Abstract
Effects of adducts of [PtCl(NH3)3]Cl or chlorodiethylenetriamineplatinum(II) on DNA stability were studied with emphasis on thermodynamic origins of that stability. Oligodeoxyribonucleotide duplexes (15-bp) containing the single, site-specific monofunctional adduct at G-residues of the central sequences TGT/ACA or 5′-AGT/5′-ACT were prepared and analyzed by differential scanning calorimetry, temperature-dependent ultraviolet absorption and circular dichroism. The unfolding of the platinated duplexes was accompanied by relatively small unfavorable free energy terms. This destabilization was enthalpic in origin. On the other hand, a relatively large reduction of melting temperature (Tm) was observed as a consequence of the monofunctional adduct in the TGT sequence, whereas Tm due to the adduct in the AGT sequence was reduced only slightly. We also examined the efficiency of the mammalian nucleotide excision repair system to remove from DNA the monofunctional adducts and found that these lesions were not recognized by this repair system. Thus, rather thermodynamic than thermal characterization of DNA adducts of monofunctional platinum compounds is a property implicated in the modulation of downstream effects such as protein recognition and repair.
INTRODUCTION
Most of the platinum anticancer drugs belong to the class of bifunctional platinum(II) or platinum(IV) compounds. Their antitumor efficacy is associated with their capability to form on DNA various types of cross-links (Brabec, 2002; Johnson et al., 1989). For instance, the first platinum antitumor drug introduced in the clinic, cis-diamminedichloroplatinum(II) (cisplatin) (Fig. 1) and its analogs form on DNA first monofunctional adducts preferentially at the guanine residues that subsequently close to intrastrand and interstrand cross-links.
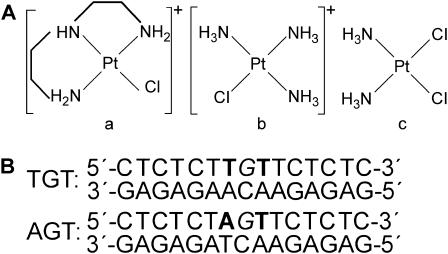
FIGURE 1.
(A) Structures of platinum complexes. Panel a, [PtCl(dien)]+; panel b, [PtCl(NH3)3]+; panel c, cisplatin. (B) The sequences of the synthetic 15-bp oligodeoxyribonucleotide duplexes used in this study with their abbreviations. The top and bottom strands in the pair of oligonucleotides are designated top and bottom, respectively, in the text. Unique and central G-residue in italics in the top strand of each duplex indicates the location of the monofunctional adduct after modification of the oligonucleotide by [PtCl(dien)]Cl or [PtCl(NH3)3]Cl in the way described in Experimental Procedures. The basepairs flanking the central G·C basepair of each duplex are depicted in bold.
Monofunctional adducts of bifunctional platinum drugs have been studied less thoroughly, although a knowledge of alterations induced in DNA by monofunctional binding of platinum complexes may also be important for better understanding how antitumor bifunctional drugs form the genotoxic cross-links. Structure-activity studies of platinum compounds often employ monodentate platinum(II) compounds such as clinically ineffective chlorodiethylenetriamineplatinum(II) or chlorotriammineplatinum(II) chlorides, ([PtCl(dien)]Cl or [PtCl(NH3)3]Cl, respectively; Fig. 1), used to afford DNA monofunctional adducts to simulate the first step of the bifunctional reaction of bidentate platinum drugs (Brabec et al., 1990). Interestingly, exploring new structural classes of platinum antitumor drugs has also resulted in the discovery of new platinum(II) complexes including those of formula cis-[PtCl(NH3)2(A)]+ (where A is a heterocyclic amine ligand) (Brabec, 2002). Thus, these formally monofunctional complexes are analogs of cisplatin containing only one leaving chloride group similar to the closely related and simpler, but inactive, platinum-triamine complexes, such as [PtCl(dien)]Cl or [PtCl(NH3)3]Cl. Examples of this class of platinum compounds are trisubstituted platinum(II) compounds in which A = pyridine, pyrimidine, purine, piperidine, or aniline (Hollis et al., 1991). These trisubstituted platinum(II) compounds form on DNA monofunctional adducts that have been suggested to be responsible for antitumor activity of these agents. The monofunctional adducts of these compounds are capable of blocking DNA replication in vitro almost as efficiently as the major adducts of cisplatin. Taken together, some platinum-triamines have been characterized as a new class of platinum anticancer agents that modify DNA differently than cisplatin. These differences have been proposed to be associated with different biological effects of these monofunctional compounds in comparison with cisplatin.
It has been shown (Brabec et al., 1994, 1990, 1992; Van Garderen et al., 1989; Vrana et al., 1986) that the monofunctional adducts of [PtCl(dien)]Cl and cisplatin, which are formed preferentially at the guanine sites, distort DNA. The conformational distortion was more pronounced in the flanking basepairs containing the base on the 5′ site of the monofunctional adduct (Brabec et al., 1994, 1992). These distortions disturb stacking interactions in double-helical DNA, involve local unwinding the duplex (unwinding angle of 6°), but no intrinsic bending is induced in DNA. Interestingly, the monofunctional adducts of [PtCl(dien)]Cl and cisplatin also reduce thermal stability of DNA (Tm, melting temperature) in a sequence-dependent manner (Brabec et al., 1992; Van Garderen et al., 1989). The most pronounced effects are observed if the platinated guanine residue is flanked by a single pyrimidine on the 5′ side.
On the other hand, changes in melting temperature are not necessarily good predictors of changes in thermodynamic stability (ΔG) because there is no simple correspondence between changes in Tm and ΔG due to the presence of a lesion in DNA (Pilch et al., 1995; Plum et al., 1999). The reason for the failure of changes of Tm values to reflect reliably the lesion-induced changes in thermodynamic stability (changes of ΔG values) is neglect of the temperature dependence of the duplex stability. Tm values reflect the behavior of the duplex at high temperature whereas the free-energy changes are evaluated for a low-temperature standard state, typically 25 or 37°C; these low-temperature standard states correspond to the temperature domain of the processes of biological significance.
