Abstract
One of the biggest problems of heart failure is the heart's inability to effectively pump blood to meet the body's demands, which may be caused by disease-induced alterations in contraction properties (such as contractile force and Young's modulus). Thus, it is very important to measure contractile properties at single cardiac myocyte level that can lay the foundation for quantitatively understanding the mechanism of heart failure and understanding molecular alterations in diseased heart cells. In this article, we report a novel single cardiac myocyte contractile force measurement technique based on moving a magnetic bead. The measuring system is mainly composed of 1), a high-power inverted microscope with video output and edge detection; and 2), a moving magnetic bead based magnetic force loading module. The main measurement procedures are as follows: 1), record maximal displacement of single cardiac myocyte during contraction; 2), attach a magnetic bead on one end of the myocyte that will move with myocyte during the contraction; 3), repeat step 1 and record contraction processes under different magnitudes of magnetic force loading by adjusting the magnetic field applied on the magnetic bead; and 4), derive the myocyte contractile force base on the maximal displacement of cell contraction and magnetic loading force. The major advantages of this unique approach are: 1), measuring the force without direct connections to the cell specimen (i.e., “remote sensing”, a noninvasive/minimally invasive approach); 2), high sensitivity and large dynamic range (force measurement range: from pico Newton to micro Newton); 3), a convenient and cost-effective approach; and 4), more importantly, it can be used to study the contractile properties of heart cells under different levels of external loading forces by adjusting the magnitude of applied magnetic field, which is very important for studying disease induced alterations in contraction properties. Experimental results demonstrated the feasibility of proposed approach.
INTRODUCTION
Heart failure is characterized by the heart's inability to effectively pump blood to meet the body's demands. Some heart diseases, even in the early stages, can impair a heart cell's ability to contract and relax (Yelamarty et al., 1992), which may result from the significant difference in contractile force between normal and heart failure cells. Thus, the information on contractile force of heart cells will be very helpful for understanding molecular alterations in diseased heart cells. In particular, understanding disease-induced alterations in contractile properties (such as contractile force and Young's modulus) at single cardiac myocyte level may lay the foundation for quantitatively understanding the mechanism of heart failure.
Although force measurements at the multicellular tissue and whole organ levels have been accomplished by using standard force or pressure transducers (Kawai et al., 1993; Roos, 1997), it is still a very challenging task to scale systems down to the smaller single cardiac myocyte level. Several approaches were proposed and summarized as follows:
The first approach is based on the bending of fine glass filament (Yoneda, 1960; Canaday and Fay, 1976). Fine glass filaments were calibrated to determine how much the filaments bent under a given load. Canaday and Fay developed such a system, in which a small glass rod connects a pair of fine glass filaments to the cell being studied. The displacement of the glass filaments is measured with a sensitive optical system. This transducer has been used to study the mechanics of single muscle cells. To a certain extent, it can give the contractile force of single muscle cells. Note that this system only applies to SMOOTH muscle cells, which are very different from cardiac cells. Furthermore, these transducers need to be physically attached to the site of interest, which is beneath the surface of the tissue. The intervening tissue, which is also in contact with the transducer, may contribute to the measured force to an unknown degree. Obviously, this may cause a measurement error. In addition, attaching myocytes to the force transducer is also a challenging job (Tarr et al., 1983). Finally, external loading force (i.e., the reacting force from filament to cell) cannot be adjusted and controlled once the filament transducer is fabricated. Thus, this approach cannot be used to study the contractile properties of cardiac cell under different magnitude of external load. Note that, in real life, to pump the blood into the entire body, there is always an external loading force (i.e., the reacting force to the heart generated by the entire blood transportation system) during heart contraction. Moreover, the magnitude of this external loading force varies from person to person as well as by physical condition of the person. Thus, it would be very useful if one could measure the contractile force of cardiac myocyte under different magnitudes of external loading force.
