Abstract
Effector binding to liganded hemoglobin (Hb) provides a new understanding of structural determinants of Hb function. L35, a bezafibrate-related compound, is one of the more potent synthetic regulators of Hb oxygen (O2) affinity. In the presence of inositol hexaphosphate and bezafibrate (or derivatives), liganded Hb at low pH (pH ∼6.5) exhibits extremely low O2 affinity and very low cooperativity. In this study, the nature of L35 binding to COHbA at pH 6.35, an altered R-state, is presented. Solution-active site-specific spectroscopic probings by front-face fluorescence and circular dichroism reveal that L35 induces a global heterogeneous conformation in COHbA at pH 6.35 that includes: a T-like structural feature at the α1β2 interface; an R-like structural feature within the heme environment; and an intermediate-like state at the central cavity. These long-range structural perturbations appear to stem from L35 binding to two classes of binding sites: the central cavity (primarily at the αα cleft) and the surface. These results indicate that L35 induces an allosteric transition species, characterized by domain-specific tertiary and quaternary-like conformation within a global R-quaternary structure.
INTRODUCTION
It has been believed that allosteric effectors function by binding to deoxy hemoglobin (Hb). However, recent studies demonstrate binding of allosteric effectors to liganded Hb, contributing a new understanding of the allosteric transition and structural determinants regulating Hb function. In the absence of heterotropic effectors, stripped Hb is a high-affinity, moderately cooperative oxygen carrier, exhibiting a Bohr effect, with limited functional diversity. In the presence of heterotropic effectors, Hb exhibits significant functional diversities; and, in vivo, endogenous red blood cell heterotropic effectors modulate Hb function in ways critical to its efficacy as an oxygen carrier (Hirsch et al., 1999; Yonetani et al., 2002; Chen et al., 2002). Such functional diversities are generated primarily through tertiary conformational constraints caused by effector interaction with Hb, especially oxyHb (Yonetani et al., 2002). These studies have led to the proposal that the classic definition of the quaternary R and T structure by Perutz (1970) is only relevant to Hb stripped of organic phosphates and other natural regulators.
Bezafibrate (BZF) derivatives, potent heterotropic allosteric effectors of Hb, are useful tools to probe Hb structure and function. These compounds exhibit anti-gelling and antihyperlipoproteinemic properties, and bind reversibly to intraerythrocytic Hb, decreasing O2 affinity (Poyart et al., 1994). The early application of BZF derivatives to Hb research and therapeutics was based on the assumption that the drug interacts only with T-state Hb (Murray et al., 1988a,b; Noble et al., 1989; Bettati et al., 1997). However, this assumption was negated by several solution and crystal studies demonstrating that BZF and its derivatives interact with R-state Hb at neutral pH and modulate Hb function (Marden et al., 1988, 1990; Coletta et al., 1995, 1999; Shibayama et al., 2002).
Laser photolysis studies of COHbA show that BZF decreases the CO association rate of R-state Hb by over a factor of 4 (Marden et al., 1988, 1990). The O2 dissociation rate constant (measured by CO displacement of fully liganded oxyHbA) plotted as a function of BZF concentration results in BZF-saturable binding site(s), indicating that oxyHbA interacts with BZF (Coletta et al., 1999). The addition of BZF to stripped oxyHb dramatically decreases O2 affinity in the absence of cooperativity, and enhances the Bohr effect—all indicative of BZF binding to R-state Hb (Tsuneshige et al., 2002). A recent high-resolution crystal structure of carbonmonoxy-Hb-BZF complex, obtained in low-ionic strength polyethylene-glycol medium at pH 6.8 acetate ammonium (50 mM), revealed a new allosteric binding site of BZF to R-state Hb located near the surface of the E-helix of each α-subunit. The complex maintains the R-state quaternary structure (Shibayama et al., 2002).
Liganded Hb at low pH (pH ∼6.5), in the presence of allosteric effectors (e.g., inositol hexaphosphate, i.e., IHP, and BZF), has been used to explore effector binding given its low O2 affinity and low cooperativity (Lalezari et al., 1990; Yonetani et al., 2002; Tsuneshige et al., 2002). However, liganded Hb in the presence of heterotropic effectors generates a variety of structural interpretations: 1), “liganded T-state with partially oxygenated Hb tetramer constrained in the T-state” (Lalezari et al., 1990; Marden et al., 1988, 1990); 2), “liganded R-state with tertiary structural constraints” (Yonetani et al., 2002); and 3), “a special state that stabilizes a tertiary T-like conformation within a quaternary R-like intermediate structure” (Coletta et al., 1999). Specifically, COHbA at pH 6.35, originally defined in the presence of organic phosphate as “an altered R-state Hb” by Scott et al. (1983), has recently been classified as “a snapshot of an R-T intermediate in the presence of inorganic phosphate” (Safo et al., 2002). Moreover, COHbA at pH 6.35 forms crystal structure intermediates R3 and RR2 (between R and R2) (Safo and Abraham, 2003).
In the present study, we aim to clarify the various structural interpretations by spectroscopically exploring the binding properties of L35, a BZF derivative (Poyart et al., 1994), to the low pH altered R-state, correlated with site-specific structural changes in both heme and globin domains (Scott et al., 1985). The altered R-state has proven useful in solution studies probing central cavity differences in variant hemoglobins and in comparative effector binding studies (e.g., Hirsch et al., 1996, 1997, 1999; Chen et al., 2002; Fablet et al., 2003). Using the low pH transition state, evidence is presented that shows L35 induces a transition species characterized by domain-specific tertiary and quaternary-like conformation within a global R-quaternary structure.
EXPERIMENTAL PROCEDURES
Materials
L35 (Fig. 1) was synthesized and prepared as used before (Lalezari et al., 1990). A stock solution was prepared by dissolving in distilled water with the addition of NaOH and titrated to the desired pH. The sodium salt of inositol hexaphosphate (IHP) was prepared as described (Chen et al., 2002). A stock solution of 8-hydroxy-l, 3, 6-pyrenetrisulfonate (HPT) was prepared in 0.05 M HEPES, pH 6.35.
FIGURE 1.
The chemical structure of L35 (Lalezari et al., 1990).
Hemoglobin purification
HEPES buffer has been employed to preserve the integral nature of stripped hemoglobins that could be compromised when using a phosphate buffer (Hirsch et al., 1996, 1999). The advantages of HEPES buffer are reviewed at length by Yonetani et al. (2002).
HbA was purified from AA hemolysates followed by DE-52 anion exchange chromatography as routinely performed in our lab (e.g., Hirsch et al., 1999; Chen et al., 2002). COHbA was prepared by gently passing chemically pure CO gas over the surface of the Hb solution, and then dialyzed overnight against 0.05 M HEPES, pH 6.35. All observations employed Hb concentrations wherein the Hb tetramer predominates (Hirsch, 1994).
Hemoglobin labeling
Binding of iodoacetamidofluorescein (5-IAF) to β93Cys was carried out as previously described (Chen et al., 2002). The purity of the Hb-AF conjugate was verified by observation of a single band on isoelectric focusing gel and a single modified β-chain peak (a mass of 16,253 compared to the normal β-chain mass of 15,867) by mass spectrometry.
Steady-state front-face fluorescence spectroscopy
Steady-state front-face fluorescence spectroscopy was performed with a SLM 8000 photon-counting spectrophotometer (SLM-Aminco, Foster City, CA) equipped with a front-face cuvette holder. A concentration of 1.0 g % COHb was used, a condition where the tetramer predominates. Front-face fluorescence is an established technique to determine the intrinsic fluorescence of Hb and extrinsic fluorophores bound to Hb (for a review of Hb front-face fluorometry, see Hirsch, 1994, 2000, 2003).