In this article, we describe our studies of the effects of the monofunctional adducts of [PtCl(dien)]Cl or [PtCl(NH3)3]Cl on DNA stability with emphasis on the thermodynamic origins of that stability. We chose for our studies these two compounds to examine also the effect of bulkiness of the nonleaving ligands of this class of platinum(II) complexes ([PtCl(dien)]Cl forms bulkier DNA adducts than [PtCl(NH3)3]Cl)). Oligodeoxyribonucleotide duplexes (15-bp) were prepared, which only differed in the two central sequences TGT/ACA or 5′-AGT/5′-ACT (duplexes TGT and AGT, respectively, in Fig. 1). These central sequences were modified so that they contained the single, site-specific monofunctional adduct of [PtCl(dien)]Cl or [PtCl(NH3)3]Cl formed at the G-residue of these central sequences. These duplexes were analyzed by differential scanning calorimetry (DSC). Importantly, the duplexes had the same base composition, only the bases in the basepair (bp) flanking the platinated G-residue on its 5′ side were reversed. Thus, these monofunctionally adducted duplexes had either pyrimidine or purine residue flanking the adduct on its 5′ side, whereas the rest of the duplexes and total composition of base residues were identical. It was shown in our previous studies (Brabec et al., 1994, 1992) that although the formation of the monofunctional adduct of [PtCl(dien)]Cl or cisplatin at guanine residue in the TGT sequence resulted in the marked reduction of the Tm value of the duplex, the formation of this adduct in the sequence AGT reduced Tm only slightly. Thus, to reveal the thermodynamic origins of the effects of the sequence context on the duplex stability due to the presence of monofunctional adducts of platinum compounds, the experiments described in this report were carried out.
EXPERIMENTAL PROCEDURES
Chemicals
[PtCl(dien)]Cl and [PtCl(NH3)3]Cl (Fig. 1) were a kind gift of Dr. G. Natile from University of Bari. Cisplatin (Fig. 1) was purchased from Sigma (Prague, Czech Republic). The synthetic oligodeoxyribonucleotides (Fig. 1) were purchased from IDT (Coralville, IA) and purified as described previously (Brabec et al., 1992). In this work the molar concentrations of the single-stranded oligonucleotides are related to the 15-mer single-stranded molecules. Molar extinction coefficients for the single-stranded oligonucleotides (related to the 15-mer strands) were determined by phosphate analysis (Murphy and Trapane, 1996). The following extinction coefficients at 260 nm and 25°C (in dm3/cm × mol strand) were obtained: 110,000 and 125,000 for the upper and lower strands of the unmodified TGT duplex, respectively; 112,000 and 139,700 for the upper and lower strands of the unmodified AGT duplex, respectively; 105,000 and 106,900 for the upper strand of the TGT duplex containing monofunctional adduct of [PtCl(dien)]Cl and [PtCl(NH3)3]Cl, respectively; 113,400 and 116,500 for the upper strand of the AGT duplex containing monofunctional adduct of [PtCl(dien)]Cl and [PtCl(NH3)3]Cl, respectively. The formation of 1:1 complexes between the top strands unmodified or containing the monofunctional adduct and bottom strands of the TGT and AGT duplexes unmodified or containing the monofunctional adduct was verified by recording isothermal ultraviolet (UV) absorbance mixing curves at 25°C (Poklar et al., 1996). In this work, the molar concentrations of the oligonucleotide duplexes are related to the double-stranded molecules 15-bp long. Acrylamide, bis(acrylamide), urea, and NaCN were from Merck KgaA (Darmstadt, Germany). Dimethyl sulfate (DMS) was from Sigma. [γ-32P]ATP was from Amersham (Arlington Heights, IL).
Platination of oligonucleotides
The single-stranded oligodeoxyribonucleotides (the top strands of the duplexes TGT or AGT) at a concentration of 6.8 × 10−4 M were reacted with either [PtCl(dien)]Cl and [PtCl(NH3)3]Cl at an input platinum-to-strand ratio of 4 for 10 min at 37°C in 10 mM NaClO4. The platinated oligonucleotides were repurified by ion-exchange fast protein liquid chromatography (FPLC). The elution patterns of the ion-exchange FPLC are shown in Fig. S1 of the Supplemental Materials. It was verified by platinum flameless atomic absorption spectrophotometry and by measurement of the optical density that the modified oligonucleotides contained one platinum atom. It was also verified using DMS footprinting of platinum on DNA (Brabec and Leng, 1993) that in the platinated top strands of the duplexes the N7 position of the single G-residue was not accessible for reaction with DMS. The unmodified or platinated top strands were allowed to anneal with nonplatinated complementary strands (the bottom strand of TGT or AGT) in 10 mM sodium phosphate (NaH2PO4/Na2HPO4) pH 7.0 and 150 mM NaCl. The annealing involved heating the mixture of the complementary oligonucleotides to 95°C for 20 min followed by slow cooling to 25°C at a rate of 30°C/h. The duplex samples were further equilibrated at 25°C for 20 min and vacuum degassed before use. Final yields of the platinations after all purification steps were ∼30%. FPLC purification and flameless atomic absorption spectrophotometry measurements were carried out on a Pharmacia Biotech (Uppsala, Sweden) FPLC system with a MonoQ HR 5/5 column and a Unicam 939 AA spectrometer equipped with a graphite furnace, respectively. Other details can be found in the literature (Brabec and Leng, 1993; Brabec et al., 1992).
Differential scanning calorimetry
Excess heat capacity (ΔCp) versus temperature profiles for the thermally induced transitions of TGT and AGT duplexes unmodified or containing a unique monofunctional adduct of [PtCl(dien)]Cl and [PtCl(NH3)3]Cl were measured using a VP-DSC Calorimeter (Microcal, Northampton, MA). In the DSC experiments the concentrations of the TGT and AGT duplexes were 30 μM, the heating rate was 60°C/h and the maximum temperature was 90°C. After reaching the maximum temperature the samples were cooled at the same rate to the starting temperature of 25°C. In this article ΔCp is defined as excess heat capacity, which is baseline subtracted and concentration normalized (Leharne and Chowdhry, 1998). The reference scans were subtracted from the sample scans to obtain ΔCp versus temperature profiles. Enthalpies (ΔHcal) and entropies (ΔS) of duplex melting were calculated from the areas under the experimental ΔCp versus T and derived ΔCp/T versus T curves, respectively, using ORIGIN v.5.0 software (Microcal). The free energy of duplex formation at 25°C (ΔG25) was calculated using the standard thermodynamic relationship given in Eq. 1 and the corresponding ΔHcal and ΔS values:
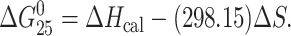 |
(1) |
The duplexes were dissolved in the buffer containing 10 mM sodium phosphate (NaH2PO4/Na2HPO4) pH 7.0 and 150 mM NaCl. It was also verified in the same way as described in previous articles (Hofr and Brabec, 2001; Hofr et al., 2001) that the melting transitions of both the platinated and unmodified duplexes were fully reversible.
