Abstract
We examine, using an analytical mean-field model, the distribution of cholesterol in a lipid bilayer. The model accounts for the perturbation of lipid packing induced by the embedded cholesterol, in a manner similar to that of transmembrane proteins. We find that the membrane-induced interactions between embedded cholesterol molecules vary as a function of the cholesterol content. Thus, the effective lipid-cholesterol interaction is concentration-dependent. Moreover, it transitions from repulsive to attractive to repulsive as the cholesterol content increases. As the concentration of cholesterol in the bilayer exceeds a critical value, phase separation occurs. The coexistence between cholesterol-rich and cholesterol-poor domains is universal for any bilayer parameters, although the composition of the cholesterol-rich phase varies as a function of the lipid properties. Although we do not assume specific cholesterol-lipid interactions or the formation of a lipid-cholesterol cluster, we find that the composition of the cholesterol-rich domains is constant, independent of the cholesterol content in the bilayer.
INTRODUCTION
Cholesterol is the most abundant molecule in mammalian plasma membranes (Gennis, 1989). The incorporation of cholesterol has been shown to modulate the packing of the phospholipid molecules in the membrane, thereby increasing bilayer rigidity and mechanical durability, and reducing passive permeability (Simons and Ikonen, 2000). Moreover, cholesterol partitioning into cholesterol- and sphingolipids-rich domains has been found to trigger the formation of “rafts”, which are implicated in such diverse membrane processes as signal transduction, protein stabilization, protein and lipid sorting, and membrane fusion (Brown and London, 1998).
Although cholesterol is essential for the proper functioning of cell membranes, excess cholesterol levels could prove detrimental: Excess cholesterol may precipitate to form cholesterol monohydrate crystals, which play a significant role in the course of diseases such as gallstones or plaque deposition in atherosclerosis (Small, 1980, 1988).
Understanding the effect of cholesterol on membrane properties and functions—as well as the triggers for the nucleation of cholesterol crystals—requires understanding of the phase behavior of cholesterol in lipid bilayers. Various studies examined cholesterol-lipid mixing in both biological and synthetic membranes (Bach and Wachtel, 2003; Finegold, 1993; McConnell and Radhakrishnan, 2003; Ohvo-Rekila et al., 2002; Silvius, 2003). As summarized in Fig. 1, in the limit of low cholesterol concentrations, lipids and cholesterol are uniformly mixed. As the concentration of cholesterol increases, the system undergoes a process reminiscent of phase separation to form cholesterol-rich domains coexisting with the dilute (gaslike) regions (Loura et al., 2001a,b; Veatch et al., 2004; Worthman et al., 1997). The composition of the cholesterol-rich domains, xo, varies as a function of the lipid type (acyl chain length, headgroup charge, and the system parameters—temperature, composition, and pressure) (Crane and Tamm, 2004; Ohvo-Rekila et al., 2002; Veatch and Keller, 2002; Veatch et al., 2004). Radhakrishnan and McConnell (2003, 1999) find that the interactions between the lipids and the cholesterol in the cholesterol-rich domains are so specific as to suggest the formation of a “molecular complex”.
FIGURE 1.
Schematic of the phase diagram of lipid-cholesterol bilayers. In the limit of dilute cholesterol concentration, the bilayer is uniformly mixed (ld). As the cholesterol concentration exceeds a critical value, domains form (lo). These have a fixed, preferred composition and they coexist with the dilute phase. With increasing cholesterol concentration, the fraction of the bilayer area taken by the cholesterol-rich domains increases until the entire bilayer is occupied by the cholesterol rich domains (namely, when the overall bilayer composition is equal to the cholesterol-rich domain composition). Above this value, a condensed cholesterol phase appears (lc). As the bilayer concentration exceeds another critical value, defined as the solubility limit, cholesterol crystals develop.
As the concentration of cholesterol in the bilayer increases, so does the fraction of the area occupied by the cholesterol domains (Crane and Tamm, 2004). Once the overall system composition reaches xo, the domains occupy the entire membrane area. However, although the composition of the cholesterol-rich domains clearly corresponds to a preferred state, it does not define the maximal cholesterol solubility in the bilayer: The overall concentration of cholesterol may be increased up to a much higher value—defined as the solubility limit—at which cholesterol crystals appear (Huang et al., 1999). The main parameters controlling the solubility limit were found to be the sample preparation method, lipid type, length, and degree of unsaturation of acyl chains, presence of charge on lipid headgroup, and interheadgroup hydrogen bonds (Bach and Wachtel, 2003; Huang et al., 1999). For example, Huang et al. (1999) recently showed that at room temperature, the maximum solubility limit of cholesterol is 66 mol % in phosphatidylcholine bilayers and 51 mol % in phosphatidylethanolamine bilayers. However, the accuracy of these values may be questioned, since the solubility limit is commonly measured through the detection of cholesterol crystallites in solution (Bach and Wachtel, 2003), although recent studies suggest that crystallites initially nucleate and exist within the membrane (Epand et al., 2003; Troup et al., 2003).
Several theoretical studies (Chiu et al., 2002, 2001; Finegold, 1993; Hjort Ipsen et al., 1987; Hofsass et al., 2003; Huang, 2002; Huang and Feigenson, 1999; Pandit et al., 2004; Pasenkiewicz-Gierula et al., 2000; Robinson et al., 1995; Scott, 1991; Smondyrev and Berkowitz, 1999; Smondyrev and Berkowitz, 2001; Tu et al., 1998) examined the phase behavior of cholesterol-lipid bilayers using atomic level simulation methods such as Monte Carlo and molecular dynamics methods. As a rule, they use a two-dimensional thermodynamic lattice model, with an interaction parameter between the cholesterol and the lipids calculated by accounting for the interactions between the lipid acyl chains and the cholesterol molecules (Chiu et al., 2002; Hjort Ipsen et al., 1987; Huang, 2002; Huang and Feigenson, 1999, and references within). Although these studies can reproduce some aspects of the system, they are limited due to the fact that mixed membranes are not a simple two-dimensional mixture. As has been shown (Aranda-Espinoza et al., 1996; Bartolo et al., 2003; Bartolo and Fournier, 2003; Biscari and Bisi, 2002; Cantor, 1997a,b, 2002; Dan et al., 1994, 1993; Dan and Safran, 1995, 1998; Fattal and Ben-Shaul, 1995; Goulian, 1996; Lague et al., 2001, 2000; Lipowsky, 2002; Marcelja, 1976; May, 2000a,b, 2002; May and Ben-Shaul, 1999, 2000; Mouritsen and Bloom, 1993; Nielsen and Andersen, 2000; Nielsen et al., 1998; Pata and Dan, 2003; Wiggins and Phillips, 2004; Sintes and Baumgaertner, 1998; Weikl, 2001, 2002; Weikl et al., 1998), mixing between lipids and inclusions in bilayers may give rise to perturbations that are expressed by changes in the bilayer density, thickness, and energy. Indeed, Kessel et al. (2001) have shown, using a semimolecular model, that, in the limit of low cholesterol content, the elastic response of the neighboring lipids predominantly determines spatial fluctuations of cholesterol in the lipid bilayer.
