Abstract
The structural pharmacophore of Taxol, responsible for binding the N terminus of the β-subunit of tubulin to arrest cell proliferation, comprises, in part, the 13-O-(N-benzoyl-3-phenylisoserinoyl) side chain. To identify the side chain transferase of Taxol biosynthesis, a set of transacylases obtained from an enriched cDNA library (constructed from mRNA isolated from Taxus cuspidata cells induced with methyl jasmonate for Taxol production) was screened. A cDNA clone (designated TAX7) encoding a taxoid C-13 O-phenylpropanoyltransferase was isolated which yielded a recombinant enzyme that catalyzes the selective 13-O-acylation of baccatin III with β-phenylalanoyl CoA as the acyl donor to form N-debenzoyl-2′-deoxytaxol. This enzymatic product was converted to 2′-deoxytaxol by chemical N-benzoylation, and the identity of this derivative was confirmed by spectrometric analyses. The full-length cDNA has an ORF of 1,335 bases and encodes a 445-aa protein with a calculated molecular weight of 50,546. Evaluation of kinetic parameters revealed Km values of 2.4 ± 0.5 μM and 4.9 ± 0.3 μM for baccatin III and β-phenylalanoyl-CoA, respectively. The pH optimum for the recombinant O-(3-amino-3-phenylpropanoyl)transferase is at 6.8. Identification of this clone completes acquisition of the five aroyl/acyltransferases involved in the biosynthesis of Taxol. Application of these transacylase genes in suitable host cells can improve the production yields of Taxol and could enable the preparation of second-generation Taxol analogs possessing greater bioactivity and improved water solubility.
Keywords: side chain attachment‖C-13 O-acyltransferase‖paclitaxel‖ baccatin III‖amino acid-CoA esters
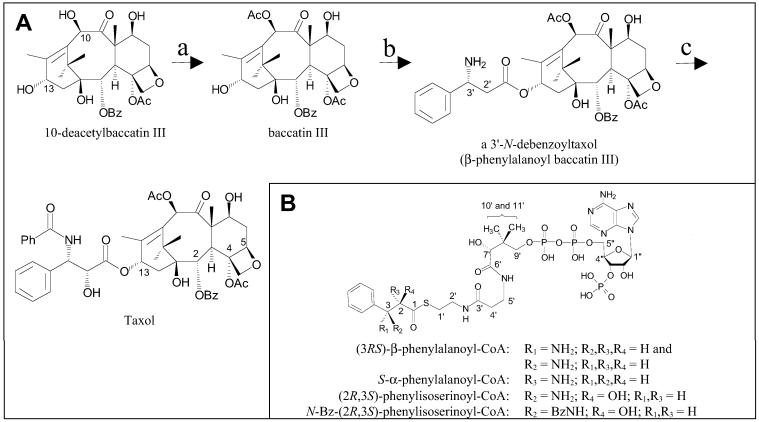
The natural product Taxol (generic name paclitaxel, Fig. 1A), produced by yew (Taxus) species, is an important drug for the treatment of several cancers, as well as AIDS-related Kaposi's sarcoma (1–3). In mitotic cells, this antineoplastic agent binds tubulin heterodimers, promotes and stabilizes microtubule assembly, and disrupts cellular division (4, 5). Structure–activity relationship studies have demonstrated that the C-2 benzoxy group, an intact oxetane ring bridged by the C-4(5) bond, and the C-13 O-[N-benzoyl (Bz) phenylisoserine] side chain of Taxol (Fig. 1A) are required for the unique tubulin-binding mode of action (6, 7).
Figure 1.
(A) Outline of the progression of advanced metabolites in Taxol biosynthesis illustrating the acetylation of 10-deacetylbaccatin III to baccatin III by 10-deacetylbaccatin III O-acetyltransferase (a), the transfer of an aminophenylpropanoyl group to C-13 of baccatin III by an O-(3-amino-3-phenylpropanoyl)transferase (b), and the benzamidation and C-2′ hydroxylation of the side chain by N-debenzoyltaxol N-benzoyltransferase and a taxoid side chain hydroxylase (c). (B) Synthetic phenylpropanoyl-CoA esters used to assess substrate specificity of the O-(3-amino-3-phenylpropanoyl)transferase.
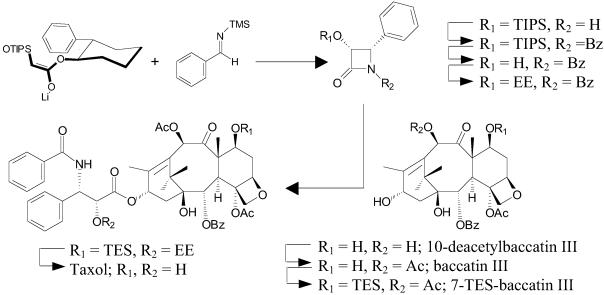
The low yield of Taxol from natural sources and the lack of a commercially viable total synthesis have rendered the semisynthetic coupling of the important phenylisoserine side chain to baccatin III (Fig. 2), a naturally occurring biosynthetic precursor of Taxol, as the principal means of producing the drug to meet increasing demand (8–10). This semisynthetic method, however, involves several steps, including silyl protection and acetylation of 10-deacetylbaccatin III to afford 7-O-protected baccatin III, synthetic attachment of the N-Bz phenylisoserine side chain at the C-13 hydroxyl, and, finally, deprotection to yield Taxol (see Fig. 2). Conceivably, a biosynthetic approach to Taxol production, by using enzyme catalysis to construct and transfer the side chain directly to baccatin III, could eliminate multistep semisynthesis of the drug and reduce production costs.
