Abstract
Intermediate filaments composed of desmin interlink Z-disks and sarcolemma in skeletal muscle. Depletion of desmin results in lower active stress of smooth, cardiac, and skeletal muscles. Structural functions of intermediate filaments in fast (psoas) and slow (soleus) skeletal muscle were examined using x-ray diffraction on permeabilized muscle from desmin-deficient mice (Des−/−) and controls (Des+/+). To examine lateral compliance of sarcomeres and cells, filament distances and fiber width were measured during osmotic compression with dextran. Equatorial spacing (x-ray diffraction) of contractile filaments was wider in soleus Des−/− muscle compared to Des+/+, showing that desmin is important for maintaining lattice structure. Osmotic lattice compression was similar in Des−/− and Des+/+. In width measurements of single fibers and bundles, Des−/− soleus were more compressed by dextran compared to Des+/+, showing that intermediate filaments contribute to whole-cell compliance. For psoas fibers, both filament distance and cell compliance were similar in Des−/− and Des+/+. We conclude that desmin is important for stabilizing sarcomeres and maintaining cell compliance in slow skeletal muscle. Wider filament spacing in Des−/− soleus cannot, however, explain the lower active stress, but might influence resistance to stretch, possibly minimizing stretch-induced cell injury.
INTRODUCTION
Desmin is an abundant cytoskeletal protein in skeletal muscle, forming intermediate filaments concentrated at the Z-disks of the sarcomeres. The intermediate filaments couple sarcomeres in the lateral direction and form connections to the nucleus and to the costameres at the sarcolemma (Lazarides et al., 1982). Desmin filaments are also found in the neuromuscular and myotendinous junctions (Askanas et al., 1990; Tidball, 1992). In cardiac muscle, desmin is associated with the intercalated discs and is highly expressed in the Purkinje fibers (Thornell and Eriksson, 1981). In smooth muscle, desmin is located in the cytoskeletal region at the dense bodies and dense plaques (Bond and Somlyo, 1982; Small and Sobieszek, 1977).
Human myopathies are often associated with aggregates of desmin, possibly due to dysfunctional desmin filaments. Mutations in the desmin gene or alterations in proteins assembling the desmin filaments have been found in myopathies (Ariza et al., 1995; Goldfarb et al., 1998; Munoz-Marmol et al., 1998; Sjöberg et al., 1999; Vicart et al., 1998; Wang et al., 2001). For a better understanding of the functions of desmin in muscle, a mouse model with a null mutation of the desmin gene has been introduced, Des−/− mice (Li et al., 1996; Milner et al., 1996). These mice develop normally without compensatory up-regulation of other known intermediate filament proteins (Li et al., 1997). An early phenotypic alteration in these mice is a cardiomyopathy with calcification and altered mitochondrial appearance (Balogh et al., 2002; Kay et al., 1997; Li et al., 1996; Milner et al., 1999). An increased expression of transforming growth factor-β, angiotensin converting enzyme, osteopontin, and decorin has been shown in the myocardium of Des−/− mice, which most likely is related to the cardiac fibrosis (Mavroidis and Capetanaki, 2002). In other muscle types of Des−/− mice the structural changes are less obvious. Electron microscopy and immunohistochemistry of skeletal muscles from Des−/− mice show that the sarcomeres are correctly assembled, although alterations in Z-lines, with misalignment and splitting, are observed (Li et al., 1997). No major changes were observed in the structure of smooth muscle cells from Des−/− mice (Sjuve et al., 1998).
A likely function of desmin intermediate filaments would be to anchor the sarcomeres or the sarcomere equivalents in smooth muscle, although the detailed mechanical and structural functions of desmin in the contractile units are not known. A lower active stress (force per cross-sectional area) of cardiac and smooth muscle is described in desmin-deficient mice (Balogh et al., 2002; Loufrani et al., 2002; Sjuve et al., 1998; Wede et al., 2002). These two muscle types have comparatively high desmin content in normal animals and the mechanical effects of desmin removal have been interpreted to reflect a misalignment of the contractile units during contraction (Balogh et al., 2002; Sjuve et al., 1998). For skeletal muscle the mechanical results are less clear. Initial studies on skeletal muscles from Des−/− mice suggested that active force generation was markedly lower (Li et al., 1997). Later studies have not been able to confirm the extensive mechanical alterations, although a general finding is that active stress of desmin-deficient skeletal muscle fibers is lowered by 20–30% (Balogh et al., 2003; Sam et al., 2000; Wieneke et al., 2000). The less pronounced effects in skeletal muscle, compared to cardiac and smooth muscle, might reflect that the concentration of desmin is lower; the concentration of desmin in fast skeletal muscle is ∼20 times lower than in the smooth (urinary bladder) muscle (Balogh et al., 2003). Slow skeletal muscle (soleus) has about twice the amount of desmin compared to fast muscle (psoas) (Balogh et al., 2003), which might suggest that the role of desmin varies between skeletal muscle types. Desmin removal has been suggested to influence other properties of skeletal muscle in addition to the active force. A shift toward a more fatigue-resistant, slow, skeletal muscle phenotype has been reported in desmin-deficient mice (Agbulut et al., 1996; Balogh et al., 2003). Mechanical stiffness of intact Des−/− soleus muscle has been reported to be increased (Anderson et al., 2001; Anderson et al., 2002b). Since this change was not observed at the single-cell level (Anderson et al., 2002a), this alteration in passive elastic properties of the intact muscle most likely is due to changes outside of the muscle fibers, e.g., fibrosis. Des−/− muscle (extensor digitorum longus, EDL) has been reported to be less vulnerable to stress injury induced by cyclic eccentric contractions or stretch (Sam et al., 2000; Shah et al., 2002), which might reflect an increased compliance of intermyofibrillar connections protecting the contractile system. A recent study on Des−/− and Des+/+ EDL fibers has suggested that the desmin intermediate filaments could be involved in regulating fiber volume and in connecting myofibrils (Shah et al., 2004). Desmin seems to be important also for the coupling of passive forces in the longitudinal and transverse directions of skeletal muscles (Boriek et al., 2001).