The energy associated with membrane perturbation due to cholesterol may be considered as an additional component of the cholesterol-lipid interaction energy that may be incorporated in a lattice model. However, as has been shown (Aranda-Espinoza et al., 1996; Bartolo et al., 2003; Bartolo and Fournier, 2003; Biscari and Bisi, 2002; Dan et al., 1994, 1993; Golestanian et al., 1996a,b; Goulian, 1996; Goulian et al., 1993a,b; Lague et al., 2000; Sintes and Baumgaertner, 1998; Taulier et al., 2002; Weikl, 2001, 2002; Weikl et al., 1998), the perturbation energy arising from inclusions is highly dependent on the inclusion spacing or density. Thus, to truly understand the mixing of lipids with cholesterol, one must examine the effect of the mixing on the local bilayer properties.
In this article, we use a mean-field approach to examine the effect of cholesterol in the bilayer on the structure and properties of a synthetic membrane. The model accounts for the asymmetric shape of the cholesterol molecules, and thus to the perturbation they impose on the bilayer lipids. As sketched in Fig. 2, lipids adjacent to a cholesterol molecule are perturbed due to both a thickness mismatch and a packing (angular) mismatch. This perturbation causes an energetic penalty that is dependent on the separation between neighboring cholesterol molecules. The model is semimolecular in the sense that it incorporates some molecular parameters (e.g., the lipid density and bilayer thickness) into a mean-field type analysis (Cantor 1999b; Dan et al., 1994, 1993; Dan and Safran, 1998; Goulian, 1996; Goulian et al., 1993a).
FIGURE 2.
Schematic of a lipid-cholesterol bilayer. Lm is the height of a monolayer and Lc is the height of a cholesterol (inclusion) molecule, 2d is the distance between inclusions, z is the distance from the inclusion boundary, and θ is the contact angle between the inclusion and the monolayer. Cholesterol perturbs the surrounding lipids due to a thickness mismatch and an angular mismatch.
One might expect that, since cholesterol perturbs the lipid packing, the membrane perturbation energy will be minimal when no cholesterol is present. However, we find that the free energy of the bilayer is minimal at a fixed cholesterol composition, xo, which varies as a function of the lipid type. As a result, increasing the cholesterol molar concentration in the bilayer above a relatively low value leads to the formation of domains whose composition is set by xo (see Fig. 1): Further addition of cholesterol simply increases the fraction of bilayer area occupied by the domains, but does not affect the domain composition. When the overall cholesterol concentration in the bilayer exceeds xo (i.e., the entire membrane is taken up by the cholesterol domains), we find a transition into a one-phase cholesterol-condensed region where cholesterol is uniformly distributed in the bilayer.
MEMBRANE MODEL
Consider a membrane section of a cholesterol/phospholipid bilayer (Fig. 2). The membrane is taken to be composed of only one type of lipid, and the cholesterol to be distributed uniformly between the two monolayers. As a result, the system is symmetrical around the bilayer midplane.
The embedded cholesterol distorts the arrangement of the lipids in two ways: the first is due to a thickness mismatch, since the thickness of a cholesterol molecule (∼1.7 nm) is smaller than that of a typical monolayer (∼2 nm) (Yeagle, 1988). The second is due to the cholesterol structure: Molecular dynamics simulations show that the hydrophobic core of cholesterol is buried in the hydrocarbon region of the bilayer and that, on average, the molecule is tilted with respect to the bilayer normal, with a tilt angle of ∼14° (Kessel et al., 2001). The rearrangement, or perturbation of membrane lipids incurred due to the thickness and angle mismatch with the cholesterol inclusion, increases the membrane energy when compared to a uniform (cholesterol-free) membrane, in the same way that protein inclusions do (Bezrukov, 2000; Dan et al., 1994, 1993; Dan and Safran, 1995, 1998; May 2000b, 2002; Nielsen et al., 1998).
For simplicity, we apply a one-dimensional model, namely, examining the perturbation as a function of distance in one dimension only (Fig. 2): Although obviously an oversimplification, previous analysis has shown that the one-dimensional model yields qualitatively and quantitatively similar results to that of the two-dimensional one in liposome-protein systems (Aranda-Espinoza et al., 1996).
The lipid bilayer is a self-assembled structure: properties such as the area per lipid 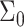 and monolayer thickness
and monolayer thickness 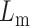 are determined by the lipid chemistry (Israelachvili, 1992), setting a free energy per lipid in the membrane of
are determined by the lipid chemistry (Israelachvili, 1992), setting a free energy per lipid in the membrane of 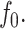 The incorporation of an inclusion may lead to a perturbation in the lipid packing, which incurs an energetic penalty. In the case of thickness mismatched inclusions, one may define the perturbation profile through a reduced thickness parameter:
The incorporation of an inclusion may lead to a perturbation in the lipid packing, which incurs an energetic penalty. In the case of thickness mismatched inclusions, one may define the perturbation profile through a reduced thickness parameter: 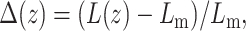 where
where 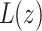 is the thickness of the perturbed monolayer,
is the thickness of the perturbed monolayer, 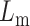 is the equilibrium monolayer thickness, and z is the distance from the inclusion boundary. At the inclusion boundary, Δ is defined by the difference between the inclusion thickness and the lipid bilayer thickness. In the case of a “packing” perturbation such as that incurred by cholesterol, the inclusion perturbs the packing of the neighboring lipids, namely, the area per molecule. However, the area per lipid and membrane thickness are coupled through an equation of state (Dan et al., 1994, 1993; Nielsen et al., 1998), so that
is the equilibrium monolayer thickness, and z is the distance from the inclusion boundary. At the inclusion boundary, Δ is defined by the difference between the inclusion thickness and the lipid bilayer thickness. In the case of a “packing” perturbation such as that incurred by cholesterol, the inclusion perturbs the packing of the neighboring lipids, namely, the area per molecule. However, the area per lipid and membrane thickness are coupled through an equation of state (Dan et al., 1994, 1993; Nielsen et al., 1998), so that 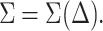 Thus, any type of membrane perturbation may be described through the local thickness profile (see Fig. 2).
Thus, any type of membrane perturbation may be described through the local thickness profile (see Fig. 2).