Circular dichroism spectroscopy
Circular dichroism spectra of COHbA in the presence and absence of allosteric effectors were recorded on a JASCO- J720 spectropolarimeter (JASCO, Tokyo, Japan) with a 0.05-cm cell-length cuvette (125 μl). A COHb concentration of 0.7 g % (0.11 mM) Hb tetramer was used. All circular dichroism (CD) measurements were performed at room temperature of 22°C with molar ellipticity in terms of Hb tetramer.
Surface plasmon resonance (SPR)
BIAcore-3000 (Biacore, Neuchâtel, Switzerland), a SPR-based biosensor, was used to determine the binding constant of L35 to COHbA. COHbA at a concentration of 0.65 g % (0.1 mM Hb tetramer) was immobilized to the carboxymethylated dextran layer of a CM5 biosensor chip (Biacore) via amine covalent coupling. Immobilization was carried out in HEPES buffer (50 mM, pH 6.35) at a flow rate of 100 μl/min. The flow-cell surface was activated by a mixture of n-hydroxysuccinimide (NHS) and n-ethyl-n′-(dimethyl-aminopropyl)-carbodiimide (EDC). An immobilization level of 4500–7500 resonance units was obtained. A nonderivatized flowcell serves as a reference surface.
L35 binding to COHbA immobilized on the biosensor chip was conducted at a flow rate of 100 μl/min. A 5-mM L35 stock solution was prepared directly in the running buffer (50 mM HEPES, pH 6.35) from which a serial twofold dilution (each in duplicate aliquots) was made and injected. To ensure complete dissociation of L35 from COHbA, a 50-mM glycine-NaOH buffer (pH 8.5) was used to regenerate the sensor surface after each L35 injection.
RESULTS
L35 binding alters the Hb intrinsic fluorescence reflective of α1β2 interface conformational changes
Long-range site-specific intramolecular conformational perturbations by ligand binding may be probed in solution by a variety of spectroscopic techniques that focus on specific molecular domains. This complements crystallography by providing access to conformational changes that may be restricted by the crystal lattice. Front-face fluorescence is useful for probing conformational differences of mutant hemoglobins in the microdomains of the central cavity and the α1β2 interface (Hirsch et al., 1996, 1999). Hb intrinsic fluorescence is primarily due to the fluorescence of β37Trp at the α1β2 interface, though it may contain some contribution by the surface Trp residues, α14 and β15Trps (Hirsch 1994, 2000, 2003). Furthermore, Hb intrinsic fluorescence serves as a reporter for quaternary transition (Hirsch et al, 1980; Hirsch and Nagel, 1981). The CO-liganded form of Hb exhibits an intrinsic fluorescence emission maximum at 322 nm. Compared to the CO-liganded form, deoxyHb exhibits increased fluorescence intensity, concomitant with a red shift of the emission maximum to 325 nm. (Although the exact wavelength may deviate by a few nanometers dependent on the spectrophotometer, the relative changes are maintained.)
Titration of COHbA with L35 causes a red shift in the emission maximum wavelength (to ∼329 nm) and a progressive decrease in the intensity of the intrinsic fluorescence of COHbA at pH 6.35 (Fig. 2 A). A shift of the intrinsic fluorescence emission of Hb to longer wavelengths indicates that Trp residues become more exposed upon L35 binding. The intrinsic fluorescence intensity changes as a function of L35 concentration generate titration curves that appear to be biphasic (Fig. 2 B). Three to five independent measurements were obtained for each data point on the curves. Given the excellent reproducibility of each point as shown by the standard deviations (see error bars, Fig. 2 B), the breaks in the curves (i.e., abrupt change of slope), although small, appear real and should not be ignored. Hence, two relatively weak binding sites are suggested (mM KD values, Table 1).
FIGURE 2.
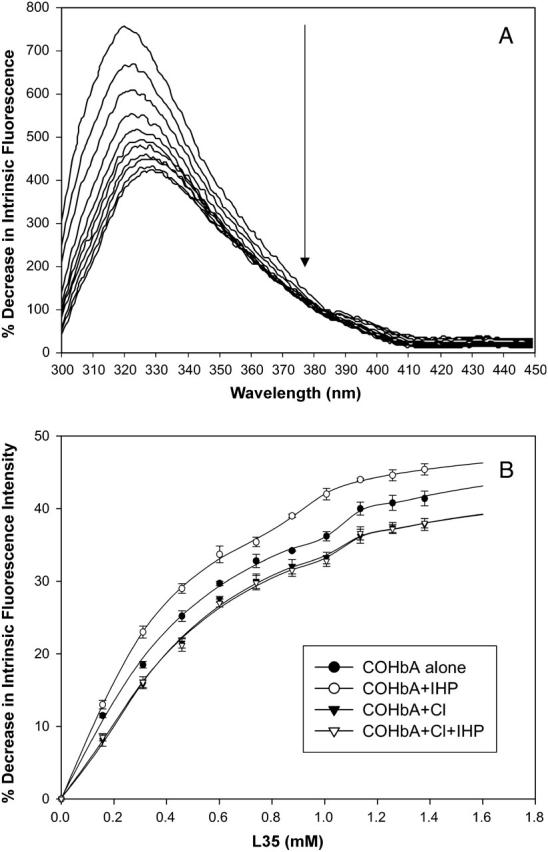
Intrinsic fluorescence emission spectra of COHbA as a function of L35 concentration (A) and L35 titration of COHbA as a function of intrinsic fluorescence intensity changes (B). Fluorescence excitation at 280 nm. (0.05 M HEPES, pH 6.35, 1.0 g % COHbA, 25°C.) Titration curves for COHbA alone and COHbA+IHP were obtained from the average of three reproducible titrations. Titration curves for COHbA+Cl and COHbA+Cl+IHP were obtained from the average of five reproducible titrations (see error bar ≤1%). Note that the symbols for COHbA+Cl (∇) and COHbA+Cl+IHP (▾) overlap in a nearly identical fashion. The derived KD values are shown in Table 1.
TABLE 1.
Binding affinities of L35 to COHbA in the presence/absence of other allosteric effectors as determined by changes in the intrinsic fluorescence of Hb
| Effectors | KD1 (mM) | KD2 (mM) | R2 |
|---|---|---|---|
| L35 | 0.41 | 0.94 | 0.998 |
| L35+IHP | 0.23 | 0.85 | 0.999 |
| L35+Cl− | 0.50 | 1.00 | 0.998 |
| L L35+Cl−+IHP | 0.47 | 1.02 | 0.998 |
Conditions: 1.0 g % COHbA, 50 mM HEPES, pH 6.35. The KD values and correlation coefficients (R2) are calculated from curve-fitting analysis using Sigma Plot.
Further support for the biphasic nature of the curves stems from the following. The KD of the second weaker binding site (KD2), obtained from fluorescence data, is consistent with the KD derived from the surface plasmon resonance study (presented below). Furthermore, as elaborated in the discussion, the suggestion of two binding sites best explains the L35-induced conformational changes probed by other spectroscopic techniques (e.g., changes in extrinsic fluorescence of Hb-bound HPT and the perturbations of the CD spectra shown below). Moreover, it is not unusual for allosteric effectors to exhibit low binding affinities to liganded Hb. For example, IHP binds to COHb (pH 7.0) at two sites (KD1 = 0.40 mM; KD2 = 6.3 mM, Coletta et al., 1993). BZF binds to liganded Hb, with a KD of 15 or 40 mM, dependent upon the heme ligand, solution conditions, and assay techniques (Ascenzi et al., 1993; Coletta et al., 1999).