Figure 2.
Outline of the semisynthesis of Taxol from the natural product 10-deacetylbaccatin III and a synthetic β-lactam precursor of the N-Bz phenylisoserine side chain. TIPS, triisopropylsilyl; TES, triethylsilyl; EE, ethoxyethyl.
Previous in vivo studies, in which ring-perdeuterated amino acids were used to elucidate the biosynthetic pathway for the C-13 side chain of Taxol (11), showed that the intact side chain (N-Bz phenylisoserine) was not incorporated into Taxol, whereas β-phenylalanine and, to a lesser extent, α-phenylalanine and phenylisoserine were incorporated. These results suggest that β-phenylalanine, as the free amine, is attached to baccatin III, and that this side chain is hydroxylated and N-benzoylated to complete the route to Taxol.
These biosynthetic results prompted a search for the Taxol pathway gene encoding a transferase that esterifies a β-phenylalanoyl intermediate with baccatin III. The isolation of this baccatin III:3-amino-3-phenylpropanoyltransferase (BAPT) was achieved by functionally screening a family (58–69% sequence identity) of previously acquired Taxus acyl/aroyltransferase cDNAs (12) by using baccatin III and β-phenylalanoyl-CoA as cosubstrates. The kinetic properties and specificity of this recombinant enzyme, which catalyzes the attachment of the biologically important Taxol side chain precursor, are described.
Materials and Methods
Substrates and Reagents.
[3H]Baccatin III was prepared by described methods (13), except that the intermediate 7-triethylsilyl-[13-3H]baccatin III was deprotected with hydrogen fluoride by standard methods (14). N-tert-Butoxycarbonyl (t-Boc)-(3RS)-β-phenylalanine and N-t-Boc-(S)-α-phenylalanine were prepared as described (12). CoA as the lithium salt was purchased from Sigma. Di-t-butyl dicarbonate, benzoyl chloride, sodium hydride, β-phenylalanine (3-amino-3-phenylpropionic acid), (S)-α-phenylalanine, N-Bz-(2R,3S)-3-phenylisoserine, and all other reagents, unless otherwise noted, were purchased from Aldrich. Authentic baccatin III was synthesized from 10-deacetylbaccatin III (Natland, Morrisville, NC) as described (15). Authentic (3′RS)-2′-deoxytaxol was prepared from the corresponding N-debenzoylated analog by described methods (12).
General Procedures.
The general procedure for the synthesis of the amino acid-CoA thioesters was adapted from literature methods (16–18) that used a mixed anhydride intermediate to facilitate transesterification with CoA. In each case, lyophilized N-protected (and O-protected when necessary) amino acid-CoA ester intermediates were typically dissolved in 5–7 ml of buffer (15 mM potassium phosphate, pH 6.9) and purified by chromatography on a C18 Sep-Pak cartridge (500 mg of C18 silica gel, Waters) that was first washed with methanol (10 ml), then with water (10 ml), and finally with phosphate buffer (10 ml). After loading the column with sample, the column was eluted with increasing concentrations of methanol (5–100%) in buffer; the pure product typically eluted in 10–20% methanol.
Synthesis of α- and β-Phenylalanoyl-CoA Esters.