UV absorption spectrophotometry
UV absorbance measurements were conducted on a Beckman DU-7400 spectrophotometer equipped with a thermoelectrically controlled cell holder and quartz cells with a pathlength of 1 cm. Absorbance versus temperature profiles were measured at 260 nm. The temperature was raised using a linear heating rate of 1.0°C/min. For each optically detected transition the melting temperature (Tm) was determined as previously described (Marky and Breslauer, 1987). The solutions of TGT and AGT duplexes were 4 μM in duplex, and contained 10 mM sodium phosphate, pH 7.0, and NaCl at concentrations in the range of 0.015–0.1 M.
Circular dichroism spectrophotometry
Circular dichroism (CD) spectra were recorded using Jasco J-720 spectropolarimeter (Easton, MD) equipped with a thermoelectrically controlled cell holder. The cell pathlength was 1 cm. Isothermal CD spectra were recorded from 220 to 320 nm in 1-nm increments with an averaging time of 5 s. The DNA concentration was 4 μM in duplex, and buffer conditions were 10 mM sodium phosphate (NaH2PO4/Na2HPO4), pH 7.0, and 150 mM NaCl. CD spectra in units of millidegrees were converted to Δɛ using the concentration of the duplex.
Nucleotide excision assay
The 148-bp substrates containing the single, central monofunctional adduct of [PtCl(dien)]Cl or [PtCl(NH3)3]Cl, or the 1,2-GG intrastrand cross-link of cisplatin were assembled from three oligonucleotide duplexes as described previously (Buschta-Hedayat et al., 1999; Matsunaga et al., 1995). Oligonucleotide excision reactions were performed in cell-free extracts (CFEs) prepared from the HeLa S3 and Chinese hamster ovary (CHO) AA8 cell lines as described (Manley et al., 1980; Reardon et al., 1999). These extracts were kindly provided by J. T. Reardon and A. Sancar from the University of North Carolina (Chapel Hill, NC). In vitro repair was measured with excision assay using these CFEs and 148-bp linear DNA substrates (vide supra) in the same way as described previously (Reardon et al., 1999). The efficiency of the CFEs used in this work to remove DNA lesions was verified using the substrate containing 1,2-GG intrastrand cross-link of cisplatin (Fig. 5, lane cisPt). The primary excision fragments were 25–29 nucleotides in length.
FIGURE 5.
Excision of the 1,2-GG intrastrand cross-link of cisplatin and the monofunctional adducts of [PtCl(dien)]Cl or [PtCl(NH3)3]Cl by rodent excinuclease. The substrates were incubated with CHO AA8 CFE for 40 min at 30°C and subsequently treated overnight with NaCN before analysis in 10% polyacrylamide/8 M urea denaturing gel. Lanes are: 1 (cisPt), the substrate containing the 1,2-GG intrastrand cross-link of cisplatin formed in the TGGT sequence; 2 (TGT-dienPt), the substrate containing the monofunctional adduct of [PtCl(dien)]Cl formed in the TGT duplex; 3 (AGT-dienPt), the substrate containing the monofunctional adduct of [PtCl(dien)]Cl formed in the AGT duplex; 4 (no Pt), control, unplatinated substrate.
RESULTS AND DISCUSSION
All thermodynamic parameters discussed in this work refer to the duplex dissociation process. Differences in the dissociation thermodynamics due to the presence of the adduct are presented as “ΔΔ” parameters. These parameters are computed by subtracting the appropriate value measured for control, unmodified duplex from the value measured for the duplex containing the single, site-specific monofunctional platinum adduct.
Fig. 2 shows the calorimetrically measured excess heat capacity (ΔCp) versus temperature profiles for the 15-bp duplexes TGT and AGT unmodified or containing single, central, site-specific monofunctional adduct of [PtCl(dien)]Cl or [PtCl(NH3)3]Cl. Each transition shows negligible changes in the heat capacities between the initial and final states, and denaturation (heating) and renaturation (cooling) curves for the unmodified and platinated duplexes were superimposable (not shown), which is consistent with the reversibility of the melting equilibrium. The interpretation of our calorimetric data described below is also based on the assumption that all thermodynamic parameters for melting of the unmodified and platinated duplexes are ascribed to differences in the initial duplex states. This implies that the final single-stranded states should be thermodynamically equivalent at the elevated temperatures at which they are formed. This assumption has been verified in earlier reports by recording identical CD spectra for the samples of nonplatinated and platinated duplexes that were heated to high temperatures (Poklar et al., 1996; Pilch et al., 2000; Hofr and Brabec, 2001; Hofr et al., 2001). Therefore, we also recorded CD spectra of the duplexes TGT, AGT containing monofunctional adducts of [PtCl(dien)]Cl or [PtCl(NH3)3]Cl and their unmodified counterparts at different temperatures (25°C and 90°C) to monitor difference in overall structure between unmodified and platinated duplexes. Although the CD spectra of the duplexes containing the monofunctional adduct at 25°C are not markedly different from those of the corresponding unmodified duplexes they exhibit a decrease in intensity of the negative and positive bands at 238 nm and 274 nm, respectively (shown in Fig. S1 of the Supplemental Materials for the duplexes containing the adduct of [PtCl(NH3)3]Cl). These results are consistent with earlier observations (Vrana et al., 1986; Van Garderen et al., 1989; Brabec et al., 1990, 1992, 1994) demonstrating that the monofunctional adducts of [PtCl(dien)]Cl affect B-conformation of DNA. Importantly, the changes in the CD spectra recorded at 25°C due to the presence of the monofunctional adduct were more pronounced if the adduct was formed in the TGT duplex. On the other hand, the CD signals of the high-temperature (denatured) states (recorded at 90°C) of the unmodified duplexes and those containing the monofunctional adduct of [PtCl(dien)]Cl or [PtCl(NH3)3]Cl agree within the noise of the measurement (shown in Fig. S1 of the Supplemental Materials for the duplexes containing the adduct of [PtCl(NH3)3]Cl), which implies that the final denatured states are not structurally and thermodynamically markedly different. Thus, validity of the assumption that the final single-stranded states of the unmodified and platinated duplexes were thermodynamically equivalent at the elevated temperatures was also verified for the duplexes investigated in this work. In aggregate, meaningful thermodynamic data from our calorimetric measurements described below could be obtained.
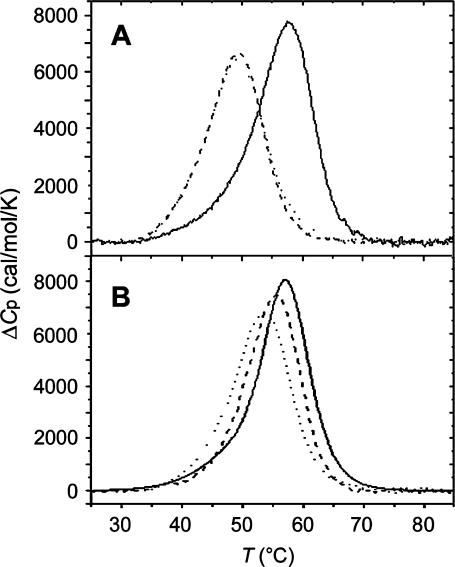
FIGURE 2.