The membrane thickness profile varies as a function of the distance from the perturbation focal point: At the inclusion boundary, it is set by the inclusion properties; far away it decays to the unperturbed membrane value, i.e., 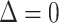 or
or 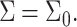 However, in membranes where the density of inclusions (e.g., proteins, cholesterol) is high and the average distance between inclusions small, the thickness perturbation may remain nonzero throughout the system. The change in monolayer energy (per inclusion) due to insertion of two inclusions, a distance 2d apart, is (Dan et al., 1993)
However, in membranes where the density of inclusions (e.g., proteins, cholesterol) is high and the average distance between inclusions small, the thickness perturbation may remain nonzero throughout the system. The change in monolayer energy (per inclusion) due to insertion of two inclusions, a distance 2d apart, is (Dan et al., 1993)
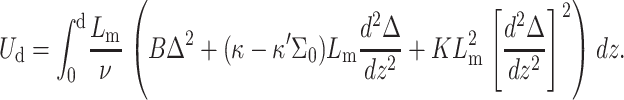 |
(1) |
The first contribution in Eq. 1 is due to packing constraints; B, the monolayer compressibility, describes the energy penalty for perturbation of the local density from equilibrium (namely, 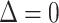 or
or 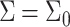 ). B is defined as
). B is defined as 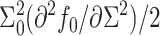 evaluated at
evaluated at 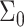 (Dan et al., 1993). The second term accounts for the energy cost when the interface curvature does not match the preferred spontaneous curvature: The monolayer spontaneous curvature,
(Dan et al., 1993). The second term accounts for the energy cost when the interface curvature does not match the preferred spontaneous curvature: The monolayer spontaneous curvature, 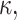 determines the sign and magnitude of the free interface curvature of the monolayer at an oil-water interface. The change in spontaneous curvature is given by
determines the sign and magnitude of the free interface curvature of the monolayer at an oil-water interface. The change in spontaneous curvature is given by 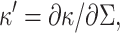 evaluated at the equilibrium bilayer surface density
evaluated at the equilibrium bilayer surface density 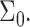 In this article, we focus on bilayer-forming lipids, and in particular on molecules for which the spontaneous curvature,
In this article, we focus on bilayer-forming lipids, and in particular on molecules for which the spontaneous curvature, 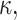 and its derivative,
and its derivative, 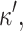 are zero. The third contribution is due to the bending energy of the monolayer, where K is the bending modulus.
are zero. The third contribution is due to the bending energy of the monolayer, where K is the bending modulus. 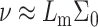 is the volume of a lipid molecule. All energies are given in units of kT, where k is the Boltzmann coefficient and T the temperature.
is the volume of a lipid molecule. All energies are given in units of kT, where k is the Boltzmann coefficient and T the temperature.
The separation, d, between the inclusions depends on their concentration in the membrane. For a single inclusion, the number of lipid molecules per inclusion is 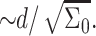 So, the total number of molecules per inclusion is
So, the total number of molecules per inclusion is 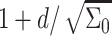 and the inclusion mole fraction is
and the inclusion mole fraction is
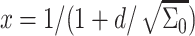 |
(2) |
To calculate the inclusion-induced bilayer perturbation profile and perturbation energy, the free energy (Eq. 1) must be minimized consistently with respect to the optimal perturbation profile. Boundary conditions for the system include a thickness mismatching condition at the inclusion/bilayer boundary (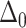 defines the reduced thickness difference) and a symmetry-enforcing condition, namely,
defines the reduced thickness difference) and a symmetry-enforcing condition, namely, 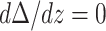 at the midpoint between two inclusions (z = d). The third boundary condition is set by the slope of the inclusion at the inclusion-monolayer boundary,
at the midpoint between two inclusions (z = d). The third boundary condition is set by the slope of the inclusion at the inclusion-monolayer boundary, 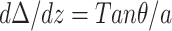 at z = 0, where a is a molecular length scale, and the fourth boundary condition is set by minimization requirements and reads
at z = 0, where a is a molecular length scale, and the fourth boundary condition is set by minimization requirements and reads
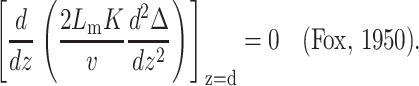 |
Equation 1 may be minimized to yield the perturbation profile and energy in a general way for any value of K, B,and the inclusion mole fraction x(d); however, although the values of the membrane thickness and surface density are well known for a variety of systems, measurements of K and B are not readily available. Thus, we use a molecular model to relate lipid properties to the bilayer parameters (Milner and Witten, 1988; Szleifer et al., 1988; Viovy et al., 1987). Although the model was developed for block copolymers (namely, both the head and tail are taken to be flexible chains), this approach has been shown (Szleifer et al., 1990, 1988) to yield qualitatively, and even reasonably quantitatively, correct results for several amphiphilic systems. For amphiphiles where the spontaneous curvature is zero (namely, 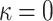 ), the free energy coefficients are given by (Milner and Witten, 1988; Szleifer et al., 1988; Viovy et al., 1987)
), the free energy coefficients are given by (Milner and Witten, 1988; Szleifer et al., 1988; Viovy et al., 1987)
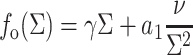 |
(3a) |
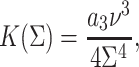 |
(3b) |
where a1 and a3 have the dimensions of a length scale of molecular size (Milner and Witten, 1988). Using the above equations and the relationship 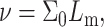 we obtain
we obtain
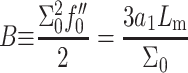 |
(4a) |
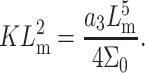 |
(4b) |
RESULTS
Minimization of the bilayer free energy with respect to Δ yields the “optimal” perturbation profile—that is, the profile that minimizes the energetic cost associated with inclusion-induced membrane perturbation. For a single inclusion (namely, cholesterol at infinite dilution), we find that the energetic penalty associated with bilayer perturbation is given by
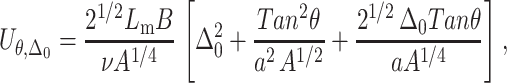 |
(5) |
where 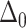 defines the reduced thickness mismatch at the cholesterol boundary and
defines the reduced thickness mismatch at the cholesterol boundary and 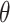 the contact angle between the membrane and the inclusion (see Fig. 1). A is the characteristic perturbation decay length—namely, the distance at which the membrane regains, more or less, its unperturbed characteristics. The perturbation length varies as a function of the membrane compressibility and bending modulus through the relationship
the contact angle between the membrane and the inclusion (see Fig. 1). A is the characteristic perturbation decay length—namely, the distance at which the membrane regains, more or less, its unperturbed characteristics. The perturbation length varies as a function of the membrane compressibility and bending modulus through the relationship 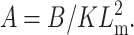 Thus, for similar bending moduli, the distance at which the bilayer regains its equilibrium thickness increases with increasing compression modulus, or resistance to area changes.
Thus, for similar bending moduli, the distance at which the bilayer regains its equilibrium thickness increases with increasing compression modulus, or resistance to area changes.