The derived N-t-Boc-α- or β-phenylalanoyl-CoA was lyophilized, and the residue was dissolved in 1 ml of water, cooled to 0°C, and 1 ml of trifluoroacetic acid was added dropwise with stirring for 1 h to deprotect the amino group. The mixture was then warmed to room temperature and stirred an additional 1 h. The progress of decarbonylation of the N-t-Boc compound was monitored by silica gel analytical TLC (1-butanol/H2O/acetic acid, 5:3:2, vol/vol/vol) with detection by UV absorbance. After complete deprotection, the reaction was diluted with 50 ml of water and concentrated to 0.5 ml under vacuum; this dilution and evaporation process was repeated three times to remove residual trifluoroacetic acid. Finally, the sample was concentrated to dryness and the residue was resuspended in 5 ml of water. The product was purified by C18 Sep-Pak cartridge chromatography as described above. The CoA esters of α- or β-phenylalanine eluted in 10–20% methanol, which was removed in vacuo, and the residue were dissolved in deuterated water (as internal reference) for analysis by 1H NMR spectrometry. The CoA ester was quantified by comparing the peak area of sample protons with that of dioxane protons (at 11 mM) added as internal standard. (3RS)-β-Phenylalanoyl-CoA was obtained in 40% yield (16 μmol at >95% purity based on 1H NMR) with respect to N-t-Boc-(3RS)-β-phenylalanine (40 μmol), and S-α-phenylalanoyl-CoA was obtained in 26% yield (11 μmol at >90% purity based on 1H NMR) with respect to N-t-Boc-(S)-α-phenylalanine (42 μmol). 1H NMR (300 MHz, D2O) for (3RS)-β-phenylalanoyl-CoA (see Fig. 1B for numbering). δ: 0.56 (s, H-10′), 0.70 (s, H-11′), 2.12 (dd, J = 6.3 and 6.9 Hz, H-4′), 2.74 (m, H-1′), 3.04 (dd, J = 5.1 and 6.0, H-2′), 3.21 (m, H-2 and H-5′), 3.37 (dd, J = 4.8 and 9.6 Hz, Ha-5"), 3.65 (dd, J = 4.8 and 9.6 Hz, Hb-5"), 3.85 (s, H-7′), 4.03 (d, J = 3.8 Hz, Ha-9′), 4.05 (d, J = 3.8 Hz, Hb-9′), 4.39 (ddd, J = 2.7 and 5.3 Hz, H-4"), 4.56–4.66 (m, H-3, H-2", and H-3"), 5.95 (two doublets; one set from each stereoisomer, J = 6.9 Hz for both, H-1"), 7.21–7.27 (phenyl protons), 8.01 (two singlets; one from each stereoisomer, adenine-CH), and 8.33 (two singlets; one from each stereoisomer, adenine-CH). 1H NMR (300 MHz, D2O) for (S)-α-phenylalanoyl-CoA (see Fig. 1B for numbering). δ: 0.54 (s, H-10′), 0.66 (s, H-11′), 2.21 (dd, J = 6.3 and 6.6 Hz, H-4′), 2.82–2.95 (m, H-3 and H-1′), 3.02–3.11 (m, H-2 and H-2′), 3.23 (dd, J = 6.3 and 6.9 Hz, H-5′), 3.34 (dd, J = 4.8 and 9.6 Hz, Ha-5"), 3.60 (dd, J = 4.8 and 9.6 Hz, Hb-5"), 3,80 (s, H-7′), 4.00 (d, J = 4.2 Hz, Ha-9′), 4.02 (d, J = 4.2 Hz, Hb-9′), 4.36 (br ddd, H-4"), 5.91 (d, J = 6.6 Hz, H-1"), 6.96–7.19 (phenyl protons), 7.97 (s, adenine-CH), 8.30 (s, adenine-CH). H-2" and H-3" proton signals obscured by solvent (H2O/D2O) signal. After NMR quantitation, the D2O was removed by evaporation; the CoA ester at 10 mM in H2O was stored at −20°C.
Synthesis of N-Bz-(2R,3S)-3-Phenylisoserinoyl-CoA.
N-Bz-(2R,3S)-3-Phenylisoserine (230 mg, 0.81 mmol) in 10 ml of tetrahydrofuran (THF) was esterified by diazomethane treatment. The crude ester was subjected to silica gel flash column chromatography (ethyl acetate/hexane, 50:50, vol/vol) to yield the pure ester derivative (0.73 mmol, 90% yield). This methyl ester (220 mg, 0.73 mmol) was dissolved in THF (15 ml) and added to a stirred suspension of sodium hydride (1.1 mmol) in diethyl ether (10 ml) under N2. To the mixture was added di-t-butyl dicarbonate (170 mg, 0.77 mmol) in THF (20 ml), and the suspension was stirred at room temperature for 20 min. The mixture was then chilled on ice, quenched with 1 ml of water, and finally filtered. The filtrate was collected and the solvent evaporated. The product was purified by silica gel flash column chromatography (ethyl acetate/hexane, 35:65, vol/vol) to yield pure N-Bz-O-t-Boc-(2R,3S)-3-phenylisoserine methyl ester (0.66 mmol, 90% yield). This methyl ester (26 mg, 65 μmol) in THF (1.2 ml) was hydrolyzed for 12 h with NaOH (65 μmol, 32.5 μl of a 2 M aqueous solution) to yield the sodium salt of N-Bz-O-t-Boc-(2R,3S)-3-phenylisoserine after solvent removal. This carboxylate sodium salt was suspended in THF (1.4 ml) to which was added ethyl chloroformate (7.0 μl, 7.8 mg, 72 μmol) under N2 to form the mixed anhydride. The mixture was stirred vigorously at room temperature for 1 h. The transesterification of the mixed anhydride with CoA, the purification of the intermediate t-Boc protected CoA ester, and the O-deprotection with trifluoroacetic acid were all performed as described above. The final product, N-Bz-(2R,3S)-3-phenylisoserinoyl-CoA, was eluted from the C18 cartridge in 15–20% methanol to give 20 μmol of product [30% yield based on the methyl N-Bz-O-t-Boc-3-phenylisoserinate (65 μmol)]. The purity of N-Bz phenylisoserinoyl-CoA was judged by 1H NMR to be >95%. 1H NMR (300 MHz, D2O) for N-Bz phenylisoserinoyl-CoA (see Fig. 1B for numbering). δ: 0.49 (s, H-10′), 0.64 (s, H-11′), 2.05 (dd, J = 6.3 and 6.6 Hz, H-4′), 2.77 (m, H-1′), 3.01 (dd, J = 4.2 and 6.0 Hz, H-2′), 3.15 (dd, J = 6.6 and 6.6 Hz, H-5′), 3.31 (dd, J = 4.8 and 9.6 Hz, Ha-5"), 3.60 (dd, J = 4.8 and 9.6 Hz, Hb-5"), 3.79 (s, H-7′), 4.02 (d, J = 3.6 Hz, Ha-9′), 4.04 (d, J = 3.6 Hz, Hb-9′), 4.37 (br ddd, H-4"), 4.57–4.65 (m, H-2, H-2", and H-3"), 5.42 (d, J = 3.3 Hz, H-3), 5.93 (d, J = 6.6 Hz, H-1"), 7.18–7.54 (phenyl protons), 7.97 (s, adenine-CH), and 8.30 (s, adenine-CH).
