Abstract
Two double-tailed pyridinium cationic amphiphiles, differing only in the degree of unsaturation of the alkyl chains, have been selected for a detailed study of their aggregation behavior, under conditions employed for transfection experiments. The transfection efficiencies of the two molecules are remarkably different, especially when combined with 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) as helper lipid. The phase behavior of the cationic amphiphile/DOPE mixtures have been studied using 31P- and 2H-NMR (on deuterated cationic amphiphiles) as main techniques, to monitor independently the behavior of the two components. In water, the lamellar organization is dominant for both the surfactants in their mixtures with the helper lipid. In HEPES saline buffer (HBS), the mixtures of the unsaturated surfactant form inverted phases and, in particular, stable HII phases for DOPE contents ≥30 mol %. By contrast, the saturated surfactant does not form homogeneously mixed inverted phases in mixtures with DOPE at room temperature. However, mixed inverted phases are observed for this system at higher temperatures and, after mixing has been achieved by heating, the metastable mixed phases remain present for several hours at 5°C. At 35°C the dominant phase is the cubic phase. The lipoplex composed of equimolar mixtures of the unsaturated surfactant with DOPE and plasmid DNA was found to be organized in highly curved bilayers.
INTRODUCTION
Combining low cytotoxicity and high transfection efficiency, cationic lipids are considered one of the most promising classes of nonviral DNA carriers for therapeutic purposes. Several studies (Koltover et al., 1998, 1999; Audouy et al., 2000; Koynova and MacDonald, 2003b; Simberg et al., 2001; Zuidam et al., 1999; Eastman et al., 1997; Hirsch-Lerner and Barenholz, 1999; Mitrakos and Macdonald, 2000; Cherezov et al., 2002) have been performed to characterize the physicochemical properties of DNA-lipid complex (lipoplex)—morphology, stability, size, and surface properties—and their relevance to reach optimal conditions for transfection. Although the relationships between the phase behavior of the carrier (with or without helper lipid) and the resulting morphology after DNA complexation are of major importance for the prediction of transfection efficiencies on the basis of the molecular structure of the carriers, these aspects have been investigated only to a smaller extent (Koynova and MacDonald, 2003a; Šmisterová et al., 2001; Zantl et al., 1999; Zuhorn et al., 2002). The importance of the ability of the lipids to condense DNA and of the balance between the colloidal stability of the lipoplex and the interaction with the cellular membrane for the success of the transfection strategy have been recognized, however. These crucial parameters are mainly determined by the phase behavior of the amphiphiles under different conditions: in pure water, where the complexation is carried out since electrostatic interactions are the main driving forces, under physiological ionic strength conditions, and by the influence of helper lipids and DNA on the properties of the aggregates.
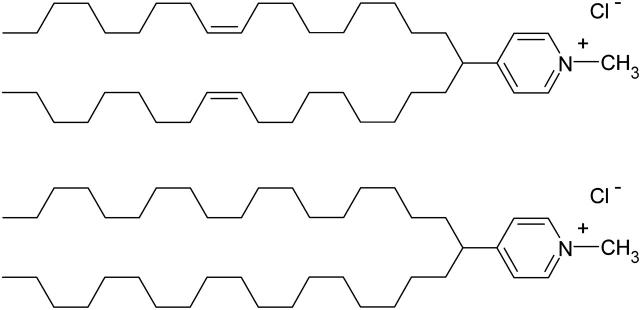
A previous study in our laboratory on two cationic pyridinium amphiphiles (Zuhorn et al., 2002), N-methyl-4-(distearyl)methylpyridinium chloride (SAINT-5) and N-methyl-4-(dioleyl)methylpyridinium chloride (SAINT-2) (Fig. 1), differing only in the degree of unsaturation of the alkyl chain, C18:0 and C18:1, respectively, revealed how such simple structural modifications give rise to dramatic differences in the transfection potential. The differences in behavior between the two molecules become even more pronounced when a helper lipid is added to the transfection cocktail; whereas 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) strongly promotes SAINT-2-mediated transfection, SAINT-5-mediated transfection remains virtually unaffected upon addition of DOPE. The results have been rationalized in terms of tighter packing of the saturated chains of SAINT-5 in comparison with the unsaturated chains of SAINT-2, resulting in a higher stiffness of the membrane formed by this amphiphile. The interaction of DNA with a poorly deformable cationic membrane leads to a less favorable lipoplex assembly, inefficient DNA translocation, and, consequently, a low(er) transfection efficiency.
FIGURE 1.
SAINT-2 (top) and SAINT-5 (bottom) cationic amphiphiles.
In this study, we have investigated the physicochemical differences between SAINT-5 and SAINT-2, as revealed by 2H-NMR, 31P-NMR, and cryo-transmission electron microscopy (TEM). The advantages of this approach arise from the possibility of carrying out experiments under conditions close to those of transfection experiments and in the complete absence of perturbative probes. Moreover, NMR experiments can be used to characterize the systems under investigation on both a molecular and a morphological level. 2H-NMR has been previously used to examine the effect of the complexation of polyadenilic acid on mixtures of cationic amphiphiles and phospholipids (Mitrakos and Macdonald, 1996) and 31P-NMR has been used to monitor the morphology of the DOTMA lipoplex with plasmid DNA (Monk and Cullis, 1997). To the best of our knowledge, however, this powerful combination of 31P- and 2H-NMR techniques has not previously been employed to study lipoplexes. Monitoring independently the phase behavior of the cationic amphiphiles (2H-NMR) and helper lipid (31P-NMR) in these mixtures, before and after complexation with DNA, yields important insights into the extent of mixing of the two lipid components. The effects of ionic strength, temperature (cycles), and different amounts of helper lipid on the phase behavior of the two pyridinium amphiphiles has been determined. The consequences of plasmid DNA binding for the properties of the aggregate are finally examined for the lipoplex with the highest transfection efficiency, i.e., the complex between the equimolar mixture of SAINT-2/DOPE with DNA at a charge ratio of +/− = 2.5.
MATERIALS AND METHODS
Synthesis
The cationic lipids SAINT-2 and SAINT-5 were synthesized as previously described (Meekel et al., 2000; Vanderwoude et al., 1997). The methyl-deuterated compounds were obtained by methylation of the alkyl pyridine using methyl iodide-d3 (Deutero GmbH, Kastellaun, Germany): the 4-substituted alkyl-pyridines were quaternized with an excess (5 equivalents) of methyl iodide-d3 in acetone for 4 h at room temperature to give the corresponding d3-methylpyridinium iodide in quantitative yields. The iodide salts were converted into the corresponding chloride salts by ion-exchange chromatography using DEAE Sephadex A-25 (Amersham Pharmacia Biotech AB, Little Chalfont, UK) with MeOH as eluent.