The mechanical changes in skeletal muscles observed in desmin-deficient muscles could be a consequence of alterations in the contractile filament arrangement. At present, information is lacking regarding the role of the intermediate filament in force transmission and structure maintenance in the lateral direction of the muscle. To examine if the intermediate filament system influences the arrangement of the contractile filaments and the elasticity in the lateral direction, we have used small-angle x-ray diffraction and fiber width measurements in combination with osmotic compression on skinned fibers and fiber preparations from soleus and psoas muscles of wild-type (Des+/+) and desmin-deficient mice (Des−/−).
METHODS
Animals
Mice with a null mutation in the desmin gene (Des−/−) were created by Li et al. (1996) with gene targeting in C57BL/6J mice (Li et al., 1996). In this study, muscles were obtained from adult (5–7 months old, 11 females and 8 males) Des−/− mice (weighing 21.7 ±0.6 g, n = 19) and compared with muscles from wild-type controls (Des+/+, weighing 29.6 ± 1.4 g, n = 16, 8 females and 8 males). Muscle preparations from these animals were also used in a separate study on intact soleus muscles and single psoas and soleus fibers (Balogh et al., 2003). Animals were killed by cervical dislocation and the soleus and psoas muscles were dissected and thereafter skinned, as described below. Two sets of experiments were performed, force and diameter measurements on isolated single muscle fibers and bundles, and x-ray diffraction measurements on fiber bundles. The project was approved by the local animal ethics committee.
Skinned muscle fiber bundles
Fiber bundles from psoas and soleus muscles were skinned with EGTA and freeze glycerination as described by Goldman et al. (1984), in a solution containing (mM): 5 EGTA, 2.5 ATP, 2.5 Mg2+, 10 imidazole, 170 K-propionate, 0.1 PMSF (phenyl methyl-sulphonyl fluoride), at pH 7.0. Bundles of fibers were mounted on silica gel plates, slightly stretched to the approximate in situ length, and skinned during gentle shaking for 24 h at 4°C in the solution above. The preparations were thereafter stored at −20°C in the same solution with 5 mM NaN3 and 50% (v/v) glycerol. In control and in later experiments leupeptin (0.5 mM) was included in the skinning and storage solutions.
To examine if the content of titin was altered by the skinning procedure or different between Des+/+ and Des−/− muscles, preparations were homogenized and analyzed using SDS-PAGE essentially as described by Tatsumi and Hattori (1995). Tissues were homogenized in 25 μl/mg wet weight and equal amounts (i.e., wet weights) were loaded on the gels. The intensity of the titin band from the skinned muscle samples was expressed relative to the corresponding intensity from intact muscle samples.
Recording of equatorial diffraction patterns
Small angle x-ray diffraction was performed using synchrotron radiation at beamline A-2 (HASY-lab, DESY, Hamburg, Germany). The beam at the muscle was ∼4 and 0.3 mm in the horizontal and vertical directions, respectively. A camera with evacuated tubes and a specimen-to-detector distance of 290 cm was used. The equatorial x-ray diffraction patterns were recorded using a 20-cm delay line detector mounted in the vertical direction or an image plate (evaluated using a Storm 820 scanner and Image Quant software from Molecular dynamics). The detector and image plate signals were calibrated using rat tail collagen samples. The detector signals were recorded online at the beamline (exposure time was 1–10 s). The equator patterns were extracted from the image plate (exposure time, 120 s) data using the analysis software. Fiber bundles (∼0.3 mm thick, corresponding to a cross-sectional area of 0.07 mm2) were mounted horizontally using aluminium clips between two metal hooks in a cuvette (volume ∼0.15 ml) made of folded Kapton membrane. The preparations were stretched to optimal sarcomere length (L0, 2.3 μm, cf. measurement on mouse flexor digitorum brevis, Westerblad et al., 1997) determined with laser diffraction. The equatorial patterns were recorded (1–10 s exposure time) at room temperature in relaxing (pCa 9) solution with the following composition (mM): 10 ATP, 11.6 MgAcetate, 5 EGTA, 30 imidazole, 12.5 phosphocreatine, and 1 mg/ml creatine kinase, at pH 6.7, with ionic strength adjusted to 125 mM using K-Methane sulphonate (modified from Kögler et al., 1998). Patterns were thereafter recorded at increasing dextran T-500 (Amersham Pharmacia, Biotech AB, Uppsala, Sweden) concentrations (1, 2, 4, 8%) in the relaxing solution. Measurements were also performed in rigor solution with 0 and 8% dextran T-500 and in relaxed muscles shortened by 20%.
Determination of fiber and muscle bundle diameter during osmotic compression
Single psoas and soleus muscle fibers were dissected and mounted using cellulose acetate glue between a fixed pin and the extended arm of an AE-801 (SensoNor AS, Horten, Norway) force transducer in a 0.5-ml cuvette on the stage of an inverted microscope (Nikon TMD, Tokyo, Japan). The fiber diameter and the sarcomere pattern were observed using a 20× objective, a CCD-camera, and an image analysis system (Argus 10, Hamamatsu, Japan). The preparations were mounted in relaxing solution (pCa 9) at 22°C and stretched to a sarcomere length of 2.3 μm. Osmotic compression of the muscle fibers was performed in the relaxation solution at increasing concentrations of dextran T-500 (1, 2, 4, and 8%). Active force and diameter were measured after activation with pCa 4.3 at 0 and 8% dextran. In experiments on fiber bundles, skinned preparations ∼0.3 μm in diameter were stretched to a sarcomere length of 2.3 μm (determined by laser diffraction) while mounted between two steel hooks in a cuvette as described. The diameter of the bundle at different dextran concentrations was analyzed as described above.
To examine if large variations in sarcomere length existed within the relaxed muscle fiber a separate set of experiments was performed where the sarcomere length in the mid-portion of the single fiber was adjusted to 2.37, 2.56, 2.83, and 3.11 μm (using the mean length of 10–12 sarcomeres). The sarcomere length in the end segments was then examined and expressed relative to that of the mid-portion for the different sarcomere lengths.