L35 titration of Hb, in the presence of IHP, results in a greater intrinsic fluorescence intensity decrease than with L35 alone. The enhancement of L35 binding by IHP is seen by a 44% increase in the L35 binding affinity for the higher affinity binding site, and a 10% increase for the lower affinity binding site (Table 1). In contrast, chloride inhibits the effect of L35 on intrinsic fluorescence changes: L35 titration of Hb in the presence of Cl− alone or Cl− with IHP minimizes the L35 effect as seen by a 6–22% decrease in the two binding affinities of L35 (Table 1). The opposing coupling effects of L35 and IHP compared to L35 and Cl− proves significant since titration of COHbA at pH 6.35 with either IHP or chloride (Cl−) alone insignificantly alters the intrinsic fluorescence of the altered R-state Hb. Notably, in the presence of Cl−, the IHP enhancement of L35 binding becomes null, as reflected in the similar KD (Table 1).
L35 affects the central cavity as seen by the extrinsic fluorescence of HPT-bound COHbA
HPT is an established fluorescent 2,3-bisphosphoglycerate (BPG) analog with an emission maximum (em. max. 385 nm when excited at 280 nm) different from that of the intrinsic Hb fluorescence (em. max. 330 nm) (MacQuarrie and Gibson, 1971, 1972; Gottfried et al., 1997). It has been established that HPT binds to the β–β cleft of the central cavity of deoxy Hb at the BPG binding pocket (MacQuarrie and Gibson, 1971, 1972; Serbanescu et al., 1998). When bound to Hb, HPT fluorescence is quenched by the heme and results in significantly reduced fluorescence. Front-face optics is advantageous in that it permits a direct observation of HPT fluorescence in the presence of Hb (for a review, see Hirsch, 2000, 2003). Several central cavity-binding effectors, such as IHP and Cl−, displace HPT from the central cavity of the altered R-state Hb as indicated by an intensity increase in HPT fluorescence (Hirsch et al., 1999; Chen et al., 2002; Fablet et al., 2003).
L35 titration to Hb-bound HPT results in an increased HPT fluorescence intensity, indicating its release from the central cavity (Fig. 3 A). In contrast, titrating L35 into a Hb-free HPT solution results in quenching of HPT fluorescence by L35. In the presence of IHP, the fluorescence of HPT is greater than in the absence of IHP (Fig. 3 B), since a significant amount of HPT has already been displaced from the central cavity by IHP. L35 titration into a mixture of IHP and HPT-bound Hb leads to a further increase in the HPT fluorescence (Fig. 3 B), indicating more HPT released from the central cavity. The synergistic effect of IHP on L35 displacement of HPT is consistent with 1), the observed IHP effect on L35 quenching of the intrinsic fluorescence of COHb (Fig. 2 B); and 2), the known synergistic action of L35 and IHP in decreasing the Hb O2 affinity (Lalezari et al., 1990).
FIGURE 3.

Extrinsic fluorescence emission spectra of COHbA-bound HPT as a function of L35 concentration (A) and L35 titration of Hb-bound HPT in the absence/presence of IHP and Cl− (B). Fluorescence excitation at 280 nm, emission at 385 nm. (0.05 M HEPES, pH 6.35, 1.0 g % COHbA, 0.31 mM IHP, 0.1 M Cl−, 25°C.)
In contrast, L35 titration of HPT–bound Hb in the presence of Cl− results in decreasing HPT fluorescence (Fig. 3 B). Compared to IHP, a greater amount of HPT is displaced out of the central cavity by Cl− before L35 titration. A decreasing HPT fluorescence indicates that some HPT rebinds to the central cavity. In the presence of both Cl− and IHP, the IHP and L35 synergistic effect is not present. Indeed, the overall effect of the Cl− and IHP combination, on L35 displacement of HPT, is almost identical to that when Cl− alone is present before L35 titration (Fig. 3 B). This is in agreement with the loss of the synergistic effect of IHP on L35 intrinsic fluorescence quenching seen in the presence of Cl− (Table 1). The competitive effect of Cl− and L35 on HPT displacement and on the intrinsic fluorescence quenching is consistent with earlier reports that Cl− and L35 act competitively to decrease Hb O2 affinity (Lalezari et al., 1990; Yonetani et al., 2002).
L35 binding perturbs β93Cys as reflected in the COHbA-AF extrinsic fluorescence changes
β93Cys is located in a conformationally flexible domain containing residues whose interactions are directly linked to allosteric properties of the Hb tetramer (Perutz, 1970, 1990; Perutz et al., 1998; Khan et al., 2001). The reactivity of β93Cys is a function of both the quaternary and tertiary structures of HbA (Riggs, 1952, 1961; Riggs and Wolbach, 1956; Vasquez et al., 1999). The Cys side chain is exposed to solvents in liganded HbA (accessible to thiol reagents for β93 modifications), but buried in the unliganded T-state (non-accessible to thiol reagent modifications). The fluorescent probe, IAF, covalently bound to oxyHbA, reports β93 local environmental changes close to the heme. In the oxy state, the probe is in a particularly hydrophilic environment and becomes less exposed upon deoxygenation (Hirsch et al., 1986). IAF modification of HbA results in a greatly increased oxygen affinity and decreased cooperativity, which constitutes the most extreme functional alteration yet reported for thiol reagent modifications (Chen et al., 2002).
A decreased fluorescein fluorescence intensity is observed upon L35 titration (Fig. 4). The titration curve, plotted as the percent decrease in fluorescein fluorescence intensity versus L35 concentration, exhibits a sigmoid shape. A KD of 0.48mM was obtained from the midpoint of the curve (Fig. 4, inset). The decrease in fluorescein fluorescence intensity may be explained by: 1), a more exposed β93Cys side chain as a function of L35 binding; or more simply, 2), L35 directly quenches fluorescein fluorescence. Since the altered R-state β93Cys side chain of COHbA appears to be less exposed than in liganded Hb at pH 7.35 (R-state) R. E. Hirsch, unpublished data), it is likely that L35 binding results in further exposure of the β93Cys side chain (toward a more R-like state), reflected in the observed decreased fluorescence. This is consistent with an L35-induced R-like heme environment supported by the CD data shown below.
FIGURE 4.
Extrinsic fluorescence emission spectra of β93 fluorescein (IAF) modified COHbA (COHbA-AF) as a function of L35 concentration. (Inset) L35 titration of COHbA-AF. Fluorescence excitation at 480 nm, emission at 520 nm. (0.05 M HEPES, pH 6.35, 1.0 g % COHbA-AF, 25°C.)
The possibility of fluorescence energy transfer from fluorescein to L35, within the range of excitation (480 nm), is improbable since the L35 absorption spectrum does not overlap with either the fluorescein excitation (absorption) or emission spectra. Furthermore, both static and dynamic quenching would require contact of the quencher with the fluorophore within the solvent shell (Lakowicz, 1999). However, there is no evidence to date that L35 or similar compounds bind at or near the β-chain heme domain.