DSC thermograms for the TGT (A) and AGT (B) duplexes unmodified (solid lines) and containing a monofunctional adduct of [PtCl(dien)]Cl or [PtCl(NH3)3]Cl (dotted or dashed lines, respectively). The concentrations of the TGT and AGT duplexes were 30 μM, and the buffer conditions were 10 mM sodium phosphate pH 7.0 and 150 mM NaCl.
The area under the calorimetrically measured excess heat capacity (ΔCp) versus temperature profiles is proportional to the total endothermic heat (ΔHcal) needed to disrupt these duplexes into single strands. The duplex melting temperatures (thermal stability parameter), Tm, and thermodynamic stability data—the duplex dissociation enthalpies, ΔHcal, and entropies, ΔS—derived from analyses of these curves are listed in Table 1, along with the corresponding 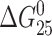 values calculated at 25°C. The presence of the monofunctional adduct of [PtCl(dien)]Cl or [PtCl(NH3)3]Cl markedly reduces the thermal stability of the duplex TGT, with ΔTm = −8.0 or −8.1°C. In contrast, the thermal stability of the duplex AGT was reduced markedly less with ΔTm falling between −1.9 and −3.9°C. Hence, the thermal stability data are in agreement with those obtained earlier using UV equilibrium melting curves for other short DNA duplexes containing the monofunctional adduct of [PtCl(dien)]Cl (Brabec et al., 1992).
values calculated at 25°C. The presence of the monofunctional adduct of [PtCl(dien)]Cl or [PtCl(NH3)3]Cl markedly reduces the thermal stability of the duplex TGT, with ΔTm = −8.0 or −8.1°C. In contrast, the thermal stability of the duplex AGT was reduced markedly less with ΔTm falling between −1.9 and −3.9°C. Hence, the thermal stability data are in agreement with those obtained earlier using UV equilibrium melting curves for other short DNA duplexes containing the monofunctional adduct of [PtCl(dien)]Cl (Brabec et al., 1992).
TABLE 1.
Calorimetrically derived thermodynamic parameters for dissociation (melting) of the 15-bp duplexes unmodified or containing a single, site-specific monofunctional adduct of [PtCl(dien)]Cl or [PtCl(NH3)3]Cl
| Duplex | Tm (°C) | ΔHcal (kcal/mol) | ΔS (cal/mol) | 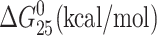 |
ΔHvH (kcal/mol) | ΔHvH/ΔHcal | 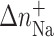 |
|---|---|---|---|---|---|---|---|
| TGT | 57.5 | 97.1 | 295.5 | 9.0 | 102.6 | 1.06 | 7.42 |
| TGT-Pt(dien) | 49.5 | 79.9 | 248.6 | 5.8 | 93.8 | 1.17 | 5.86 |
| TGT-Pt(NH3)3 | 49.4 | 81.9 | 254.2 | 6.1 | 93.0 | 1.14 | 5.94 |
| AGT | 57.5 | 94.9 | 288.8 | 8.8 | 105.1 | 1.11 | 7.14 |
| AGT-Pt(dien) | 55.6 | 88.7 | 271.1 | 7.9 | 101.7 | 1.15 | 5.74 |
| AGT-Pt(NH3)3 | 53.6 | 85.0 | 262.6 | 6.7 | 91.2 | 1.07 | 5.46 |
The ΔH, ΔS, and 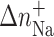 values are averages derived from three independent experiments. The experimental uncertainties of the parameters are as follows: Tm (±0.5°C), ΔHcal (±2%), ΔS (±3%),
values are averages derived from three independent experiments. The experimental uncertainties of the parameters are as follows: Tm (±0.5°C), ΔHcal (±2%), ΔS (±3%), 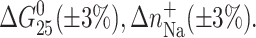
We just described the impact of the monofunctional adduct of [PtCl(dien)]Cl or [PtCl(NH3)3]Cl on duplex thermal stability. However, the thermal melting is not a thermodynamic parameter. Interestingly, introduction of the monofunctional adduct of [PtCl(dien)]Cl or [PtCl(NH3)3]Cl decreases the 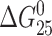 of both TGT and AGT duplexes only slightly and roughly to the same extent (Table 1).
of both TGT and AGT duplexes only slightly and roughly to the same extent (Table 1).
Inspection of Table 1 shows that the melting of each duplex accompanied by unfavorable free energy terms results from characteristic compensation of unfavorable enthalpy and favorable entropy terms. In general, the unfavorable enthalpy terms correspond mainly to the disruption of basepair stacks whereas the favorable entropy terms arise from contributions of the favorable dissociation of two strands and release of counterions and water molecules. In short, relative to the unmodified parent TGT duplex, its transition enthalpy can be perturbed by as much as 17 kcal/mol by the monofunctional platinum adduct. The magnitude of this effect is remarkable because it represents a loss of ∼18% of the total enthalpy of dissociation of the parent duplex, when only 1 of 15 basepairs is chemically altered. On the basis of nearest neighbor predictions (SantaLucia, 1998), complete loss of stacking on both sides of the platinated guanine residue of the TGT duplex is expected to reduce ΔHcal considerably less. The observed endothermic enthalpies result primarily from the endothermic heats for disrupting basepairs and base-base stacks of the duplex. Hence, the formation of the monofunctional platinum adduct in the TGT sequence of the duplex can be more deleterious energetically than the complete loss of stacking on both sides of the unmodified basepair at that site. On the other hand, relative to the unmodified parent AGT duplex, its transition enthalpy can be perturbed by only 6–10 kcal/mol by the monofunctional platinum adduct. This observation may be explained in terms of a smaller decrease in stacking interactions by the modified duplex AGT than by the modified duplex TGT, resulting from conformational changes induced by the monofunctional adduct.
The impact of the monofunctional adduct of [PtCl(dien)]Cl formed at the guanine residue in the TGT and AGT sequences on the conformation of DNA has been already compared using various techniques of molecular biophysics (Brabec et al., 1994, 1992). Consistent with the previous suggestion, the geometry of the double helix was found altered over several basepairs around the monofunctional platinum adducts and in particular on the 5′ side of the adduct in the platinated strand and, importantly, the conformational changes induced by this adduct were more extensive if these adducts were formed in the sequence TGT in comparison with those formed in the sequence AGT.