The second approach is based on recently developed microelectromechanical systems (MEMS) technology (Lin et al., 2000; Tan et al., 2002). MEMS technology offers the ability to shrink the force transducer down to a size comparable to that of a cardiac myocyte. In the method described by G. Lin, cell attachment and measurement of contractile forces have been demonstrated with a commercially fabricated surface-micromachined hinged polysilicon device. Two freestanding polysilicon clamps, each suspended by a pair of microbeams, hold each end of heart cell. When the cell contracts, the beams bend and force is determined from the measured deflection and the spring constant in the beams. The average maximal contractile force was measured to be ∼12.6 μN, which is compatible with the data obtained by other methods (Tarr et al., 1983). However, this technical approach involves cell manipulations with probes, gluing, and clamping, which may have some unknown effect on the cell ends and their function. Disruption of some of the contractile filaments likely occurred at the cell ends. Since the topology of the cell ends varies from cell to cell, the amount of disruption will be difficult to quantify, which limits the accuracy of the measurement. In the method described by Tan, cells are put on top of an array of micrometer scale elastomeric posts. Each post in the array independently bends in response to local forces exerted by the attached cell. The degree and direction of the bending of each individual post reflects the magnitude and direction of the force exerted by the cell at that particular location. However, the hard contact between the cell and a bed of needles (i.e., posts) may again have some effect on the cell membrane and myocyte function. Furthermore, both Lin's and Tan's approaches cannot be used to measure the contractile properties of cardiac cell under different magnitudes of external load since external loading force is fixed once the micro transducer is fabricated.
The third approach is based on viscous loading. Cells are immersed in the liquid and contractile properties are investigated under different viscous loading of liquid. Different viscous loading can be realized by changing the viscosity of liquid around the cell (Kent et al., 1989; Yin et al., 1996) or by changing the concentrations of microspheres added to the liquid (Wang et al., 1997). The major advantages of this approach are: 1), it is a noninvasive/minimal invasive approach due to the soft contact between the cell and surrounding liquid medium; and 2), one can change the external loading force by changing the viscous loading. However, due to the sophisticated nature of fluid movement, it is very difficult to calculate the exact value of viscous loading force. This method may provide qualitative relationships between the contractile properties and viscous loadings (such as the relationship between the velocity of shortening and relative resistive load). But, it is difficult to provide exact value of contractile force.
The fourth approach is based on remotely added external loading force. One way of realizing remotely added external loading force may be using optical tweezers technology (Ashkin and Dziedzic, 1971). For example, optical tweezers have been successfully used to estimate the force of mitochondrial transport by the proteins kinesin and dynein inside living amoeba. However, to avoid damaging the living tissue by the strong light beam, the maximal force generated by optical tweezers is usually <1 nN (typically, hundreds of pN), which is too small as compared with contractile force of cardiac myocyte (>1 μN, in general). Another possible way is using remotely added magnetic force (Guilford and Gore, 1992). In the method described by Guilford, a magnetic microsphere is attached to the specimen being studied and is positioned between two electromagnets. Video microscopy and edge detection are used to monitor small movements of the microsphere that occur when the specimen generates force. An automatic control system adjusts the current through the electromagnets to keep the microsphere stationary. Since the microsphere is in a stationary state, the magnitude of specimen-generated force equals to the magnitude of magnetic force on the microsphere. Thus, the specimen-generated force can be derived by calculating the magnetic force on the microsphere via the magnetic field intensity and properties of magnetic microsphere. This method could measure the stationary force generated by specimen, similar to isometric muscle contraction. However, to study physiological contractile properties (isometric and isotonic) of cardiac myocytes, one would need to know the contractile force during the myocyte contraction and relaxation processes. Due to the movement of specimen (i.e., cardiac myocyte), the method described by Guilford cannot be applied.
To overcome limitations of the previous approaches, in this article, we report a novel single cardiac myocyte contractile force measurement technique based on moving a magnetic bead. The major advantages of this approach are: 1), measuring the contractile force without direct transducer connection to the cell specimen (i.e., “remote sensing”, a noninvasive/minimal invasive approach); 2), high sensitivity and large dynamic range (force measurement range: from pN to μN); 3), it can be used to study the contractile properties of heart cells under different levels of external loading force, which is very important because there is always resistive force (or called “external loading force”) when blood is pumped by heart contraction; and 4), applied magnetic loading force can be much smaller than that of the contractile force itself, which leads to minimized perturbation to the contracting myocyte. Thus, this unique technique could become a very useful tool in studying disease-induced alterations in contractile properties at single cardiac myocyte level.