Synthesis of Phenylisoserinoyl-CoA.
A described transamidation method (19) was used to convert N-Bz phenylisoserine to N-t-Boc phenylisoserine for subsequent, facile N-deprotection to liberate the free amine. In brief, to methyl N-Bz-(2R,3S)-3-phenylisoserinate (470 mg, 1.6 mmol) (see above) dissolved in CH2Cl2/THF (5:2, vol/vol, 28 ml) under N2 were added (N,N-dimethylamino)pyridine (370 mg, 1.7 mmol) in CH2Cl2 (1.6 ml) and benzyl chloroformate (244 μl, 290 mg, 1.7 mmol), and the mixture was stirred at room temperature for 1 h. The solvents were evaporated, and the product was purified by silica gel flash column chromatography (ethyl acetate/hexane, 35:65, vol/vol) to yield pure N-Bz-O-benzyloxycarbonyl-(2R,3S)-3-phenylisoserine methyl ester (1.5 mmol, 90% yield). To the methyl ester (1.5 mmol) dissolved in CH3CN (10 ml) under N2 were added 10 ml of CH3CN containing (N,N-dimethylamino)pyridine (780 mg, 3.6 mmol) and 20 ml of CH3CN containing di-t-butyl dicarbonate (3.5 g, 16 mmol), and the mixture was stirred for 24 h at room temperature. The solvent was evaporated, and the residue was dissolved in 100 ml of ethyl acetate. The organic layer was washed with brine, dried over Na2SO4, and evaporated under vacuum. The resulting product was purified by silica gel flash column chromatography (ethyl acetate/hexane, 25:75, vol/vol) to yield pure N-Bz-N-t-Boc-O-benzyloxycarbonyl-(2R,3S)-3-phenylisoserine methyl ester (0.3 mmol, 20% yield). To the methyl phenylisoserinate (0.3 mmol) stirred in 20 ml of methanol was added 20 ml of a 6% solution of magnesium methoxide in methanol. The mixture was stirred for 1 h at room temperature, then diluted with 300 ml of ethyl acetate. The organic layer was washed with brine, water, and again with brine, then dried over Na2SO4, and the solvent evaporated. The product was purified by silica gel flash chromatography (15–35% ethyl acetate gradient in hexane) to yield pure methyl N-t-Boc-(2R,3S)-3-phenylisoserinate (0.24 mmol, 80% yield). The procedure for protecting the C-2 hydroxyl of the phenylisoserine methyl ester is described in the previous section. The yield of methyl N,O-bis-(t-Boc)-(2R,3S)-3-phenylisoserinate was 0.2 mmol (80% yield). The hydrolysis of this methyl ester with NaOH, transesterification of the liberated carboxylate with CoA, purification of the target CoA ester, and elimination of t-Boc groups with trifluoroacetic acid were performed as described above to yield (2R,3S)-phenylisoserinoyl-CoA, which eluted from the C18 cartridge in 10% methanol [2.9 μmol, 10% yield based on the methyl N,O-bis-(t-Boc)-3-phenylisoserinate (30 μmol)]. The purity of phenylisoserine-CoA was judged by 1H NMR to be ≈80%. 1H NMR (300 MHz, CD3OD) for phenylisoserine-CoA (see Fig. 1B for numbering). δ: 0.83 (s, H-10′), 1.05 (s, H-11′), 2.44 (dd, J = 6.5 and 6.7 Hz, H-4′), 2.79 (m, H-1′), 3.45 (m, H-2′), 3.47 (dd, J = 6.6 Hz, H-5′), 3.57 (dd, J = 6.6 and 10.5 Hz, Ha-5"), 3.98 (dd, J = 5.4 and 9.9 Hz, Hb-5"), 4.06 (s, H-7′), 4.23 (d, J = 3.8 Hz, Ha-9′), 4.25 (d, J = 3.8 Hz, Hb-9′), 4.49 (br ddd, H-4"), 4.69–4.90 (m, H-2, H-3, H-2", and H-3"), 6.13 (d, J = 6.0 Hz, H-1"), 7.25–7.41 (phenyl protons), 8.18 (s, adenine-CH), and 8.57 (s, adenine-CH).
Heterologous Expression, Transferase Assay, and Product Analysis.
