Abstract
The magnetotactic multicellular prokaryote (MMP), a motile aggregate of bacterial cells, is known to exhibit an unusual “ping-pong” motility in magnetic fields greater than the earth's field. This motility is characterized by rapid excursions, opposite the direction of an applied magnetic field, and slower returns along the direction of the magnetic field. We have carried out detailed observations of the time and spatial dependence of the ping-pong motility and find 1), the outward and return excursions exhibit a uniform deceleration and acceleration, respectively; 2), the probability per unit time of an MMP undergoing a ping-pong excursion increases monotonically with the field strength; and 3), the outward excursions exhibit a very unusual distance distribution which is dependent on the magnetic field strength. At any given field strength, a characteristic distance is observed, below which very few excursions occur. Beyond this distance, there is a rapid increase in the number of excursions with an exponentially decaying distribution. These observations cannot be explained by conventional magnetotaxis, i.e., a physical directing torque on the organism, and suggest a magnetoreceptive capability of the MMP.
INTRODUCTION
The issue of biomagnetic sensing, or magnetoreception, has been a topic of interest among scientists and the public for many years. This interest has been pursued in terms of the navigational advantages for species to use the earth's magnetic field to find their optimal niches. However, the discussion of proposed mechanisms for magnetoreception has mainly been restricted to the presence of magnetic crystals in higher life forms, i.e., organisms having neural systems (Thorsten et al., 2002). The simplest organisms that exhibit a response to a magnetic field are magnetotactic bacteria (Blakemore, 1975), which represent a morphological variety of prokaryotes containing “magnetosomes”, intracellular single domain magnetic crystals enveloped by phospholipid membranes (Gorby et al., 1988). The majority of single-celled magnetotactic bacteria have magnetosomes comprised of single crystal magnetite, Fe3O4 (Frankel et al., 1979). These magnetosomes give rise to a robust magnetic dipole moment due to the close north-south polar juxtaposition of the magnetite crystals, which are aligned in a chain in the cell's interior. The resulting “compass needle” enables the organism to be steered by a physical torque determined by the direction of an external magnetic field (Esquivel and Lins de Barros, 1986). “Magnetotaxis” was the term coined to describe this steering response although this taxis is merely the result of a physical torque, with no magnetoreception mechanism apparently involved.
One of the most interesting magnetotactic microbes is the magnetotactic multicellular prokaryote (MMP; Farina et al., 1983), an aggregate of ∼20 Gram-negative cells in a spherical arrangement, multiflagellated on the surface exposed to the environment. Rodgers et al. (1990) reported the presence of intercellular connections in the form of apposed outer membranes in the MMP under investigation. The authors suggested that the intercellular connections described could be functionally important in the motility and magnetotaxis of the organism. Intercellular connections were also found in an MMP isolated in Brazil (Keim et al., 2004). This isolate contains an internal acellular compartment containing filaments linking the cells. Based on the novel and unusual cellular arrangement and behavior of the MMP studied, both groups suggested the term “magnetotactic multicellular prokaryote”.
The MMP studied generally contain greigite, Fe3S4 (Pósfai et al., 1998); however, the ability to form either magnetite or greigite, depending on environmental conditions, has been reported (Keim et al., 2003). The magnetosomes of the MMP possess a spatial distribution in the individual cells that is less ordered than in most single-celled magnetotactic bacteria. MMP polarity, i.e., the direction of its net magnetic dipole moment with respect to its axis of motility (Frankel, 1984), can be reversed, as well as nullified, by a 60-Hz magnetic field with a peak amplitude of several hundred gauss (Rodgers et al., 1990). This implies a net magnetic moment less robust than the compass needle of single-celled magnetotactic bacteria. Nevertheless, it has been commonly accepted that magnetotaxis is the correct way to classify the effect of a magnetic field on the motility of the MMP since its direction of motility is determined by the direction of the magnetic field. In this article, we will only be discussing, with no loss of generality, the north-seeking MMPs whose normal motion is in the same direction as the applied magnetic field.