Finally, we would like to emphasize that our technique is the only one that could readily allow variation in loading force, in contrast to previous techniques. This is one of the major advantages of our technique in which effects of changing external load on contractility can be studied in the same cell easily. It is much more difficult to change solutions (especially viscous ones) and try to study the same myocyte.
MEASUREMENT PRINCIPLE
Build cell contraction mathematical model
To obtain contractile force of single cardiac myocyte using moving a magnetic bead-based remote sensing technology, first, a quantitative mathematic model is constructed. For the purpose of simplicity (but very close to the real case), the contraction of single cardiac myocyte is modeled by the longitudinal vibration of a one-dimensional spring, which can mathematically be described by the following standard partial differential equation (Andrei, 2002; Rao, 2003):
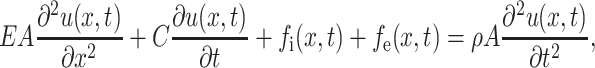 |
(1) |
where
E = cell elastic modulus;
A = cell cross-sectional area;
u(x, t) = position function of the unit cell at time t, which is originally (t = 0) located at position x;
C = viscosity damping factor;
fi(x, t) = internal force (i.e., cell contraction force);
fe(x, t) = external force load (i.e., force generated by the magnetic bead);
ρ = cell density
One the left-hand side, there are four terms: the first term is the net elastic force caused by deformation of the unit cell in the x-direction; the second term is viscosity damping force, which is a linear function of unit cell velocity; the third term is internal cellular force (i.e., cell contraction force); the fourth term is external loading force load (i.e., magnetic loading generated by the magnetic field on the moving magnetic bead). The term on the right-hand side represents acceleration force of the unit cell.
There are totally seven parameters. A can be directly measured, ρ can be found in the published literature, u(x, t) can be obtained by a high-resolution microscope with a real-time video output, and fe(x, t) is a known magnetic loading function . So there are only three unknowns: E, C, and fi(x, t).
The initial and boundary conditions can be expressed by the following equations:
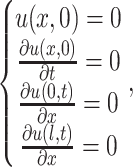 |
(2) |
where l denotes the length of the cell.
Equation 1 is actually a telegraph equation, and has an exact analytical solution under the above boundary conditions. For simplicity, Eq. 1 is rewritten into
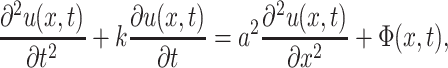 |
(3) |
where
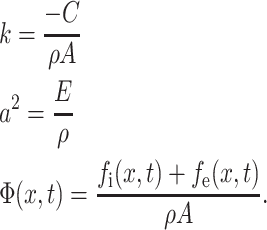 |
The solution of Eq. 3 under the boundary condition of Eq. 2 can be shown to be (Rao, 2003)
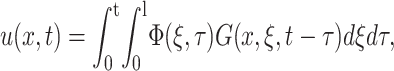 |
(4) |
where
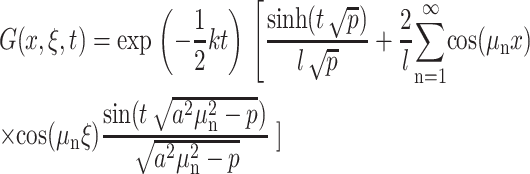 |
(5) |
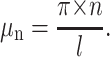 |
(6) |
If the inequality 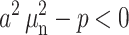 holds for several first values n = 1……m, then the expressions
holds for several first values n = 1……m, then the expressions 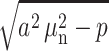 should be replaced by
should be replaced by 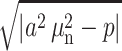 and the sine functions be replaced by hyperbolic sine functions in the corresponding terms of the series.
and the sine functions be replaced by hyperbolic sine functions in the corresponding terms of the series.