N-methyl{d3}-4-(dioleyl)methylpyridinium chloride
1H NMR (200 MHz): δ = 0.87 (t, 3J = 6.5, 6H, CH3), 1.25 (m, 44H, CH2), 1.55 (m, 4H, CH2CH3), 1.75 (m, 4H, CH(CH2)2), 2.00 (m, 8H, CH2CH=CHCH2), 2.77 (m, 1H, CH), 5.33 (m, 4H, CH), 7.72 (d, 3JAB = 6.4, 2H, CHar), 9.47 (d, 3JAB = 6.4, 2H, CHar). 13C-NMR (50.28 MHz) δ = 13.9, 22.4, 27.0, 27.2, 28.9, 29.0, 29.l, 29.3, 29.4, 29.5, 31.7, 35.5, 46.4, 126.8, 129.5, 129.8, 130.1, 130.3, 145.0, 167.1.
N-methyl{d3}-4-(distearyl)methylpyridinium chloride
1H NMR (200 MHz): δ = 0.87 (t, 3J = 6.5, 6H, CH3), 1.24 (m, 64H, CH2), 1.48–1.78 (m, 4H, CH(CH2)2), 2.77 (m, 1H, CH), 7.72 (d, 3JAB = 6.3, 2H, CHar), 9.38 (d, 3JAB = 6.5, 2H, CHar). 13C-NMR (50.28 MHz) δ = 13.8, 22.4, 27.1, 29.1, 29.3, 29.4, 31.6, 35.4, 46.4, 126.8, 144.9, 167.1.
Sample preparation
DOPE and 1,2-dioleoyl-sn-glycero -3-[phospho-L-serine] (DOPS) were purchased from Avanti Polar Lipids (Alabaster, AL). All other chemicals were of analytical grade or purified according to standard procedures (Vogel, 1962).
A sample of 7.1-kilobase DNA was isolated from Escherichia coli using the Sigma-Aldrich GenElute HP Maxiprep kit (Sigma-Aldrich, Zwijndrecht, The Netherlands). The plasmid concentration was determined spectrophotometrically by measuring the absorption at λ = 260 nm using the relation 1.0 A = 50 μg/ml. Typically, the A260/A280 values were ∼1.85.
A solution of the respective SAINT and the desired amount of DOPE in dichloromethane was dried under a stream of nitrogen and, hereafter, any residual solvent was removed under high vacuum. For the study of the lipid phase behavior in the absence of DNA, the films were hydrated with bidistilled water or HEPES saline buffer (15 mM HEPES, 150 mM NaCl, pH 7.4) to a total lipid concentration of 0.1 M. The lipids were then vortexed and freeze-thawed five times to ensure homogeneous mixing.
To obtain small unilamellar vesicles, the lipid films were hydrated to a final total lipid concentration of 5 mM (unless stated otherwise) and, after vigorous vortexing, the suspension was tip-sonicated for 2 min (Branson Sonifier B15-P, Danbury, CT).
Lipoplexes in water were prepared by addition of the plasmid DNA to a vesicular suspension, reaching a final charge ratio of positive/negative (+/−) = 2.5.
NMR spectroscopy
31P-NMR spectroscopy
31P-NMR spectra were recorded on a Varian Unity-Plus 500 (Varian, Palo Alto, CA) operating at 202.653 MHz for the phosphorus channel. Data were acquired with single pulse excitation under high-power broadband proton decoupling. The 90° pulse length was 28 μs, the recycle delay 1.3 s, the spectral width 40.588 kHz and the data size 16,384. Typically, 4000 transients were signal-averaged and exponentially multiplied with a 50- or 100-Hz line broadening before Fourier transformation. Spectra were measured at different temperatures, and samples were allowed to equilibrate at each temperature for at least 30 min before data collection.
2H-NMR spectroscopy
2H-NMR experiments were recorded on a Bruker Avance 500 WB (Bruker BioSpin, Billerica, MA) NMR spectrometer (operating frequency 76.8 MHz), using a quadrupolar echo technique (Davis et al., 1976). The recycling delay was 200 ms, echo delay was 30 μs, the 90° pulse was 6.15 μs, the spectral width 500 kHz, and 20,000–50,000 scans were collected. Typically, before Fourier transformation, an exponential multiplication with a line-broadening factor of 100 Hz was used. Spectra were measured at different temperatures, and samples were allowed to equilibrate at each temperature for at least 30 min before data collection.
Differential scanning calorimetry
Differential scanning calorimetry (DSC) scans on SUVs were taken on a NANO II – DSC (Calorimetry Sciences, Lindon, UT) with a scan rate of 1°C/s. Eight heating scans were performed between 1 and 90°C. The reference cell was filled with water. The samples were allowed to equilibrate for 30 min. at 1°C between successive scans.
Cryo-TEM
A drop of the lipid suspension was deposited on a glow discharged holey carbon-coated grid. After blotting away the excess of lipid, the grids were plunged in liquid ethane. The frozen specimens were mounted in a Gatan (model 626) cryostage and examined in a Philips CM 120 cryoelectron microscope (Philips, Eindhoven, The Netherlands) operating at 120 KV. Micrographs were taken under low-dose conditions.
RESULTS
SAINT-2 mixed with DOPE
The phase behavior of mixtures of the cationic amphiphiles with DOPE has been studied combining 31P-NMR and 2H-NMR (of the N-methyl deuterated SAINTs) to monitor independently the properties of both components in the mixture.
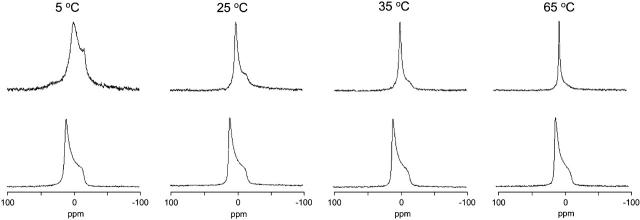
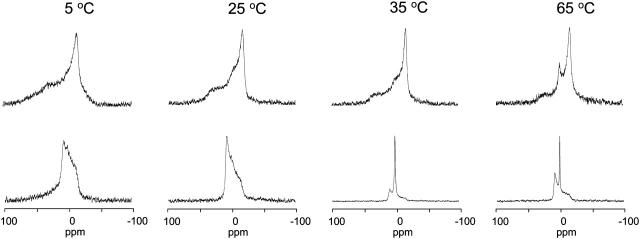
The 31P-NMR spectra of equimolar mixtures of SAINT-2/DOPE 1/1 in water at different temperatures are shown in Fig. 2 (top row). The spectrum at 5°C consists of a superposition of a signal characterized by a high-field peak and a low-field shoulder, indicating the presence of an Lα phase (chemical shift anisotropy (CSA) = +54 ppm), and a symmetric peak centered at 0 ppm (isotropic peak), of which the contribution increases with temperature. The averaging of CSA leading to the isotropic peak can be due to the tumbling of small aggregates, lateral diffusion around small diameter particles or by the organization of the lipids in an isotropic phase (e.g., a cubic phase) (Cullis and De Kruyff, 1979).