In magnetic fields several times the earth's field, an MMP will demonstrate an unusual “ping-pong” motility (Rodgers et al., 1990), a behavior that is not described by the simple passive torque model. This motility has been distinguished from normal motility along magnetic field lines by a rapid excursion against the direction of the magnetic field, followed by a slower return. We report the results of a detailed investigation of the effects of applied magnetic fields on the ping-pong phenomenon, which reveal unexpected features of MMPs subjected to stronger-than-earth magnetic fields. The nature of these features falls well outside of the phenomenon of conventional magnetotaxis and suggests a magnetoreceptive capability for this organism.
MATERIALS AND METHODS
The MMP samples were extracted from a salt marsh pond at Wood Neck Beach in Woods Hole, MA. The samples were kept in a rectangular 100-L vat in the dark until extraction for an experiment. Occasionally, hydrogen sulfide was vented using a ventilation pump. Before extraction, the large vat was stirred and a 400-mL scoop of liquid and sediment was removed from the container into a beaker. The sediment was allowed to settle, and a bar magnet was placed ∼1 cm above the sediment line for 30–60 min. Occasionally, a dense pellet of MMPs would form against the glass of the beaker. The edge of the beaker was scraped with the tip of a Pipetman, and a 20-μl sample was extracted then placed on a coverslip, which was inverted onto an o-ring resting on a slide. The hanging drop generated an air-water interface at the edge of the droplet. The applied magnetic field was in the plane of the coverslip and was directed perpendicular to the interface from the water to the air (from right to left in Figs. 1 and 3). This is the geometry used to investigate the north-seeking MMPs since the normal magnetotactic motility directs the MMPs toward the air-water interface. The south-seeking (reverse polarity) MMPs at the opposite side of the drop undergo ping-pongs reversed in direction, i.e., along the direction of the magnetic field. Viability of the organism was not affected at the highest field investigated (1.7 mT).
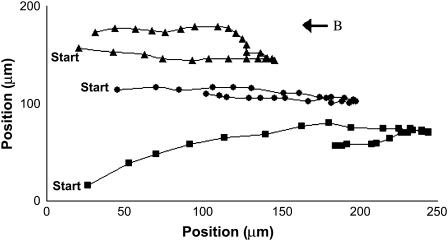
FIGURE 1.
Time lapses of MMP ping-pong trajectories at 1.7 mT. Successive images are taken 0.1 s apart at near the air-water interface. The interface is at ∼20 μm from the right of the figure with air on the left and water on the right. A uniform magnetic field of 1.7 mT is present in the direction right to left. The paths were followed until the MMPs swam below the plane of focus.
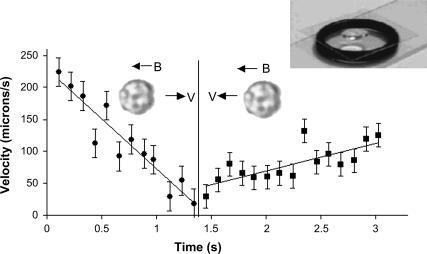
FIGURE 3.
Typical velocity versus time of an MMP during a ping-pong at 0.8 mT. Successive data points were taken 0.1 s apart. Best fit line for acceleration gives an acceleration of 160 μm/s2 against the field and 40 μm/s2 along the field. The average outgoing excursion velocity is ∼170 μm/s. The upper left inset shows the suspended drop on a coverslip, supported by an o-ring.
A large (3.1m × 3.1m × 3.1m) set of Helmholtz coils (one set for each axis) was used to zero out the earth's field in the vicinity of the microscope by adjusting the current and monitoring the field using a hall probe. Samples on the microscope stage were situated between two 33-cm diameter Helmholtz coils. The smaller coils allowed us to apply fields of up to 1.7 mT using a current regulated power supply. The applied field over the effective sample sizes was homogeneous to within 0.5% over 1 cm. The magnetic field homogeneity is important since it is the only source of an external force on these microbes. A gradient of 0.5%/cm at 1.7 mT would result in a force which is 7 orders of magnitude less than the frictional force on an MMP moving in a fluid at a typical swimming velocity of 100 μm/s.