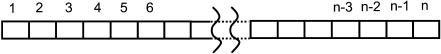
In the numerical analysis, a cardiac myocyte is modeled as a one-dimensional spring and is divided into (n − 1) subcellular units by n nodes, as shown in Fig. 1, and force fe(x, t) can only be applied on the nodes. To reserve the boundary condition 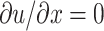 at both ends (Eq. 2), there should be no force applied on nodes 1 and n. Since the cell has no bulky movement, the net force of Φ(x, t) should be zero, which means Φ(x, t) will always be applied in pairs with opposite direction.
at both ends (Eq. 2), there should be no force applied on nodes 1 and n. Since the cell has no bulky movement, the net force of Φ(x, t) should be zero, which means Φ(x, t) will always be applied in pairs with opposite direction.
FIGURE 1.
Schematic diagram of subcellular structure.
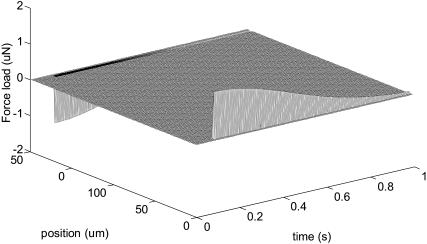
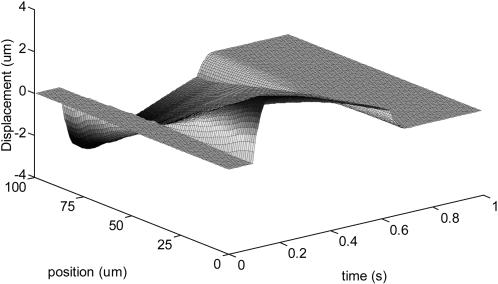
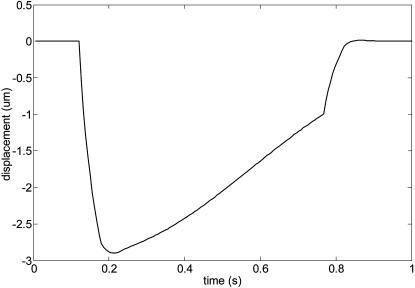
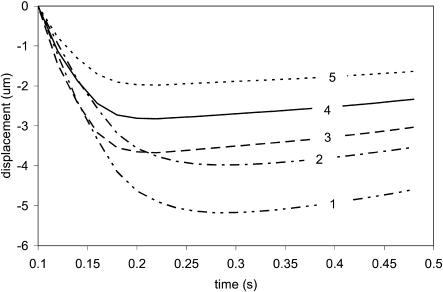
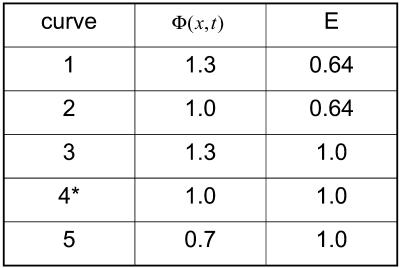
Figs. 2 and 3 represent force load Φ(x, t) and corresponding cell displacement as a function of location and time, respectively. Fig. 4 denotes the displacement of one end of the cardiac myocyte as a function of time, which is consistent with the experimentally measured curve as described in literature 1. Figs. 5 and 6 show the displacement of one end of cardiac myocyte under different levels of external forces and corresponding conditions, respectively. Curves 3 and 4 in Fig. 5 correspond to 100% magnitude of Φ(x, t) and 70% magnitude of Φ(x, t), respectively. It is found that the corresponding peak displacement values are also 100% and 70%, respectively. Thus, there is a linear relationship between the peak displacement value and the magnitude of force load Φ(x, t).
FIGURE 2.
Force load Φ(x, t).
FIGURE 3.
Cell displacement under force load Φ(x, t).
FIGURE 4.
Displacement of one end of the cardiac myocyte.
FIGURE 5.
Displacement of one end of the cardiac cell.
FIGURE 6.
Simulation input data condition.