Statistics
All data are given as mean ± SE. Analysis of x-ray diffraction patterns was performed using the OTOKO program (M. Koch, EMBL, c/o DESY, Hamburg, Germany). All other curve fitting and statisitcal analysis were performed using SigmaPlot 8 and SigmaStat 3 for Windows (SPSS, Science Software, Gmb, Erkrath, Germany).
RESULTS
Equatorial x-ray diffraction patterns and effects of osmotic compression on filament spacing
Equatorial x-ray diffraction patterns were recorded from skinned fiber bundles of psoas and soleus. The equatorial patterns of muscle fibers, recorded as reflections in the vertical direction when the muscle is mounted horizontally, reflect the hexagonal array of thick and thin filaments in the sarcomere. Two distinct reflections are usually recorded, 1.1 and 1.0. The spacing of these is inversely related to the lateral distance between filaments. Wider filament spacing results in an inward movement of the reflections. The origin of these reflections and their detailed interpretation is described in several comprehensive reviews (e.g., Millman, 1998). Fig. 1 shows original recordings of the equatorial patterns from the control and desmin-deficient psoas and soleus muscles, in relaxing solution. The 1.1 and 1.0 reflections were identified and after background subtraction the center positions of the peaks were determined. The insets in Fig. 1 show that osmotic compression with dextran T-500 reduces the filament distances, i.e., 1.1 and 1.0 peaks move away from the center with increasing dextran concentration. In Fig. 2 the calculated filament spacing of the 1.1 reflections is shown for skinned psoas and soleus muscles at increasing dextran concentration. For both muscle types the lattice spacing was compressed with dextran. However, in the Des−/− soleus muscle the spacing was significantly wider (∼4%; p < 0.001, ANOVA) compared to that of the Des+/+ soleus at all concentrations of dextran. In rigor, the 1.1 spacing was reduced in both Des+/+ and Des−/− soleus compared to the relaxed state (squares in Fig. 2). The effect was slightly larger in Des−/− soleus. At 8% dextran the filament spacing was reduced in rigor by a larger relative extent in the Des−/− soleus compared to the Des+/+ soleus (Fig. 2).
FIGURE 1.
Original recording of equatorial x-ray diffraction pattern from Des+/+ (A and C) and Des−/− (B and D) psoas (A and B) and soleus (C and D) muscle fiber bundles. The inserted diagrams indicate the effect of osmotic compression with dextran on the patterns on one side: at 0% (upper) and 8% (lower) dextran T-500. Data are shown as relative intensity units.
FIGURE 2.
Filament spacing, calculated from the 1.1 equatorial x-ray diffraction pattern (d11) from skinned fiber bundles of Des+/+ (open symbols) and Des−/− (solid symbols) psoas (A and B) and soleus (C and D) muscle preparations. Circles show data from relaxed fibers and squares from fibers in rigor. The spacing of the relaxed Des−/− soleus muscle (circles in C) was significantly larger compared to the Des+/+ group (ANOVA, p = 0.001). Panels B and D show values expressed relative to the spacing at 0% dextran. The relative spacing of Des−/− soleus muscle fibers in rigor at 8% dextran (squares in D) was significantly smaller than that of the corresponding Des+/+ group (p < 0.05, Student's t-test), n = 6–8.
The results using the 1.0 reflections (not illustrated) were similar to those observed for the 1.1 reflections. No difference in 1.0 spacing was observed between the Des+/+ and Des−/− psoas fibers at 0 and 8% dextran (Des+/+: 0% dextran, 39.9 ± 0.5 nm; 8% dextran, 34.8 ± 0.9 nm; Des−/−: 0% dextran, 39.7 ± 0.9 nm; 8% dextran, 34.7 ± 0.9 nm; n = 8). For the soleus fibers the 1.0 spacing was wider in the Des−/− compared to the Des+/+ group at all dextran concentrations (Des+/+: 0% dextran, 40.8 ± 1.2 nm; 8% dextran, 34.5 ± 1.0 nm; Des−/−: 0% dextran, 42.1 ± 1.0 nm; 8% dextran, 37.1 ± 1.2 nm; n = 6–7). The spacing of the 1.0 reflection was significantly wider in the Des−/− soleus compared to the Des+/+ group (p < 0.01, ANOVA for data at 0–8% dextran). No difference in the widths at half-height (in detector channel units) of the 1.1 and 1.0 reflections could be detected between the Des+/+ and Des−/− groups for psoas (1.1 reflection: Des+/+, 12.9 ± 0.5 channels, Des−/−, 13.1 ± 0.6 channels; 1.0 reflection: Des+/+, 7.6 ± 0.6 channels, Des−/−, 7.0 ± 0.4 channels, n = 16) and soleus (1.1 reflection: Des+/+, 17.8 ± 0.9 channels, Des−/−, 18.0 ± 0.6 channels; 1.0 reflection: Des+/+, 9.7 ± 0.3 channels, Des−/−, 8.8 ± 0.6 channels, n = 15–28) muscles. Since background scattering from the fiber bundle and beamline was comparatively high close to the back-stop and camera length was limited by the physical dimensions of the laboratory, determination of the amplitude of the 1.0 peak required subtraction of the background and was difficult to determine unequivocally. However, we estimated the 1.1/1.0 amplitude ratios and found no difference between the Des−/− and Des+/+ psoas (Des+/+, 1.7 ± 0.1; Des−/−, 1.7 ± 0.1, n = 9–10) and soleus (Des+/+, 1.9 ± 0.3; Des−/−, 2.2 ± 0.2, n = 6–7) muscles. We examined the changes in 1.1/1.0 ratio during stretching from 2.3 to 2.9 μm. In the psoas muscles the 1.1/1.0 ratio was slightly lower in the stretched muscles, with no difference between Des+/+ and Des−/− (1.1/1.0 amplitude ratio at 2.9 μm sarcomere length: Des+/+, 1.1 ± 0.4, n = 4; Des−/−, 1.2 ± 0.3, n = 4). For the soleus muscles the ratio did not change significantly when stretched (Des+/+, 1.8 ± 0.2, n = 6; Des−/−, 1.6 ± 0.3, n = 7).