The samples were examined via video microscopy using an Olympus (Melville, NY) BX-51 microscope at 100× and 400× magnifications. The images were collected using a CCD video camera (Javelin Electronics, Torrance, CA) and were recorded onto DVD disks. The number and excursion distances of ping-pongs were manually counted from the DVD recordings. The DVD data were also streamed into a Scion frame grabbing board. The data could then be analyzed using Scion Image (Scion Image Software, Frederick, MD), the PC port of NIH Image. Using Scion Image, the population was estimated and the position of a given MMP as a function of time could be carefully recorded. Data were also analyzed using Sonic Foundry Vegas Video (Sonic Factory, Pittsburgh, PA) to measure the excursion times and distances. In both cases, the distance scales of the digitized images were calibrated using a calibrated graticule in the specimen plane and imaged in the viewing plane of the CCD camera.
RESULTS
We tested the MMP for oxygen tolerance in an open 20-μl drop in which it survived for ∼15 min. In the hanging drop setup used (inset in Fig. 3), the organism remained viable and active for at least 2 h. All of our data were obtained in the first 35 min or less after removing the MMPs from the final collection jars.
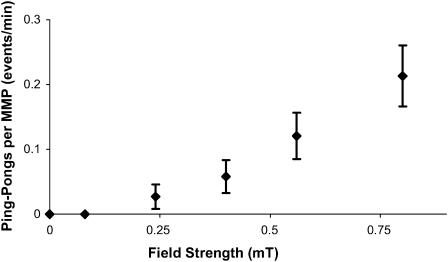
At magnetic fields approaching earth's field (≈0.05 mT), the MMPs swim at a constant velocity in a looping motion at the air-water interface. At higher fields, the ping-pong motility emerges. The backward and forward motion of a ping-pong is much more aligned with the field than the looping motion. The looping and ping-pong motions can be viewed in the online supplemental video clip. Time-lapsed digitized positions of typical MMP ping-pongs are shown in Fig. 1. Fig. 2 shows the rate of ping-pong events per MMP of an ensemble of MMPs versus the magnetic field. Measurements carried out on individual MMPs revealed the same behavior but with larger statistical errors.
FIGURE 2.
Probability, per minute, of an MMP undergoing a ping-pong event as a function of the magnetic field. The probabilities for single MMPs followed individually or chosen randomly from an ensemble, as shown here, were the same.
The average velocity of the MMPs swimming against the field during a ping-pong exhibits a narrow distribution, 170 μm/s with a full-width-at-half-maximum of 25 μm/s, which was observed to be independent of the magnetic field. A typical analysis of the time dependence of the outgoing and returning ping-pong speeds for an MMP (solid circle points in Fig. 1) is shown in Fig. 3. The velocities were determined by dividing the metric distances separating neighboring position points in Fig. 1 by the corresponding time interval 0.1 s. The quantitative validity of this analysis is based on an assumed constant velocity for each time interval. Taking into account the small changes in the velocity per 0.1 s interval shown by the accelerations in Fig. 3 would add only 8 μm/s and 2 μm/s, respectively, to the outgoing and returning times. These corrections were not made to the velocity data shown in Fig. 3 to show the data in its rawest form. Note the uniform deceleration and acceleration in the round trip. Since we examine the MMPs in a highly uniform magnetic field, which cannot exert a net force on a magnetic dipole, the uniform decelerations and accelerations at low Reynolds Number (R < 0.001) can only be attributed to variations in the propelling force of the MMPs' flagella during this motion. Thus the flagellar propulsion is stronger and decreases uniformly with time when the organism travels against the field direction. When it returns along the field, there is a slight but discernible increase in the flagellar propulsions.