Specific binding of L35 to sensorchip-immobilized COHbA as detected by surface plasmon resonance
Surface plasmon resonance (SPR)-based biosensors, such as BIAcore, are used to determine binding constants for macromolecular interactions (Myszka, 1997; Myszka et al., 1999; Rich and Myszka, 2000; Day et al., 2002). Immobilization of Hb to the CM5 sensorchip surface uses primary amines (the N-terminal amines and surface lysines) for covalent coupling. Therefore, this Hb immobilization should restrict access to the N-terminal β1Val (which lies at the entrance of the central cavity ββ cleft), and α1Val (located in the entrance of the αα cleft). We demonstrate here that the central cavity entrance is blocked using SPR methodology: the N-terminal amino acids are covalently bound to the carboxymethylated dextran layer of the sensorchip surface, imposing steric hindrance for the accessible of effectors such as IHP and Cl−. This becomes advantageous by serving as a control to test the proposed L35 binding site(s) indicated by the fluorescence results.
The observations support the prediction of a blocked Hb central cavity entrance both at the αα and ββ clefts when COHbA is immobilized to the sensorchip surface, since, uncharacteristically, IHP and Cl− show no binding to hemoglobin (Fig. 5, A and B insets). L35 only shows a low-affinity binding, equivalent to the secondary binding site as identified by the intrinsic fluorescence titration (Fig. 5 A). Also, Cl− or IHP has no effect on L35 binding to the sensor surface (Fig. 5 B). These various controls are consistent with a model that L35 binds to a low-affinity noncentral cavity-binding site, most likely the secondary binding site indicated by intrinsic fluorescence (Fig. 2).
FIGURE 5.

Surface plasmon resonance raw sensorgrams and binding curves for the interaction of sensorchip-immobilized COHbA with effectors. (Conditions: 0.65 g% COHbA or COHbA-AF, 50 mM HEPES, pH 6.35.) (A) Titration of L35 alone; (B) L35+IHP and L35+Cl. (Conditions: 1.0 mM IHP; 0.1 M Cl.) The binding curves of L35 alone (triangle and solid line), L35 + IHP (circle and dashed line), and L35+chloride (square and dotted line), plotted by taking the plateau value as a function of L35 concentration are shown in C. (Inset) A comparison of L35 binding curves for COHbA and COHbA-AF. The points on the curve represent the highest resonance units (plateau value) for the respective effector(s) concentration.
The surface plasmon resonance response of the L35 interaction with COHbA is that of a complete binding cycle, achieving the expected association plateau and subsequent dissociation toward the baseline (Fig. 5, A and B). A rapid downfall of the dissociation curve provides further evidence that this is not a high-affinity binding. Plotting the maximum resonance response as a function of L35 concentration generates a sigmoid binding curve (Fig. 5 C). The dissociation constant (KD = 1.5 mM) of L35 binding to COHbA can be derived from the midpoint value of the curve (Fig. 5 C). The KD determined from the biosensor is comparable to the secondary L35 binding obtained by the solution-based front-face florescence data (Table 1, KD2 = 0.94 mM), with the small difference in KD attributed to the difference in methods. Moreover, a control study employing COHbA-AF immobilized onto the sensorchip surface shows that L35 exhibits a significantly weakened noncentral cavity binding to COHbA-AF compared to unmodified COHbA (KD = 10 mM as derived from the midpoint of the binding curve; Fig. 5 C, inset). This low-affinity binding is too weak (a KD of 10 mM) to be reflected in the L35 titration range (0–1.5 mM) of the COHbA-AF monitored by fluorescein fluorescence (Fig. 4). Thus, the L35 titration of COHbA-AF results in a sigmoid-shape titration curve (Fig. 4), implying a low-affinity L35 binding site with a KD of 0.48 mM. In contrast, L35 titration of unbound COHbA (monitored in solution by the intrinsic fluorescence) results in the appearance of a biphasic-like curve, indicating two classes of binding affinities (Fig. 2 B).
L35 effect on COHbA conformation at the heme and α1β2 interface as probed by CD
CD spectra in the near-UV region (250–300 nm) arise from tryptophanyl, tyrosyl, and phenylalanyl residues, the S-S bridge, and the heme groups (Timasheff, 1970). CD bands at ∼260 nm stem from the phenylalanyl residues. They are the least sensitive to alterations in their environment but are very responsive to the state of the heme (Simon and Cantor, 1969). Specifically, the 270–290 nm region CD band provides a sensitive probe for human Hb quaternary structure at the α1β2 interface (Perutz et al., 1974a,b,c; Perutz, 1976). The 280–290 nm CD bands reflect the local environments of β37Trp, α42Tyr, α140Tyr, and β145Tyr (Plese and Amma, 1977; Plese et al., 1977; Baldwin and Chothia, 1979; Abraham et al., 1991; Li et al., 2000a,b), which are located at the α1β2 interface. The sharp negative trough in the CD spectrum at ∼280–290 nm is characteristic of the globin quaternary structural changes, but independent of the state of ligation of the heme and the accompanying changes in globin chain tertiary structure (Perutz et al., 1974a,b,c). However, the relative contributions of these residues to the 280-nm region UV CD changes have not been assigned (Li et al., 2000a,b).
The CD spectra of COHbA (stripped 50 mM HEPES at pH 6.35) in the near-UV and Soret regions in the absence and presence of L35 are shown in Figs. 6–7. (For a comparison, the effect of other allosteric effectors such as IHP and Cl− on the CD spectra of COHbA is also presented; see Figs. 6–7, insets.) IHP and Cl− alone do not exhibit CD bands in the spectral region 250–650 nm. L35 has a weak CD band at 259 nm, which may interfere with the much stronger Hb CD band in the 260-nm region. However, there is no interference with the Hb 280-nm region CD band.
FIGURE 6.
Near UV region CD spectra of COHbA in the presence of L35 and other effectors: COHbA alone, COHbA+L35, COHbA+L35+IHP, COHbA+L35+Cl−, and COHbA+L35+IHP +Cl−. (Inset) CD spectra of COHbA alone, COHbA+IHP, and COHbA+Cl−. (0.05 M HEPES, pH 6.35, 0.65 g % COHbA, 0.7 mM L35, 0.7 mM IHP, 70 mM Cl−, 23°C.)
FIGURE 7.
Soret region CD spectra of COHbA in the presence of L35 and other effectors: COHbA alone, COHbA+L35, COHbA+L35+IHP, COHbA+L35+Cl−, and COHbA+L35+IHP+Cl−. (Inset) CD spectra of COHbA alone, COHbA+IHP, and COHbA+Cl−. (0.05 M HEPES, pH 6.35, 0.65 g % COHbA, 0.7 mM L35, 0.7 mM IHP, 70 mM Cl−, 23°C.)
Notably, L35 induces a deep negative trough at ∼280 nm in COHbA, indicative of a T-like quaternary structure feature at the α1β2 interface (Perutz et al., 1974a,b,c). This is consistent with the observed L35-induced red-shift in intrinsic fluorescence emissions, a T-like feature, since the emission maximum of the intrinsic fluorescence of deoxyHbA red-shifts to 325 nm compared to COHbA at pH 7.35 in phosphate buffer (321 nm) (Hirsch and Nagel, 1981). However, the simultaneous presence of L35 and IHP results in a complete loss of the negative trough at 280 nm, whereas the presence of L35 and Cl− together induces only a diminished negative trough (Fig. 6). A complete loss of the negative trough at 280 nm occurs with the simultaneous presence of L35, IHP, and Cl−, similar to the absence of the negative trough observed in the COHbA-L35-IHP complex (Fig. 6).
To ensure that the L35-induced COHbA UV CD changes are a quaternary conformational event, two different liganded Hb controls were performed. COHbA-AF was chosen as a control since AF modification of COHbA at the β93Cys stabilizes the R-state (Riggs and Wolbach, 1956; Hirsch et al., 1986). The second control employs COHbA at pH 7.35. In the presence of L35, the negative trough within the 280-nm region is not observed for COHbA-AF and for COHbA at pH 7.35 (data not shown).