Equation 5 defines the energetic penalty for embedding a single cholesterol inclusion in a membrane. It constitutes three contributions that correspond to the three terms: The first is due to the thickness mismatch between the membrane and the inclusion. The second is due to the packing mismatch (i.e., “contact angle”) between the membrane and the inclusion, and the last is a cross term that accounts for the interrelationship between the two types of penalties. In the case of cholesterol inclusions, we may assign some values to the different terms in Eq. 5:  is typically of order −0.15 (Yeagle, 1988) and θ is of order
is typically of order −0.15 (Yeagle, 1988) and θ is of order 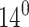 (Tu et al., 1998). The membrane correlation length, A, depends on the lipid type, but is usually of order 1 nm−4 (Nagle and Tristram-Nagle, 2000). Thus, we find that, for cholesterol, the dominant term in Eq. 5 by far is the
(Tu et al., 1998). The membrane correlation length, A, depends on the lipid type, but is usually of order 1 nm−4 (Nagle and Tristram-Nagle, 2000). Thus, we find that, for cholesterol, the dominant term in Eq. 5 by far is the 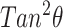 term. In further discussions, we will therefore neglect the contributions arising from the thickness mismatch, focusing instead on the contact angle.
term. In further discussions, we will therefore neglect the contributions arising from the thickness mismatch, focusing instead on the contact angle.
The perturbation profile associated with inclusion of a cholesterol molecule into a membrane, neglecting the thickness mismatch, is then given by
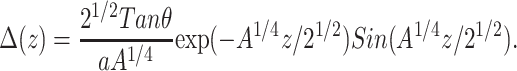 |
(6) |
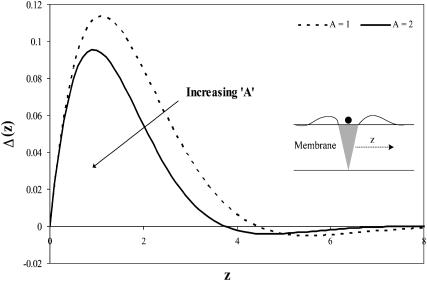
In Fig. 3, we plot the thickness profile of a monolayer containing a single cholesterol inclusion. Despite the fact that we neglect the thickness mismatch between the cholesterol and the lipid monolayer, we see that the thickness of the monolayer is indeed perturbed by the cholesterol inclusion. This is due to the coupling between the membrane packing (surface area per molecule) and the thickness. Indeed, as in the case of thickness-mismatched inclusion/membrane systems (Aranda-Espinoza et al., 1996; Dan et al., 1993; Nielsen et al., 1998), the membrane thickness decays to its unperturbed value within a distance of order (3–4 Lm) from the cholesterol boundary, which typically corresponds to 6–8 nm. Thus, in systems where the cholesterol spacing in the bilayer is >∼8 nm (which corresponds to ∼0.08 mol fraction cholesterol), interactions between the cholesterol molecules may be neglected. However, above this relatively low cholesterol content, interactions between the cholesterol inclusions must be accounted for.
FIGURE 3.
Perturbation profile of a monolayer containing a single cholesterol molecule (Eq. 6), as a function of distance from the inclusion boundary, z. The thickness profile of the monolayer is defined as 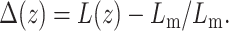 Despite the thickness matching, there is a monolayer thickness perturbation near the inclusion, the amplitude of which depends on A, the ratio of the monolayer compression modulus to the bending stiffness.
Despite the thickness matching, there is a monolayer thickness perturbation near the inclusion, the amplitude of which depends on A, the ratio of the monolayer compression modulus to the bending stiffness.
Calculating the membrane (optimal) perturbation profile for membranes containing cholesterol molecules that are separated by a distance 2d yields
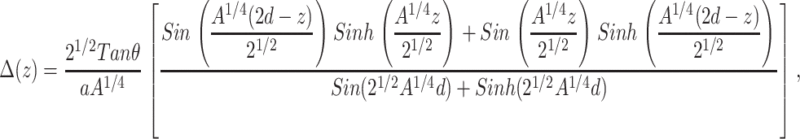 |
(7) |
where 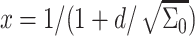 is the cholesterol mole fraction. Substituting this profile into the expression for the energy (relative to the dilute limit) yields
is the cholesterol mole fraction. Substituting this profile into the expression for the energy (relative to the dilute limit) yields
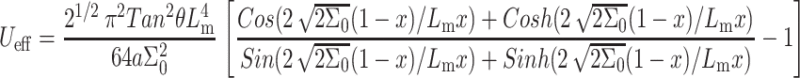 |
(8) |
Ueff defines the membrane energetic penalty, per inclusion, due to the incorporation of x mole fraction cholesterol. It is composed of two contributions: one is the energetic penalty associated with the insertion of the individual cholesterol into the bilayer (i.e., Eq. 5); the other component is due to the membrane-induced interactions between cholesterol molecules.
In Fig. 4, we plot the membrane perturbation energy as a function of the cholesterol mole fraction (which is inversely proportional to the average distance between cholesterol molecules). The value of Ueff = 0.708 kT/nm defines the energy required to insert a single cholesterol molecule (Eq. 5). We see that for low cholesterol mole fractions, Ueff remains constant, thereby indicating that the energetic penalty associated with cholesterol incorporation remains constant, or that the cholesterol perturbation domains do not overlap (i.e., no membrane-induced interactions between cholesterol molecules). As x increases, we see a very slight increase in the energy corresponding to a region where the perturbed areas of the membrane start to overlap. Quite surprisingly, however, we see a wide region (in the case shown in Fig. 4, it corresponds to 0.22 ≤ x ≤ 0.5) where Ueff decreases below the value of the dilute, single inclusion, reaching a minimum at x = xo (≈0.36 in this case). The location of this minimum depends on the bilayer characteristics, and is given approximately by xo ≈ (1 + 1/√2Σ0/Lm)−1.
FIGURE 4.
Membrane perturbation penalty per inclusion, Eq. 8, as a function of the cholesterol mole fraction, x. For a given membrane, the free energy of the system is at a global minimum at a finite composition, defined by xo, which depends on the type of lipid. Here Lm = 1.7 nm and Σo = 0.4 nm2. The inset shows the membrane perturbation profile at x = 0.36, namely, where Ueff is minimal. The perturbation decay length is taken to be A = 1 nm−4.
The minimum in Ueff as a function of x indicates an effectively attractive interaction between the embedded cholesterol molecules—namely, that the penalty associated with membrane perturbation is reduced due to interactions between the perturbation profiles of neighboring cholesterol inclusions.
Why would the bilayer, which is obviously perturbed by the cholesterol inclusions, favor a moderately close packing rather than complete phase separation (between pure cholesterol and pure lipid domains)? To understand this, we must reexamine the membrane thickness profile. As shown in Fig. 3, in the dilute cholesterol case, the membrane thickness increases and then decreases to the unperturbed value. However, if the cholesterol density is higher (see inset in Fig. 4), the membrane thickness does not decay back to the unperturbed value, thereby reducing the curvature penalty.