FIGURE 2.
31P-NMR spectra as a function of increasing temperature of samples of SAINT-2 mixed with DOPE (1:1 molar ratio, 100 mM total lipid concentration) in water (top row) and in HBS (bottom row).
Increasing the ionic strength of the solution to physiological values has a dramatic effect on the phase behavior of the system, as indicated by the 31P-NMR line shapes (Fig. 2, bottom row), which are now characterized by a low-field peak and a high-field shoulder, with a span of the anisotropy (CSA = −27 ppm) equal to half the one observed for the lamellar phase. The inversion of the asymmetry and the reduction of the CSA upon going from water to HBS indicate a transition from the Lα phase to the HII phase and is determined by the fast diffusion of the molecules around the small-diameter tubes that constitute this inverted phase. The spectra indicate a quantitative conversion to the HII phase. The remarkable thermal stability of the HII phase is indicated by the absence of significant variations in the line shapes in the whole range of temperatures explored.
Compared to a 150 mM aqueous NaCl solution, buffering of the pH to physiological values (pH 7.4) does not have a measurable effect on the phase behavior of the mixture.
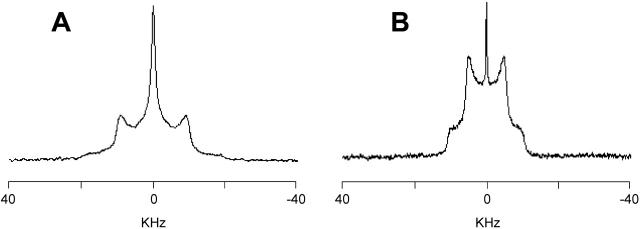
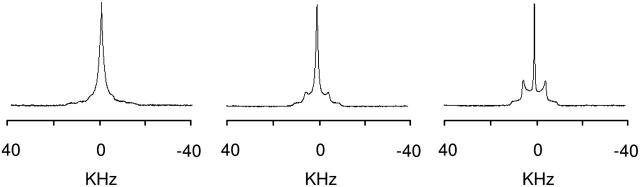
To determine whether the phases observed with 31P-NMR arise from homogeneous mixtures of the two lipid components or from domains of unmixed DOPE, 2H-NMR spectra have been recorded on samples consisting of DOPE and deuterated cationic lipids to determine the behavior of the SAINT amphiphile independently (Fig. 3). The cationic amphiphile produces in water a Pake pattern line shape, characterized by the presence of two maxima, superimposed to a broad isotropic peak; the separation between the maxima of the Pake pattern (quadrupolar splitting, ΔνQ) is 18 kHz. Also the spectra of the samples in HBS present a well-resolved Pake pattern, with a quadrupolar splitting of ΔνQ = 9.8 kHz, about half the quadrupolar splitting observed for the same sample in water, and a very sharp isotropic peak, which can be assigned to the deuterium naturally present in water. For the samples in water, the signals are consistent with aggregates of different dimensions in the Lα phase. The reduction of the quadrupolar splitting upon going from water to HBS confirms the quantitative conversion to the HII phase and the absence of significant variation in the line shape of the 2H-NMR spectra recorded in HBS as a function of temperature (data not shown) confirms the stability of the HII assembly, as already deduced from the 31P-NMR spectra.
FIGURE 3.
2H-NMR spectra of samples of SAINT-2 deuterated on the N-methyl mixed with DOPE (1:1 molar ratio, 100 mM total lipid concentration) in water (A) and in HBS (B) at 25°C.
Decreasing the amount of DOPE to 30 mol % has no effect on the phase behavior in HBS, whereas a further decrease in the helper lipid content leads to the formation of a cubic phase; in SAINT-2/DOPE 80:20 (mol %) solely a cubic phase is observed in the temperature range explored (confirmed by small angle x-ray scattering (SAXS), data not shown).
SAINT-5 mixed with DOPE
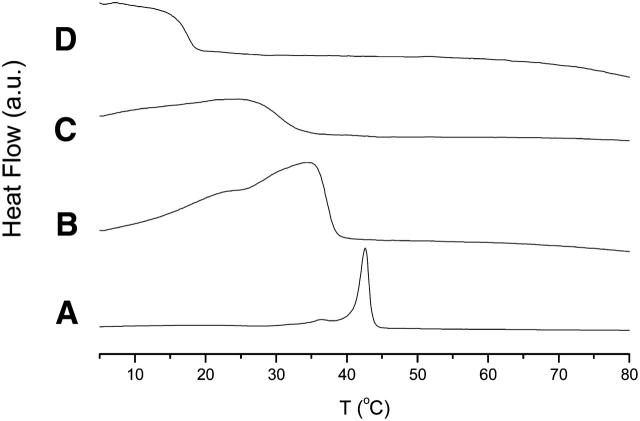
DSC measurements were performed to investigate the influence of DOPE on the main phase transition temperature of SAINT-5, the surfactant with saturated tails (Fig. 4). The temperature for the transition from the Lβ to the Lα phase (Tm) is 43°C for pure SAINT-5. Addition of DOPE decreases the Tm and broadens the coexistence region between the gel and the liquid-crystalline regions. Although the transition to the liquid-crystalline phase for SAINT-5/DOPE 70:30 (mol %) is complete at 40°C, the equimolar mixture (optimal for transfection) is in the fluid state at 35°C (approximately the transfection temperature).
FIGURE 4.
DSC scans of SAINT-5/DOPE vesicles (5 mM total lipid concentration) in water at (A) 100:0, (B) 70:30, (C) 50:50, (D) 30:70 mol %.
The 31P-NMR spectra recorded on equimolar mixtures of SAINT-5/DOPE in water (Fig. 5) show that the lamellar organization is predominant in the region of the phase diagram under investigation and, in contrast with the surfactant with unsaturated chains, the isotropic signal is only clearly visible at 65°C.
FIGURE 5.
31P-NMR spectra as a function of increasing temperature of samples of SAINT-5 mixed with DOPE (1:1 molar ratio, 100 mM total lipid concentration) in water (top row) and HBS (bottom row).