Based on this linear relationship, the unknown cell internal force, fi(x, t), can be derived. Mathematically, the linear relationship between the maximal displacement value, dmax, and the magnitude of force load, Φmax, can be expressed as
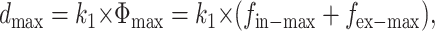 |
(7) |
where k1 is a constant. To get 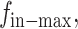 first, we measure the maximal displacement without external force (i.e.,
first, we measure the maximal displacement without external force (i.e., 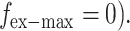 dmax-1 denotes the displacement in this case, as given by
dmax-1 denotes the displacement in this case, as given by
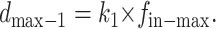 |
(8) |
Then, we measure the displacement with a known external force 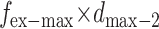 denotes the displacement in this new case, as given by
denotes the displacement in this new case, as given by
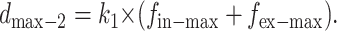 |
(9) |
By solving Eqs. 8 and 9 simultaneously, the value of fin-max and k1 can be found as follows:
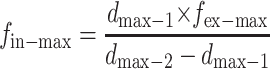 |
(10a) |
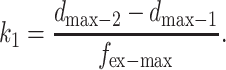 |
(10b) |
Generate external loading force by applying magnetic field on a magnetic bead attached on one end of cardiac myocyte
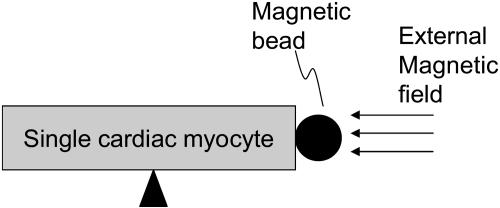
To generate external loading force, a magnetic bead (such as a nickel bead) is attached to one end of a cardiac myocyte loosely attached to the bottom of the laminin-precoated cover slip and the center of myocyte is fixed on the bottom, as illustrated in Fig. 7.
FIGURE 7.
External loading force by adding magnetic field on a magnetic bead attached on one end of cardiac myocyte.
Again, the idea of measuring contractile force based on the stationary magnetic microsphere has been pursued by Guilford. Here, we overcome the limitation of the stationary magnetic bead technology. Contractile force can be measured even when magnetic microsphere is moving, which not only makes the measurement more flexible but also offers the capability of studying the contractile properties under different levels of external loading force.
The magnetic force added on a magnetic bead, Fm, can be calculated by the following equation (Suzuki and Kasagi, 2003):
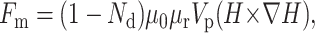 |
(12) |
where H denotes magnetic field, Nd is the demagnetizing factor (0.33 for a sphere), μ0 is the permeability in vacuum, μr is the relative permeability, and Vp is the volume of magnetic material.
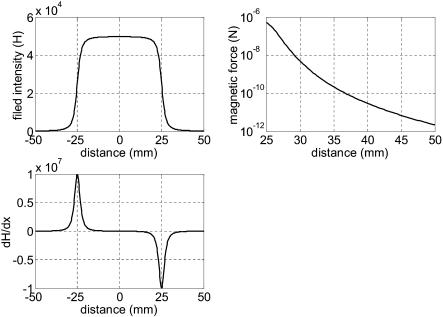
To have a numerical feeling about Fm, assume that the magnetic field is generated by a thin coil solenoid with the following parameters: number of turns: N = 5000, diameter of solenoid: D = 5 mm, length of solenoid, L = 50 mm, and the current passing through solenoid: I = 0.5 A. The magnetic bead is made of nickel with 20 μm diameter and 600 relative permeability, respectively. Fig. 8 shows the calculated magnetic loading force as a function of distance between the solenoid and the magnetic bead. It can be seen that the range of magnetic loading force is relatively huge (from pN to μN), which is a perfect candidate as external loading force.
FIGURE 8.
Magnetic loading force as a function of distance between the magnetic bead and solenoid with the following parameters: number of turns of solenoid: N = 5000; diameter of solenoid: D = 5 mm; length of solenoid, L = 50 mm; and the current passing through solenoid: I = 0.5 A. The magnetic bead is made of nickel with 20 μm diameter and 600 relative permeability.
EXPERIMENTAL PROCEDURES AND RESULTS
Single cardiac myocyte preparation
Chronic ischemic cardiomyopathy model. Male Sprague-Dawley rats (250–300 g) are anesthetized (2% isoflurane), intubated, and hearts externalized via thoracotomy. The left coronary artery is ligated ∼3–5 mm from its origin and the heart repositioned with the chest. Sham-operated animals are treated in identical manner except that the suture around the coronary artery is not tied. Survivors are allowed to recover for three weeks before myocyte isolation (Zhang et al., 1999).