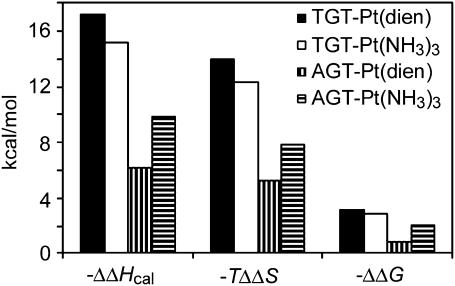
The changes in thermodynamic stability in the duplexes TGT and AGT 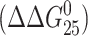 due to the formation of the single, site-specific monofunctional adduct of [PtCl(dien)]Cl or [PtCl(NH3)3]Cl reflect a combination of enthalpic (ΔΔHcal) and entropic (ΔΔS) effects. The magnitudes of these effects vary with the sequence context. The relative contributions of the adduct-induced changes in the enthalpy and entropy terms for disturbance of the duplexes TGT and AGT can be seen in Fig. 3. Interestingly, the differences in transition free-energy change
due to the formation of the single, site-specific monofunctional adduct of [PtCl(dien)]Cl or [PtCl(NH3)3]Cl reflect a combination of enthalpic (ΔΔHcal) and entropic (ΔΔS) effects. The magnitudes of these effects vary with the sequence context. The relative contributions of the adduct-induced changes in the enthalpy and entropy terms for disturbance of the duplexes TGT and AGT can be seen in Fig. 3. Interestingly, the differences in transition free-energy change 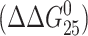 observed upon formation of the monofunctional adduct are significantly smaller than the observed differences in transition enthalpy change (ΔΔHcal) (Fig. 3). The value of ΔΔHcal ranges from −6.2 to −17.2 kcal/mol, whereas the value of
observed upon formation of the monofunctional adduct are significantly smaller than the observed differences in transition enthalpy change (ΔΔHcal) (Fig. 3). The value of ΔΔHcal ranges from −6.2 to −17.2 kcal/mol, whereas the value of 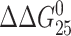 ranges from only −0.9 to −3.2 kcal/mol. Regardless of the magnitude of ΔΔHcal, there is a considerable, but not complete compensating change in entropy term. Interestingly, the higher transition enthalpy change due to the adduct formed in the TGT sequence is accompanied by the higher entropical compensation. The impact of the monofunctional adducts of [PtCl(dien)]Cl or [PtCl(NH3)3]Cl on the enthalpy is always destabilizing, whereas the entropy term is always stabilizing. The compensation does not result in invariant stability with respect to sequence context and the type of the monofunctional platinum complex. In addition, we tried to answer the question whether sequence has any effect on the magnitude of the enthalpy-driven destabilization. As the TGT and AGT duplexes only differ in basepair stacks to which the adduct is attached, TG/CA versus AG/CT and the next-neighbor basepair stack toward the 5′ end of the modified strand, TT/AA versus TA/TA, we considered the enthalpy contributions of these basepairs stacks. DNA nearest-neighbor parameters (SantaLucia, 1998) indicate an enthalpy difference of 1.4 kcal/mol between these two sets of basepair stacks, in a reasonable agreement with the measured enthalpy difference of 2.2 kcal/mol of the unmodified duplexes (ΔHcal(TGT) − ΔHcal(AGT)) (Table 1). On the other hand, the enthalpy difference between the two duplexes modified by [PtCl(dien)]Cl or [PtCl(NH3)3]Cl is equal to −8.8 or −3.1 kcal/mol, respectively (Table 1), which indicates that the monofunctional adduct in the TGT sequence induces more extensive unstacking interactions than the adduct in the AGT sequence, yielding a higher exposure of the nonpolar surface to the solvent. This allows us to predict that the inclusion of the monofunctional adduct of [PtCl(dien)]Cl or [PtCl(NH3)3]Cl in the TGT sequence yields more pronounced conformational alterations at the site of the adduct, in particular on its 5′ side, which could be detected by chemical probes of DNA conformation. This prediction is in excellent agreement with the results obtained in our previous work (Brabec et al., 1992, 1994).
ranges from only −0.9 to −3.2 kcal/mol. Regardless of the magnitude of ΔΔHcal, there is a considerable, but not complete compensating change in entropy term. Interestingly, the higher transition enthalpy change due to the adduct formed in the TGT sequence is accompanied by the higher entropical compensation. The impact of the monofunctional adducts of [PtCl(dien)]Cl or [PtCl(NH3)3]Cl on the enthalpy is always destabilizing, whereas the entropy term is always stabilizing. The compensation does not result in invariant stability with respect to sequence context and the type of the monofunctional platinum complex. In addition, we tried to answer the question whether sequence has any effect on the magnitude of the enthalpy-driven destabilization. As the TGT and AGT duplexes only differ in basepair stacks to which the adduct is attached, TG/CA versus AG/CT and the next-neighbor basepair stack toward the 5′ end of the modified strand, TT/AA versus TA/TA, we considered the enthalpy contributions of these basepairs stacks. DNA nearest-neighbor parameters (SantaLucia, 1998) indicate an enthalpy difference of 1.4 kcal/mol between these two sets of basepair stacks, in a reasonable agreement with the measured enthalpy difference of 2.2 kcal/mol of the unmodified duplexes (ΔHcal(TGT) − ΔHcal(AGT)) (Table 1). On the other hand, the enthalpy difference between the two duplexes modified by [PtCl(dien)]Cl or [PtCl(NH3)3]Cl is equal to −8.8 or −3.1 kcal/mol, respectively (Table 1), which indicates that the monofunctional adduct in the TGT sequence induces more extensive unstacking interactions than the adduct in the AGT sequence, yielding a higher exposure of the nonpolar surface to the solvent. This allows us to predict that the inclusion of the monofunctional adduct of [PtCl(dien)]Cl or [PtCl(NH3)3]Cl in the TGT sequence yields more pronounced conformational alterations at the site of the adduct, in particular on its 5′ side, which could be detected by chemical probes of DNA conformation. This prediction is in excellent agreement with the results obtained in our previous work (Brabec et al., 1992, 1994).
FIGURE 3.
The contributions of enthalpic and entropic effects to the stability (free energy change) of the 15-bp duplexes TGT (solid and open bars) and AGT (vertical and horizontal striped bars) containing the monofunctional adduct of [PtCl(dien)]Cl (solid bars; vertical striped bars) or [PtCl(NH3)3]Cl (open bars; horizontal striped bars). The units for each parameter (ΔΔHcal, TΔΔS, and ΔΔG25) are kcal/mol and T = 25°C.
