Abstract
Decapping of mRNA is a critical step in eukaryotic mRNA turnover, yet the proteins involved in this activity remain elusive in mammals. We identified the human Dcp2 protein (hDcp2) as an enzyme containing intrinsic decapping activity. hDcp2 specifically hydrolyzed methylated capped RNA to release m7GDP; however, it did not function on the cap structure alone. hDcp2 is therefore functionally distinct from the recently identified mammalian scavenger decapping enzyme, DcpS. hDcp2-mediated decapping required a functional Nudix (nucleotide diphosphate linked to an X moiety) pyrophosphatase motif as mutations in conserved amino acids within this motif disrupted the decapping activity. hDcp2 is detected exclusively in the cytoplasm and predominantly cosediments with polysomes. Consistent with the localization of hDcp2, endogenous Dcp2-like decapping activity was detected in polysomal fractions prepared from mammalian cells. Similar to decapping in yeast, the presence of the poly(A) tail was inhibitory to the endogenous decapping activity, yet unlike yeast, competition of cap-binding proteins by cap analog did not influence the efficiency of decapping. Therefore the mammalian homologue of the yeast Dcp2 protein is an mRNA decapping enzyme demonstrated to contain intrinsic decapping activity.
The important role of mRNA stability in the regulation of eukaryotic gene expression has been increasingly recognized in recent years. Significant progress has been made in our understanding of the pathways of mRNA turnover in eukaryotes. In yeast, the major mRNA decay pathway involves a deadenylation-dependent decapping mechanism in which mRNA is initially deadenylated, then decapped by the Dcp1p decapping enzyme (1, 2), and subsequently degraded by a highly processive 5′ to 3′ exoribonuclease (3, 4). A deadenylation-independent mRNA turnover pathway has also been well characterized in yeast, whereby premature translation termination triggers decapping of the mRNA independent of the removal of the poly(A) tail (5–7). In an alternative decay pathway, the deadenylated mRNA is degraded by the exosome in a 3′ to 5′ direction (4, 8, 9). Interestingly, this pathway is also involved in the nonstop decay mechanism (10, 11).
In mammals, the initial step of mRNA decay also involves removal of the poly(A) tail (12–19). Unlike yeast, however, deadenylated mRNA seems to be predominately degraded from the 3′ end as demonstrated in vitro (13, 18, 20–23) and in cells with a transfected RNA (18), a viral mRNA (24), and an endogenous mRNA (18). The 3′ end decay is mediated by the exosome in association with the scavenger decapping activity that hydrolyzes the residual cap structure to release m7GMP (18). Mammalian 5′ to 3′ exoribonuclease decay activities have been observed both in vitro (18, 21) and in cells (18). The detection of full-length mRNA with a short poly(A) tail and a 5′ monophosphate provide indirect evidence for the existence of a deadenylation-dependent decay pathway in mammalian cells (14). However, additional studies are still required to elucidate this pathway.
At present, two eukaryotic decapping enzymes have been cloned and characterized. The first is the yeast Dcp1p that preferentially hydrolyzes methylated cap on an mRNA longer than 25 nt to release m7GDP (2, 25). mRNA decapping activity in yeast cells also requires the product of the DCP2 gene. Dcp2p contains a nucleotide diphosphate linked to an X moiety (Nudix) pyrophosphatase motif (previously referred to as MutT motif) and coimmunopurifies with Dcp1p (26). Surprisingly, despite its requirement for decapping, Dcp2p itself has not been demonstrated to possess intrinsic decapping activity (26). The second eukaryotic decapping enzyme identified is the scavenger decapping enzyme, DcpS. DcpS contains a histidine triad (HIT) motif and hydrolyzes the residual methylated cap structure after 3′ to 5′ decay of the mRNA (18, 27).
Although a decapping activity that functions on intact RNA has been reported in mammalian cells (14), the enzyme remains unidentified. Here we report the identification of a second mammalian decapping enzyme, the human Dcp2 protein (hDcp2). Using a bioinformatics approach, we cloned a mammalian cDNA encoding the human homologue of yeast Dcp2p and demonstrated that it contains intrinsic mRNA decapping activity. In addition, we observed the presence of endogenous Dcp2-like decapping activity in mammalian extract. The identification of additional decapping enzymes and activities is a critical step in defining the pathways and mechanisms regulating mRNA turnover in mammalian cells.
Materials and Methods
Plasmids and Expression of Recombinant Protein.