Isolation of cardiac ventricular myocytes. Cardiac cells from adult rat ventricles are isolated by successive perfusion with collagenase and hyaluronidase (Anand et al., 1997; Zhang et al., 1999). For postinfarction hearts, the infarct scar is excised before the final enzymatic digestion step. For Sham hearts, anterior LV free walls corresponding to infarct location in myocardial infarction rats are excised. Freshly isolated myocytes are seeded on laminin-coated glass cover slips, bathed in serum-free medium 199 with supplements (creatine, L-carnitine, and taurine), and allowed to adhere for 2 h before field-stimulated contraction studies.
Myocytes shortening measurements. Myocytes bathed in 0.6 ml of air and temperature equilibrated (37°C), HEPES — buffered (20 mM, pH 7.4) medium 199 are placed on a temperature-controlled stage (37°C) of an inverted microscope. Myocytes are field-stimulated to contract between platinum wire electrodes spaced 2 mm apart. [Ca2+]o is varied between 1.1 to 2.0 mM, and pacing frequency is 1.0 Hz. Myocytes viewed through an Olympus DApo UV ×40/1.30 NA oil objective are imaged by a high-resolution CCD video camera, and myocyte length, width, and motion measurements are acquired by a personal computer with interface and software custom-designed for this project.
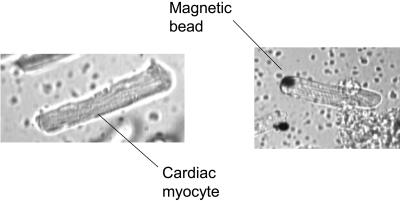
As an example, Fig. 9 a shows an isolated single cardiac myocyte obtained from normal adult male Sprague-Dawley rats.
FIGURE 9.
Isolated single adult rat cardiac myocyte (a) without an attached magnetic bead and (b) with an attached magnetic bead.
Measuring single cardiac myocyte contractile force
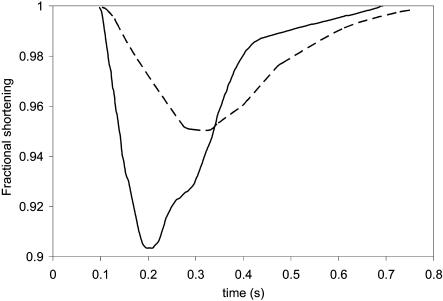
First, we recorded the field-stimulated contraction of single cardiac myocyte as a function of time without attaching the magnetic bead and applying magnetic field. The solid line in Fig. 10 shows myocyte shortening as a function of time.
FIGURE 10.
Shortening distance as a function of time for single adult rat cardiac myocyte. (Solid line) Without magnetic loading force. (Dashed line) With magnetic loading force.
Second, a laminin-precoated magnetic bead (≅10 μm diameter) was attached on one end of myocyte, as shown in Fig. 9 b, but without applying magnetic field. We again recorded the contraction of single cardiac myocyte as a function of time. It was found that there was almost no change in the contraction behavior. It may be due to the fact that the inertia of this magnetic bead (nN) is too small as compared with contraction force (μN).
Finally, a magnetic field was applied on the magnetic bead. In our preliminary experiment, a permanent magnetic rod (with 2 mm in diameter and 20 mm in length) is used to generate the magnetic field. The distance between the magnetic bead and the tip of rod is ∼5 mm. In this case, the magnetic loaded force was estimated ∼5 μN. We again recorded the contraction of cardiac myocyte as a function time, as shown by the dashed line in Fig. 10. It was found that there was a 50% reduction in contraction. Substituting these data into Eq. 10 a, the estimated contraction force is ∼10 μN, which is similar to the other reported data (Tarr et al., 1983; Lin et al., 2000; Shepherd et al., 1990). These experimental results demonstrated that, indeed, our proposed method could be used to measure the contractile force of single cardiac myocyte.
References
- Anand, I. S., D. Liu, S. S. Chugh, A. J. Prahash, S. Gupta, R. John, F. Popescu, and Y. Chandrashekhar. 1997. Isolated myocyte contractile function is normal in postinfarction remodeled rat heart with systolic dysfunction. Circulation. 96:3974–3984. [DOI] [PubMed] [Google Scholar]
- Andrei, D. 2002. Polyanin, Handbook of Linear Partial Differential Equations for Engineers and Scientists. Chapman & Hall/CRC, Boca Raton, FL.