Shape analysis of the experimental DSC curves allows calculating model-dependent ΔHvH enthalpies (Marky and Breslauer, 1987). The ΔHvH/ΔHcal ratio makes it possible to inspect if duplex unfolding takes place in two-state transitions or through the formation of intermediates. If the ΔHvH/ΔHcal ratio is equal to 1 then the transition takes place in an all-or-none fashion (Marky and Breslauer, 1987). As shown in Table 1, we obtained ΔHvH/ΔHcal ratios in the range of 1.06–1.17 confirming that each duplex examined in this work is unfolding in a two-state transition. Hence, despite affecting thermal and thermodynamic parameters of the unfolding of the host duplexes, the monofunctional adduct formation does not markedly change the cooperativity of their melting transition. This result demonstrates that neither the monofunctional adduct nor the identity of the base flanking the adduct on its 5′ site alters the ability of the duplex to propagate those interactions required for cooperative melting.
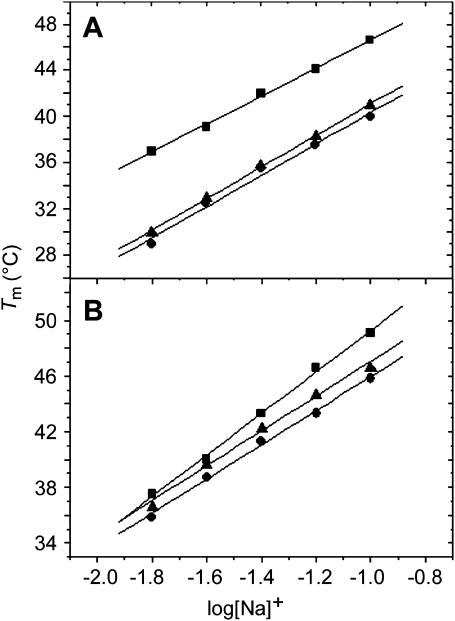
UV melting curves at several salt concentrations were also measured to examine thermodynamic release of counterions. The increase in the salt concentration resulted in a shift of Tm values to higher temperatures. The Tm dependence on salt concentration is shown in Fig. 4 for each duplex. From the slopes of these lines (δTm/δln[Na+]) (Rentzeperis et al., 1991) we obtained the thermodynamic release of counterions 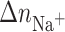 for the unmodified duplexes in the range of 7.1–7.4 whereas those for the duplexes containing the monofunctional adduct of [PtCl(dien)]Cl or [PtCl(NH3)3]Cl were in the range 5.5–5.9. The overall effect is that the presence of the platinum monofunctional adduct significantly decreases the overall counterion release by ∼1.5 mol Na+/mol duplex, but approximately to the same extent in both duplexes AGT and TGT modified by either [PtCl(dien)]Cl or [PtCl(NH3)3]Cl. This decrease correlates with entropy changes (Table1), which indicates that release of counterions is an important thermodynamic factor contributing to the stability of the duplex modified by the adducts of both monofunctional platinum compounds tested in this work. On the other hand, the observation that the formation of the platinum monofunctional adduct decreases the overall counterion release approximately to the same extent in both duplexes AGT and TGT implies that release of counterions is not apparently the factor responsible for the different impact of the monofunctional adduct of [PtCl(dien)]Cl or [PtCl(NH3)3]Cl formed at the AGT and TGT duplexes on their thermodynamic parameters.
for the unmodified duplexes in the range of 7.1–7.4 whereas those for the duplexes containing the monofunctional adduct of [PtCl(dien)]Cl or [PtCl(NH3)3]Cl were in the range 5.5–5.9. The overall effect is that the presence of the platinum monofunctional adduct significantly decreases the overall counterion release by ∼1.5 mol Na+/mol duplex, but approximately to the same extent in both duplexes AGT and TGT modified by either [PtCl(dien)]Cl or [PtCl(NH3)3]Cl. This decrease correlates with entropy changes (Table1), which indicates that release of counterions is an important thermodynamic factor contributing to the stability of the duplex modified by the adducts of both monofunctional platinum compounds tested in this work. On the other hand, the observation that the formation of the platinum monofunctional adduct decreases the overall counterion release approximately to the same extent in both duplexes AGT and TGT implies that release of counterions is not apparently the factor responsible for the different impact of the monofunctional adduct of [PtCl(dien)]Cl or [PtCl(NH3)3]Cl formed at the AGT and TGT duplexes on their thermodynamic parameters.
FIGURE 4.
Dependence of Tm on NaCl concentration in 10 mM phosphate buffer, pH 7.0. Tm values were obtained from UV melting curves of 15-bp duplexes TGT (A) or AGT (B) nonmodified (▪) or containing single, site-specific monofunctional adduct of [PtCl(dien)]Cl (▴) or [PtCl(NH3)3]Cl (•). The concentration of the duplexes was 4 μM.
It has been suggested (Geacintov et al., 2002; Plum and Breslauer, 1994; Plum et al., 1999) that the initial recognition event of the DNA lesion repair process is dependent on the lesion-induced alterations of duplex energetics. Nucleotide excision repair (NER) is a pathway used by human cells for the removal of damaged nucleotides from DNA (Sancar, 1996; Wood, 1999). In mammalian cells, this repair pathway is an important mechanism for the removal of DNA adducts, such as those generated by various chemotherapeutics including platinum antitumor drugs (Reardon and Sancar, 1998). For instance, efficient repair of various cross-links produced by several bifunctional antitumor platinum drugs has been reported by various NER systems including human and rodent excinucleases (Zamble et al., 1996; Koberle et al., 1997; Reardon et al., 1999; Malina et al., 2002; Kasparkova et al., 2003; Novakova et al., 2003).
We have also examined whether the adduct of [PtCl(dien)]Cl or [PtCl(NH3)3]Cl formed at the guanine residue in the TGT and AGT sequence is a substrate for mammalian excinucleases. No excision fragments were noticed if the monofunctional adduct of [PtCl(dien)]Cl or [PtCl(NH3)3]Cl in the sequence AGT or TGT was used as a substrate for human and rodent excinucleases (shown in Fig. 5, lanes TGT-dienPt and AGT-dienPt for rodent excinuclease and the adduct of [PtCl(dien)]Cl). This observation is consistent with the view that monofunctional lesions induced in DNA of eukaryotic cells treated with monofunctional adducts of platinum compounds, such as those of [PtCl(dien)]Cl or [PtCl(NH3)3]Cl, are not recognized by the NER enzymes. Similarly, monofunctional adducts of [PtCl(dien)]Cl are bypassed by DNA and RNA polymerases (Brabec and Leng, 1993; Corda et al., 1993; Novakova et al., 2003; Zaludova et al., 1997).
The impact of both [PtCl(dien)]Cl or [PtCl(NH3)3]Cl, which differ in bulkiness of their nonleaving ligands, on physical properties of DNA (such as thermodynamic, thermal, and conformational properties) and nucleotide excision repair of their DNA adducts were almost identical (vide supra). Hence, it is reasonable to suggest that the bulkiness of the DNA adducts of the monofunctional platinum(II) compounds tested in this work plays no marked role in the effects observed in this work.