Once the concentration of cholesterol increases above a critical value (in this case, when x ≈ 0.5), the perturbation energy increases sharply, indicating a region where increasing the cholesterol concentration is highly unfavorable, or, alternately, where the membrane-induced cholesterol-cholesterol interactions become strongly repulsive.
The membrane perturbation energy, Ueff, indicates that there is an effectively attractive interaction between cholesterol molecules embedded in a lipid bilayer, which favors regions with a specific cholesterol mole fraction: The energy associated with the membrane perturbation may be reduced by forming cholesterol domains whose composition is given by xo. This suggests that, if the overall cholesterol content in the bilayer is lower than xo, the membrane will phase-separate into domains whose composition corresponds to xo and regions where the cholesterol content is low.
To correctly evaluate the mixing phase diagram of cholesterol in the bilayer, we cannot focus only on the membrane-induced interactions. We need to calculate the overall system free energy, which includes the interaction energy, Ueff, the two-dimensional mixing entropy, and the direct interactions between cholesterol molecules in the bilayer.
The direct interactions between membrane-embedded cholesterol molecules are composed of two contributions: The first, which is attractive, is due to van der Waals forces (Yeagle, 1988). The second, which is effectively repulsive, is due to an interfacial penalty associated with the increased cholesterol exposure to water in cholesterol-only domains when compared to the lipid “shielding” effect (Huang and Feigenson, 1999). Both of these are negligible when compared to the membrane perturbation penalty, except in extremely high concentrations when x ≈ 1 or d ≈ 0. Since in this analysis we focus on moderate cholesterol concentrations, we neglect these contributions. Thus, the membrane mixing free energy per unit width is given by
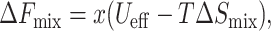 |
(9) |
where 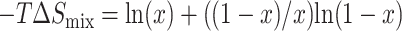 is the dimensionless entropy of mixing in a two-component system, per inclusion (see, for example, Dill and Bromberg, 2002).
is the dimensionless entropy of mixing in a two-component system, per inclusion (see, for example, Dill and Bromberg, 2002).
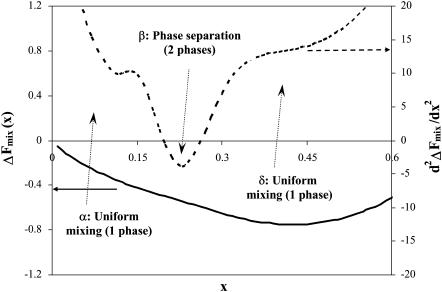
The mixing behavior, or phase diagram at a fixed temperature T of the cholesterol-lipid systems, is set by the value of 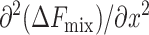 at the given mixture composition, x: If the second derivative is positive, the mixed system is stable. If it is negative, the system separates into coexisting domains. As shown in Fig. 5, we find that (as expected from Ueff) in the limit of either dilute cholesterol limit or high concentrations, the system favors mixing (regions α and δ). However, in an intermediate range, we find a region, β, where the system undergoes phase separation into cholesterol-rich and cholesterol-poor domains. The composition of the cholesterol-rich domains is set by xo, the composition at which the bilayer energy is minimal. (The composition of the coexisting phases may be calculated using the Maxwell construction. Due to the distinct minimum in Ueff at xo, which dominates ΔFmix, we find that the composition of the cholesterol-rich phase is closely associated with xo.) The range of compositions at which domain coexistence appears varies as a function of the lipid characteristics, namely, area per molecule and bilayer thickness.
at the given mixture composition, x: If the second derivative is positive, the mixed system is stable. If it is negative, the system separates into coexisting domains. As shown in Fig. 5, we find that (as expected from Ueff) in the limit of either dilute cholesterol limit or high concentrations, the system favors mixing (regions α and δ). However, in an intermediate range, we find a region, β, where the system undergoes phase separation into cholesterol-rich and cholesterol-poor domains. The composition of the cholesterol-rich domains is set by xo, the composition at which the bilayer energy is minimal. (The composition of the coexisting phases may be calculated using the Maxwell construction. Due to the distinct minimum in Ueff at xo, which dominates ΔFmix, we find that the composition of the cholesterol-rich phase is closely associated with xo.) The range of compositions at which domain coexistence appears varies as a function of the lipid characteristics, namely, area per molecule and bilayer thickness.
FIGURE 5.
Second derivative of the system free energy (Eq. 9) as a function of cholesterol mole fraction x for 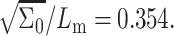
As the average mole fraction of cholesterol in the phase-separated region increases, so does the fraction of bilayer area occupied by the cholesterol-rich domains. Once x exceeds that of the cholesterol-rich domains (∼xo), the membrane enters another uniformly mixed phase—the cholesterol-dense phase. As shown by Fig. 4, in this region the membrane perturbation energy per cholesterol increases almost linearly with the cholesterol mole fraction.
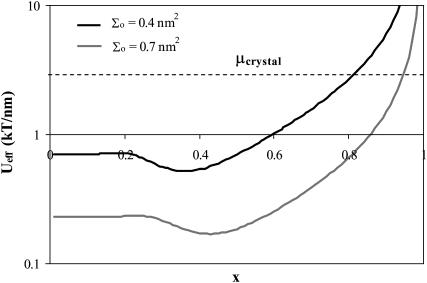
One issue of interest is the nucleation of cholesterol crystals. Crystallite nucleation can take place via two routes. In the first one, excess cholesterol remains in solution, thereby nucleating crystallites in the bulk suspension (Huang et al., 1999; Huang and Feigenson, 1999). Alternately, the excess cholesterol may segregate in the bilayer into condensed cholesterol domains, which coalesce with time and precipitate into solution over time (Troup et al., 2003). In either case, cholesterol will accumulate in the bilayer until the chemical potential of the cholesterol in the membrane becomes equal to that of cholesterol in the monohydrate crystals, or (neglecting the mixing entropy in all phases) when Ueff =μcrystal. Unfortunately, estimating the chemical potential of cholesterol in monohydrate crystals (whether within the bilayer or in bulk suspension) is outside the domain of this study: It accounts for a combination of the molecular, short-range cholesterol-cholesterol interactions as a function of the cholesterol organization in the crystal, as well as the interfacial tension between the crystal and the surrounding solution. However, we may qualitatively estimate the effect of bilayer characteristics on the maximum solubility limit through evaluation of the effect of these parameters on Ueff in the limit of high cholesterol content. As shown in Fig. 6, we find that (for a given bilayer thickness) Ueff decreases with increasing lipid surface density 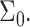 Thus, we conclude that the critical solubility limit shifts toward higher values for lipids with a larger headgroup area
Thus, we conclude that the critical solubility limit shifts toward higher values for lipids with a larger headgroup area 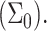 This result is in qualitative agreement with the experimental results of Huang et al. (1999), where it was shown that, comparing phosphatidylcholines versus phosphatidylethanolamine bilayers, the maximum solubility limit increases with the headgroup area.