The coding region sequence of hDcp2 was PCR-amplified from the reverse-transcribed product of human K562 cell total RNA with a 5′ primer (5′-AAC CTG GAT CCA TGG AGA CCA AAC GGG TGG-3′) and 3′ primer (5′-CAA TAC TCG AGC TGC TAT CAA AGG TCC AAG-3′). The PCR product was cut with BamHI and XhoI and inserted into the same sites of pET28a and pGEX6p-1 to generate pET28-hDcp2 and pGEX-hDcp2, respectively. The plasmid pET-hDcp21–349 contains a stop codon after amino acid 349 and was generated by PCR with the same 5′ primer as above and the following 3′ primer (5′-ATT CGA AGC TTC ATC AAA GAA TTC TGC TGC-3′) and inserted into pET28a as above. pET-hDcp2Q147/8 substitutes glutamines for the glutamic acids at amino acids 147 and 148 by using the QuikChange mutagenesis system according to the manufacturer (Stratagene) with the following 5′ primer (5′-GTG CTG CTA GAG AGG TCT TTC AAC AAA CTG GTT TTG ATA TCA AAG-3′) and 3′ primer (5′-CTT TGA TAT CAA AAC CAG TTT GTT GAA AGA CCT CTC TAG CAG CAC-3′). All plasmids were confirmed by sequencing. His-tagged proteins (generated from the pET series of plasmids) and the GST-fusion protein (pGEX-hDcp2) were expressed and purified according to the manufacturers' instructions (Novagen and Amersham Pharmacia, respectively).
Extract Preparation.
Human erythroleukemia K562 cells were obtained from the National Cell Culture Center at Cellex Biosciences (Minneapolis). Cells were resuspended in buffer A (10 mM Tris⋅HCl, pH 7.5/1 mM potassium acetate/1.5 mM magnesium acetate/2 mM DTT, 1.5 ml per 108 cells) and lysed with 25 strokes of a type-B Dounce homogenizer, and nuclei were removed with a 10-min, 2,000-g centrifugation as described (28). The supernatant was centrifuged again at 15,000 g for 30 min to generate S15. Cytosolic S130 extract and polysomes fractions were prepared as described (23, 28).
RNA Production.
RNAs corresponding to the pcDNA3 polylinker containing a poly(G) track at the 3′ end (pcP-G16) were transcribed with SP6 RNA polymerase from a template generated by using the SP6 primer and a T7 primer followed by 16 cytosines. RNAs containing 60 adenosines at the 3′ end after the G16 track (pcP-G16-A60) were transcribed with SP6 RNA polymerase from a template generated by using the SP6 primer and a T7 primer followed by 16 cytosines and 60 thymidines. Generation of cap-labeled RNAs containing or lacking a N-7 methyl group and production of labeled cap analog were as described (17, 18). All RNAs were gel-purified before use (18).
In Vitro RNA Decay Assays.
In vitro RNA decay assays were carried out at 37°C with cap-labeled RNA (10 Kcpm) and the indicated amount of extracts or purified proteins for the specified times as described (17). Decapping products were resolved by polyethyleneimine-cellulose TLC plates as described in Wang and Kiledjian (18) except the TLC was developed in 0.75 M LiCl. Cold standards and 32Pi were loaded alongside the samples and visualized by UV shadowing or autoradiography, respectively. The nucleotide diphosphate kinase (NDPK) reactions were carried out as described (18). The TLC plates were exposed to Kodak BioMax film. All quantitations were conducted with a Molecular Dynamics PhosphorImager (Storm860) with IMAGEQUANT-5 software.
In-Gel Decapping Assay.
The indicated recombinant proteins (1 μg each) were loaded onto a standard 12.5% SDS/PAGE gel or a 12.5% SDS/PAGE gel containing cap-labeled pcP-G16 RNA (1 × 106 cpm) immobilized in the running gel. The two SDS/PAGE gels were subjected to electrophoresis under identical conditions, and proteins in the unlabeled gel were visualized by Coomassie blue staining. Proteins in the cap-labeled RNA-containing gel were renatured at 4°C and assayed for decapping activity at 37°C as described by LaGrandeur and Parker (2). The gel was subsequently dried and exposed to Kodak x-ray film.
Antibody Generation and Western Blotting.
The antiserum to hDcp2 was commercially generated (Cocalico Biologicals, Reamstown, PA) by immunizing rabbits with recombinant His-hDcp2. The polyclonal sera was affinity-purified with GST-hDcp2 coupled to an NHS column according to the manufacturer (Amersham Pharmacia). A 1:100 dilution of the affinity-purified antibody was used for Western analysis and visualized by ECL with a horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:5,000 dilution; Cappel).
Results
Identification of the Human Dcp2 Protein.
We used a genomics approach to identify a mammalian decapping enzyme that was able to hydrolyze intact capped mRNA similar to the yeast Dcp1 protein. We reasoned that because the decapping reaction involves a pyrophosphatase activity, proteins containing a pyrophosphatase domain would be likely candidates. In vivo, yeast Dcp2p is essential but not sufficient for decapping and is a member of a class of pyrophosphatases designated the Nudix hydrolases (29). The human expressed sequence tag database was searched with the 23-aa Nudix sequence of yeast Dcp2p and a single sequence of 570 nt was identified. This sequence was in turn used to assemble a contiguous cDNA of 2,548 nt. The presence of such a transcript was confirmed by oligo(dT) reverse-transcribed mRNA derived from human K562 erythroleukemia cells, which PCR-amplified a band analogous to the predicted size of the mRNA (data not shown). The deduced protein contained 420 aa possessing the 23-aa consensus Nudix motif (29) within a 109-aa Nudix fold (30) region (see Fig. 5, which is published as supporting information on the PNAS web site, www.pnas.org). The sequence of the protein was most similar to yeast Dcp2p yet considerably smaller (420 aa versus 970 aa), with the highest degree of conservation at the amino (N)-terminal 300 aa (36% identity, 54% similarity). Interestingly, the N-terminal 300 aa of yeast Dcp2p is sufficient to complement yeast Dcp2p function in vivo, demonstrating the functional significance of this region for decapping (26). A search of the National Center for Biotechnology Information LocusLink database indicated that the gene encoding this human protein is contained on chromosome 5q22–23.