It has been often assumed that changes in Tm can be used as predictors of changes in thermodynamic stability and consequently also as predictors of recognition by cellular repair proteins and efficiency of repair mechanisms to remove the lesion from DNA. A relatively large change of Tm was observed at least as a consequence of the formation of monofunctional adducts of [PtCl(dien)]Cl or [PtCl(NH3)3]Cl in the sequence TGT (ΔTm = −8°C; Table 1). This change of Tm is comparable to that induced by the adducts of other compounds that were readily repaired (Geacintov et al., 2002; Plum and Breslauer, 1994; Gunz et al., 1996; Plum et al., 1999; Pilch et al., 2000). Then, one could expect that at least the monofunctional adduct of [PtCl(dien)]Cl or [PtCl(NH3)3]Cl formed in the sequence TGT is efficiently removed from DNA by NER systems or is not tolerated by DNA or RNA polymerases. As the results of this (Fig. 5) and previous work (Brabec and Leng, 1993; Corda et al., 1993; Novakova et al., 2003; Zaludova et al., 1997) are inconsistent with this assumption, it is apparent that in the case of DNA adducts of [PtCl(dien)]Cl or [PtCl(NH3)3]Cl the values of Tm are not good predictors of inefficiency of repair systems and DNA or RNA polymerases to recognize these adducts. On the other hand, relatively small reductions of free energy (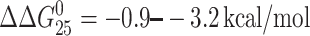 (−2.3 kcal/mol on average)) were only observed as a consequence of the formation of these monofunctional adducts (Table 1) independently of the sequence context. Thus, the results of this work represent an experimental support for the view that thermodynamic rather than thermal characterization of DNA modified by monofunctional platinum(II) compounds is a property implicated in the modulation of downstream effects such as protein recognition and repair. Generalization of this conclusion to other types of DNA lesion will require additional systematic study.
(−2.3 kcal/mol on average)) were only observed as a consequence of the formation of these monofunctional adducts (Table 1) independently of the sequence context. Thus, the results of this work represent an experimental support for the view that thermodynamic rather than thermal characterization of DNA modified by monofunctional platinum(II) compounds is a property implicated in the modulation of downstream effects such as protein recognition and repair. Generalization of this conclusion to other types of DNA lesion will require additional systematic study.
SUPPLEMENTAL MATERIALS
An online supplement to this article can be found by visiting BJ Online at http://www.biophysj.org.
The elution patterns of the ion-exchange FPLC of platinated oligonucleotides and CD spectra for the duplexes unplatinated or containing a single, site-specific monofunctional adduct of [PtCl(NH3)3]Cl are available.
Supplementary Material
Acknowledgments
The authors acknowledge that their participation in the EC COST Chemistry Action D20 enabled them to exchange regularly the most recent ideas in the field of platinum anticancer drugs with several European colleagues.
This research was supported by the Grant Agency of the Czech Republic (grants No. 204/03/H016 and No. 305/02/1552) and the Grant Agency of the Academy of Sciences of the Czech Republic (grants No. B5004301 and No. 5004101). J.K. is the international research scholar of the Howard Hughes Medical Institute.
References
- Brabec, V. 2002. DNA modifications by antitumor platinum and ruthenium compounds: their recognition and repair. Prog. Nucleic Acid Res. Mol. Biol. 71:1–68. [DOI] [PubMed] [Google Scholar]
- Brabec, V., V. Boudny, and Z. Balcarova. 1994. Monofunctional adducts of platinum(II) produce in DNA a sequence-dependent local denaturation. Biochemistry. 33:1316–1322. [DOI] [PubMed] [Google Scholar]
- Brabec, V., V. Kleinwächter, J. L. Butour, and N. P. Johnson. 1990. Biophysical studies of the modification of DNA by antitumour platinum coordination complexes. Biophys. Chem. 35:129–141. [DOI] [PubMed] [Google Scholar]
- Brabec, V., and M. Leng. 1993. DNA interstrand cross-links of trans-diamminedichloroplatinum(II) are preferentially formed between guanine and complementary cytosine residues. Proc. Natl. Acad. Sci. USA. 90:5345–5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabec, V., J. Reedijk, and M. Leng. 1992. Sequence-dependent distortions induced in DNA by monofunctional platinum(II) binding. Biochemistry. 31:12397–12402. [DOI] [PubMed] [Google Scholar]
- Buschta-Hedayat, N., T. Buterin, M. T. Hess, M. Missura, and H. Naegeli. 1999. Recognition of nonhybridizing base pairs during nucleotide excision repair of DNA. Proc. Natl. Acad. Sci. USA. 96:6090–6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corda, Y., C. Job, M.-F. Anin, M. Leng, and D. Job. 1993. Spectrum of DNA-platinum adduct recognition by prokaryotic and eukaryotic DNA-dependent RNA polymerases. Biochemistry. 32:8582–8588. [DOI] [PubMed] [Google Scholar]
- Geacintov, N. E., S. Broyde, T. Buterin, H. Naegeli, M. Wu, S. X. Yan, and D. J. Patel. 2002. Thermodynamic and structural factors in the removal of bulky DNA adducts by the nucleotide excision repair machinery. Biopolymers. 65:202–210. [DOI] [PubMed] [Google Scholar]
- Gunz, D., M. T. Hess, and H. Naegeli. 1996. Recognition of DNA adducts by human nucleotide excision repair: evidence for a thermodynamic probing mechanism. J. Biol. Chem. 271:25089–25098. [DOI] [PubMed] [Google Scholar]
- Hofr, C., and V. Brabec. 2001. Thermal and thermodynamic properties of duplex DNA containing site-specific interstrand cross-link of antitumor cisplatin or its clinically ineffective trans isomer. J. Biol. Chem. 276:9655–9661. [DOI] [PubMed] [Google Scholar]
- Hofr, C., N. Farrell, and V. Brabec. 2001. Thermodynamic properties of duplex DNA containing a site-specific d(GpG) intrastrand crosslink formed by an antitumor dinuclear platinum complex. Nucleic Acids Res. 29:2034–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis, L. S., W. I. Sundquist, J. N. Burstyn, W. J. Heiger-Bernays, S. F. Bellon, K. J. Ahmed, A. R. Amundsen, E. W. Stern, and S. J. Lippard. 1991. Mechanistic studies of a novel class of trisubstituted platinum(II) antitumor agents. Cancer Res. 51:1866–1875. [PubMed] [Google Scholar]
- Johnson, N. P., J.-L. Butour, G. Villani, F. L. Wimmer, M. Defais, V. Pierson, and V. Brabec. 1989. Metal antitumor compounds: the mechanism of action of platinum complexes. Prog. Clin. Biochem. Med. 10:1–24. [Google Scholar]
- Kasparkova, J., O. Novakova, N. Farrell, and V. Brabec. 2003. DNA binding by antitumor trans-[PtCl2(NH3)(thiazole)]. Protein recognition and nucleotide excision repair of monofunctional adducts. Biochemistry. 42:792–800. [DOI] [PubMed] [Google Scholar]
- Koberle, B., K. A. Grimaldi, A. Sunters, J. A. Hartley, L. R. Kelland, and J. R. W. Masters. 1997. DNA repair capacity and cisplatin sensitivity of human testis tumour cells. Int. J. Cancer. 70:551–555. [DOI] [PubMed] [Google Scholar]
- Leharne, S. A., and B. Z. Chowdhry. 1998. Thermodynamic background to differential scanning calorimetry. In Biocalorimetry: Applications of Calorimetry in the Biological Science. J. E. Ladbury and B. Z. Chowdhry, editors. J. Wiley & Sons, Chichester, UK. 157–182.