This result is in qualitative agreement with the experimental results of Huang et al. (1999), where it was shown that, comparing phosphatidylcholines versus phosphatidylethanolamine bilayers, the maximum solubility limit increases with the headgroup area.
FIGURE 6.
Ueff as a function of x for two different lipid surface densities (but Lm is the same). The maximal solubility limit is defined as the x value at which Ueff exceeds the chemical potential of the cholesterol in the crystal. We see that (regardless of the value of μcrystal) the solubility limit will be higher for the higher density bilayer. Note that the y axis is on a log scale.
DISCUSSION AND CONCLUSIONS
In this article, we develop a mean-field model for cholesterol-lipid bilayers. The model accounts for the cholesterol-induced perturbation of the bilayer, which gives rise to an energetic penalty whose magnitude is sensitive to the cholesterol content in the bilayer. One of the main conclusions from our analysis is that the enthalpy of mixing between cholesterol and lipids in the bilayer cannot be accurately described using a constant interaction potential that is concentration-independent, as done in standard mean-field lattice models (i.e., writing the energy as Ux(1 − x), where U is independent of x) (Chiu et al., 2002; Huang, 2002; Huang and Feigenson, 1999; Pasenkiewicz-Gierula et al., 2000). In fact, we find that the nature of the cholesterol interactions, let alone their numerical value, changes as a function of the cholesterol content: At low cholesterol concentrations, the membrane-induced interactions between cholesterol molecules are repulsive, favoring uniform distribution. As the concentration exceeds above a certain value, the interactions turn positive, favoring a specific distance between cholesterol molecules embedded in the bilayer, thereby leading to phase separation between cholesterol-rich and cholesterol-poor domains. As the concentration increases above a second critical value, the interactions become repulsive again, favoring uniform mixing.
The composition of the cholesterol-rich domains, 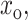 is found to vary as a function of the lipid type (Fig. 4), but to be largely independent of the overall cholesterol composition in the system. This is in agreement with the experiments of Radhakrishnan and McConnell (McConnell and Radhakrishnan, 2003; Radhakrishnan et al., 2000; Radhakrishnan and McConnell, 1999), who find that the interactions between the lipids and the cholesterol in the domains formed in monolayers at the air-liquid interface are so specific as to suggest the formation of a “molecular complex”. This is in qualitative agreement with our analysis, which demonstrates that the system energy is greatly minimized upon the formation of regions characterized by a specific cholesterol/lipid ratio. In summary, we developed a model to understand the influence of membrane characteristics on domain formation and phase separation in binary mixtures of lipids and cholesterol. We find that the nature and magnitude of the membrane-mediated interactions between cholesterol molecules change as a function of the cholesterol content. As a result (Fig. 7), we find that the cholesterol-lipid bilayer transitions from a homogeneous, dilute (gaslike) at low cholesterol content to a phase-separated coexistence between cholesterol-poor and cholesterol-rich domains, followed at higher cholesterol concentrations by another transition to a uniform phase. The composition of the cholesterol-rich domains in the phase-separated regime is largely set by the lipid characteristics and is independent of the cholesterol concentration.
is found to vary as a function of the lipid type (Fig. 4), but to be largely independent of the overall cholesterol composition in the system. This is in agreement with the experiments of Radhakrishnan and McConnell (McConnell and Radhakrishnan, 2003; Radhakrishnan et al., 2000; Radhakrishnan and McConnell, 1999), who find that the interactions between the lipids and the cholesterol in the domains formed in monolayers at the air-liquid interface are so specific as to suggest the formation of a “molecular complex”. This is in qualitative agreement with our analysis, which demonstrates that the system energy is greatly minimized upon the formation of regions characterized by a specific cholesterol/lipid ratio. In summary, we developed a model to understand the influence of membrane characteristics on domain formation and phase separation in binary mixtures of lipids and cholesterol. We find that the nature and magnitude of the membrane-mediated interactions between cholesterol molecules change as a function of the cholesterol content. As a result (Fig. 7), we find that the cholesterol-lipid bilayer transitions from a homogeneous, dilute (gaslike) at low cholesterol content to a phase-separated coexistence between cholesterol-poor and cholesterol-rich domains, followed at higher cholesterol concentrations by another transition to a uniform phase. The composition of the cholesterol-rich domains in the phase-separated regime is largely set by the lipid characteristics and is independent of the cholesterol concentration.
FIGURE 7.
Schematic of the coexisting phases in a lipid-cholesterol bilayer: (a) at low concentrations, cholesterol is uniformly distributed in the bilayer; (b) as the concentration is increased, lipid-cholesterol domains start to form; and (c) above a certain cholesterol concentration, cholesterol-rich domains coexist with the lipid-cholesterol domains.
Acknowledgments
The support of the Louis and Bessie Stein Foundation and the Pennsylvania Nanotechnology Institute (administered by the Ben Franklin Associates) is acknowledged.
References
- Aranda-Espinoza, H., A. Berman, N. Dan, P. Pincus, and S. Safran. 1996. Interaction between inclusions embedded in membranes. Biophys. J. 71:648–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach, D., and E. Wachtel. 2003. Phospholipid/cholesterol model membranes: formation of cholesterol crystallites. BBA-Biomembranes. 1610:187–197. [DOI] [PubMed] [Google Scholar]
- Bartolo, D., A. Ajdari, and J. B. Fournier. 2003. Effective interactions between inclusions in complex fluids driven out of equilibrium. Phys. Rev. E. 67:061112. [DOI] [PubMed] [Google Scholar]
- Bartolo, D., and J. B. Fournier. 2003. Elastic interaction between “hard” or “soft” pointwise inclusions on biological membranes. Eur. Phys. J. E. 11:141–146. [DOI] [PubMed] [Google Scholar]
- Bezrukov, S. M. 2000. Functional consequences of lipid packing stress. Curr. Opin. Colloid In. 5:237–243. [Google Scholar]
- Biscari, P., and F. Bisi. 2002. Membrane-mediated interactions of rod-like inclusions. Eur. Phys. J. E. 7:381–386. [DOI] [PubMed] [Google Scholar]
- Brown, D. A., and E. London. 1998. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14:111–136. [DOI] [PubMed] [Google Scholar]
- Cantor, R. S. 1997. Lateral pressures in cell membranes: a mechanism for modulation of protein function. J. Phys. Chem. B. 101:1723–1725. [Google Scholar]
- Cantor, R. S. 1999a. The influence of membrane lateral pressures on simple geometric models of protein conformational equilibria. Chem. Phys. Lipids. 101:45–56. [DOI] [PubMed] [Google Scholar]
- Cantor, R. S. 1999b. Lipid composition and the lateral pressure profile in bilayers. Biophys. J. 76:2625–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor, R. S. 2002. Size distribution of barrel-stave aggregates of membrane peptides. Biophys. J. 82:2520–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu, S. W., E. Jakobsson, R. J. Mashl, and H. L. Scott. 2002. Cholesterol-induced modifications in lipid bilayers: a simulation study. Biophys. J. 83:1842–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu, S. W., E. Jakobsson, and H. L. Scott. 2001. Combined Monte Carlo and molecular dynamics simulation of hydrated lipid-cholesterol lipid bilayers at low cholesterol concentration. Biophys. J. 80:1104–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane, J. M., and L. K. Tamm. 2004. Role of cholesterol in the formation and nature of lipid rafts in planar and spherical model membranes. Biophys. J. 86:2965–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan, N., A. Berman, P. Pincus, and S. A. Safran. 1994. Membrane-induced interactions between inclusions. J. Phys. II. 4:1713–1725. [Google Scholar]
- Dan, N., P. Pincus, and S. A. Safran. 1993. Membrane-induced interactions between inclusions. Langmuir. 9:2768–2771. [Google Scholar]
- Dan, N., and S. A. Safran. 1995. Solubilization of proteins in membranes. Isr. J. Chem. 35:37–40. [Google Scholar]
- Dan, N., and S. A. Safran. 1998. Effect of lipid characteristics on the structure of transmembrane proteins. Biophys. J. 75:1410–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill, K. A., and S. Bromberg. 2002. Molecular Driving Forces. Garland Science, New York.