- Ashkin, A., and J. M. Dziedzic. 1971. Optical levitation by radiation pressure. Appl. Phys. Lett. 19:283–285. [Google Scholar]
- Canaday, P. G., and F. S. Fay. 1976. An ultrasensitive isometric force transducer for single smooth muscle cell mechanics. J. Appl. Physiol. 40:243–246. [DOI] [PubMed] [Google Scholar]
- Guilford, W. H., and R. W. Gore. 1992. A novel remote-sensing isometric force transducer for micromechanics studies. Am. J. Physiol. 263:C700–C707. [DOI] [PubMed] [Google Scholar]
- Kawai, M., Y. Saeki, and Y. Zhao. 1993. Crossbridge scheme and the kinetic constants of elementary steps deduced from chemically skinned papillary and trabecular muscles of the ferret. Circ. Res. 73:35–50. [DOI] [PubMed] [Google Scholar]
- Kent, R. L., D. L. Mann, Y. Urabe, R. Hisano, K. Hewett, M. Loughnane, and G. Cooper. 1989. Contractile function of isolated feline cardiocytes in response to viscous loading. Am. J. Physiol. 257:H1717–H1727. [DOI] [PubMed] [Google Scholar]
- Lin, G., K. Pister, and K. Roos. 2000. Surface micromachined polysilicon heart cell force transducer. IEEE J. MEMS . 9:9–17. [DOI] [PubMed] [Google Scholar]
- Rao, S. 2003. Mechanical Vibrations, 4th ed. Pearson Prentice Hall, Upper Saddle River, NJ.
- Roos, K. P. 1997, Mechanics of force production. In The Myocardium, 2nd ed. G. A. Langer, editor. Academic, New York. 235–323.
- Shepherd, N., M. Vornanen, and G. Isenberg. 1990. Force measurements from voltage-clamped guinea pig ventricular myocytes. Am. J. Physiol. 258:H452–H459. [DOI] [PubMed] [Google Scholar]
- Suzuki, H., and N. Kasagi. 2003. Chaotic mixing of magnetics beads in micro cell separator. Proc. 3rd Int. Symp. Turbulence and Shear Flow Phenomena. 817–822.
- Tan, J. L., J. Tien, K. Bhadriraju, D. Pirone, D. Gray, and C. S. Chen. 2002. Feel the force: using a bed of needles to map single cell generated traction forces. Proc. 2nd Joint EMBS/BMES Conference. Houston, TX. 1648–1649.
- Tarr, M., J. W. Trank, and K. K. Goertz. 1983. Effect of external force on relaxation kinetics in single frog atrial cardiac cells. Circ. Res. 52:161–169. [DOI] [PubMed] [Google Scholar]
- Wang, Z., C. Lam, R. Mukhersee, L. Hebbar, Y. Wang, and F. Spinale. 1997. Relationship between external load and isolated myocyte contractile function with CHF in pigs. Am. J. Physiol. 273:H183–H191. [DOI] [PubMed] [Google Scholar]
- Yelamarty, R. V., R. L. Moore, F. Yu, M. Elensky, A. Semanchick, and J. Cheung. 1992. Relaxation abnormalities in single cardiac myocytes from renovascular hypertensive rats. Am. J. Physiol. 262:C980–C990. [DOI] [PubMed] [Google Scholar]
- Yin, S., C. Chen, and J. Cheung. 1996. Using optical particle image velocimetry for the nondestructive measurement of the single-cell contractile force. Microw. Opt. Techn. Let. 13:31–34. [Google Scholar]
- Yoneda, M. 1960. Force exerted by a single cilium of Mytilus edulis. J. Exp. Biol. 37:461–469. [Google Scholar]
- Zhang, X.-Q., T. I. Musch, R. Zelis, and J. Y. Cheung. 1999. Effects of impaired Ca2+ homeostasis on contraction in postinfarction myocytes. J. Appl. Physiol. 86:943–950. [DOI] [PubMed] [Google Scholar]