A schematic of the putative Dcp2 homologues from diverse organisms is presented in Fig. 1. clustalw analysis identified a 230-aa region that was highly conserved among the different proteins. This region contains three distinct domains. There is a 38-aa domain (box A) present in all of the putative Dcp2 proteins at the N terminus, followed by the 109-aa Nudix fold region containing the highly conserved 23-aa Nudix motif. The third domain (box B) is curiously present in five of the six homologues listed in Fig. 1. The high degree of aa conservation and retention of specific domains among these proteins suggests they are homologues of yeast Dcp2p. The human Dcp2 homologue will be referred to as hDcp.
Figure 1.
Schematic of Dcp2 proteins from different organisms. Dcp2 proteins from diverse species are schematically represented and aligned by clustalw relative to the Nudix motif. Three distinct regions of high homology are noted. An N-terminal conserved 38-aa box A, a 109-aa Nudix fold with the central 23-aa Nudix motif region, and a 20-aa box B segment are indicated (see Fig. 5). The aa position of the conserved regions within each protein is denoted above the protein schematics and the total number of amino acids in each protein is shown on the right. The degree of identity within the Nudix motif among the different Dcp2 proteins relative to the human protein is as follows: 98% with mouse (GenBank accession no. AK017809); 44% with Caenorhabditis elegans (GenBank accession no. NM_070208), 53% with Drosophila melanogaster (GenBank accession no. AE003529), 47% with Schizosaccharomyces pombe (GenBank accession no. CAB11648), and 38% with Saccharomyces cerevisiae (GenBank accession no. P53550).
hDcp2 Contains Intrinsic mRNA Decapping Activity.
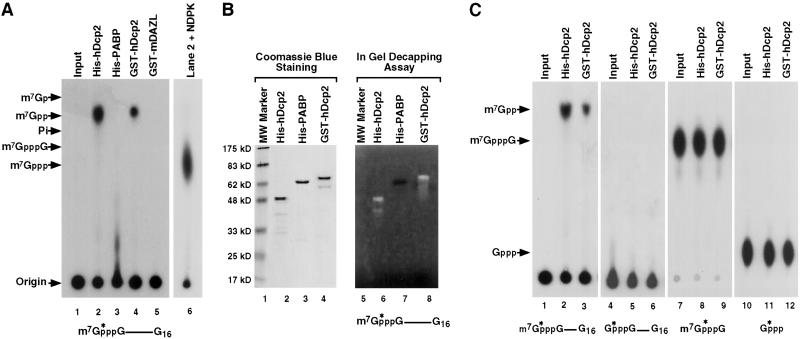
The ability of the hDcp2 protein to decap RNA was tested. The protein was expressed as a histidine-tagged recombinant protein in bacteria (His-hDcp2), purified on a nickel column, and tested for its potential to decap. 32P-cap-labeled RNA corresponding to the pcDNA3 polylinker containing a G16 track (pcP-G16) at the 3′ end to minimize 3′ to 5′ exoribonuclease activity (18) was used as a substrate in our standard in vitro decay reaction conditions (17) with the recombinant protein instead of cell extract. A modified polyethyleneimine-cellulose TLC was used to develop the reaction products in 0.75 M LiCl. We found that this running buffer resolved m7GDP from 32Pi, which otherwise comigrate in the standard 0.45 M (NH4)2SO4 buffer (18). As shown in Fig. 2A, recombinant hDcp2 protein was capable of decapping the capped RNA and released m7GDP product (lane 2). The identity of the m7GDP reaction product was further confirmed with the use of NDPK, which specifically converts m7GDP, but not m7GMP, into m7GTP (Fig. 2A, lane 6). Decapping was not detected with a control His-tagged protein (Fig. 2A, lane 3). Furthermore, decapping was detected with recombinant hDcp2 fused to GST (Fig. 2A, lane 4), but not a control GST fusion protein (Fig. 2A, lane 5), indicating that the hDcp2 protein and not the fused tag or a copurifying bacterial contaminant was responsible for the decapping.
Figure 2.