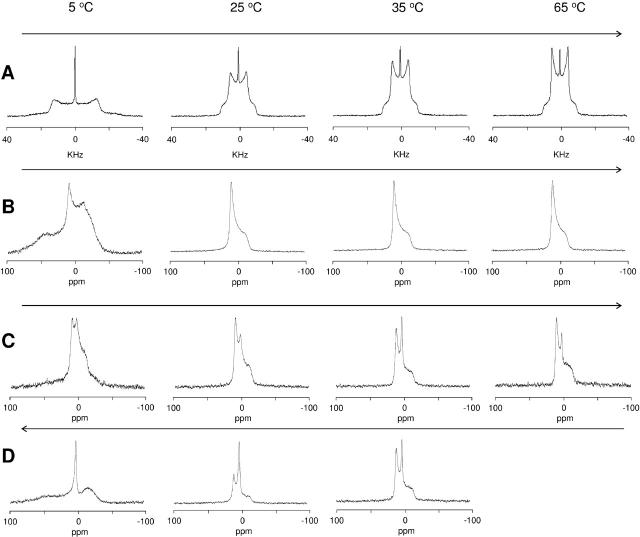
The phase behavior of the mixture, however, appears to be more complex in HBS. At 5°C, the broad signal indicates the coexistence of Lβ, HII, and cubic phases. Upon increasing the temperature to 25°C, the contribution of a HII phase becomes predominant. At 35°C the line shape is determined by the superposition of an isotropic peak and a weak signal arising from a HII phase. The contribution of the HII signal increases again with a further increase in temperature. The reversibility of the high-temperature transitions has been established by decreasing the temperature to 35°C and studying the cooling behavior. When the temperature is decreased to 25°C (Fig. 6, left column), the line shape appears to be different from the one obtained at the same temperature in the heating array, showing only an isotropic peak (and no features due to HII phases). At 5°C the spectrum presents a broad, badly resolved component and is still dominated by the isotropic peak; after 24 h of equilibration at 5°C, the broad component evolves in the typical line shape for the Lα phase. The 2H-NMR spectra recorded in the cooling array (Fig. 6, right column) show the same phases as detected by 31P-NMR, suggesting the presence of a perfectly mixed system. The analysis of the 2H-NMR spectra recorded in the heating array (Fig. 7) reveals that at 25°C the cationic amphiphile is mainly organized in a cubic phase (isotropic signal); from the comparison with the 31P-NMR spectra, we can conclude that the mixing of the cationic surfactant with the helper lipid is incomplete at room temperature and the HII phase observed with 31P-NMR at 25°C is due to domains of unmixed DOPE. From 35°C to 65°C, the correspondence between the 31P-NMR and 2H-NMR data indicates that the observed cubic and HII phases are mixed lipid phases. Interestingly, in the cooling array, demixing is not observed after several hours at 5°C.
FIGURE 6.
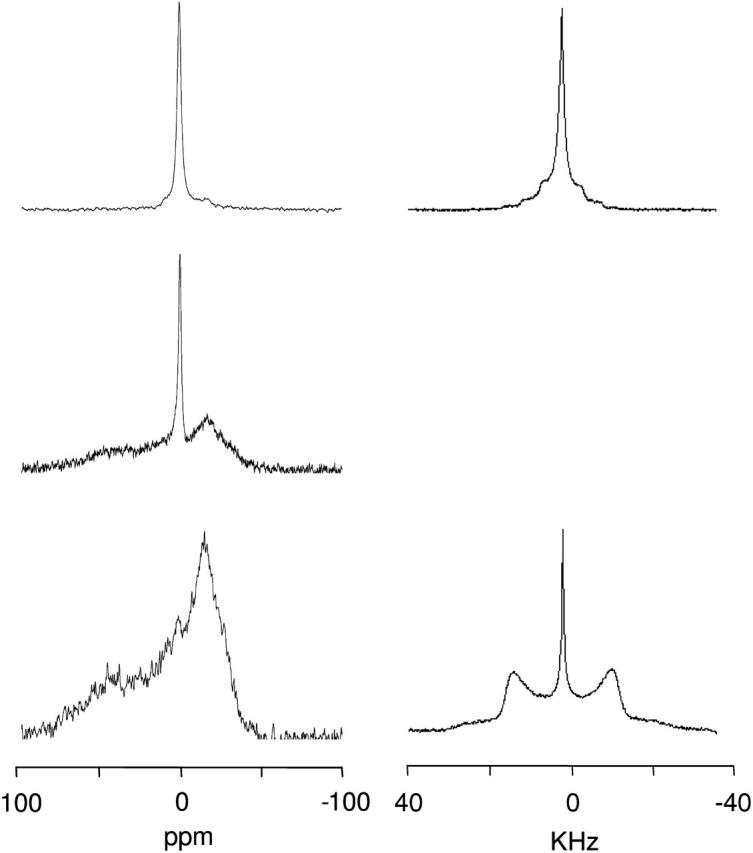
31P-NMR (left column) and 2H-NMR (right column) spectra of SAINT-5 mixed with DOPE (1:1 molar ratio) in HBS after equilibration of the sample at 65°C for 1 day. At 25°C (top), 5°C after 30 min of equilibration (middle), and 5°C after 1 day of equilibration (bottom).
FIGURE 7.
2H-NMR spectra of samples of SAINT-5 mixed with DOPE (1:1 molar ratio, 100 mM total lipid concentration) in HBS at 25°C (left), 35°C (middle), 65°C (right).
Decreasing the amount of DOPE leads to a smaller contribution to the line shape of the high-temperature HII phase with respect to the cubic phase; in SAINT-5/DOPE 90:10 (mol %), this mixed HII phase is no longer detectable (data not shown).
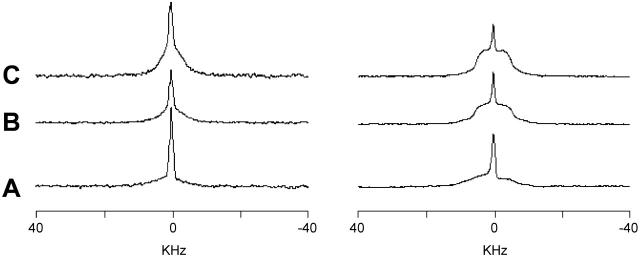
It was found previously that lipoplexes of SAINT-5/DOPE can assume the HII morphology after interaction with anionic vesicles (Zuhorn et al., 2002). Experiments on mixtures of SAINT-2/DOPE/DOPS 2.5/2.5/1 (Fig. 8) confirm the effect of the negatively charged phospholipid. Whereas at 5°C different phases are present, at temperatures above 25°C only the HII phase is detectable. The spectra recorded for this sample in the cooling array showed complete reversibility of the transitions, in contrast with observations in the absence of the negatively charged phospholipid.
FIGURE 8.
2H (A) and 31P-NMR (B) spectra of SAINT-5/DOPE/PS 2.5:2.5:1 in HBS. Heating (C) and cooling (D) 31P-NMR spectra array of SAINT-5/DOPE 2.5:3.5. The arrow above each spectrum array indicates the direction of the temperature variation.