Subcloning of the original Taxus transacylase cDNAs from pCW into pSBET was performed as described (20), using the indicated sticky-end primers [Pair 1, TX7NDE1F (5′-TGA AGA AGA CAG GTT CGT TTG C-3′) and TX7BAM1R (5′-GAT CCT CAT AAC TTT GAC GGA CAC AC-3′); Pair 2, TX7NDE2F (5′-TAT GAA GAA GAC AGG TTC GTT TGC-3′) and TX7BAM2R (5′-CTC ATA ACT TTG ACG GAC ACA C-3′)] to install 5′-NdeI and 3′-BamHI terminal overhangs for directional ligation into the pSBETa vector. Procedures for expression in Escherichia coli BL21(DE3) and for preparing the corresponding soluble enzyme extracts also have been reported (20). A 0.1-ml aliquot (≈0.5 mg of total protein) of each soluble enzyme preparation was incubated for 3 h at 31°C with 100 μM acyl-CoA cosubstrate and 70 μM (0.5 μCi; 1 Ci = 37 GBq) [13-3H]baccatin III. The reaction mixture was basified (pH ≈ 9) with saturated sodium bicarbonate solution, and then treated with benzoyl chloride (7 μmol) for 0.5 h at 25°C to effect N-benzoylation (14). After derivatization, the mixture was extracted as described for other transferase assays (12, 18), and the products were analyzed by radio-HPLC (12). Of the nine enzyme preparations evaluated, only that from an E. coli transformant bearing the clone designated TAX7 generated a single radioactive product (with a coincident absorbance at 254 nm) with a retention time identical to that of the authentic standard [i.e., (3′RS)-2′-deoxytaxol in the case of β-phenylalanoyl-CoA and baccatin III as cosubstrates].
Sufficient enzyme was obtained from large-scale cultures of E. coli transformed with TAX7 for preparative conversion of substrate to product for characterization. The resulting N-debenzoylated product (≈1 mg) was chemically benzoylated as before, extracted into ethyl acetate, and purified by TLC (0.5-mm silica gel, EtOAc/hexane, 50:50, vol/vol) (12). The material that comigrated with authentic (3′RS)-2′-deoxytaxol (Rf = 0.15) was analyzed by 1H NMR as described (12). The TLC-purified product was also analyzed by combined LC-MS on a Hewlett–Packard Series 1100 MSD system in the atmospheric pressure chemical ionization mode. The sample was dissolved in acetonitrile, loaded onto a Supelcosil Discovery HS F5 column (5 μm, 4.6 × 250 mm, Sigma-Aldrich), and eluted with acetonitrile/water (50:50, vol/vol) at 1 ml/min, with the effluent directed to the atmospheric pressure chemical ionization mass detector in the positive-ion mode.
Partial Purification and Characterization of Recombinant T. cuspidata Phenylpropanoyltransferase.
Overexpression of TAX7 from pSBET in E. coli resulted largely in the formation of inclusion bodies, and yielded the target phenylpropanoyltransferase at about 1% of the total soluble bacterial protein (as determined by SDS/PAGE). Partial purification of this soluble extract (12) afforded only 15–20% recovery of activity, presumably because of the instability of the enzyme. Therefore, the crude soluble fraction was used as the enzyme source to determine kinetic parameters, and for preparative assays to generate sufficient product for confirmation by spectrometric methods.
Standard assays were performed as described, after evaluation of optimal protein concentration and reaction time (12). KALEIDAGRAPH (Version 3.08, Synergy Software, Reading, PA) was used for calculation of kinetic parameters by double-reciprocal plotting of each data set, and the equation for the best-fit line (R2 = 0.99) was determined. The pH optimum for this O-acyltransferase was assessed in assay mixtures containing 0.44 mg of total bacterial protein, each diluted with 200 μl of buffer comprising either 25 mM sodium acetate (pH 4.6), 2-(N-morpholino)ethanesulfonic acid (pH 5.6–7.0), potassium phosphate (pH 6.2–7.4), or N,N-bis[2-hydroxyethyl]-glycine (pH 7.6–9.2).
Results and Discussion
Cloning and Expression.
The biosynthetic origin of the Taxol C-13 side chain was previously proposed to involve conversion of phenylalanine, by means of β-phenylalanine, to phenylisoserine, followed by transfer of this side chain to baccatin III (to yield N-debenzoyltaxol), and final benzamidation (11). Stable-isotope feeding studies, however, demonstrated that β-phenylalanine was more efficiently incorporated into Taxol than was phenylisoserine (or N-Bz phenylisoserine; ref. 11). These experimental observations, coupled to the recent discovery of an N-benzoyltransferase from Taxus that preferentially catalyzes the N-benzoylation of β-phenylalanine baccatin III ester (i.e., N-debenzoyl 2′-deoxytaxol; ref. 12), suggested that β-phenylalanine is the side chain precursor that is attached directly to baccatin III (the last taxane core intermediate before C-13 O-acylation) and is followed by modification of the side chain (hydroxylation and N-benzoylation) to yield Taxol.
Several intermediates en route to Taxol contain free hydroxyl or amine groups that are enzymatically O- and N-acylated by the corresponding acyl/aroyl CoA thioester donor (12, 21), thereby suggesting that the critical C-13 side chain O-acyltransferase is also acyl-CoA thioester-dependent. The considerable deduced sequence identity (58–69%) between the currently defined acyl and aroyl N- and O-transferases of a family of genes previously isolated from Taxus (20) suggested that the target taxoid C-13 O-phenylpropanoyltransferase resided within this group of clones.