Recombinant hDcp2 contains intrinsic decapping activity specific to methylated capped RNA. (A) In vitro decay assays were carried out by incubating 0.5 μg of the indicated histidine-tagged (His-hDcp2 and His-PABP) or GST-fused (GST-hDcp2 and GST-mDAZL) recombinant proteins with cap-labeled pcP-G16 RNA at 37°C for 30 min. Reaction products were resolved by polyethyleneimine-TLC developed in 0.75 M LiCl. An aliquot of the reaction product used in lane 2 was treated with NDPK and revolved by TLC (lane 6) to confirm the original product was m7GDP. Standards were simultaneously developed and their positions are denoted on the left. The substrate used in the assay is schematically denoted at the bottom. The asterisks represent the position of the 32P-labeling and the line denotes the RNA. (B) One microgram of each recombinant protein was separated either on a standard SDS/PAGE (Left) or an identical gel containing 32P-cap-labeled pcP-G16 RNA polymerized into the gel (Right). Migration of the proteins was visualized by Coomassie blue staining (Left). An in-gel decapping activity assay was carried out with the gel containing 32P-labeled-capped RNA as described in Materials and Methods, and an autoradiography of the gel is shown (Right). Decapping of the RNA produces a clearing of the radioactive background upon release of the labeled cap substrate that diffuses out of the gel. Both the His-hDcp2 (lane 6) and GST-hDcp2 (lane 8) are capable of decapping whereas the His-PABP control (lane 7) was not. Protein size markers are shown on the left. (C) In vitro decay assays were carried out similar to A but used different substrates. Cap-labeled RNA lacking the N-7 methyl moiety was used in lanes 4–6, labeled methylated cap analog dinucleotide in lanes 7–9, and [α-32P]GTP in lanes 10–12. hDcp2 efficiently hydrolyzed methylated cap-labeled RNA (lanes 2 and 3) but not the other three substrates. The input substrates are shown in lanes 1, 4, 7, and 10. The recombinant protein used for the assays are indicated at the top.
To further confirm the recombinant hDcp2 protein decapping activity, an in-gel decapping assay was performed. His-hDcp2 and GST-hDcp2 recombinant proteins were purified and resolved on an SDS-polyacrylamide gel containing 32P-cap-labeled pcP RNA polymerized within the gel (2). The gel was subsequently denatured in 6 M guanidine and gradually renatured by a stepwise reduction of guanidine followed by incubation in decapping buffer. Intrinsic decapping activity of the renatured hDcp2 protein was confirmed by the presence of a zone of radioactive signal clearing in the region of the gel that corresponded to hDcp2. The zone of clearing, which appears as a white band, arises upon the release of the radiolabeled m7GDP product, which readily diffuses from the radioactively labeled gel. As shown in Fig. 2B, the area around His-hDcp2 (lane 2) contained a reduction of radioactive signal (lane 6). Similar results were obtained with GST-hDcp2 (Fig. 2B, lanes 4 and 8). Decapping activity of proteolytic fragments of the recombinant hDcp2 proteins was also observed as bands smaller than the full-length protein. Decapping was not detected with a control protein (Fig. 2B, lane 3) as indicated by the lack of any detected clearing around this protein (Fig. 2B, lane 7). Taken together, these data indicate that the hDcp2 protein contains a decapping activity that functions on capped RNA to release m7GDP.
The specificity of hDcp2 decapping substrates was determined by the use of unmethylated capped RNA. Neither His-tagged nor GST-tagged hDcp2 fusion proteins efficiently hydrolyzed cap-labeled RNA lacking an N-7 methyl group (Fig. 2C, lanes 5 and 6), although a faint signal can be detected upon overexposure (data not shown). The decapping was specific to capped RNA and did not hydrolyze the cap structure alone (Fig. 2C, lanes 8 and 9) or GTP (Fig. 2C, lanes 11 and 12). Therefore, hDcp2 protein is a specific pyrophosphatase that can decap intact mRNA to release m7GDP and is distinct from the recently described mammalian DcpS scavenger decapping enzyme that functions on cap structure to release m7GMP (18, 27).
hDcp2 Contains a Functional Nudix Motif.
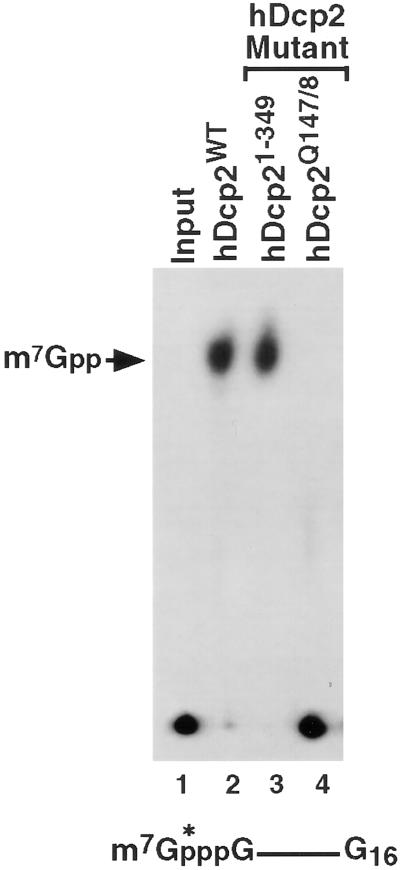
The Nudix motif was originally identified in a class of pyrophosphatase proteins and shown to be critical for pyrophosphatase activity (31, 32). The Nudix motif consists of a highly conserved 23-aa sequence, GX5EX7REUXEEXGU, where X can be any amino acid and U is an aliphatic hydrophobic amino acid (31, 32). To test whether the hDcp2 Nudix motif was functional, the two conserved glutamic acid residues (E147 and E148) within this motif were substituted by glutamine. These two glutamic acids are critical for metal ion coordination (30, 33) and were shown to be critical for pyrophosphatase activity of Nudix proteins (26, 34, 35). As seen in Fig. 3, the two aa substitutions disrupted the decapping function of the resulting recombinant protein (lane 4). However, truncation of 71 aa from the C terminus of the protein, leaving the Nudix motif intact, resulted in a recombinant protein that maintained decapping activity (Fig. 3, lane 3). These data demonstrate that hDcp2 contains a functional Nudix motif required for decapping.