- Epand, R. M., D. W. Hughes, B. G. Sayer, N. Borochov, D. Bach, and E. Wachtel. 2003. Novel properties of cholesterol-dioleoylphosphatidylcholine mixtures. BBA-Biomembranes. 1616:196–208. [DOI] [PubMed] [Google Scholar]
- Fattal, D.R., and A. Ben-Shaul. 1995. Lipid chain packing and lipid-protein interaction in membranes. Physica A. 220:192–216. [Google Scholar]
- Finegold, L. 1993. Cholesterol in Membrane Models. CRC Press, Boca Raton, FL.
- Fox, C. 1950. An introduction to the calculus of variations. Oxford Press, Oxford, UK.
- Gennis, R. B. 1989. Biomembranes: Molecular Structure and Function. Springer-Verlag, New York.
- Golestanian, R., M. Goulian, and M. Kardar. 1996a. Fluctuation-induced interactions between rods on a membrane. Phys. Rev. E. 54:6725–6734. [DOI] [PubMed] [Google Scholar]
- Golestanian, R., M. Goulian, and M. Kardar. 1996b. Fluctuation-induced interactions between rods on membranes and interfaces. Europhys. Lett. 33:241–245. [DOI] [PubMed] [Google Scholar]
- Goulian, M. 1996. Inclusions in membranes. Curr. Opin. Colloid In. 1:358–361. [Google Scholar]
- Goulian, M., R. Bruinsma, and P. Pincus. 1993a. Long-range forces in heterogeneous fluid membranes. Europhys. Lett. 22:145–150. [Google Scholar]
- Goulian, M., R. Bruinsma, and P. Pincus. 1993b. Long-range forces in heterogeneous fluid membranes. Europhys. Lett. 23:155–155. [Google Scholar]
- Hjort Ipsen, J., G. Karlstrom, O. G. Mourtisen, H. Wennerstrom, and M. J. Zuckermann. 1987. Phase equilibria in the phosphatidylcholine-cholesterol system. BBA-Biomembranes. 905:162–172. [DOI] [PubMed] [Google Scholar]
- Hofsass, C., E. Lindahl, and O. Edholm. 2003. Molecular dynamics simulations of phospholipid bilayers with cholesterol. Biophys. J. 84:2192–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J. 2002. Exploration of molecular interactions in cholesterol superlattices: effect of multibody interactions. Biophys. J. 83:1014–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J. Y., J. T. Buboltz, and G. W. Feigenson. 1999. Maximum solubility of cholesterol in phosphatidylcholine and phosphatidylethanolamine bilayers. BBA-Biomembranes. 1417:89–100. [DOI] [PubMed] [Google Scholar]
- Huang, J. Y., and G. W. Feigenson. 1999. A microscopic interaction model of maximum solubility of cholesterol in lipid bilayers. Biophys. J. 76:2142–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelachvili, J. N. 1992. Intermolecular and Surface Forces. Academic Press, London.
- Kessel, A., N. Ben-Tal, and S. May. 2001. Interactions of cholesterol with lipid bilayers: the preferred configuration and fluctuations. Biophys. J. 81:643–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lague, P., M. J. Zuckermann, and B. Roux. 2000. Lipid-mediated interactions between intrinsic membrane proteins: a theoretical study based on integral equations. Biophys. J. 79:2867–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lague, P., M. J. Zuckermann, and B. Roux. 2001. Lipid-mediated interactions between intrinsic membrane proteins: dependence on protein size and lipid composition. Biophys. J. 81:276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipowsky, R. 2002. Domains and rafts in membranes: hidden dimensions of self organization. J. Biol. Phys. 28:195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loura, L. M. S., A. Fedorov, and M. Prieto. 2001a. Exclusion of a cholesterol analog from the cholesterol-rich phase in model membranes. BBA-Biomembranes. 1511:236–243. [DOI] [PubMed] [Google Scholar]
- Loura, L. M. S., A. Fedorov, and M. Prieto. 2001b. Fluid-fluid membrane microheterogeneity: a fluorescence resonance energy transfer study. Biophys. J. 80:776–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelja, S. 1976. Lipid-mediated protein interactions in membranes. Biophys. Biochim. Acta. 455:1–7. [DOI] [PubMed] [Google Scholar]
- May, S. 2000a. Protein-induced bilayer deformations: the lipid tilt degree of freedom. Eur. Biophys. J. 29:17–28. [DOI] [PubMed] [Google Scholar]
- May, S. 2000b. Theories on structural perturbations of lipid bilayers. Curr. Opin. Colloid In. 5:244–249. [Google Scholar]
- May, S. 2002. Membrane perturbations induced by integral proteins: role of conformational restrictions of the lipid chains. Langmuir. 18:6356–6364. [Google Scholar]
- May, S., and A. Ben-Shaul. 1999. Molecular theory of lipid-protein interaction and the Lα-HII transition. Biophys. J. 76:751–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, S., and A. Ben-Shaul. 2000. A molecular model for lipid-mediated interaction between proteins in membranes. Phys. Chem. Chem. Phys. 2:4494–4502. [Google Scholar]
- McConnell, H. M., and A. Radhakrishnan. 2003. Condensed complexes of cholesterol and phospholipids. BBA-Biomembranes. 1610:159–173. [DOI] [PubMed] [Google Scholar]
- Milner, S. T., and T. A. Witten. 1988. Bending moduli of polymeric surfactant interfaces. J. Phys-Paris. 49:1951–1962. [Google Scholar]
- Mouritsen, O. G., and M. Bloom. 1993. Models of lipid-protein interactions in membranes. Annu. Rev. Biophys. Biomol. Struct. 22:145–171. [DOI] [PubMed] [Google Scholar]
- Nagle, J. F., and S. Tristram-Nagle. 2000. Structure of lipid bilayers. BBA-Rev. Biomembranes. 1469:159–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, C., and O. S. Andersen. 2000. Inclusion-induced bilayer deformations: effects of monolayer equilibrium curvature. Biophys. J. 79:2583–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, C., M. Goulian, and O. S. Andersen. 1998. Energetics of inclusion-induced bilayer deformations. Biophys. J. 74:1966–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohvo-Rekila, H., B. Ramstedt, P. Leppimaki, and J. P. Slotte. 2002. Cholesterol interactions with phospholipids in membranes. Prog. Lipid Res. 41:66–97. [DOI] [PubMed] [Google Scholar]