SAINT-2/DOPE lipoplex in H2O
The effect of plasmid DNA complexation on the morphology of SAINT-2/DOPE mixtures in water and in HBS has been investigated by monitoring the 31P CSA and 2H quadrupolar splittings of the lipoplexes. The 31P-NMR spectra of the mixture in water (data not shown) are dominated by an isotropic component in the complete temperature range explored. At 5°C the isotropic signal is superimposed to a bilayer component, with a line shape characteristic of a system in which the CSA is partially averaged (Gorenstein, 1984). The 2H-NMR spectrum (Fig. 9, left) at 5°C shows an isotropic signal together with a broad component. Raising the temperature leads to the narrowing of the broad component. The 2H-NMR line shapes indicate the presence of an anisotropic phase in which a mechanism of averaging of the quadrupolar interaction is active. These effects on the line shape increase with temperature.
FIGURE 9.
2H-NMR spectra of lipoplex of equimolar mixtures of Saint-2-d3/DOPE with plasmid DNA (+/− charge ratio was 2.5) in water (left) and HBS (right), at 5°C (A), 35°C (B), and 65°C (C).
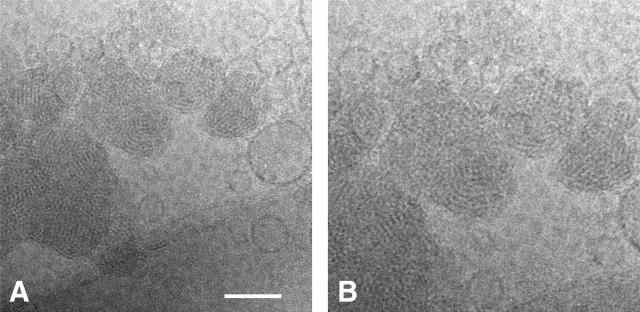
High-resolution cryo-TEM pictures have been recorded of the same sample (Fig. 10). The results confirm the lamellar organization previously observed for the pelleted lipoplex (Šmisterová et al., 2001). The high quality of the pictures clearly establishes the absence of extended lamellae in the complex. The bilayers are folded to form small-diameter loops. This particular organization has been previously observed for different surfactants (Bell et al., 2003; Lasic et al., 1997; Schmutz et al., 1999) and does not seem to depend on the type of DNA used for the lipoplex preparation (Schmutz et al., 1999). This finding helps to rationalize the NMR results, since lateral diffusion around these structures is certainly an effective source of averaging of the CSA and of the quadrupolar interaction (see Discussion). It is also evident from the cryo-TEM data that under conditions of the presence of such a large excess of positive relative to negative charges, some of the vesicles remain unbound to the DNA.
FIGURE 10.
Cryo-TEM picture of the lipoplex formed from equimolar mixtures of SAINT-2/DOPE and plasmid DNA (+/− charge ratio was 2.5). The bar represents 100 nm. On the right, an expansion is shown to facilitate the visualization of the characteristic features.
Cryo-TEM pictures also reveal that lipoplexes obtained at an excess of negative charge (charge ratio 0.5, data not shown) assume morphologies analogous to the complex at the optimal charge ratio for transfection, and free vesicles are no longer present.
In HBS, at 5°C, a 2H-NMR signal similar to the one in water was observed. Increasing the temperature leads to an irreversible transition to the HII phase. The line shape of the 31P-NMR spectra (data not shown) confirms the presence of the HII phase.
DISCUSSION
This study focused on the polymorphism of two well-studied cationic pyridinium amphiphiles belonging to the SAINT family (Meekel et al., 2000; Shi et al., 2002; Vanderwoude et al., 1997; Šmisterová et al., 2001; Pijper et al., 2003; Roosjen et al., 2002; Zuhorn et al., 2002) in mixtures with the uncharged phospholipid DOPE. Besides the remarkable transfection efficiency and low cytotoxicity displayed by a number of SAINT amphiphiles, this class of molecules is particularly interesting because of the simplicity of introducing structural modifications, which allows investigation of the effects of essential molecular structural elements that determine transfection efficiency and also permits attempts to control the lipoplex morphology on a molecular basis (Hulst et al., 2004).
The aim of the present study was to gain a better understanding of the physical properties of the mixtures to further rationalize the ∼2 times higher transfection efficiency of SAINT-2 compared with that of SAINT-5. The difference between the two DNA carriers becomes even more pronounced when DOPE is added: whereas the transfection efficiency of SAINT-2 is more than doubled, the SAINT-5-mediated transfection remains unaffected. The transfection efficiency of the equimolar mixture of SAINT-2/DOPE is ∼10 times higher than that of the equimolar mixture of SAINT-5/DOPE (Zuhorn et al., 2002).
The observed differences in the molecular assemblies of SAINT-5 and SAINT-2 and the effect of physiological salt concentrations and temperature can be qualitatively rationalized on the basis of simple shape concepts, expressed in the dimensionless packing parameter (Israelachvili et al., 1976), 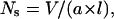 which is proportional to the hydrophobic chain volume (V) and inversely proportional to the optimal cross-sectional head group area (a) and to the length of the hydrophobic tails (l). When ½ < Ns ≤ 1, a lamellar organization is favored, and when Ns > 1, inverted structures are preferred. Often cubic phases are intermediates in the transition between Lα and HII phases. The presence of unsaturations in the tails implies a higher packing parameter for SAINT-2 and, consequently, a higher propensity for the formation of inverted structures in comparison with SAINT-5.
which is proportional to the hydrophobic chain volume (V) and inversely proportional to the optimal cross-sectional head group area (a) and to the length of the hydrophobic tails (l). When ½ < Ns ≤ 1, a lamellar organization is favored, and when Ns > 1, inverted structures are preferred. Often cubic phases are intermediates in the transition between Lα and HII phases. The presence of unsaturations in the tails implies a higher packing parameter for SAINT-2 and, consequently, a higher propensity for the formation of inverted structures in comparison with SAINT-5.