The Hb Soret region CD bands (410–430 nm) stem from the π→ π* transition of the heme. Studies show that the CD bands in the Soret region, as well as in the 500–600-nm range, are sensitive to the oxidation state of the heme, ligand specificity, and subunit interactions (Goodall and Shooter, 1969; Nagai et al., 1969; Geraci and Parkhurst, 1981). Liganded Hb has a characteristic CD band at ∼420 nm (COHb at 422 nm, oxyHb at 419 nm), which is distinct from that of deoxy Hb (431 nm) and met Hb (410 nm). Upon L35 binding, both an increase and a merely detectable red-shift in the ellipticity of COHbA at 422 nm are observed, concurrent with an increased shoulder at ∼407 nm (Fig. 7). The L35-induced 422-nm region ellipticity increase resembles the BPG-induced ellipticity increase at 420 nm reported for oxyHb and deoxy Hb (Sugita et al., 1971). The 407-nm shoulder is similar to that seen in the COHbA Soret CD spectrum in the presence of IHP (Fig. 7, inset) as reported by others (Bucci et al., 1978).
In the absence of L35, Cl− alone results in an ellipticity increase at ∼422 nm and a slight shift of the 422-nm CD band to a shorter wavelength. In the presence of L35, Cl− significantly diminishes the L35 effect, whereas IHP enhances the L35-induced perturbation in the Soret region, further shifting the 422-nm CD band to a longer wavelength (Fig. 7). In the presence of both IHP and Cl−, there is an alteration of the L35-induced perturbation that appears as a small but significant shift of the CD band to longer wavelengths toward the T-state, but within the R-state assignments. All these results are in agreement with the reported heterotropic effector-induced tertiary structural changes in the heme environment (Yonetani et al., 2002; Scott et al., 1983, 1985), which will be further discussed in this article.
DISCUSSION
Effector binding to liganded Hb provides insight into Hb allosteric transition. Compared to organic and inorganic phosphate-induced tertiary structural changes (Scott et al., 1983, 1985; Safo et al., 2002), L35 induces a global heterogeneous conformation in COHbA at pH 6.35: a T-like structural feature at the α1β2 interface, R-like structural features within the heme environment, and an intermediate-like state at the central cavity. Therefore, L35 may induce a transition species characterized by the domain-specific tertiary and quaternary-like conformation within a global R-quaternary structure.
The unique L35-induced structural perturbation stems from a specific binding of L35 to COHbA, as indicated by: 1), saturable titration curves (Figs. 2–5); and 2), solution- and surface-based KD determinations that, taken together, suggest two classes of binding sites. Although L35 binding releases Hb-bound HPT, the synergistic effect of L35 and IHP on HPT displacement (Fig. 3) may indicate that L35 does not bind specifically at the ββ cleft BPG pocket. Considering that the Cl−-binding channel runs through the central cavity (Ueno and Manning, 1992), the competitive effect of Cl− alone or Cl− and IHP on L35 displacement of HPT (Fig. 3) further suggests that L35 binding sites lie within the central cavity, but not at the BPG site. In deoxyHb, L35 binds at the interface of the α- and β-subunits deep in the waterfilled central cavity at a distance from the heme and the organic phosphate binding sites (Lalezari et al., 1990; Poyart et al., 1994). It is possible that L35 binds deeply in the central cavity of COHbA and causes a conformational perturbation transmitted to the ββ cleft BPG site, weakening HPT binding. This possibility is supported by our SPR data (Fig. 5), wherein immobilization of COHbA (effecting a blocked central cavity ββ cleft entrance) results in only one low-affinity noncentral cavity binding site, correlated with the secondary binding site detected by intrinsic fluorescence (Fig. 2 B). Based upon a recent crystallization study at pH 6.8, showing specific binding of BZF to horse COHb surface (i.e., the surface of each α-subunit E-helix) (Shibayama et al., 2002), it is likely that the secondary binding site of L35 also resides on the surface, since both L35 and BZF have chlorobenzene and methyl groups that hydrophobically interact with the Hb hydrophobic pocket between the E-helix and the α-chain heme edge. Therefore, for COHbA at low pH, an altered R-state, L35 may have two classes of binding sites, one in the central cavity and the other on the Hb surface, resembling binding characteristics intermediate to both that of the deoxy and CO forms of Hb.
The mixed binding characteristic of L35 mentioned above may be due to the fact that we are studying an R-like intermediate (COHb at low pH). A similar notion has been presented by Safo et al. (2002), showing that COHbA in phosphate buffer at pH 6.4 may be “a snapshot of an intermediate” in the allosteric transition. This could be explained by the L35-induced domain-specific heterogeneous conformational changes reported by spectroscopic probing. In contrast to the T-like structural feature at the α1β2 interface, the heme is essentially in an R-state conformation, even though L35 induces a modest red-shift CD band in the Soret region. This is further supported by 1), the L35 induction of an R-like β93Cys microenvironment seen in COHbA-AF (Fig. 4); and 2), previous reports demonstrating heterotropic effector-induced tertiary R-state structural changes in the heme environment (Scott et al., 1983, 1985; Yonetani et al., 2002).
The issue of domain-dependent R- and T-like structural features raises a question: how does L35 induce a T-like structural feature at the α1β2 interface, but not in the heme environment? Similar domain-dependent R- and T-like structural features have been previously reported. UV CD spectra of metal hybrid Hb, such as αFe→Zn βCO, αCO βFe→Zn, and αFe→Zn βFe→Zn, show a significant negative ellipticity band centered at 280 nm, analogous to T-state Hb (Fiechtner et al., 1980; Simolo et al., 1986), whereas other regions, such as Val (E11) and Asp (β89)-Tyr (α42), probed by NMR resonance and exchangeable proton, resemble the oxy R structure (Simolo et al., 1986). Additionally, studying the allosteric effector-induced changes of oxyHbA at the near-UV and Soret region CD spectra changes, Coletta et al. (1999) showed that the α1β2 interface of oxyHbA in the simultaneous presence of IHP and BZF mostly maintains a quaternary R-tetrameric structure, whereas the heme conformation conforms to that typical of the low-affinity structure at neutral pH. They demonstrated that IHP and BZF together induce a stabilization of a tertiary T-like conformation within a quaternary R-like structure (Coletta et al., 1999). Consistent with this finding is that the stepwise progress of the Hb allosteric pathway starts with a rearrangement of the contacts at the hinge region and ends with a rearrangement involving the switch region of the interface (Goldbeck et al., 2002). Therefore, the L35 structural perturbation unique to COHbA at low pH may be explained by the kinetic data of Goldbeck et al. (2002), demonstrating that β37Trp at the α1β2 interface is the obligatory conformational change that gives rise to an intermediate conformer in sequence to the quaternary transition. It is possible that L35 induces an intermediate conformation wherein the quaternary structural changes in the hinge region are not fully transmitted to the switch region of the α1β2 interface and the heme environment.
Based upon the low pH COHbA crystal structure (Safo et al., 2002), we propose a model of L35-induced intramolecular communication pathways, to explain some of the L35-induced domain-specific perturbations reported by these spectroscopic probings. Since L35 binds to different sites than IHP, and competes with Cl−, possible L35 binding contacts are the α141Arg and α126Asn at the αα cleft, the well-known Cl− binding sites. This serves as the basis for the proposed communication pathways shown in Scheme I–Scheme III. Hence, L35 binding at α141Arg could perturb the α140Tyr and β37Trp in the hinge region of the α1β2 interface and the α-heme proximal Histidine α87His, consistent with the fluorescence and CD data. In addition, L35 binding may invoke other long-range effects on α42Tyr and β145Tyr, located in the switch region of the interface.