Evaluation of the remaining clones of the extant group required the syntheses of β-phenylalanoyl-CoA ester (and other possible candidate CoA esters) and [3H]baccatin III as cosubstrates. A synthetic procedure using a mixed anhydride intermediate was chosen to prepare β-phenylalanoyl-CoA as a cosubstrate for functional screening of the set of recombinant transferases. In brief, β-phenylalanine was converted to N-t-Boc-β-phenylalanine by standard methods (22) and then esterified with CoA by using established procedures (16–18) that involve formation of a mixed anhydride between an alkyl/aryl carboxylic acid and ethyl formic acid. Facile removal of the t-Boc group by trifluoroacetic acid treatment, followed by rapid purification of the product by reversed-phase chromatography, afforded pure β-phenylalanoyl-CoA in high yield. CoA esters of α-phenylalanine, phenylisoserine, and N-Bz phenylisoserine were similarly prepared, except that the latter two amino acids required t-Boc protection of the C-2 hydroxyl before conversion to the mixed anhydride. [3H]Baccatin III was prepared by modification of an established procedure (13).
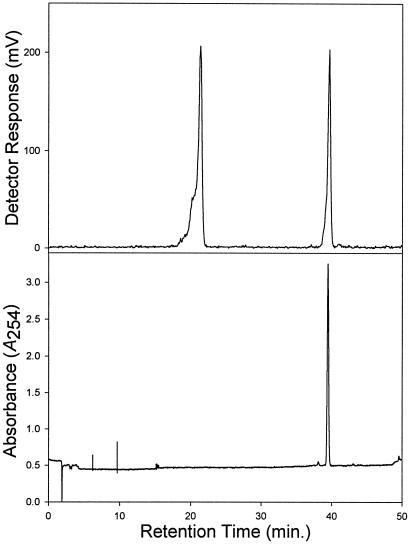
The five remaining full-length, but still functionally uncharacterized, Taxus transacylase genes from a family of nine such cDNA clones were assessed by expression as described (12). The derived enzyme preparations were assayed under standard conditions (12) with each synthetic amino phenylpropanoyl-CoA and [3H]baccatin III as cosubstrates. After incubation for 3 h at 31°C, each enzyme preparation (except where N-Bz phenylisoserinoyl-CoA was used as cosubstrate) was subjected to the Schotten–Baumann method (14) by using benzoyl chloride as the acyl donor to N-benzoylate selectively the amino phenylpropanoyl baccatin III product; this N-derivatization allowed selective organic solvent extraction and subsequent reversed-phase chromatography for purification. Only the recombinant enzyme expressed from the clone designated TAX7 catalyzed the formation of a product that (after N-benzoylation) chromatographed on radio-HPLC with the same retention time (39.6 ± 0.1 min) as that of authentic (3′RS)-2′-deoxytaxol (Fig. 3). No product was detected in assays without either cosubstrate, or in control assays with the enzyme extract of E. coli carrying empty vector in the presence of both cosubstrates at apparent saturation, or in complete assays in which the chemical benzamidation step was excluded.
Figure 3.
Radio-HPLC analysis of the biosynthetic product (after chemical N-benzoylation; Rt = 39.6 ± 0.1 min) generated by the recombinant O-phenylpropanoyltransferase by using [13-3H]baccatin III and β-phenylalanoyl-CoA as cosubstrates. The upper trace shows the radioactivity profile (in millivolts) and the lower trace shows the absorbance profile (A254) of authentic (3′RS)-2′-deoxytaxol. The radioactivity peak at 21.3 min is residual substrate.
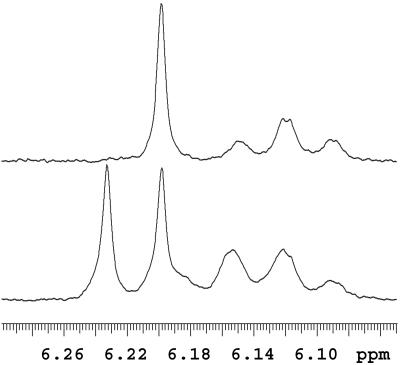
The molecular weight of the TAX7-encoded enzyme is calculated at 50,546, consistent with the size of the recombinant TAX7 transferase assessed at ≈50 kDa by SDS/PAGE. Crude preparations of the soluble TAX7 enzyme were used for large-scale conversion of the cosubstrates to the putative β-phenylalanoyl baccatin III ester, which was N-benzoylated as before. The product was purified by TLC (>95% pure by UV-HPLC) and analyzed by 1H NMR. The sample was judged to contain a single 3′-epimer of 2′-deoxytaxol (Fig. 4) by comparison with a 1H NMR spectrum of an authentic (3′RS)-2′-deoxytaxol standard; the stereochemistry of this epimer has not yet been assigned, but is likely the same as that of Taxol at this position (i.e., C-3′). The identity of the benzoylated product was also confirmed by atmospheric pressure chemical ionization MS and comparison of the mass spectrum with that of the authentic standard (Fig. 5). Taken together, these spectrometric data confirm that this TAX7 enzyme encodes the baccatin III 13-O-(3-amino-3-phenylpropanoyl)transferase responsible for side chain attachment en route to Taxol.