Aggregation properties in water
Taking advantage of the powerful combination of 31P- and 2H-NMR, detailed studies have been made of the aggregates morphologies in both the absence and presence of DNA. In water, the two cationic amphiphiles in equimolar mixtures with DOPE present similar morphologies: both phase diagrams are dominated by a lamellar organization. Nevertheless, although the equimolar mixture SAINT-2/DOPE is in the liquid-crystalline state at temperatures >0°C (Lα phase), the main phase transition of the mixture with the saturated amphiphile SAINT-5 is complete only above room temperature. These observations are consistent with the lower dynamic character of saturated alkyl chains with respect to unsaturated ones (Iwahashi et al., 2000). Compared to the Lα phase, the Lβ phase gives rise to a broader 31P-NMR signal with a less well-defined low-field shoulder, due to the motional restriction of the phospholipids in the gel state. According to DSC data, the main phase transition is complete at 35°C, in agreement with the variations in the line shape of the 31P-NMR spectra. Visual inspection and SAXS results on pelleted vesicles suggest that the isotropic peak observed in water results from lipids in a bilayer organization, as characterized by short correlation times, and not from the transition toward an isotropic phase; this conclusion is consistent with the reversibility of the line-shape variations observed in monitoring the cooling behavior, as well as with short equilibration times. The higher isotropic contribution to the line shape in the mixture of SAINT-2 compared to that of SAINT-5 provides further evidence for the higher fluidity of the mixtures containing the unsaturated amphiphile. Studies of the interaction of SAINT-5 monolayers with p-DNA showed that the recruitment of the lipid onto the lipoplex surface is hampered by the inability of the cationic surfactant to dissociate from the lipid monolayer. The presence of the helper lipid leads only to a partial relief of this structural rigidity of the membrane and is insufficient to avoid deformation and decondensation of the DNA. This leads to inefficient DNA translocation which results in a poor transfection efficiency (Zuhorn et al., 2002). Our DCS and 31P-NMR data are consistent with these suggestions; the only partial increase in fluidity of the equimolar mixture of SAINT-5/DOPE finds an explanation in the fact that when the complexation with DNA is carried out at room temperature, lipids are present in coexisting gel and liquid-crystalline phases.
The effect of ionic strength
Moving from water to HBS, the solution conditions are considerably different since pH and ionic strength are changed. The effect of the pH variation has been checked by comparing the spectra of the suspensions in HBS with those performed on samples hydrated with aqueous solution containing 150 mM NaCl (data not shown). The perfect correspondence between the line shapes in the two media indicates that the degree of protonation of the headgroup of DOPE does not change significantly upon the increase in pH.
31P- and 2H-NMR spectra of the SAINT-2/DOPE mixture in HBS show that DOPE and SAINT-2, respectively, are present in an HII phase and that the line shapes of both 2H- and 31P-NMR reveal the same independence from temperature. These observations, together with the fact that the lipoplex of SAINT-2 in the absence of DOPE at physiological ionic strength exhibits lamellar morphology (Šmisterová et al., 2001), represent a strong indication for the homogeneous mixing of the unsaturated cationc amphiphile with DOPE.
The quantitative transition to the HII phase observed for SAINT-2/DOPE mixtures is induced by the increase in ionic strength. The main consequence of the presence of NaCl is the screening of the electrostatic repulsion between the headgroups of the charged amphiphile. This effect leads to a decrease of a and, consequently, an increase of Ns, consistent with the transition to the inverted phase.
Decreasing the amount of DOPE decreases the average Ns of the mixture and 20 mol % of DOPE is not sufficient to stabilize the HII phase with respect to the cubic phase.
Besides the reduction of the cross-sectional headgroup area, the salt-induced reduction of the electrostatic repulsion leads to demixing of the lipids. According to the regular solution model (Lee, 1977), the enthalpic contribution, ΔHm, to the Gibbs energy of mixing (ΔGm) of the molecules A with the molecules B, depends on the effective interaction parameter, 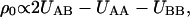 which relates to the difference in energy between unlike and like pair interactions. Negative values of ρ0 favor mixing, resulting from unlike pair interactions stronger than the average interaction between like pairs. It follows that going from water to HBS the intermolecular repulsive interactions between SAINT-5 molecules are decreased to such an extent that domains of different lipid compositions are formed. Upon increasing the temperature, the favorable entropic contribution to the Gibbs energy of mixing is increased, leading to ΔGm < 0 only above 25°C.
which relates to the difference in energy between unlike and like pair interactions. Negative values of ρ0 favor mixing, resulting from unlike pair interactions stronger than the average interaction between like pairs. It follows that going from water to HBS the intermolecular repulsive interactions between SAINT-5 molecules are decreased to such an extent that domains of different lipid compositions are formed. Upon increasing the temperature, the favorable entropic contribution to the Gibbs energy of mixing is increased, leading to ΔGm < 0 only above 25°C.
When the amphiphiles are mixed at 35°C, the HII phase coexists with a dominating isotropic phase. A further increase in temperature increases the contribution of the HII phase. The transition is consistent with an increase in Ns; at high(er) temperatures the cross-sectional headgroup area decreases as a consequence of the decrease in the hydration shell and the hydrophobic volume increases due to the increased mobility of the alkyl chains. The coexistence of different phases in the same lipoplex preparation occurs when the phases have a similar Gibbs energy or if a high kinetic barrier for the transition is present. The reversibility of the observed transition, within short equilibration times, indicates that the coexisting phases have similar Gibbs energies.
The effect of DOPS on S5/DOPE mixtures
When the lipoplex interacts with the cellular membrane, the composition of the lipoplex can vary as a consequence of the recruitment of membrane lipids; the exchange of lipids can have considerable effects on the morphology of the aggregates, with important implications for the transfection efficiency. Major reasons may involve the translocation of the DNA across the cellular membrane and endosomal escape. As previously shown by SAXS (Zuhorn et al., 2002), pelleted SAINT-5/DOPE lipoplexes at a charge ratio of +/− = 2.5 are unable to adopt an HII phase in HBS at room temperature. Incubation of SAINT-5/DOPE lipoplexes with vesicles containing anionic phospholipids leads to the formation of the HII phase at room temperature. The 2H and 31P-NMR experiments shown in Fig. 8, A and B, respectively, clearly indicate that an amount of negative phospholipid that introduces the same number of negative charges as the DNA at the optimal charge ratio for transfection, leads to quantitative HII-phase formation at room temperature. The correspondence between the data obtained by monitoring the cationic amphiphile and the phospholipids independently using 2H and 31P-NMR, respectively, and the complete reversibility of the transitions observed within short equilibration times in the cooling array are consistent with a good mixing of the components. To verify that the variation in the phase behavior observed after the introduction of DOPS is due to the presence of the negative charge in the headgroup region and not to the increased number of unsaturated alkyl chains, the experiments reported in Fig. 8, C and D, were performed. Substituting DOPS with the same amount of DOPE does not lead to a significant change with respect to the equimolar mixture SAINT-5/DOPE. The negatively charged phospholipid has therefore both an effect on the packing parameter, favoring HII-phase formation, and on the mixing of the lipids.
Lipoplex morphology
New insight into the morphology of the lipoplex composed by the equimolar mixture of SAINT-2/DOPE and plasmid DNA at a +/− charge ratio of 2.5, which is the most efficient transfection cocktail, has been gained by 31P-NMR, 2H-NMR, and cryo-TEM. SAXS measurements (Šmisterová et al., 2001) previously indicated a lamellar organization for this lipoplex in water. The NMR experiments show that the anisotropic interactions are averaged to a large extent with the anisotropic contribution being clearly observable only at 5°C, i.e., the reorientation of the molecules is fast with respect to the NMR timescale. Analogous 31P-NMR line shapes were obtained for DOTMA/DOPE lipoplexes (Monk and Cullis, 1997) but they were not assigned to a specific lipid structure. Cryo-TEM pictures clearly reveal the presence of small vesicles (diameter <100 nm) unbound to the DNA in water. The tumbling in solution and the lateral diffusion of the lipids around the highly curved surfaces of these aggregates average out completely the anisotropy leading to the isotropic peak present even at low temperatures.