SCHEME I.
L35-induced intramolecular communication pathway to the α140Tyr at the hinge region of the α1β2 interface. Asterisk (*) indicates residues forming side-chain contacts based on PDB1ljw (Safo et al., 2002).
SCHEME II.
L35-induced intramolecular communication pathway to the β37Trp at hinge region of the α1β2 interface. See Scheme I for notes.
SCHEME III.
L35-induced intramolecular communication pathway to the α87His at the α-heme environment. See Scheme I for notes.
The L35-induced domain-specific heterogeneous conformational changes are differentially affected by IHP and Cl−. This is summarized in Table 2, showing the effect of IHP and Cl− on L35-induced perturbations in selected domains, as reported by fluorescence and CD probings. Cl− weakens whereas IHP enhances the L35-induced conformational perturbations in the central cavity and the heme environment, suggesting long-range intramolecular communication pathways from the central cavity to the heme environment. These observations underlie the importance of coupled interactions among effectors and their corresponding central cavity domains in modulating Hb conformation and functionality. This may explain why Cl− and L35 act competitively, whereas IHP and L35 act synergistically in decreasing Hb O2 affinity. Note that spectroscopic probings by intrinsic fluorescence and UV CD show opposing effects of IHP on the L35-induced perturbations at the α1β2 interface. This may be explained by considering that the intrinsic fluorescence primarily originates from β37Trp (Hirsch and Nagel, 1981), whereas the near UV CD includes the contributions of β37Trp, α42Tyr, α140Tyr, and β145Tyr (Li et al., 2000a,b; Perutz et al., 1974a,b,c). One possibility is that IHP binding affects certain aromatic residues at the α1β2 interface, contributing to the UV-CD but not to the intrinsic fluorescence where tryptophan predominates. Indeed, β145Tyr is one of such aromatic residues. As shown for inorganic phosphate (Safo et al., 2002), IHP may bind to the COHbA central cavity ββ cleft (β139Asn, β143His, and β146His) and thus significantly alter the β145Tyr as shown in Scheme IV.
TABLE 2.
Overall effects of IHP and Cl− on L35-induced perturbations in COHbA
| Domains | Cl− | Effectors IHP | IHP+Cl− |
|---|---|---|---|
| Intrinsic fluorescence/α1β2 interface | Reduced | Enhanced | Reduced |
| Extrinsic HPT fluorescence/central cavity | Reduced | Enhanced | Reduced |
| UV CD 280-nm region/α1β2 interface | Reduced | Negated | Negated |
| Soret CD/heme environment | Reduced intensity (shift is negated) | Enhanced | Reduced |
Conditions: 1.0 g % COHbA, 50 mM HEPES, pH 6.35.
SCHEME IV.
IHP binding or β93Cys alteration may each communicate to α145Tyr at the switch region of the α1β2 interface. See Scheme I for notes.
Similarly, since β93Cys hydrophobically interacts with β145Tyr (Safo et al., 2002, PDB 1ljw), perturbation of β145Tyr by L35 may also explain the lack of a negative trough for the CD 280-nm region (assigned to the α1β2 interface R-state) in COHbA-AF complexes used as controls. This is consistent with the long-range L35 effect resulting in a greater exposure of β93Cys in COHbA-AF (Fig. 4).
In summary, it is proposed that L35 induces a transition species characterized by the domain-specific R- and T-like tertiary and quaternary conformations within a global R-quaternary structure. This unifying model may clarify the variety of structural interpretations of liganded Hb in the presence of heterotropic allosteric effectors.
Acknowledgments
We thank Mr. Huiyong Cheng for processing the Biacore data, and Dr. Argyrides Argyrou, PhD, for helpful discussions related to the data analysis.
This work is supported in part by the American Heart Association, Heritage Affiliate, Grant-in-Aid No. 0256390T; National Institutes of Health NHLBI, Bronx Comprehensive Sickle Cell Center and Sickle Cell Scholar Award NIH HL 070994; DK R21 06423; and NCRR 12248.
Abbreviations used: COHbA-AF, acetamidofluorescein covalently bound to COHbA at β93Cys; HbA, human adult hemoglobin; L35, 2-[4-(3,5-dichlorophenylureido) phenoxy]-2-methylpropionic acid.
References
- Abraham, D. J., A. S. Mehanna, F. C. Wireko, J. Whitney, R. P. Thomas, and E. P. Orringer. 1991. Vanillin, a potential agent for the treatment of sickle cell anemia. Blood. 77:1334–1341. [PubMed] [Google Scholar]
- Ascenzi, P., A. Bertollini, M. Coletta, A. Desideri, B. Giardina, F. Polizio, R. Santucci, R. Scatena, and G. Amiconi. 1993. Cooperative effect of inositol hexakisphosphate, bezafibrate, and clofibric acid on the spectroscopic properties of the nitric oxide derivative of ferrous human hemoglobin. J. Inorg. Biochem. 50:263–272. [DOI] [PubMed] [Google Scholar]
- Bettati, S., L. D. Kwiatkowski, J. S. Kavanaugh, A. Mozzarelli, A. Arnone, G. L. Rossi, and R. W. Noble. 1997. Structure and oxygen affinity of crystalline des-His-146β human hemoglobin in the T state. J. Biol. Chem. 272:33077–33084. [DOI] [PubMed] [Google Scholar]
- Baldwin, J., and C. Chothia. 1979. Haemoglobin: the structural changes related to ligand binding and its allosteric mechanism. J. Mol. Biol. 129:175–220. [DOI] [PubMed] [Google Scholar]
- Bucci, E., A. Salahuddin, J. Bonaventura, and C. Bonaventura. 1978. Characterization of the ionizable groups interacting with anionic allosteric effectors of human hemoglobin. J. Biol. Chem. 253:821–827. [PubMed] [Google Scholar]
- Chen, Q., C. Bonaventura, R. L. Nagel, and R. E. Hirsch. 2002. Distinct domain responses of R-state human hemoglobins A, C, and S to anions. Blood Cells Mol. Dis. 29:119–132. [DOI] [PubMed] [Google Scholar]
- Coletta, M., P. Ascenzi, R. Santucci, A. Bertollini, and G. Amiconi. 1993. Interaction of inositol hexakisphosphate with liganded ferrous human hemoglobin. Direct evidence for two functionally operative binding sites. Biochim. Biophys. Acta. 1162:309–314. [DOI] [PubMed]
- Coletta, M., P. Ascenzi, M. Castagnola, and B. Giardina. 1995. Functional and spectroscopic evidence for a conformational transition in ferrous liganded human hemoglobin. J. Mol. Biol. 249:800–803. [DOI] [PubMed] [Google Scholar]
- Coletta, M., M. Angeletti, P. Ascenzi, A. Bertollini, L. S. Della, G. De Sanctis, A. M. Priori, R. Santucci, and G. Amiconi. 1999. Coupling of the oxygen-linked interaction energy for inositol hexakisphosphate and bezafibrate binding to human HbA0. J. Biol. Chem. 274:6865–6874. [DOI] [PubMed] [Google Scholar]