Figure 4.
Partial 1H NMR spectra (recorded in deuterated chloroform) of the biosynthetic product (after chemical N-benzoylation) derived by the recombinant O-phenylpropanoyltransferase (TAX7) with baccatin III and β-phenylalanoyl-CoA as cosubstrates (Upper) and of authentic (3′RS)-2′-deoxytaxol (Lower). The remaining spectrum of the N-benzoylated biosynthetic product was identical to that of a single stereoisomer in the 2′-deoxytaxol standard, thus demonstrating the formation of a single epimer at the 3′ position.
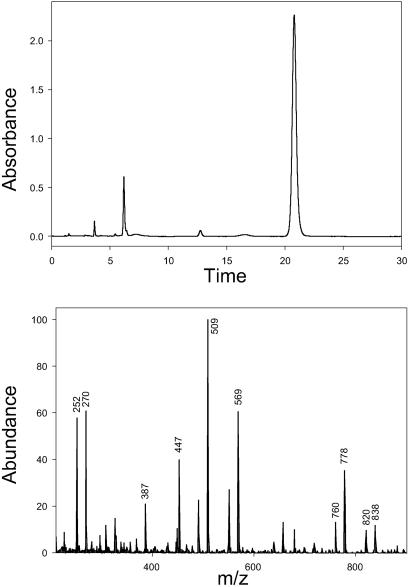
Figure 5.
Coupled reversed-phase HPLC atmospheric pressure chemical ionization MS analysis demonstrating that the biosynthetic product (after chemical N-benzoylation; Rt = 20.8 ± 0.1; Upper) generated by the recombinant phenylpropanoyltransferase with baccatin III and β-phenylalanoyl-CoA as cosubstrates is identical to authentic (3′RS)-2′-deoxytaxol (Rt = 20.8 ± 0.1). Diagnostic ions in the mass spectrum (Lower) are m/z 838 (PH+), 820 (PH+ − H2O), 778 (PH+ − CH3CO2H), 760 (m/z 778 − H2O), 569 [PH+ − PhCH(BzNH)CH2CO2H], 509 (m/z 569 − CH3CO2H), and 270 [PhCH(BzNH)CH2CO2H + H+].
Functional Characterization of Recombinant Phenylpropanoyltransferase.
The calculated Km values [Lineweaver–Burk plotting (R2 = 0.99)] for the recombinant enzyme, designated BAPT, are 2.4 ± 0.5 μM and 4.9 ± 0.3 μM for baccatin III and β-phenylalanoyl-CoA, respectively, and the pH optimum was found to be at pH 6.8. This enzyme catalyzes regiospecific acylation at the C-13 hydroxyl of baccatin III (no transfer to C-1 or C-7 was observed), and it is selective for 3-amino-3-phenylpropanoyl-CoA esters with a preference for β-phenylalanoyl-CoA (Vrel = 1.0) over 3-phenylisoserinoyl-CoA (Vrel = 0.4); α-phenylalanoyl-CoA and N-Bz phenylisoserinoyl-CoA were not productive acyl donors (Vrel < 0.01).
Sequence Analysis.
BAPT (accession no. AY082804) contains 1,335 nucleotides and encodes a 445-aa protein (calculated molecular weight of 50,546). The amino acid level similarity is between 71 and 74% among BAPT and the four other acyl/aroyltransferases involved directly in Taxol biosynthesis, including taxadien-5α-ol O-acetyltransferase (TAT, accession no. AF190130), taxane 2α-O-benzoyltransferase (TBT, accession no. AF297618), 10-deacetylbaccatin III 10-O-acetyltransferase (DBAT, accession no. AF193765), and N-debenzoyltaxol N-benzoyltransferase (DBTNBT, accession no. AF466397). The BAPT sequence, however, is the only one of the five that contains a G163XXXDA168 motif instead of the typical acyltransferase HXXXDG, or less frequent HXXXDA, element (23), of which the His and Asp side chains along with a conserved upstream cysteine residue (Cys-95) form a suggested catalytic triad involved in acyl group transfer. The Gly-163 for His-163 substitution in BAPT would likely disrupt the proposed triad function; however, the free β-amine of the CoA ester cosubstrate in this instance could, through hydrogen bonding, function as a surrogate intramolecular general acid/base in place of the normal histidine at this position.
Conclusions
The identification of the BAPT completes the acquisition of all of the acyl/aroyltransferases required for Taxol biosynthesis. The more than 2-fold greater selectivity of this enzyme for β-phenylalanoyl over phenylisoserinoyl transfer suggests that the biogenesis of the side chain begins with the isomerization of α-phenylalanine, by an aminomutase (24), to β-phenylalanine, which is converted by a ligase to the reactive CoA ester and transferred by BAPT to the C-13 hydroxyl of baccatin III to yield 13-O-β-phenylalanoyl baccatin III (see Fig. 1A). Consideration of this assembly scheme, including the transformation of a β-amino taxoid precursor by a recently acquired N-debenzoyl-2′-deoxytaxol N-benzoyltransferase to form 2′-deoxytaxol (12), implicates β-phenylalanoyl baccatin III as a true intermediate on the pathway to Taxol. Thus, the remaining steps in Taxol biosynthesis would follow by side chain C-2′ hydroxylation and N-benzoylation. The order of these final side chain modifications remains to be determined.