To exclude that proteolytic degradation of the samples could be contributing to a difference in filament spacing between Des+/+ and Des−/− soleus muscles, we also performed experiments on muscles skinned in the presence of leupeptin. Also in these samples the filament lattice spacing was wider (p < 0.05) in the Des−/− soleus (1.1 spacing: 23.5 ± 0.2 nm, n = 7) compared to the Des+/+ soleus (22.9 ± 0.2 nm, n = 7). For the psoas fibers no difference could be detected (1.1 spacing: Des+/+, 22.9 ± 0.6 nm, n = 11; Des−/−, 22.8 ± 0.5 nm, n = 7). Further, the content of titin was not decreased after skinning (psoas: 1.35 ± 0.20, n = 4; 1.10 ± 0.05, n = 8; soleus: 0.93 ± 0.05, n = 3; 0.90 ± 0.06, n = 8, without and with leupeptin, respectively; values expressed relative to the titin content of intact muscle). No difference was noted between the Des+/+ and Des−/− muscles (data not shown). To exclude that large variations in sarcomere length existed in the muscle fibers, we examined sarcomere length in the distal part relative to the central part in relaxed single-fiber preparations at sarcomere lengths 2.37, 2.56, 2.83, and 3.11 μm. These data show that the sarcomere length of the distal part, relative to the central part, was similar at these sarcomere lengths (Des+/+ psoas: 1.02 ± 0.01, 1.02 ± 0.01, 1.02 ± 0.01, and 1.03 ± 0.01; Des−/− psoas: 1.03 ± 0.01, 1.03 ± 0.01, 1.03 ± 0.01, and 1.02 ± 0.01; Des+/+ soleus: 1.03 ± 0.01, 1.02 ± 0.01, 1.03 ± 0.01, and 1.05 ± 0.01; Des−/− soleus: 1.04 ± 0.01, 1.04 ± 0.02, 1.04 ± 0.01, and 1.03 ± 0.02, n = 6). These data show that extensive sarcomere inhomogeneity is not present in the muscle fibers.
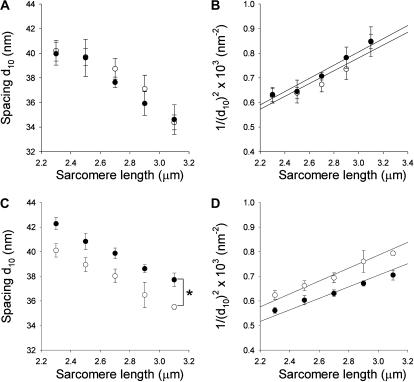
To consider if variation in the length dependence of the filament structure could explain the difference in filament spacing between Des+/+ and Des−/− soleus muscles, we examined x-ray patterns at L0 (sarcomere length 2.3 μm) and in preparations shortened by 20%. In both Des+/+ and Des−/− soleus preparations the relative 1.1 spacing increases in a similar manner (Des+/+, 5.1 ± 1.2 increase and Des−/−, 5.2 ± 2.1% increase, n = 5–6). The corresponding value for the 1.0 reflections were for Des+/+ 7.6 ± 1.8 and for Des−/− 6.2 ± 3.0%, n = 5–6. These data show that the length dependence of the 1.1 spacing was similar in the two groups and that ∼20% difference in the degree of stretch is necessary to explain the difference in spacing between the Des−/− and Des+/+ soleus muscles. Since all fiber preparations had visible laser diffraction patterns and were stretched to equal optimal lengths (2.3 μm) a systematic error of this magnitude can be ruled out. To obtain an estimate of sarcomere volume we also examined muscles stretched to different sarcomere lengths. The data presented in Fig. 3 show that the filament 1.0 spacing decreased with stretch of the muscles. The relations were similar in Des+/+ and Des−/− psoas. For soleus muscles the spacing of the Des−/− was wider at all sarcomere lengths. In the panels B and D of Fig. 3 the inverse of the square of the 1.0 spacing was plotted against sarcomere length (cf. Millman, 1998) to obtain estimates of the sarcomere volume. The value for Des−/− soleus was ∼12% larger than that of the Des+/+ soleus.
FIGURE 3.
Filament 1.0 equatorial spacing determined in x-ray diffraction measurements on psoas (A and B) and soleus (C and D) fiber bundles from Des+/+ (open symbols) and Des−/− (solid symbols) mice. In A and C, the 1.0 spacing (d10) is plotted against sarcomere length and in B and D, 1/(d10)2 is plotted against sarcomere length. Linear regressions were used to calculate apparent lattice volumes (Millman, 1998): Des+/+ psoas, 3.2 × 10−3 μm3; Des−/− psoas, 3.1 × 10−3 μm3; Des+/+ soleus, 3.2 × 10−3 μm3; Des−/− soleus, 3.6 × 10−3 μm3 per sarcomere. The spacing of the relaxed Des−/− soleus muscle (solid circles in C) was significantly larger compared to the Des+/+ group (ANOVA, p < 0.05).
Effects of osmotic compression on width and force of skinned fibers
Striation patterns were visible in all single skinned muscle fibers and laser diffraction patterns were observed in the fiber bundles in the relaxed state of Des+/+ and Des−/− psoas and soleus muscles. The single skinned muscle fibers and muscle bundles were osmotically compressed using increasing concentration of dextran T-500. The fiber and bundle diameters were reduced by the increased osmotic pressure in both psoas and soleus fibers (Fig. 4). In the experiments on single fibers, the initial fiber diameters were similar in Des−/− and Des+/+ psoas muscles (Des+/+, 54.9 ± 4.5 μm; Des−/−, 55.1 ± 4.9 μm; n = 7–9). Diameter of Des−/− soleus fibers was slightly, not significantly, smaller compared to Des+/+ (Des+/+, 50.8 ± 3.9 μm; Des−/−, 47.6 ± 4.3 μm, n = 8–9). The soleus muscle fibers from Des−/− mice were significantly more compressed at high concentrations of dextran compared to the Des+/+ soleus fibers (Fig. 4 B). Active contraction (pCa 4.3) reduced fiber width compared to the relaxed state both at 0 and 8% dextran (squares in Fig. 4, A and B). The reduction in fiber width during contraction, relative to the width in the relaxed state, was similar in psoas fibers from Des−/− and Des+/+ mice (Fig. 4 A). In contrast the activation-induced reduction of fiber width was significantly lower in Des−/− soleus muscles (Fig. 4 B). To examine if compression of the filament lattice by dextran influenced active force generation in Des−/− and Des+/+ soleus muscle in a different manner, possibly related to the difference in filament spacing, muscle fibers were activated at 0 and 8% dextran. The active force at 8% dextran was expressed relative to that at 0% dextran. Absolute values for force and muscle fiber diameter of single-muscle preparations from Des+/+ and Des−/− muscles have been reported previously (Balogh et al., 2003). Active force in the presence of 8% dextran, relative to the force at 0% dextran, was not significantly different between the two groups (psoas: Des+/+, 97.9 ± 3.8%; Des−/−, 105.5 ± 10.4%; n = 7–9;soleus: Des+/+, 94.7 ± 6.3%; Des−/−, 90.8 ± 7.8%; n = 8–9).