- Day, Y. S., C. L. Baird, R. L. Rich, and D. G. Myszka. 2002. Direct comparison of binding equilibrium, thermodynamic, and rate constants determined by surface- and solution-based biophysical methods. Protein Sci. 11:1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fablet, C., Q. Chen, V. Baudin-Creuza, M. C. Marden, R. L. Nagel, J. Pagnier, and R. E. Hirsch. 2003. β7E-β132K salt bridge and sickle haemoglobin stability and conformation. Br. J. Haematol. 122:317–325. [DOI] [PubMed] [Google Scholar]
- Fiechtner, M. D., G. McLendon, and M. W. Bailey. 1980. Assignment of the quaternary structure of Fe/Zn hybrid hemoglobins: implications for allosteric theories. Biochem. Biophys. Res. Commun. 96:618–625. [DOI] [PubMed] [Google Scholar]
- Geraci, G., and L. J. Parkhurst. 1981. Circular dichroism spectra of hemoglobins. Methods Enzymol. 76:262–275. [DOI] [PubMed] [Google Scholar]
- Goldbeck, R. A., R. M. Esquerra, and D. S. Kliger. 2002. Hydrogen bonding to Trp β37 is the first step in a compound pathway for hemoglobin allostery. J. Am. Chem. Soc. 124:7646–7647. [DOI] [PubMed] [Google Scholar]
- Goodall, P. T., and E. M. Shooter. 1969. Changes in heme environment due to subunit interaction in hemoglobin. J. Mol. Biol. 39:675–678. [DOI] [PubMed] [Google Scholar]
- Gottfried, D. S., L. J. Juszczak, N. A. Fataliev, A. S. Acharya, R. E. Hirsch, and J. M. Friedman. 1997. Probing the hemoglobin central cavity by direct quantification of effector binding using fluorescence lifetime methods. J. Biol. Chem. 272:1571–1578. [DOI] [PubMed] [Google Scholar]
- Hirsch, R. E., R. S. Zukin, and R. L. Nagel. 1980. Intrinsic fluorescence emission of intact oxy hemoglobins. Biochem. Biophys. Res. Commun. 93:432–439. [DOI] [PubMed] [Google Scholar]
- Hirsch, R. E., and R. L. Nagel. 1981. Conformational studies of hemoglobins using intrinsic fluorescence measurements. J. Biol. Chem. 256:1080–1083. [PubMed] [Google Scholar]
- Hirsch, R. E., R. S. Zukin, and R. L. Nagel. 1986. Steady-state fluorescence emission from the fluorescent probe, 5-iodoacetamidofluorescein, bound to hemoglobin. Biochem. Biophys. Res. Commun. 138:489–495. [DOI] [PubMed] [Google Scholar]
- Hirsch, R. E. 1994. Front-face fluorescence spectroscopy of hemoglobins. Methods Enzymol. 232:231–246. [DOI] [PubMed] [Google Scholar]
- Hirsch, R. E., M. J. Lin, G. J. Vidugiris, S. Huang, J. M. Friedman, R. L. Nagel, and G. V. Vidugirus. 1996. Conformational changes in oxyhemoglobin C (Glu β6→ Lys) detected by spectroscopic probing. J. Biol. Chem. 271:372–375. [DOI] [PubMed] [Google Scholar]
- Hirsch, R. E., A. C. Rybicki, N. A. Fataliev, M. J. Lin, J. M. Friedman, and R. L. Nagel. 1997. A potential determinant of enhanced crystallization of HbC: spectroscopic and functional evidence of an alteration in the central cavity of oxyHbC. Br. J. Haematol. 98:583–588. [DOI] [PubMed] [Google Scholar]
- Hirsch, R. E., L. J. Juszczak, N. A. Fataliev, J. M. Friedman, and R. L. Nagel. 1999. Solution-active structural alterations in liganded hemoglobins C (β6 Glu → Lys) and S (β6 Glu → Val). J. Biol. Chem. 274:13777–13782. [DOI] [PubMed] [Google Scholar]
- Hirsch, R. E. 2000. Heme-protein fluorescence. In Topics in Fluorescence Spectroscopy, Vol. 6. J.R. Lakowicz, editor. Kluwer Academic/Plenum Publishers, New York.
- Hirsch, R. E. 2003. Hemoglobin fluorescence. Methods Mol. Med. 82:133–154. [DOI] [PubMed] [Google Scholar]
- Khan, I., D. Dantsker, U. Samuni, A. J. Friedman, C. Bonaventura, B. Manjula, S. A. Acharya, and J. M. Friedman. 2001. β93-modified hemoglobin: kinetic and conformational consequences. Biochemistry. 40:7581–7592. [DOI] [PubMed] [Google Scholar]
- Lalezari, I., P. Lalezari, C. Poyart, M. Marden, J. Kister, B. Bohn, G. Fermi, and M. F. Perutz. 1990. New effectors of human hemoglobin: structure and function. Biochemistry. 29:1515–1523. [DOI] [PubMed] [Google Scholar]
- Lakowicz, J. R. 1999. Principles of Fluorescence Spectroscopy. Kluwer Academic Publishers, New York.
- Li, R., Y. Nagai, and M. Nagai. 2000a. Changes of tyrosine and tryptophan residues in human hemoglobin by oxygen binding: near- and far-UV circular dichroism of isolated chains and recombined hemoglobin. J. Inorg. Biochem. 82:93–101. [DOI] [PubMed] [Google Scholar]
- Li, R., Y. Nagai, and M. Nagai. 2000b. Contribution of α140Tyr and β37Trp to the near-UV CD spectra on quaternary structure transition of human hemoglobin A. Chirality. 12:216–220. [DOI] [PubMed] [Google Scholar]
- MacQuarrie, R., and Q. H. Gibson. 1971. Use of a fluorescent analogue of 2,3-diphosphoglycerate as a probe of human hemoglobin conformation during carbon monoxide binding. J. Biol. Chem. 246:5832–5835. [PubMed] [Google Scholar]
- MacQuarrie, R., and Q. H. Gibson. 1972. Ligand binding and release of an analogue of 2,3-diphosphoglycerate from human hemoglobin. J. Biol. Chem. 247:5686–5694. [PubMed] [Google Scholar]
- Marden, M. C., J. Kister, B. Bohn, and C. Poyart. 1988. T-state hemoglobin with four ligands bound. Biochemistry. 27:1659–1664. [DOI] [PubMed] [Google Scholar]
- Marden, M. C., B. Bohn, J. Kister, and C. Poyart. 1990. Effectors of hemoglobin. Separation of allosteric and affinity factors. Biophys. J. 57:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, L. P., J. Hofrichter, E. R. Henry, M. Ikeda-Saito, K. Kitagishi, T. Yonetani, and W. A. Eaton. 1988a. The effect of quaternary structure on the kinetics of conformational changes and nanosecond geminate rebinding of carbon monoxide to hemoglobin. Proc. Natl. Acad. Sci. USA. 85:2151–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, L. P., J. Hofrichter, E. R. Henry, and W. A. Eaton. 1988b. Time-resolved optical spectroscopy and structural dynamics following photodissociation of carbonmonoxyhemoglobin. Biophys. Chem. 29:63–76. [DOI] [PubMed] [Google Scholar]
- Myszka, D. G. 1997. Kinetic analysis of macromolecular interactions using surface plasmon resonance biosensors. Curr. Opin. Biotechnol. 8:50–57. [DOI] [PubMed] [Google Scholar]
- Myszka, D. G., S. J. Wood, and A. L. Biere. 1999. Analysis of fibril elongation using surface plasmon resonance biosensors. Methods Enzymol. 309:386–402. [DOI] [PubMed] [Google Scholar]