Figure 3.
Significance of the hDcp2 Nudix motif in decapping. In vitro decay assays were carried out by using cap-labeled RNA and 0.5 μg of the indicated recombinant protein at 37°C for 30 min. Wild-type hDcp2 protein, the truncated protein lacking the C-terminal 71 aa (hDcp21–349), and the double point mutation substituting glutamine for glutamic acid at amino acids 147 and 148 (hDcp2Q147/8) are indicated. The resulting decapping products were resolved on TLC plates and the labeling is as described in the legend to Fig. 1.
hDcp2 Is a Cytoplasmic Protein.
To determine in which cellular compartments hDcp2 resides, we tested its presence in different fractions by Western analysis. Rabbit polyclonal antisera raised against His-tagged hDcp2 was affinity-purified with GST-hDcp2 and used for Western analysis. Consistent with the predicted size of hDcp2, the antibody detects a specific protein of 45 kDa in K562 total extract (Fig. 4A, lane 2). As expected, this size is slightly smaller than the His-tagged hDcp2 (Fig. 4A, lane 1). Support for a cytoplasmic role for hDcp2 is provided by the presence of this protein in the cytoplasmic fraction (Fig. 4A, lane 4) and the lack of detection in the nuclear fraction (Fig. 4A, lane 3). Furthermore, a comparison of the soluble postpolysomal S130 extract and the polysomal pellet indicated that hDcp2 cosediments with the polysomal fraction (Fig. 4A, compare lanes 5 and 6) and mostly dissociates from the polysome in a 500 mM KCl ribosomal salt wash (RSW). These data imply that hDcp2 is a cytoplasmic protein primarily associated with the polysome and therefore likely involved in the regulation of cytoplasmic mRNA turnover.
Figure 4.
Endogenous decapping activity cosediments with polysomes. (A) Western analysis using affinity-purified antibody directed against hDcp2 was carried out. Fifty nanograms of His-hDcp2 was used in lanes 1, and extract derived from 1 × 106 K562 cells was fractionated and resolved in lanes 2–7 as indicated. The protein size markers are shown on the left. (B) An in vitro decay assay was carried out by incubating cap-labeled pcP-G16 RNA with 30 μg of the indicated K562 cell extract in the absence or presence of 100 μM cap analog. The obtained products were resolved by TLC as shown in lanes 2–9. The products of lanes 5–9 were treated with NDPK and then resolved by TLC (lanes 10–14). (C) In vitro decay assay was carried out by incubating 30 μg of K562 RSW fraction with cap-labeled pcP-G16 RNA or pcP-G16-A60 RNA in the absence or presence of 500 ng of cold poly(A) competitor at 37°C for 30 min. The obtained products were resolved by TLC as shown in lanes 2, 3, 5, and 6.
Detection of Endogenous mRNA Decapping Activity.
To determine whether Dcp2-like decapping activity that releases m7GDP could be detected in mammalian extract, cap-labeled RNA was used in an in vitro decay assay with total cytoplasmic extract (S15), soluble postpolysomal cytosolic extract (S130), polysomal extract, or RSW. Using the pcP-G16 RNA in our standard in vitro decay assay conditions, a decapping activity that released m7GDP was not detected in S15 or S130 extract (Fig. 4B, lanes 2 and 4). To determine whether the lack of activity was a result of cap-binding proteins that protected the cap from hydrolysis, cap analog competitor was added to the reaction. As seen in Fig. 4B, lane 3, addition of cap analog did not stimulate m7GDP formation even though cap-binding proteins are sequestered with this concentration of competitor (36, 37). Therefore, the lack of detected decapping activity to produce m7GDP was not caused by the inaccessibility to the cap even though addition of recombinant eIF4E protein can efficiently inhibit the decapping activity in vitro as would be expected (data not shown). The only decapping activity detected was that of DcpS, which produced m7GMP, and the released free phosphate. Upon addition of cold cap analog competitor, DcpS activity is inhibited and a new reaction product is observed (Fig. 4B, lanes 3 and 5) (18). This new product corresponds to labeled cap analog that accumulates after decay of the RNA from the 3′ end by the exosome because the subsequent hydrolysis of the cap analog by DcpS is blocked (18). These data are consistent with our previous report that DcpS is the major decapping activity detected in mammalian extract.