At the concentration used, the colloidal stability of the complexes is low and formation of macroscopic aggregates is observed after a short time. Besides the large size of the complexes, both 31P and 2H-NMR clearly showed that an effective mechanism of averaging of the anisotropy was active. The high-resolution cryo-TEM pictures recorded to investigate this mechanism gave new insights into the morphology of the lamellar aggregates. As pointed out earlier, the DNA is not sandwiched between flat extended bilayers, but the bilayers bound to the DNA are arranged in highly curved loops with different diameters. On the basis of the line-shape study we conclude that the lateral diffusion of the lipids is not slow on the NMR timescale, even after interaction with DNA, and leads to different degrees of averaging as a function of temperature. The particular morphology observed in the lipoplex of SAINT-2 is not a unique feature of this amphiphile. Cryo-TEM pictures of lipoplexes of sugar-based gemini surfactants developed in our group (Bell et al., 2003) and of other transfection cocktails (Schmutz et al., 1999; Lasic et al., 1997) present similar characteristics. In Schmutz at al., 1999, the lipoplex morphology presented is also characterized by highly curved lamellae and a concentric “ring-like” pattern is recognizable. Some ring-like structures are easily identifiable in our system as well. It has been suggested that this particular morphology arises from cationic-liposome-induced DNA compactation which appears to occur through a concentric winding, where DNA is absorbed onto the cationic bilayers (Schmutz et al., 1999). The characteristic pattern seems to be related to the structural changes DNA molecules undergo during DNA condensation. It has already been mentioned that analogous structures have been observed with plasmid DNA, linear DNA, and oligodeoxyribonucleotide (Schmutz et al., 1999). Our results show that similar patterns with bilayers folded in loops are recognizable in lipoplexes formed by structurally different cationic amphiphiles. We contend that the observed morphology, characterized by highly curved bilayers, is the general aggregate organization for complexes between condensed nucleic acids and bilayer-forming cationic amphiphiles. When the solution conditions are changed in a way to induce an increase in the packing parameter of the amphiphile, the aggregates undergo a transition toward the HII phase. The measurements performed on the lipoplexes in HBS showed how the hexagonal morphology preferred by the equimolar SAINT-2/DOPE mixture in the absence of DNA remains the thermodynamically stable phase even after interaction with the polyanion. The reason for the superior transfection efficiency of a lipoplex that can assume the hexagonal morphology has not been unambiguosly identified, but has been suggested to be related to an easier endosomal escape of the transfected gene (Zuhorn et al., 2002).
CONCLUSIONS
We have performed an NMR-based investigation of the differences between the mixtures of two structurally related cationic amphiphiles, differing only in the degree of unsaturation of the alkyl chains, in combination with DOPE under conditions relevant for gene transfection. The unsaturated surfactant, SAINT-2, shows a higher dynamic character of the membranes formed. The different effects induced by an increase of the ionic strength to physiological values has been determined and rationalized on the basis of the packing parameter: whereas the mixtures of the unsaturated surfactant (SAINT-2) form inverted phases and, in particular, stable HII phases for DOPE contents ≥30 mol %, the saturated surfactant (SAINT-5) does not form homogeneously mixed inverted phases in mixtures with DOPE at room temperature. However, mixed inverted phases are observed for this system at higher temperatures and, after mixing has been achieved by heating, metastable mixed phases are present for several hours even at 5°C. At 35°C the cubic phase is dominant.
The morphology of the lipoplex formed from an equimolar mixture of SAINT-2/DOPE and plasmid DNA is characterized by highly curved bilayers. We suggest that this organization is a general characteristic of complexes of bilayer-forming amphiphiles with DNA present in the condensed form, irrespective of the nature of the headgroup of the amphiphile.
Finally it has been established that the HII morphology of the same lipoplex preparation under physiological ionic strength conditions is thermodynamically stable.
Our results demonstrate the power and convenience of the combination of 2H and 31P-NMR experiments in studies of complex lipid/phospholipid mixtures, in particular when multiple phases coexist in the same preparation.
References
- Audouy, S., G. Molema, L. De Leij, and D. Hoekstra. 2000. Serum as a modulator of lipoplex-mediated gene transfection: dependence of amphiphile, cell type and complex stability. J. Gene Med. 2:465–475. [DOI] [PubMed] [Google Scholar]
- Bell, P. C., M. Bergsma, I. P. Dolbnya, W. Bras, M. C. A. Stuart, A. E. Rowan, M. C. Feiters, and J. B. F. N. Engberts. 2003. Transfection mediated by gemini surfactants: engineered escape from the endosomal compartment. J. Am. Chem. Soc. 125:1551–1558. [DOI] [PubMed] [Google Scholar]
- Cherezov, V., H. Qiu, V. Pector, M. Vandenbranden, J. M. Ruysschaert, and M. Caffrey. 2002. Biophysical and transfection studies of the diC(14)-amidine/DNA complex. Biophys. J. 82:3105–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullis, P. R., and B. De Kruyff. 1979. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim. Biophys. Acta. 559:399–420. [DOI] [PubMed] [Google Scholar]
- Davis, J. H., K. R. Jeffrey, M. Bloom, M. I. Valic, and T. P. Higgs. 1976. Quadrupolar echo deuteron magnetic resonance spectroscopy in ordered hydrocarbon chains. Chem. Phys. Lett. 42:390–394. [Google Scholar]
- Eastman, S. J., C. Siegel, J. Tousignant, A. E. Smith, S. H. Cheng, and R. K. Scheule. 1997. Biophysical characterization of cationic lipid:DNA complexes. Biochim. Biophys. Acta. 1325:41–62. [DOI] [PubMed] [Google Scholar]
- Gorenstein, D. G. 1984. Phosphorus-31 NMR. Academic Press, New York.