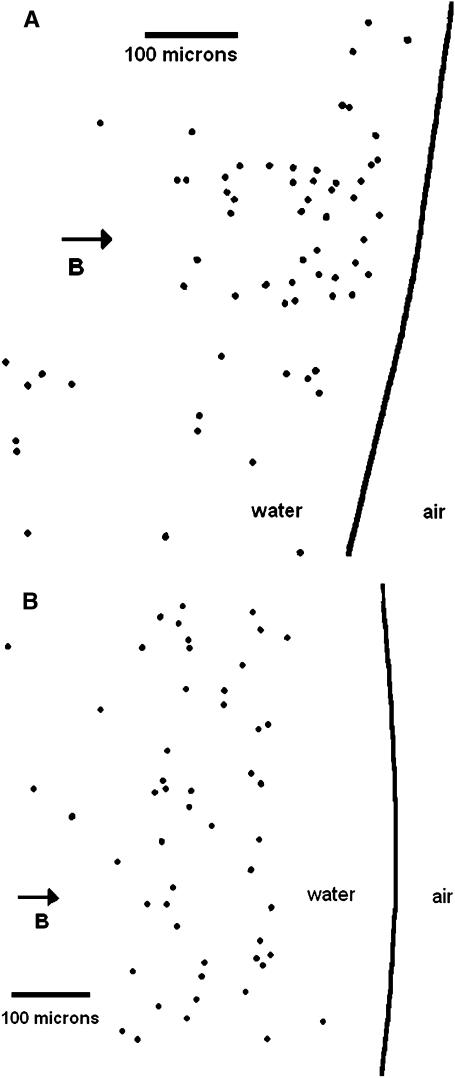
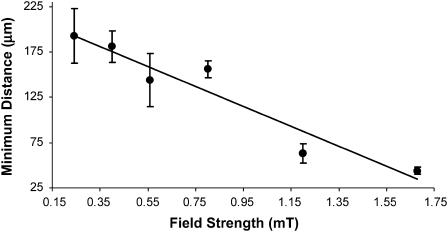
Another unusual feature of the ping-pongs is the distribution of the maximum outgoing excursion distances of each MMP as a function of field strength. The excursion distances can be seen for three ping-pongs in Fig. 1 for a field of 0.8 mT. To enable the measurements of many more excursions without having to generate frame by frame time dependent analysis, the following method of analysis was used. First, MMP ping-pongs were recorded for different magnetic fields. Second, a plastic sheet was then taped to the playback screen and a dot was placed on the sheet at the point of the terminus of each outgoing excursion, i.e., when the MMP came to rest before starting its return journey. Care was taken to insure that no ping-pongs were missed or double counted. Fig. 4, A and B, shows the resulting distribution of outgoing termination dots at 1.2 mT and 0.5 mT, respectively. The most striking feature of this distribution is the “deserts” where very few MMP excursions are terminated and that the termination distance depends strongly on the magnetic field. We define an “onset distance” do for the terminations by averaging 10 values of the shortest excursion distances in the onset region of histogrammed excursions. The results are shown in Fig. 5, to be discussed in the “Discussion” section.
FIGURE 4.
(A) Outward excursion termination dots recorded for a 2 min interval at 1.2 mT. The vertical direction is not necessarily the actual vertical position of the recorded ping-pongs. The solid line denotes the starting points of the ping-pongs, i.e., essentially the air-water interface. A video clip of the MMP motility from which this figure was generated is given in the online supplemental materials. (B) Outward excursion termination dots for a magnetic field of 0.55 mT.
FIGURE 5.
Onset distance, do, as a function of field strength. The onset was measured at fields varying from 0.08 to 1.7 mT.
We also investigated whether or not the initiation of a ping-pong occurring might be due to some form of a magneto-aerotactic response (Frankel et al., 1997). The average rate of ping-pongs per MMP in an ensemble accumulated at the interface was measured to be 1.4 ± 0.3 events/min in a field of 1.7 mT. The ensemble was then induced to travel across the droplet by reversing the direction of the magnetic field. By tracking individual MMPs in transit and normalizing to the population of MMPs being tracked, we observed a rate of 1.2 ± 0.1 events/min, per MMP, at 1.7 mT. Thus, there is no discernable difference between the rate of ping-pongs initiated at the center or at the edge of the water drop. We were also able to track the position versus time of an individual MMP ping-pong as it moved across the drop and saw no significant variation from the deceleration-acceleration behavior observed for ping-pongs initiated near the interface (Fig. 1).
DISCUSSION
It is not presently known what mechanism initiates the ping-pong motility, other than its relation to the magnetic field, as shown in Fig. 2. This behavior rarely occurs at lower fields and thus can be regarded as an emergent property of the MMP in which the magnetic field is a major factor. Further, the asymmetry in the outward and return excursion accelerations cannot be explained by the passive torque model. The unusual distribution of the outward excursion distances shown in Fig. 3 and the field dependency of the onset distances shown in Fig. 5 also belie any conventional models of magnetotaxis. Finally, the fact that the excursions do not begin to terminate until a minimum distance has elapsed suggests a magnetoreceptive internal cellular regulator whose switching is determined by the magnetic field. Since the MMP velocities are essentially field independent, the two factors that would play a role in determining do are the acceleration and/or the time duration of the outward excursions as a function of field. Further work in the future will be necessary to resolve this issue.