FIGURE 4.
Fiber and fiber bundle diameter at increasing concentrations of dextran T-500. Panel A shows results from single skinned psoas fibers and panel B shows results from single skinned soleus fibers. Des+/+ fibers are indicated with open symbols and Des−/− with solid symbols. Circles show data obtained in relaxation solution (pCa 9) and squares data in contraction solution (pCa 4.3). Data in contraction solution at 8% dextran are plotted with a slight offset in the x axis to increase clarity, n = 7–9. Panels C and D show corresponding data for fiber bundle preparations from skinned psoas muscle (C; initial diameter Des+/+, 0.29 ± 0.03 mm; n = 4; Des−/−, 0.29 ± 0.04 mm, n = 5) and skinned soleus muscles (D; initial diameter Des+/+, 0.29 ± 0.04 mm; n = 8; Des−/−, 0.28 ± 0.03 mm, n = 10).
In the compression experiments on skinned fiber bundles the diameter was reduced by the increased osmotic pressure in both psoas and soleus fibers (Fig. 4, C and D), in a similar manner as in the single cells. The fiber bundles from Des−/− soleus muscles were more compressed at al increasing dextran concentrations compared to soleus Des+/+ fiber bundles. There was no difference in diameter compression between psoas Des+/+ and Des−/− fiber bundles.
DISCUSSION
The desmin intermediate filaments have connections both to the Z-disks of the sarcomere and to the sarcolemma, which suggests that these structures have a role in the organization and mechanical coupling of the contractile filaments in muscle. We used desmin-deficient mice (Des−/−) and their wild type controls (Des+/+) to evaluate effects of intermediate filament depletion on contractile filament spacing. Low-angle x-ray diffraction measurements of the equatorial reflections were used to determine filament spacing. The experiments were performed on skinned preparations and we have previously confirmed that the tissue contents of contractile proteins and desmin are not altered by the skinning procedure, and that desmin is completely absent in the Des−/− muscles (Balogh et al., 2003). Unaltered myosin and actin content of both intact and skinned preparations from Des−/− and Des+/+ (Balogh et al., 2003), and unaltered titin content (this study, and Anderson et al., 2002) exclude that major degeneration is occurring in the muscles. Since both laser diffraction and distinct 1.1 and 1.0 equatorial reflections were present in the Des−/− muscles and since sarcomere length did not differ between central and distal parts of single fibers, we conclude that desmin depletion is not associated with a dramatic change in sarcomere structure. This is consistent with microscopy data showing essentially normally assembled sarcomeres (Li et al., 1997). The lattice spacing of the 1.1 and 1.0 reflections in the mouse soleus and psoas muscles correspond well with those previously reported for skinned rabbit skeletal muscles (Kawai et al., 1993). The spacing of the equatorial reflections from the skinned mouse soleus and psoas muscles is similar to data from living relaxed mouse soleus and EDL muscles (Kurg et al., 1982; Zappe and Maeda, 1985). The low-angle x-ray diffraction measurements revealed that the lattice in soleus of desmin-deficient mice is wider compared to that of the wild-type soleus. The larger filament spacing at L0 in the Des−/− group would correspond to an ∼12% larger lattice volume. The increased spacing in Des−/− soleus fibers suggests that the intermediate filaments have a structural role in determining the lateral arrangement of the contractile filaments. We could not detect a similar change in lattice spacing in the Des−/− psoas fibers. This difference in effect of desmin depletion on filament arrangement between psoas and soleus muscles could reflect several factors including differences in the content of desmin, muscle architecture, loading, or innervation. It is possible that the soleus muscle, which has a twofold higher amount of desmin (Balogh et al., 2003), exhibits a stronger dependence on intermediate filaments for maintaining sarcomere structure.
Filament spacing increases if sarcomeres are shortened and decreases in stretched muscles (cf. Millman, 1998). Our measurements were performed at a defined sarcomere length (L0, 2.3 μm) determined for each preparation, excluding a difference in sarcomere length as a reason for the difference in filament spacing between Des−/− and Des+/+ soleus fibers. Shortening to 80% (to 1.84 μm) of the optimal length (L0) resulted in a similar increase in filament lattice spacing in Des−/− and Des+/+ fiber bundles. Stretching reduced the spacing. Using the equatorial spacing data from soleus muscles shortened to 1.84 μm and stretched up to 2.9 μm we estimate, based on the relation between sarcomere length and filament spacing, that an ∼20% difference in sarcomere length between Des−/− and Des+/+ fibers would have been required to explain the difference in spacing at L0.
It could be possible that inhomogeneities in the sarcomere length are present and that different parts of the myofibril have different sarcomere lengths and filament lattice spacing in Des−/− muscles. However, laser diffraction was recorded from each preparation and the 1.1 and 1.0 reflections were equally sharp in the Des+/+ and Des−/− preparations. Light microscopy of isolated single soleus fibers did not reveal large differences in sarcomere length along the fibers. Also the widths of the 1.1 and 1.0 peaks and the 1.1/1.0 intensity ratio were similar in the Des+/+ and Des−/− muscles, excluding major differences in the order of the filament lattice. However, during contraction, the lack of desmin intermediate filaments could introduce sarcomere length inhomogeneities. At present, information on sarcomere structure of contracting Des−/− muscles is not available. A contraction-induced inhomogeneity in sarcomere length could be an important mechanism contributing to the lower active stress of Des−/− muscles, as suggested previously (Balogh et al., 2002, 2003; Li et al., 1997).