- Hirsch-Lerner, D., and Y. Barenholz. 1999. Hydration of lipoplexes commonly used in gene delivery: follow-up by laurdan fluorescence changes and quantification by differential scanning calorimetry. Biochim. Biophys. Acta. 1461:47–57. [DOI] [PubMed] [Google Scholar]
- Hulst, R., I. Muizebelt, P. Oosting, C. van der Pol, A. Wagenaar, J. Šmisterová, E. Bulten, C. Driessen, D. Hoekstra, and J. B. F. N. Engberts. 2004. Sunfish amphiphiles: Conceptually new carriers for DNA delivery. Eur. J. Org. Chem. 4:835–849. [Google Scholar]
- Israelachvili, J. N., D. J. Mitchell, and B. W. Ninham. 1976. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J. Chem. Soc., Faraday Trans. 2. 72:1525–1568. [Google Scholar]
- Iwahashi, M., Y. Kasahara, H. Matsuzawa, K. Yagi, K. Nomura, H. Terauchi, Y. Ozaki, and M. Suzuki. 2000. Self-diffusion, dynamical molecular conformation, and liquid structures of n-saturated and unsaturated fatty acids. J. Phys. Chem. B. 104:6186–6194. [Google Scholar]
- Koltover, I., T. Salditt, J. O. Radler, and C. R. Safinya. 1998. An inverted hexagonal phase of cationic liposome-DNA complexes related to DNA release and delivery. Science. 281:78–81. [DOI] [PubMed] [Google Scholar]
- Koltover, I., T. Salditt, and C. R. Safinya. 1999. Phase diagram, stability, and overcharging of lamellar cationic lipid-DNA self-assembled complexes. Biophys. J. 77:915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koynova, R., and R. C. MacDonald. 2003a. Mixtures of cationic lipid O-ethylphosphatidylcholine with membrane lipids and DNA: Phase diagrams. Biophys. J. 85:2449–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koynova, R., and R. C. MacDonald. 2003b. Cationic O-ethylphosphatidylcholines and their lipoplexes: phase behavior aspects, structural organization and morphology. Biochim. Biophys. Acta. 1613:39–48. [DOI] [PubMed] [Google Scholar]
- Lasic, D. D., H. Strey, M. C. A. Stuart, R. Podgornik, and P. M. Frederik. 1997. The structure of DNA-liposome complexes. J. Am. Chem. Soc. 119:832–833. [Google Scholar]
- Lee, A. G. 1977. Lipid phase-transitions and phase-diagrams. II. Mixtures involving lipids. Biochim. Biophys. Acta. 472:285–344. [DOI] [PubMed] [Google Scholar]
- Meekel, A. A. P., A. Wagenaar, J. Šmisterová, J. E. Kroeze, P. Haadsma, B. Bosgraaf, M. C. A. Stuart, A. Brisson, M. H. J. Ruiters, D. Hoekstra, and J. B. F. N. Engberts. 2000. Synthesis of pyridinium amphiphiles used for transfection and some characteristics of amphiphile/DNA complex formation. Eur. J. Org. Chem. 4:665–673. [Google Scholar]
- Mitrakos, P., and P. M. Macdonald. 1996. DNA-induced lateral segregation of cationic amphiphiles in lipid bilayer membranes as detected via H-2 NMR. Biochemistry. 35:16714–16722. [DOI] [PubMed] [Google Scholar]
- Mitrakos, P., and P. M. Macdonald. 2000. Nucleotide chain length and the morphology of complexes with cationic amphiphiles: (31)P-NMR observations. Biochim. Biophys. Acta. 1463:355–373. [DOI] [PubMed] [Google Scholar]
- Monk, K. W. C., and P. R. Cullis. 1997. Structural and fusogenic properties of cationic liposomes in the presence of plasmid DNA. Biophys. J. 73:2534–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijper, D., E. Bulten, J. Šmisterová, A. Wagenaar, D. Hoekstra, J. B. F. N. Engberts, and R. Hulst. 2003. Novel biodegradable pyridinium amphiphiles for gene delivery. Eur. J. Org. Chem. 22:4406–4412. [Google Scholar]
- Roosjen, A., J. Šmisterová, C. Driessen, J. T. Anders, A. Wagenaar, D. Hoekstra, R. Hulst, and J. B. F. N. Engberts. 2002. Synthesis and characteristics of biodegradable pyridinium amphiphiles used for in vitro DNA delivery. Eur. J. Org. Chem. 7:1271–1277. [Google Scholar]
- Schmutz, M., D. Durand, A. Debin, Y. Palvadeau, A. Etienne, and A. R. Thierry. 1999. DNA packing in stable lipid complexes designed for gene transfer imitates DNA compaction in bacteriophage. Proc. Natl. Acad. Sci. USA. 96:12293–12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, F. X., L. Wasungu, A. Nomden, M. C. A. Stuart, E. Polushkin, J. B. F. N. Engberts, and D. Hoekstra. 2002. Interference of poly(ethylene glycol)-lipid analogues with cationic-lipid-mediated delivery of oligonucleotides; role of lipid exchangeability and non-lamellar transitions. Biochem. J. 366:333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simberg, D., D. Danino, Y. Talmon, A. Minsky, M. E. Ferrari, C. J. Wheeler, and Y. Barenholz. 2001. Phase behavior, DNA ordering, and size instability of cationic lipoplexes. Relevance to optimal transfection activity. J. Biol. Chem. 276:47453–47459. [DOI] [PubMed] [Google Scholar]
- Šmisterová, J., A. Wagenaar, M. C. A. Stuart, E. Polushkin, G. ten Brinke, R. Hulst, J. B. F. N. Engberts, and D. Hoekstra. 2001. Molecular shape of the cationic lipid controls the structure of cationic lipid/dioleylphosphatidylethanolamine-DNA complexes and the efficiency of gene delivery. J. Biol. Chem. 276:47615–47622. [DOI] [PubMed] [Google Scholar]
- Vanderwoude, I., A. Wagenaar, A. A. P. Meekel, M. B. A. Terbeest, M. H. J. Ruiters, J. B. F. N. Engberts, and D. Hoekstra. 1997. Novel pyridinium surfactants for efficient, nontoxic in vitro gene delivery. Proc. Natl. Acad. Sci. USA. 94:1160–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel, A. I. 1962. A Text-book of Practical Organic Chemistry. Longmans, London.
- Zantl, R., L. Baicu, F. Artzner, I. Sprenger, G. Rapp, and J. O. Radler. 1999. Thermotropic phase behavior of cationic lipid-DNA complexes compared to binary lipid mixtures. J. Phys. Chem. B. 103:10300–10310. [Google Scholar]
- Zuhorn, I. S., V. Oberle, W. H. Visser, J. B. F. N. Engberts, U. Bakowsky, E. Polushkin, and D. Hoekstra. 2002. Phase behavior of cationic amphiphiles and their mixtures with helper lipid influences lipoplex shape, DNA translocation, and transfection efficiency. Biophys. J. 83:2096–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuidam, N. J., D. Hirsch-Lerner, S. Margulies, and Y. Barenholz. 1999. Lamellarity of cationic liposomes and mode of preparation of lipoplexes affect transfection efficiency. Biochim. Biophys. Acta. 1419:207–220. [DOI] [PubMed] [Google Scholar]