Concentration of hDcp2 in the polysome (Fig. 4A) prompted us to test for endogenous decapping activity within polysomal extract. As seen in lane 6 of Fig. 4B, m7GDP was detected with polysomal extract and this activity was unaffected by the addition of cap analog competitor (Fig. 4B, lane 7). Similarly, a m7GDP decapping product was also detected in the polysomal RSW fraction (Fig. 4B, lane 8). Interestingly, addition of cap analogue competitor did not further augment decapping (Fig. 4B, lane 9). These data imply that cap-binding proteins play a minimal regulatory role for this decapping step. The identity of the spot comigrating with the m7GDP in Fig. 4B, lanes 6–9 was unambiguously confirmed by treating an aliquot from each of these samples with NDPK. The m7GDP spot was converted to m7GTP (Fig. 4B, lanes 11 and 14) whereas the DcpS product (m7GMP) or cap analog were unaffected. Fig. 4B, lane 5, which contained S130 extract that did not produce m7GDP, was used as a negative control and as expected m7GTP was not produced (Fig. 4B, lane 10). These data confirm the presence of an endogenous decapping activity capable of releasing m7GDP in mammalian extract and demonstrated that this activity is not influenced by cap-binding proteins in this extract.
Decapping Is Inhibited by the Poly(A) Tail.
The decapping experiments described above were carried out with unadenylated RNAs. We next addressed whether the presence of a poly(A) tail influenced decapping in mammalian extract. The pcP-G16 RNA containing a G track at the 3′ end was compared with an identical RNA that contained a poly(A) tail (pcP-G16-A60). These RNAs were subjected to a standard in vitro decay assay with RSW extract. Decapping products were resolved by TLC. Consistent with Fig. 4B, m7GDP was detected as a decapping product by using an unadenylated RNA (Fig. 4C, lane 2) and the level of decapping was minimally affected (0.3-fold) by the addition of poly(A) competitor (Fig. 4C, lane 3). However, the presence of a poly(A) tail reduced the level of decapping by more than 2-fold (Fig. 4C, lane 5). The inhibition of decapping was reversed upon the addition of poly(A) competitor (Fig. 4C, lane 6), at levels sufficient to induce deadenylation (data not shown). These data indicate that the poly(A) tail exerts a negative influence on the endogenous decapping activity observed and this repression is relieved upon deadenylation.
Discussion
We present the identification of a mammalian decapping enzyme that surprisingly is the homologue of the yeast Dcp2 protein. hDcp2 contains a functional Nudix pyrophosphatase motif that is required for decapping activity and hDcp2 is predominantly polysome-associated.
Dcp2p was originally isolated as a high copy suppressor of a temperature-sensitive dcp1 allele and shown to be required for decapping (26). However, the catalytic component of decapping is thought to be mediated by Dcp1p (2). It was therefore unexpected that hDcp2p contained inherent mRNA decapping activity independent of any other protein. The reason for the lack of intrinsic decapping activity by yeast Dcp2p is currently unknown.
Decapping in yeast has been shown to be intimately associated with deadenylation (4). We have detected deadenylation-dependent decapping in mammalian extract as well and observed a reproducible 2-fold lower rate of decapping of an adenylated RNA verses unadenylated RNA (Fig. 4C). The inhibitory affect of the poly(A) tail was relieved upon the addition of poly(A) competitor to sequester poly(A)-binding proteins. However, considering we have not detected a cap-binding protein dependence on decapping, it is unclear how the poly(A) tail influences the fate of the 5′ cap independent of cap-binding proteins. In the absence of an association between the eIF4F cap binding complex and DABP, an alternative means of interaction between the two ends of the RNA may exist, as proposed (37, 38), and may regulate decapping.
Cosedimentation of hDcp2 with polysomes indicates a potential link between translation and decapping reminiscent of the nonsense-mediated decay (NMD) pathway. NMD is a surveillance pathway that detects and degrades aberrant transcripts containing premature termination codons by a deadenylation-independent decapping mechanism in yeast (7). Upf1 is one of the critical proteins involved in NMD both in yeast (39, 40) and mammals (41, 42). The yeast Dcp2p can interact with the Upf1 protein (43) (referred to as NMD1) and hDcp2 can interact with human Upf1 protein (F. Lejeune, M.K., and L. E. Maquat, unpublished observations). This finding suggests that hDcp2 might function in NMD in mammals as well. It will be interesting to determine whether association of hDcp2 with Upf1 is functionally relevant and whether hDcp2 is directly involved in NMD in mammalian cells.
Recently, a Dcp1-like decapping activity present in the postpolysomal S100 extract was reported. This activity was detected only in the presence of cap analog competition on an RNA containing an A + U-rich instability element (36). It is unlikely the reported activity is the same as that presented in this study. We are unable to detect such an activity in postpolysomal extract and do not see a significant effect of cap analog competitor. Considering the fortuitous comigration of m7GDP with that of inorganic phosphate (32Pi) that is hydrolyzed from the m7GMP DcpS decapping product, it is unclear whether the reported decapping was caused by a Dcp1-like decapping activity or DcpS.
To date there are at least two distinct pyrophosphatase domains that have been identified, the Nudix motif and the HIT motif. The evolutionarily conserved Nudix motif is found in a large number of proteins that use a diverse spectrum of substrates (29). The HIT domain is found in a family of proteins that contain a core hexapeptide of three histidines separated by hydrophobic amino acids (44). HIT proteins are nucleotide-binding proteins that can hydrolyze a pyrophosphate bond and have been proposed to be involved in the regulation of dinucleotide signaling molecules (45). Interestingly, DcpS, contains a HIT motif (27). Therefore, the two decapping enzymes each evolved to use two distinct pyrophosphatase domains to carry out the two different decapping activities involved in mRNA turnover. In the case of hDcp2, it contains the Nudix motif and functions only on intact mRNA (Fig. 2), whereas DcpS contains the HIT motif and functions only on cap structures or cap structures containing fewer than 10 nt (27). Whether either of these proteins can be regulated to change their substrate specificity or influence each other's activity is currently unknown.