Osmotic compression of the skinned fibers was associated with a compression of the filament lattice. The relation between 1.1 spacing and dextran concentration in Des+/+ muscles was similar to that reported for skinned rabbit psoas fibers (Kawai et al., 1993). For the psoas fibers no differences could be noted between Des−/− and Des+/+ groups. In the soleus muscles the 1.1 and 1.0 filament spacing remained wider in the Des−/− group at all degrees of osmotic compression. This suggests that depletion of desmin intermediate filaments in the soleus muscles is not associated with increased sarcomere compliance in the lateral direction. The lack of intermediate filaments would thus rather be associated with a loss of compressing structures outside of the sarcomeres.
We did not observe any major differences in the skinned single fiber diameter between the Des−/− and Des+/+ groups (Balogh et al., 2003). It should be noted that our results were obtained from skinned preparations and that some mechanical functions of the intermediate filaments in the intact muscle cells might not be completely retained in the permeabilized muscle. However, the Des−/− skinned soleus fibers were more compressed by osmotic forces compared to the Des+/+ group. Since the relative changes in filament lattice spacing, induced by osmotic compression, were similar in Des+/+ and Des−/− soleus muscles, the larger extent of compression of Des−/− soleus muscle cells is most likely due to increased compliance of structures outside of the sarcomere, i.e., between the myofibrils and the sarcolemma. Since the increased compliance during osmotic compression of single Des−/− soleus cells also was observed in fiber bundles, it is likely that the larger compliance of the Des−/− soleus muscle width is not due to changes in extracellular matrix properties. Thus, the intermediate filaments are required for maintaining resistance to compression of the whole muscle cell. During active contraction, fiber width decreased less in the Des−/− soleus fibers compared to the Des+/+ group. These results are consistent with a model where intermediate filaments are important for the mechanical coupling between sarcomeres and sarcolemma.
Attachment of cross-bridges in rigor leads to a compression of the filament lattice of the mouse soleus muscle. Compared to the relaxed state, the filament lattice was less compressed by dextran during rigor. These results are similar to data from skinned rabbit psoas muscles (Kawai et al., 1993). This suggests that rigor attachment of cross-bridges introduces a force in the lateral direction and lowers the lateral compliance of the sarcomere. Both at 0 and 8% dextran the rigor-induced decrease in 1.1 lattice spacing was larger in the Des−/− soleus muscles compared to the Des+/+ group. This could reflect that the intermediate filament system is important for preventing compression of the lattice during cross-bridge attachment. A loss of the coupling in the lateral direction could also explain the smaller decrease in fiber diameter during active contraction.
It has previously been shown that active force of skeletal muscle fibers in desmin-deficient animals is lowered by ∼30% (Balogh et al., 2003). The mechanical role of the intermediate filament system would be coupled to its structural functions. The main structural change observed in this study of desmin-deficient mice is a wider contractile filament lattice spacing in the slow soleus fibers. The increase in spacing was ∼4%. Theoretically an increased distance between actin and myosin filaments can under some model assumptions decrease the axial force of the muscle (Schoenberg, 1980). In a study by Godt and Maughan (1981) skinned skeletal muscle fibers were compressed with 5% Dextran T-500 by ∼20% to near the in situ width. This compression did not influence maximum force. At higher dextran concentration (10%) active force was reduced (Godt and Maughan, 1981). In our experiments we compressed fibers with 8% dextran and observed a similar reduction in active force in both Des−/− and Des+/+. We could thus not increase active force in the soleus Des−/− group by compressing the muscle filament lattice. According to these data and the considerations above it seems unlikely that the increased lattice spacing per se contributes to the lower force of the Des−/− muscles. Force of psoas Des−/− muscles cells was lowered (Balogh et al., 2003), although filament lattice spacing was not changed. This is consistent with our interpretation that the lower active force is not due to the wider filament lattice spacing. We do not find a difference in the Ca2+-sensitivity between skinned fibers of Des−/− and Des+/+ skeletal muscles (Balogh et al., 2003). These results show that the 4% wider filament spacing does not influence Ca2+-sensitivity. In a study by Edman (1999) on intact frog muscle fibers, increased filament width induced by lowered solution tonicity did not lower active force generation. However the force enhancement during stretch of active contractions, possibly reflecting the binding strength of the cross-bridge, was markedly dependent on fiber width. In swollen cells the stretch-induced force enhancement was reduced. One consequence of the wider filament spacing in desmin-deficient mice could thus be a lowered resistance to stretch during active contraction. This mechanism, together with an increased compliance of structures coupling sarcomeres to the sarcolemma, might be important for the lower vulnerability to stretch-induced injury observed in Des−/− muscles (Sam et al., 2000).
In summary, we interpret the results according to a model where desmin intermediate filaments connect the myofilaments in the lateral direction and provide anchoring to the sarcolemma. The desmin intermediate filaments are important for maintaining lateral filament distance in the slow soleus skeletal muscle, since depletion of desmin results in a wider filament lattice. This effect was not observed in the fast psoas muscle, which could reflect the lower desmin content in this muscle type, although other differences between the two muscle types could also contribute to the different effects of intermediate filament removal on the lateral filament arrangement. The increased filament spacing in Des−/− soleus muscle does not influence Ca2+ sensitivity and is not responsible for the decreased active stress generation in Des−/− muscles. The intermediate filaments in the slow soleus are responsible for the whole cell compliance in the lateral direction. In their absence the cell becomes more compliant and lateral forces generated by cross-bridge attachment are not opposed to the same extent as in normal cells. Both the wider filament lattice and the increased lateral compliance of the Des−/− soleus fibers would lower the resistance to stretch and possibly provide a physiological mechanism protecting against injury.
Acknowledgments
We thank Dr. Sergio Funari for the competent technical support at the beamline, DESY A2, Hamburg.
This work was supported by grants from the Swedish Research Council (04X-8268), the Swedish Heart Lung Foundation, the Medical Faculty of Lund University, and AFM (French association against myopathies).