- Nagai, M., Y. Sugita, and Y. Yoneyama. 1969. Circular dichroism of hemoglobin and its subunits in the Soret region. J. Biol. Chem. 244:1651–1653. [PubMed] [Google Scholar]
- Noble, R. W., A. DeYoung, S. Vitale, M. Cerdonio, and E. E. DiIorio. 1989. Spin equilibria in human methemoglobin: effects of bezafibrate and inositol hexaphosphate as measured by susceptometry and visible spectroscopy. Biochemistry. 28:5288–5292. [DOI] [PubMed] [Google Scholar]
- Perutz, M. F. 1970. Stereochemistry of cooperative effects in haemoglobin. Nature. 228:726–739. [DOI] [PubMed] [Google Scholar]
- Perutz, M. F., A. R. Fersht, S. R. Simon, and G. C. Roberts. 1974a. Influence of globin structure on the state of the heme. II. Allosteric transitions in methemoglobin. Biochemistry. 13:2174–2186. [DOI] [PubMed] [Google Scholar]
- Perutz, M. F., E. J. Heidner, J. E. Ladner, J. G. Beetlestone, C. Ho, and E. F. Slade. 1974b. Influence of globin structure on the state of the heme. III. Changes in heme spectra accompanying allosteric transitions in methemoglobin and their implications for heme-heme interaction. Biochemistry. 13:2187–2200. [DOI] [PubMed] [Google Scholar]
- Perutz, M. F., J. E. Ladner, S. R. Simon, and C. Ho. 1974c. Influence of globin structure on the state of the heme. I. Human deoxyhemoglobin. Biochemistry. 13:2163–2173. [DOI] [PubMed] [Google Scholar]
- Perutz, M. F. 1976. Haemoglobin: structure, function and synthesis. Br. Med. Bull. 32:193–194. [DOI] [PubMed] [Google Scholar]
- Perutz, M. F. 1990. Mechanisms regulating the reactions of human hemoglobin with oxygen and carbon monoxide. Annu. Rev. Physiol. 52:1–25. [DOI] [PubMed] [Google Scholar]
- Perutz, M. F., A. J. Wilkinson, M. Paoli, and G. G. Dodson. 1998. The stereochemical mechanism of the cooperative effects in hemoglobin revisited. Annu. Rev. Biophys. Biomol. Struct. 27:1–34. [DOI] [PubMed] [Google Scholar]
- Plese, C. F., and E. L. Amma. 1977. Circular dichroism as a probe of the allosteric R in equilibrium T transformation in hemoglobins and modified hemoglobins. Biochem. Biophys. Res. Commun. 76:691–697. [DOI] [PubMed] [Google Scholar]
- Plese, C. F., E. L. Amma, and P. F. Rodesiler. 1977. Conformational state and R to and from T transformation in Mn(II) and Mn(III) hemoglobins and azide Mn(III) hemoglobin. Biochem. Biophys. Res. Commun. 77:837–844. [DOI] [PubMed] [Google Scholar]
- Poyart, C., M. C. Marden, and J. Kister. 1994. Bezafibrate derivatives as potent effectors of hemoglobin. Methods Enzymol. 232:496–513. [DOI] [PubMed] [Google Scholar]
- Rich, R. L., and D. G. Myszka. 2000. Advances in surface plasmon resonance biosensor analysis. Curr. Opin. Biotechnol. 11:54–61. [DOI] [PubMed] [Google Scholar]
- Riggs, A. F. 1952. Sulfhydryl groups and the interaction between the hemes in hemoglobin. J. Gen. Physiol. 36:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs, A. F., and R. A. Wolbach. 1956. Sulfhydryl groups and the structure of hemoglobin. J. Gen. Physiol. 39:585–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs, A. 1961. The binding of n-ethylmaleimide by human hemoglobin and its effect upon the oxygen equilibrium. J. Biol. Chem. 236:1948–1954. [PubMed] [Google Scholar]
- Safo, M. K., J. C. Burnett, F. N. Musayev, S. Nokuri, and D. J. Abraham. 2002. Structure of human carbonmonoxyhemoglobin at 2.16 Å: a snapshot of the allosteric transition. Acta Crystallogr. D Biol. Crystallogr. 58:2031–2037. [DOI] [PubMed] [Google Scholar]
- Safo, M. K., and D. J. Abraham. 2003. X-ray crystallography of hemoglobins. Methods Mol. Med. 82:1–19. [DOI] [PubMed] [Google Scholar]
- Simon, S. R., and C. R. Cantor. 1969. Measurement of ligand-induced conformational changes in hemoglobin by circular dichroism. Proc. Natl. Acad. Sci. USA. 63:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbanescu, R., L. Kiger, C. Poyart, and M. C. Marden. 1998. Fluorescent effector as a probe of the allosteric equilibrium in methemoglobin. Biochim. Biophys. Acta. 1363:79–84. [DOI] [PubMed] [Google Scholar]
- Scott, T. W., J. M. Friedman, M. Ikeda-Saito, and T. Yonetani. 1983. Subunit heterogeneity in the structure and dynamics of hemoglobin. A transient Raman study. FEBS Lett. 158:68–72. [DOI] [PubMed] [Google Scholar]
- Scott, T. W., J. M. Friedman, and V. W. MacDonald. 1985. Distal and proximal control of ligand reactivity: a transient Raman comparison of COHbA and COHbC. J. Am. Chem. Soc. 107:3702–3705. [Google Scholar]
- Shibayama, N., S. Miura, J. R. Tame, T. Yonetani, and S. Y. Park. 2002. Crystal structure of horse carbonmonoxyhemoglobin-bezafibrate complex at 1.55-Å resolution. A novel allosteric binding site in R-state hemoglobin. J. Biol. Chem. 277:38791–38796. [DOI] [PubMed] [Google Scholar]
- Simolo, K., Z. R. Korszun, G. Stucky, K. Moffat, G. McLendon, and G. Bunker. 1986. Extended x-ray absorption fine structure studies of Zn2Fe2 hybrid hemoglobins: absence of heme bond length changes in half-ligated species. Biochemistry. 25:3773–3778. [DOI] [PubMed] [Google Scholar]
- Sugita, Y., M. Nagai, and Y. Yoneyama. 1971. Circular dichroism of hemoglobin in relation to the structure surrounding the heme. J. Biol. Chem. 246:383–388. [PubMed] [Google Scholar]
- Timasheff, S. N. 1970. The Enzyme, 3rd Ed. P.D. Boyer, editor. Academic Press, New York.
- Tsuneshige, A., S. Park, and T. Yonetani. 2002. Heterotropic effectors control the hemoglobin function by interacting with its T and R states—a new view on the principle of allostery. Biophys. Chem. 98:49–63. [DOI] [PubMed] [Google Scholar]
- Ueno, H., and J. M. Manning. 1992. The functional, oxygen-linked Cl− binding sites of hemoglobin are contiguous within a channel in the central cavity. J. Protein Chem. 11:177–185. [DOI] [PubMed] [Google Scholar]
- Vasquez, G. B., M. Karavitis, X. Ji, I. Pechik, W. S. Brinigar, G. L. Gilliland, and C. Fronticelli. 1999. Cysteines β93 and β112 as probes of conformational and functional events at the human hemoglobin subunit interfaces. Biophys. J. 76:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonetani, T., S. I. Park, A. Tsuneshige, K. Imai, and K. Kanaori. 2002. Global allostery model of hemoglobin. Modulation of O2 affinity, cooperativity, and Bohr effect by heterotropic allosteric effectors. J. Biol. Chem. 277:34508–34520. [DOI] [PubMed] [Google Scholar]