The demonstration that hDcp2 is a mammalian decapping enzyme raises an interesting question as to whether yeast Dcp2p could also play a more direct role in the pyrophosphatase enzymatic activity than previously thought. Conversely, the presence of two distantly related Dcp1 genes present in the mammalian database (unpublished observations) suggests that key components involved in yeast deadenylation-dependent mRNA turnover are conserved in mammals. Consistent with a conservation of decapping enzymes in both organisms, DcpS is also present in yeast and mammals (18, 27). Identification of the various mammalian decapping enzymes will now provide the groundwork to begin addressing regulated decapping and turnover in mammalian cells.
Supplementary Material
Acknowledgments
We thank A. Puzio for the construction of the pET-hDcp2Q147/8 plasmid and R. Davies for communication of unpublished sequence information. We also thank members of the Kiledjian lab for helpful discussions and critical reading of the manuscript. This work was supported by National Institutes of Health Grant DK51611 (to M.K.).
Abbreviations
- HIT
histidine triad
- NDPK
nucleotide diphosphate kinase
- NMD
nonsense-mediated decay
- Nudix
nucleotide diphosphate linked to an X moiety
- RSW
ribosomal salt wash
Note Added in Proof.
We have recently demonstrated that the yeast Dcp2p also contains intrinsic decapping activity (unpublished work), suggesting that this enzyme is also directly involved in yeast decapping. The GenBank annotated sequence for the C. elegans Dcp2 presented in Fig. 1 appears to be incomplete. A more recent annotation of the sequence is longer and contains a B box (Richard Davies, City University of New York Graduate Center, personal communication).
Footnotes
References
- 1.Beelman C A, Stevens A, Caponigro G, LaGrandeur T E, Hatfield L, Fortner D M, Parker R. Nature (London) 1996;382:642–646. doi: 10.1038/382642a0. [DOI] [PubMed] [Google Scholar]
- 2.LaGrandeur T E, Parker R. EMBO J. 1998;17:1487–1496. doi: 10.1093/emboj/17.5.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larimer F W, Hsu C L, Maupin M K, Stevens A. Gene. 1992;120:51–57. doi: 10.1016/0378-1119(92)90008-d. [DOI] [PubMed] [Google Scholar]
- 4.Muhlrad D, Decker C J, Parker R. Genes Dev. 1994;8:855–866. doi: 10.1101/gad.8.7.855. [DOI] [PubMed] [Google Scholar]
- 5.Czaplinski K, Ruiz-Echevarria M J, Gonzalez C I, Peltz S W. BioEssays. 1999;21:685–696. doi: 10.1002/(SICI)1521-1878(199908)21:8<685::AID-BIES8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 6.Hilleren P, Parker R. RNA. 1999;5:711–719. doi: 10.1017/s1355838299990519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muhlrad D, Parker R. Nature (London) 1994;370:578–581. doi: 10.1038/370578a0. [DOI] [PubMed] [Google Scholar]
- 8.Anderson J S J, Parker R P. EMBO J. 1998;17:1497–1506. doi: 10.1093/emboj/17.5.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell P, Petfalski E, Shevchenko A, Mann M, Tollervey D. Cell. 1997;91:457–466. doi: 10.1016/s0092-8674(00)80432-8. [DOI] [PubMed] [Google Scholar]
- 10.Frischmeyer P A, van Hoof A, O'Donnell K, Guerrerio A L, Parker R, Dietz H C. Science. 2002;295:2258–2261. doi: 10.1126/science.1067338. [DOI] [PubMed] [Google Scholar]
- 11.van Hoof A, Frischmeyer P A, Dietz H C, Parker R. Science. 2002;295:2262–2264. doi: 10.1126/science.1067272. [DOI] [PubMed] [Google Scholar]
- 12.Bernstein P, Peltz S W, Ross J. Mol Cell Biol. 1989;9:659–670. doi: 10.1128/mcb.9.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brewer G. J Biol Chem. 1998;273:34770–34774. doi: 10.1074/jbc.273.52.34770. [DOI] [PubMed] [Google Scholar]
- 14.Couttet P, Fromont-Racine M, Steel D, Pictet R, Grange T. Proc Natl Acad Sci USA. 1997;94:5628–5633. doi: 10.1073/pnas.94.11.5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford L P, Watson J, Keene J D, Wilusz J. Genes Dev. 1999;13:188–201. doi: 10.1101/gad.13.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shyu A B, Belasco J G, Greenberg M E. Genes Dev. 1991;5:221–231. doi: 10.1101/gad.5.2.221. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Day N, Trifillis P, Kiledjian M. Mol Cell Biol. 1999;19:4552–4560. doi: 10.1128/mcb.19.7.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z, Kiledjian M. Cell. 2001;107:751–762. doi: 10.1016/s0092-8674(01)00592-x. [DOI] [PubMed] [Google Scholar]