A novel mechanism has been proposed (Kirschvink et al., 2001) that supports the possibility of magnetoreception at the microbial level. The proposed mechanism features a magnetosome (at the terminus of a magnetosome chain) anchored via a cytoskeleton filament to a mechanically activated transmembrane ion channel. The magnetoreceptive mechanism proposed is “torque from the magnetosome, if properly applied, could cause the transient opening of the channel and lead to membrane depolarization” (Kirschvink et al., 2001). Sufficiently high resolution transmission electron microscope tomography is needed to further investigate the MMP as a candidate for this mechanism. Such resolution could possibly explain the origin of the biomagnetic behavior that we have observed. Other scenarios that are worth considering are magnetosome-protein coupling to ion channels or a more elaborate role of the torque on magnetosome interactions with cytoplasmic components in general.
SUPPLEMENTARY MATERIAL
An online supplement to this article can be found by visiting BJ Online at http://www.biophysj.org.
Supplementary Material
Acknowledgments
We acknowledge valuable discussions with Dennis Bazylinski and Lacra Bintu as well as the data analysis assistance of Joseph Lee.
This work was supported by the Kransberg Fund.
Michael Greenberg's present address is Physics Department, Brown University, Providence, RI 02912.
References
- Blakemore, R. P. 1975. Magnetotactic bacteria. Science. 190:377–379. [DOI] [PubMed] [Google Scholar]
- Esquivel, D., and H. G. P. Lins de Barros. 1986. Motion of magnetotactic microorganisms. J. Exp. Biol. 121:153–163. [Google Scholar]
- Farina, M., H. G. P. Lins de Barros, D. Esquivel, and J. Danon. 1983. Ultrastructure of a magnetotactic microorganism. Biol. Cell. 48:85–88. [Google Scholar]
- Frankel, R. B. 1984. Guidance of organisms. Ann. Rev. Biophys. 13:85–103. [DOI] [PubMed] [Google Scholar]
- Frankel, R. B., D. A. Bazylinski, M. S. Johnson, and B. L. Taylor. 1997. Magneto-aerotaxis in marine coccoid bacteria. Biophys. J. 73:994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel, R. B., R. P. Blakemore, and R. S. Wolfe. 1979. Magnetite in freshwater magnetotactic bacteria. Science. 203:1355–1356. [DOI] [PubMed] [Google Scholar]
- Gorby, Y. A., T. J. Beveridge, and R. P. Blakemore. 1988. Characterization of the bacterial magnetosome membrane. J. Bacteriol. 170:834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keim, C., U. Lins, and M. Farina. 2003. Iron oxide and iron sulfide crystals in magnetotactic multicellular aggregates. Acta Microscopica. 12:3–4. [Google Scholar]
- Keim, C. N., F. Abreu, U. Lins, H. G. P. Lins de Barros, and M. Farina. 2004. Cell organization and ultrastructure of a magnetotactic multicellular organism. J. Struct. Biol. 145:245–262. [DOI] [PubMed] [Google Scholar]
- Kirschvink, J., M. Walker, and C. Diebel. 2001. Magnetite-based magnetoreception. Neurobiol. 11:462–467. [DOI] [PubMed] [Google Scholar]
- Pósfai, M., P. R. Buseck, D. A. Bazylinkski, and R. B. Frankel. 1998. Reaction sequence of iron sulfide minerals in bacteria and their use as biomarkers. Science. 280:880–883. [DOI] [PubMed] [Google Scholar]
- Rodgers, F., R. Blakemore, N. Blakemore, R. Frankel, D. Bazylinski, D. Maratea, and C. Rodgers. 1990. Intercellular junctions, motility and magnetosome structure in a multicellular magnetotactic prokaryote. In Iron Biominerals. R. Frankel and R. Blakemore, editors. Plenum Press, New York. 231–238.
- Thorsten, R., D. Dommer, and J. Phillips. 2002. Shedding light on vertebrate magnetoreception. Neuron. 34:503–506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.