- Pandit, S. A., D. Bostick, and M. L. Berkowitz. 2004. Complexation of phosphatidylcholine lipids with cholesterol. Biophys. J. 86:1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasenkiewicz-Gierula, M., T. Rog, K. Kitamura, and A. Kusumi. 2000. Cholesterol effects on the phosphatidylcholine bilayer polar region: a molecular simulation study. Biophys. J. 78:1376–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pata, V., and N. Dan. 2003. The effect of chain length on protein solubilization in polymer-based vesicles (polymersomes). Biophys. J. 85:2111–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan, A., T. G. Anderson, and H. M. McConnell. 2000. Condensed complexes, rafts, and the chemical activity of cholesterol in membranes. Proc. Natl. Acad. Sci. USA. 97:12422–12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan, A., and H. M. McConnell. 1999. Condensed complexes of cholesterol and phospholipids. Biophys. J. 77:1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, A., W. Richards, P. Thomas, and M. Hann. 1995. Behavior of cholesterol and its effect on headgroup and chain conformations in lipid bilayers: a molecular dynamics study. Biophys. J. 68:164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, H. 1991. Lipid-cholesterol interactions. Monte Carlo simulations and theory. Biophys. J. 59:445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvius, J. R. 2003. Role of cholesterol in lipid raft formation: lessons from lipid model systems. BBA-Biomembranes. 1610:174–183. [DOI] [PubMed] [Google Scholar]
- Simons, K., and E. Ikonen. 2000. Cell biology: how cells handle cholesterol. Science. 290:1721–1726. [DOI] [PubMed] [Google Scholar]
- Sintes, T., and A. Baumgaertner. 1998. Interaction of wedge-shaped proteins in flat bilayer membranes. J. Phys. Chem. B. 102:7050–7057. [Google Scholar]
- Small, D. M. 1980. Cholesterol nucleation and growth in gallstone formation. N. Engl. J. Med. 302:1305–1307. [DOI] [PubMed] [Google Scholar]
- Small, D. M. 1988. Progression and regression of atherosclerotic lesions. Insights from lipid physical biochemistry. Arteriosclerosis. 8:103–129. [DOI] [PubMed] [Google Scholar]
- Smondyrev, A. M., and M. L. Berkowitz. 1999. Structure of dipalmitoylphosphatidylcholine/cholesterol bilayer at low and high cholesterol concentrations: molecular dynamics simulation. Biophys. J. 77:2075–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smondyrev, A. M., and M. L. Berkowitz. 2001. Molecular dynamics simulation of the structure of dimyristoylphosphatidylcholine bilayers with cholesterol, ergosterol, and lanosterol. Biophys. J. 80:1649–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szleifer, I., D. Kramer, A. Benshaul, W. M. Gelbart, and S. A. Safran. 1990. Molecular theory of curvature elasticity in surfactant films. J. Chem. Phys. 92:6800–6817. [Google Scholar]
- Szleifer, I., D. Kramer, A. Ben-Shaul, D. Roux, and W. M. Gelbart. 1988. Curvature elasticity of pure and mixed surfactant films. Phys. Rev. Lett. 60:1966–1969. [DOI] [PubMed] [Google Scholar]
- Taulier, N., M. Waks, T. Gulik-Krzywicki, and W. Urbach. 2002. Interactions between transmembrane proteins embedded in a lamellar phase, stabilized by steric interactions. Europhys. Lett. 59:142–148. [Google Scholar]
- Troup, G. M., T. N. Tulenko, S. P. Lee, and S. P. Wrenn. 2003. Detection and characterization of laterally phase separated cholesterol domains in model lipid membranes. Colloid. Surface. B. 29:217–231. [Google Scholar]
- Tu, K. C., M. L. Klein, and D. J. Tobias. 1998. Constant-pressure molecular dynamics investigation of cholesterol effects in a dipalmitoylphosphatidylcholine bilayer. Biophys. J. 75:2147–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch, S. L., and S. L. Keller. 2002. Organization in lipid membranes containing cholesterol. Phys. Rev. Lett. 89:268101–268104. [DOI] [PubMed] [Google Scholar]
- Veatch, S. L., I. V. Polozov, K. Gawrisch, and S. L. Keller. 2004. Liquid domains in vesicles investigated by NMR and fluorescence microscopy. Biophys. J. 86:2910–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viovy, J. L., W. M. Gelbart, and A. Ben-Shaul. 1987. Scaling properties of chain interactions in amphiphilic aggregates. J. Chem. Phys. 87:4114–4125. [Google Scholar]
- Weikl, T. R. 2001. Fluctuation-induced aggregation of rigid membrane inclusions. Europhys. Lett. 54:547–553. [Google Scholar]
- Weikl, T. R. 2002. Dynamic phase separation of fluid membranes with rigid inclusions. Phys. Rev. E. 66:061915. [DOI] [PubMed] [Google Scholar]
- Weikl, T. R., M. M. Kozlov, and W. Helfrich. 1998. Interaction of conical membrane inclusions: Effect of lateral tension. Physical Review E. 57:6988–6995. [Google Scholar]
- Wiggins, P., and R. Phillips. 2004. Analytic models for mechanotransduction: gating a mechanosensitive channel. Proc. Natl. Acad. Sci. USA. 101:4071–4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthman, L. A. D., K. Nag, P. J. Davis, and K. M. W. Keough. 1997. Cholesterol in condensed and fluid phosphatidylcholine monolayers studied by epifluorescence microscopy. Biophys. J. 72:2569–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeagle, P. L. 1988. Biology of Cholesterol. CRC Press, Boca Raton, FL.