- 19.Wilson T, Treisman R. Nature (London) 1988;336:396–399. doi: 10.1038/336396a0. [DOI] [PubMed] [Google Scholar]
- 20.Chen C Y, Gherzi R, Ong S E, Chan E L, Raijmakers R, Pruijn G J, Stoecklin G, Moroni C, Mann M, Karin M. Cell. 2001;107:451–464. doi: 10.1016/s0092-8674(01)00578-5. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee D, Gao M, O'Connor J P, Raijmakers R, Pruijn G, Lutz C S, Wilusz J. EMBO J. 2002;21:165–174. doi: 10.1093/emboj/21.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross J, Kobs G. J Mol Biol. 1986;188:579–593. doi: 10.1016/s0022-2836(86)80008-0. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Kiledjian M. EMBO J. 2000;19:295–305. doi: 10.1093/emboj/19.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thoma C, Hasselblatt P, Kock J, Chang S F, Hockenjos B, Will H, Hentze M W, Blum H E, von Weizsacker F, Offensperger W B. Mol Cell. 2001;8:865–872. doi: 10.1016/s1097-2765(01)00364-1. [DOI] [PubMed] [Google Scholar]
- 25.Stevens A. Mol Cell Biol. 1988;8:2005–2010. doi: 10.1128/mcb.8.5.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunckley T, Parker R. EMBO J. 1999;18:5411–5422. doi: 10.1093/emboj/18.19.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H, Rodgers N D, Jiao X, Kiledjian M. EMBO J. 2002;21:4699–4708. doi: 10.1093/emboj/cdf448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiledjian M, Day N, Trifillis P. Methods. 1999;17:84–91. doi: 10.1006/meth.1998.0710. [DOI] [PubMed] [Google Scholar]
- 29.Bessman M J, Frick D N, O'Handley S F. J Biol Chem. 1996;271:25059–25062. doi: 10.1074/jbc.271.41.25059. [DOI] [PubMed] [Google Scholar]
- 30.Gabelli S B, Bianchet M A, Bessman M J, Amzel L M. Nat Struct Biol. 2001;8:467–472. doi: 10.1038/87647. [DOI] [PubMed] [Google Scholar]
- 31.Koonin E V. Nucleic Acids Res. 1993;21:4847. doi: 10.1093/nar/21.20.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mejean V, Salles C, Bullions L C, Bessman M J, Claverys J P. Mol Microbiol. 1994;11:323–330. doi: 10.1111/j.1365-2958.1994.tb00312.x. [DOI] [PubMed] [Google Scholar]
- 33.Abeygunawardana C, Weber D J, Gittis A G, Frick D N, Lin J, Miller A F, Bessman M J, Mildvan A S. Biochemistry. 1995;34:14997–5005. doi: 10.1021/bi00046a006. [DOI] [PubMed] [Google Scholar]
- 34.Lin J, Abeygunawardana C, Frick D N, Bessman M J, Mildvan A S. Biochemistry. 1996;35:6715–6726. doi: 10.1021/bi953071x. [DOI] [PubMed] [Google Scholar]
- 35.Safrany S T, Caffrey J J, Yang X, Bembenek M E, Moyer M B, Burkhart W A, Shears S B. EMBO J. 1998;17:6599–6607. doi: 10.1093/emboj/17.22.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao M, Wilusz C J, Peltz S W, Wilusz J. EMBO J. 2001;20:1134–1143. doi: 10.1093/emboj/20.5.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilusz C J, Gao M, Jones C L, Wilusz J, Peltz S W. RNA. 2001;7:1416–1424. [PMC free article] [PubMed] [Google Scholar]
- 38.Vilela C, Velasco C, Ptushkina M, McCarthy J E. EMBO J. 2000;19:4372–4382. doi: 10.1093/emboj/19.16.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cui Y, Hagan K W, Zhang S, Peltz S W. Genes Dev. 1995;9:423–436. doi: 10.1101/gad.9.4.423. [DOI] [PubMed] [Google Scholar]
- 40.Leeds P, Peltz S W, Jacobson A, Culbertson M R. Genes Dev. 1991;5:2303–2314. doi: 10.1101/gad.5.12a.2303. [DOI] [PubMed] [Google Scholar]
- 41.Lykke-Andersen J, Shu M D, Steitz J A. Cell. 2000;103:1121–1131. doi: 10.1016/s0092-8674(00)00214-2. [DOI] [PubMed] [Google Scholar]
- 42.Sun X, Perlick H A, Dietz H C, Maquat L E. Proc Natl Acad Sci USA. 1998;95:10009–10014. doi: 10.1073/pnas.95.17.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He F, Jacobson A. Genes Dev. 1995;9:437–454. doi: 10.1101/gad.9.4.437. [DOI] [PubMed] [Google Scholar]
- 44.Seraphin B. DNA Seq. 1992;3:177–179. doi: 10.3109/10425179209034013. [DOI] [PubMed] [Google Scholar]
- 45.Kisselev L L, Justesen J, Wolfson A D, Frolova L Y. FEBS Lett. 1998;427:157–163. doi: 10.1016/s0014-5793(98)00420-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.