References
- Agbulut, O., Z. Li, V. Mouly, and G. S. Butler-Browne. 1996. Analysis of skeletal and cardiac muscle from desmin knock-out and normal mice by high resolution separation of myosin heavy-chain isoforms. Biol. Cell. 88:131–135. [PubMed] [Google Scholar]
- Anderson, J., V. Joumaa, L. Stevens, C. Neagoe, Z. Li, Y. Mounier, W. A. Linke, and F. Goubel. 2002a. Passive stiffness changes in soleus muscles from desmin knockout mice are not due to titin modifications. Pflugers Arch. 444:771–776. [DOI] [PubMed] [Google Scholar]
- Anderson, J., Z. Li, and F. Goubel. 2002b. Models of skeletal muscle to explain the increase in passive stiffness in desmin knockout muscle. J. Biomech. 35:1315–1324. [DOI] [PubMed] [Google Scholar]
- Anderson, J., Z. Li, and F. Goubel. 2001. Passive stiffness is increased in soleus muscle of desmin knockout mouse. Muscle Nerve. 24:1090–1092. [DOI] [PubMed] [Google Scholar]
- Ariza, A., J. Coll, M. T. Fernandez-Figueras, M. D. Lopez, J. L. Mate, O. Garcia, A. Fernandez-Vasalo, and J. J. Navas-Palacios. 1995. Desmin myopathy: a multisystem disorder involving skeletal, cardiac, and smooth muscle. Hum. Pathol. 26:1032–1037. [DOI] [PubMed] [Google Scholar]
- Askanas, V., A. Bornemann, and W. K. Engel. 1990. Immunocytochemical localization of desmin at human neuromuscular junctions. Neurology. 40:949–953. [DOI] [PubMed] [Google Scholar]
- Balogh, J., Z. Li, D. Paulin, and A. Arner. 2003. Lower active force generation and improved fatigue resistance in skeletal muscle from desmin deficient mice. J. Muscle Res. Cell Motil. 24:453–459. [DOI] [PubMed] [Google Scholar]
- Balogh, J., M. Merisckay, Z. Li, D. Paulin, and A. Arner. 2002. Hearts from mice lacking desmin have a myopathy with impaired active force generation and unaltered wall compliance. Cardiovasc. Res. 53:439–450. [DOI] [PubMed] [Google Scholar]
- Bond, M., and A. V. Somlyo. 1982. Dense bodies and actin polarity in vertebrate smooth muscle. J. Cell Biol. 95:403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boriek, A. M., Y. Capetanaki, W. Hwang, T. Officer, M. Badshah, J. Rodarte, and J. G. Tidball. 2001. Desmin integrates the three-dimensional mechanical properties of muscles. Am. J. Physiol. Cell Physiol. 280:C46–C52. [DOI] [PubMed] [Google Scholar]
- Edman,K. A. 1999. The force bearing capacity of frog muscle fibres during stretch: its relation to sarcomere length and fibre width. J. Physiol. 519:515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt, R. E., and D. W. Maughan. 1981. Influence of osmotic compression on calcium activation and tension in skinned muscle fibers of the rabbit. Pflugers Arch. 391:334–337. [DOI] [PubMed] [Google Scholar]
- Goldfarb, L. G., K. Y. Park, L. Cervenakova, S. Gorokhova, H. S. Lee, O. Vasconcelos, J. W. Nagle, C. Semino-Mora, K. Sivakumar, and M. C. Dalakas. 1998. Missense mutations in desmin associated with familial cardiac and skeletal myopathy. Nat. Genet. 19:402–403. [DOI] [PubMed] [Google Scholar]
- Goldman, Y. E., M. G. Hibberd, and D. R. Trentham. 1984. Initiation of active contraction by photogeneration of adenosine-5′-triphosphate in rabbit psoas muscle fibres. J. Physiol. 354:605–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai, M., J. S. Wray, and Y. Zhao. 1993. The effect of lattice spacing change on cross-bridge kinetics in chemically skinned rabbit psoas muscle fibers. I. Proportionality between the lattice spacing and the fiber width. Biophys. J. 64:187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay, L., Z. Li, M. Mericskay, J. Olivares, L. Tranqui, E. Fontaine, T. Tiivel, P. Sikk, T. Kaambre, J. L. Samuel, L. Rappaport, Y. Usson, X. Leverve, D. Paulin, and V. A. Saks. 1997. Study of regulation of mitochondrial respiration in vivo. An analysis of influence of ADP diffusion and possible role of cytoskeleton. Biochim. Biophys. Acta. 1322:41–59. [DOI] [PubMed] [Google Scholar]
- Kögler, H., C. Plathow, E. Al Hillawi, I. P. Trayer, and J. C. Ruegg. 1998. Replacement of troponin-I in slow-twitch skeletal muscle alters the effects of the calcium sensitizer EMD 53998. Pflugers Arch. 436:398–406. [DOI] [PubMed] [Google Scholar]
- Kurg, T., R. H. Stinson, and B. M. Millman. 1982. X-ray diffraction from striated muscles and nerves in normal and dystrophic mice. Muscle Nerve. 5:238–246. [DOI] [PubMed] [Google Scholar]
- Lazarides,E. 1982. Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annu. Rev. Biochem. 51:219–250. [DOI] [PubMed] [Google Scholar]
- Lazarides, E., B. L. Granger, D. L. Gard, C. M. O'Connor, J. Breckler, M. Price, and S. I. Danto. 1982. Desmin- and vimentin-containing filaments and their role in the assembly of the Z disk in muscle cells. Cold Spring Harb. Symp. Quant. Biol. 46:351–378. [DOI] [PubMed] [Google Scholar]
- Li, Z., E. Colucci-Guyon, M. Pincon-Raymond, M. Mericskay, S. Pournin, D. Paulin, and C. Babinet. 1996. Cardiovascular lesions and skeletal myopathy in mice lacking desmin. Dev. Biol. 175:362–366. [DOI] [PubMed] [Google Scholar]