Acknowledgments
This investigation was supported in part by Grant CA-55254 from the National Institutes of Health, by eXegenics, Inc. (Dallas), and by McIntire-Stennis Project 0967 from the Washington State University Agricultural Research Center. S.F. was in part supported by the Department of Biomolecular Science, Toho University, Japan.
Abbreviations
- t-Boc
tert-butoxycarbonyl
- Bz
benzoyl
- THF
tetrahydrofuran
- BAPT
baccatin III:3-amino-3-phenylpropanoyltransferase
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AY082804).
References
- 1.Rowinsky E K, Citardi M J, Noe D A, Donehower R C. J Cancer Res Clin Oncol. 1993;119:727–733. doi: 10.1007/BF01195344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dieras V. Semin Oncol. 1998;25:18–22. [PubMed] [Google Scholar]
- 3.Sgadari C, Toschi E, Palladino C, Barillari G, Carlei D, Cereseto A, Ciccolella C, Yarchoan R, Monini P, Sturzl M, Ensoli B. J Immunol. 2000;165:509–517. doi: 10.4049/jimmunol.165.1.509. [DOI] [PubMed] [Google Scholar]
- 4.Schiff P B, Horwitz S B. Proc Natl Acad Sci USA. 1980;77:1561–1565. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rowinsky E K, Donehower R C, Jones R J, Tucker R W. Cancer Res. 1988;48:4093–5100. [PubMed] [Google Scholar]
- 6.Parness J, Kingston D G I, Powell R G, Harracksingh C, Horwitz S B. Biochem Biophys Res Commun. 1982;105:1082–1089. doi: 10.1016/0006-291x(82)91080-4. [DOI] [PubMed] [Google Scholar]
- 7.Lataste H, Senilh V, Wright M, Guénard D, Potier P. Proc Natl Acad Sci USA. 1984;81:4090–4094. doi: 10.1073/pnas.81.13.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Georg G I, Ali S, Zygmunt J, Jayasinghe L R. Exp Opin Ther Patents. 1994;4:109–120. [Google Scholar]
- 9.Guénard D, Guéritte-Voegelein F, Potier P. Acc Chem Res. 1993;26:160–167. [Google Scholar]
- 10.Ojima I, Habus I, Zhao M, Zucco M, Park Y H, Sun C M, Brigaud T. Tetrahedron. 1992;48:6985–7012. [Google Scholar]
- 11.Floss H G, Mocek U. In: Taxol: Science and Applications. Suffness M, editor. Boca Raton, FL: CRC; 1995. pp. 191–208. [Google Scholar]
- 12.Walker K, Long R, Croteau R. Proc Natl Acad Sci USA. 2002;99:9166–9171. doi: 10.1073/pnas.082115799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor G F, Thornton S S, Tallent C R, Kepler J A. J Label Compd Radiopharm. 1993;33:501–515. [Google Scholar]
- 14.Georg G I, Boge T C, Cheruvallath Z S, Harriman G C B, Hepperle M, Park H, Himes R H. Bioorg Med Chem Lett. 1994;4:335–338. [Google Scholar]
- 15.Cravallee C, Didier E, Pecquet P. Tetrahedron Lett. 1998;39:4263–4266. [Google Scholar]
- 16.Rasmussen J T, Boerchers T, Knudsen J. Biochem J. 1990;265:849–855. doi: 10.1042/bj2650849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva M F B, Ruiter J P N, Ijlst L, Allers P, ten Brink H J, Jakobs C, Duran M, Tavares de Almeida I, Wanders R J A. Anal Biochem. 2001;290:60–67. doi: 10.1006/abio.2000.4947. [DOI] [PubMed] [Google Scholar]
- 18.Walker K, Croteau R. Proc Natl Acad Sci USA. 2000;97:13591–13596. doi: 10.1073/pnas.250491997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jagtap P G, Kingston D G I. Tetrahedron Lett. 1999;40:189–192. [Google Scholar]
- 20.Walker K, Schoendorf A, Croteau R. Arch Biochem Biophys. 2000;374:371–380. doi: 10.1006/abbi.1999.1609. [DOI] [PubMed] [Google Scholar]
- 21.Walker K, Croteau R. Phytochemistry. 2001;58:1–7. doi: 10.1016/s0031-9422(01)00160-1. [DOI] [PubMed] [Google Scholar]
- 22.Tarbell D S, Yamamoto Y, Pope B M. Proc Natl Acad Sci USA. 1972;69:730–732. doi: 10.1073/pnas.69.3.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown N F, Anderson R C, Caplan S L, Foster D W, McGarry J D. J Biol Chem. 1994;269:19157–19162. [PubMed] [Google Scholar]
- 24.Walker K D, Floss H G. J Am Chem Soc. 1998;120:5333–5334. [Google Scholar]