- Malina, J., J. Kasparkova, G. Natile, and V. Brabec. 2002. Recognition of major DNA adducts of enantiomeric cisplatin analogs by HMG box proteins and nucleotide excision repair of these adducts. Chem. Biol. 9:629–638. [DOI] [PubMed] [Google Scholar]
- Manley, J. L., A. Fire, A. Cano, P. A. Sharp, and M. L. Gefter. 1980. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc. Natl. Acad. Sci. USA. 77:3855–3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marky, L. A., and K. J. Breslauer. 1987. Calculating thermodynamic data for transitions of any molecularity from equilibrium melting curves. Biopolymers. 26:1601–1620. [DOI] [PubMed] [Google Scholar]
- Matsunaga, T., D. Mu, C.-H. Park, J. T. Reardon, and A. Sancar. 1995. Human DNA repair excision nuclease. J. Biol. Chem. 270:20862–20869. [DOI] [PubMed] [Google Scholar]
- Murphy, J. H., and T. L. Trapane. 1996. Concentration and extinction coefficient determination for oligonucleotides and analogs using a general phosphate analysis. Anal. Biochem. 240:273–282. [DOI] [PubMed] [Google Scholar]
- Novakova, O., J. Kasparkova, J. Malina, G. Natile, and V. Brabec. 2003. DNA-protein cross-linking by trans-[PtCl2(E-iminoether)2]. A concept for activation of the trans geometry in platinum antitumor complexes. Nucleic Acids Res. 31:6450–6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilch, D. S., S. U. Dunham, E. R. Jamieson, S. J. Lippard, and K. J. Breslauer. 2000. DNA sequence context modulates the impact of a cisplatin 1,2-d(GpG) intrastrand cross-link on the conformational and thermodynamic properties of duplex DNA. J. Mol. Biol. 296:803–812. [DOI] [PubMed] [Google Scholar]
- Pilch, D. S., G. E. Plum, and K. J. Breslauer. 1995. The thermodynamics of DNA structures that contain lesions or guanine tetrads. Curr. Opin. Struct. Biol. 5:334–342. [DOI] [PubMed] [Google Scholar]
- Plum, G. E., and K. J. Breslauer. 1994. DNA lesions: a thermodynamic perspective. Ann. N. Y. Acad. Sci. 726:45–56. [DOI] [PubMed] [Google Scholar]
- Plum, G. E., C. A. Gelfand, and K. J. Breslauer. 1999. Physicochemical approaches to structural elucidation. Effects of 3,N4-ethenodeoxycytidine on duplex stability and energetics. In Exocyclic DNA Adducts in Mutagenesis and Carcinogenesis. B. Singer and H. Bartsch, editors. IARC, Lyon, France. 169–177. [PubMed]
- Poklar, N., D. S. Pilch, S. J. Lippard, E. A. Redding, S. U. Dunham, and K. J. Breslauer. 1996. Influence of cisplatin intrastrand crosslinking on the conformation, thermal stability, and energetics of a 20-mer DNA duplex. Proc. Natl. Acad. Sci. USA. 93:7606–7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon, J. T., and A. Sancar. 1998. Molecular mechanism of nucleotide excision repair in mammalian cells. In Advances in DNA Damage and Repair. M. Dizdaroglu and A. Karakaya, editors. Plenum, New York. 377–393.
- Reardon, J. T., A. Vaisman, S. G. Chaney, and A. Sancar. 1999. Efficient nucleotide excision repair of cisplatin, oxaliplatin, and bis-aceto-ammine-dichloro-cyclohexylamine-platinum(IV) (JM216) platinum intrastrand DNA diadducts. Cancer Res. 59:3968–3971. [PubMed] [Google Scholar]
- Rentzeperis, D., D. P. Kharakoz, and L. A. Marky. 1991. Coupling of sequential transitions of any molecularity from equilibrium melting curves. Biochemistry. 30:6276–6283. [DOI] [PubMed] [Google Scholar]
- Sancar, A. 1996. DNA excision repair. Annu. Rev. Biochem. 65:43–81. [DOI] [PubMed] [Google Scholar]
- SantaLucia, J., Jr. 1998. A unified view of polymer, dumbbell, and oligonucleotide DNA nearest-neighbor thermodynamics. Proc. Natl. Acad. Sci. USA. 95:1460–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Garderen, C. J., H. Van den Elst, J. H. Van Boom, J. Reedijk, and L. P. A. Van Houte. 1989. A double-stranded DNA fragment shows a significant decrease in double-helix stability after binding of monofunctional platinum amine compounds. J. Am. Chem. Soc. 111:4123–4125. [Google Scholar]
- Vrana, O., V. Brabec, and V. Kleinwächter. 1986. Polarographic studies on the conformation of some platinum complexes: relations to anti-tumour activity. Anticancer Drug Des. 1:95–109. [PubMed] [Google Scholar]
- Wood, R. D. 1999. DNA damage recognition during nucleotide excision repair in mammalian cells. Biochimie. 81:39–44. [DOI] [PubMed] [Google Scholar]
- Zaludova, R., A. Zakovska, J. Kasparkova, Z. Balcarova, O. Vrana, M. Coluccia, G. Natile, and V. Brabec. 1997. DNA modifications by antitumor trans-[PtCl2(E-iminoether)2]. Mol. Pharmacol. 52:354–361. [DOI] [PubMed] [Google Scholar]
- Zamble, D. B., D. Mu, J. T. Reardon, A. Sancar, and S. J. Lippard. 1996. Repair of cisplatin-DNA adducts by the mammalian excision nuclease. Biochemistry. 35:10004–10013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.