- Li, Z., M. Mericskay, O. Agbulut, G. Butler-Browne, L. Carlsson, L. E. Thornell, C. Babinet, and D. Paulin. 1997. Desmin is essential for the tensile strength and integrity of myofibrils but not for myogenic commitment, differentiation, and fusion of skeletal muscle. J. Cell Biol. 139:129–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loufrani, L., K. Matrougui, Z. Li, B. I. Levy, P. Lacolley, D. Paulin, and D. Henrion. 2002. Selective microvascular dysfunction in mice lacking the gene encoding for desmin. FASEB J. 16:117–119. [DOI] [PubMed] [Google Scholar]
- Mavroidis, M., and Y. Capetanaki. 2002. Extensive induction of important mediators of fibrosis and dystrophic calcification in desmin-deficient cardiomyopathy. Am. J. Pathol. 160:943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millman, B. M. 1998. The filament lattice of striated muscle. Physiol. Rev. 78:359–391. [DOI] [PubMed] [Google Scholar]
- Milner, D. J., G. E. Taffet, X. Wang, T. Pham, T. Tamura, C. Hartley, A. M. Gerdes, and Y. Capetanaki. 1999. The absence of desmin leads to cardiomyocyte hypertrophy and cardiac dilation with compromised systolic function. J. Mol. Cell. Cardiol. 31:2063–2076. [DOI] [PubMed] [Google Scholar]
- Milner, D. J., G. Weitzer, D. Tran, A. Bradley, and Y. Capetanaki. 1996. Disruption of muscle architecture and myocardial degeneration in mice lacking desmin. J. Cell Biol. 134:1255–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Marmol, A. M., G. Strasser, M. Isamat, P. A. Coulombe, Y. Yang, X. Roca, E. Vela, J. L. Mate, J. Coll, M. T. Fernandez-Figueras, J. J. Navas-Palacios, A. Ariza, and E. Fuchs. 1998. A dysfunctional desmin mutation in a patient with severe generalized myopathy. Proc. Natl. Acad. Sci. USA. 95:11312–11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sam, M., S. Shah, J. Friden, D. J. Milner, Y. Capetanaki, and R. L. Lieber. 2000. Desmin knockout muscles generate lower stress and are less vulnerable to injury compared with wild-type muscles. Am. J. Physiol. Cell Physiol. 279:C1116–C1122. [DOI] [PubMed] [Google Scholar]
- Schoenberg, M. 1980. Geometrical factors influencing muscle force development. II. Radial forces. Biophys. J. 30:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, S. B., J. Davis, N. Weisleder, I. Kostavassili, A. D. McCulloch, E. Ralston, Y. Capetanaki, and R. L. Lieber. 2004. Structural and functional roles of desmin in mouse skeletal muscle during passive deformation. Biophys. J. 86:2993–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, S. B., F. C. Su, K. Jordan, D. J. Milner, J. Friden, Y. Capetanaki, and R. L. Lieber. 2002. Evidence for increased myofibrillar mobility in desmin-null mouse skeletal muscle. J. Exp. Biol. 205:321–325. [DOI] [PubMed] [Google Scholar]
- Sjöberg, G., C. A. Saavedra-Matiz, D. R. Rosen, E. M. Wijsman, K. Borg, S. H. Horowitz, and T. Sejersen. 1999. A missense mutation in the desmin rod domain is associated with autosomal dominant distal myopathy, and exerts a dominant negative effect on filament formation. Hum. Mol. Genet. 8:2191–2198. [DOI] [PubMed] [Google Scholar]
- Sjuve, R., A. Arner, Z. Li, B. Mies, D. Paulin, M. Schmittner, and J. V. Small. 1998. Mechanical alterations in smooth muscle from mice lacking desmin. J. Muscle Res. Cell Motil. 19:415–429. [DOI] [PubMed] [Google Scholar]
- Small, J. V., and A. Sobieszek. 1977. Studies on the function and composition of the 10-NM(100-A) filaments of vertebrate smooth muscle. J. Cell Sci. 23:243–268. [DOI] [PubMed] [Google Scholar]
- Tatsumi, R., and A. Hattori. 1995. Detection of giant myofibrillar proteins connectin and nebulin by electrophoresis in 2% polyacrylamide slab gels strengthened with agarose. Anal. Biochem. 224:28–31. [DOI] [PubMed] [Google Scholar]
- Thornell, L. E., and A. Eriksson. 1981. Filament systems in the Purkinje fibers of the heart. Am. J. Physiol. 241:H291–H305. [DOI] [PubMed] [Google Scholar]
- Tidball, J. G. 1992. Desmin at myotendinous junctions. Exp. Cell Res. 199:206–212. [DOI] [PubMed] [Google Scholar]
- Vicart, P., A. Caron, P. Guicheney, Z. Li, M. C. Prevost, A. Faure, D. Chateau, F. Chapon, F. Tome, J. M. Dupret, D. Paulin, and M. Fardeau. 1998. A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat. Genet. 20:92–95. [DOI] [PubMed] [Google Scholar]
- Wang, X., H. Osinska, G. W. Dorn, M. Nieman, J. N. Lorenz, A. M. Gerdes, S. Witt, T. Kimball, J. Gulick, and J. Robbins. 2001. Mouse model of desmin-related cardiomyopathy. Circulation. 103:2402–2407. [DOI] [PubMed] [Google Scholar]
- Wede, O. K., M. Lofgren, Z. Li, D. Paulin, and A. Arner. 2002. Mechanical function of intermediate filaments in arteries of different size examined using desmin deficient mice. J. Physiol. 540:941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad, H., J. D. Bruton, and J. Lannergren. 1997. The effect of intracellular pH on contractile function of intact, single fibres of mouse muscle declines with increasing temperature. J. Physiol. 500:193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieneke,S., R. Stehle, Z. Li, and H. Jockusch. 2000. Generation of tension by skinned fibers and intact skeletal muscles from desmin-deficient mice. Biochem. Biophys. Res. Commun. 278:419–425. [DOI] [PubMed] [Google Scholar]
- Zappe, H. A., and Y. Maeda. 1985. X-ray diffraction study of fast and slow mammalian skeletal muscle in the live relaxed state. J. Mol. Biol. 185:211–214. [DOI] [PubMed] [Google Scholar]