veenhoff, L. M., E. R. Geertsma, J. Knol, and B. Poolman. 2000. Close approximation of putative α-helices II, IV, VII, X, and XI in the translocation pathway of the lactose transport protein of Streptococcus thermophilus. J. Biol. Chem. 275:23834–23840. [DOI] [PubMed] [Google Scholar]
- Veenhoff, L. M., E. H. M. L. Heuberger, and B. Poolman. 2001. The lactose transport protein is a cooperative dimer with two sugar translocation pathways. EMBO J. 20:3056–3062. [DOI] [PMC free article] [PubMed] [Google Scholar]