Abstract
Previous work showed that human β-globin mRNAs harboring a premature termination codon are degraded in the erythroid tissues of mice to products that lack sequences from the mRNA 5′ end but contain a 5′ cap-like structure. Whether these decay products are the consequence of endonucleolytic or 5′-to-3′ exonucleolytic activity is unclear. We report that this β-globin mRNA decay pathway is recapitulated in cultured mouse erythroleukemia (MEL) cells and targets nonsense-free mRNA to a lesser extent than nonsense-containing mRNA. S1 nuclease mapping and primer extension demonstrated that 70–80% of decay product 5′ ends contain a UG dinucleotide. Detection of upstream counterparts of these decay products indicates that they are generated by endonucleolytic activity. Both crude and partially purified polysome extracts prepared from MEL cells contain an endonucleolytic activity that generates decay products comparable to those observed in vivo. These data suggest that an endonuclease with preference for UG dinucleotides is involved in the degradation of nonsense-containing and, to a lesser extent, nonsense-free human β-globin mRNAs in mouse erythroid cells.
Keywords: nonsense codon‖UG dinucleotides‖mRNA endonuclease‖polysomes
Messenger RNA decay is a regulated process that affects gene expression in all organisms. Despite its importance, however, the process by which most mammalian mRNAs are degraded remains unknown. In theory, mRNA decay may be initiated by an exoribonuclease or an endoribonuclease, the activities of which are generally influenced by other proteins (reviewed in refs. 1 and 2). Endoribonucleases are responsible for the decay of a number of mRNAs, including those for human insulin-like growth factor II (3), human transferrin receptor (4), chicken apolipoprotein II (5), human α-globin (6), human c-myc (7), and Xenopus vitellogenin (8). In the case of Xenopus vitellogenin mRNA, an unstructured sequence in the 3′ untranslated region is cleaved by polysome ribonuclease 1 (PMR1) provided that vigilin is not bound. Estrogen-induced Xenopus liver PMR1 also hydrolyzes serum protein mRNAs, preferentially at UG sites. The sequence hydrolyzed most efficiently within albumin mRNA is AYUGA (Y = C or U) (9, 10). Other UG sites are also hydrolyzed. However, mutagenesis studies demonstrated that the UG dinucleotide of AYUGA is critical for efficient cleavage.
Experiments performed more than a decade ago indicate that the decay of mammalian β-globin mRNA also involves an endoribonuclease; an activity from mouse sarcoma 180 cells that cosediments with polysomes as well as free messenger ribonucleoprotein particles cleaves β-globin mRNA primarily at UG sites but also at UC sites (11). However, the relationship of this activity to the one that elicits the faster decay of nonsense-containing β-globin mRNA in the cytoplasm of erythroid cells of mice transgenic for one of several human β0-thalassemic β-globin alleles (12–14) is unknown. Remarkably, nonsense-mediated mRNA decay (NMD) was accompanied by the accumulation of human β-globin transcripts that are polyadenylated but lack sequences from the 5′ end of full-length mRNA (12–14). There is no evidence for aberrant transcription initiation or pre-mRNA splicing, indicating that the truncated transcripts are mRNA decay products.
In general, nonsense-containing mRNAs in mammalian cells are abnormally short-lived (reviewed in ref. 15). Observations report human β-globin transcripts in transgenic mice are notable in being the only detectable NMD products. Also remarkable, these decay products bind cap antibody with the same efficiency as full-length mRNA in a way that is prevented by exposure to tobacco acid pyrophosphatase, suggesting the presence of a cap-like structure at their 5′ ends (13). It was proposed that these 5′ ends acquire a cap after an appropriate modification. In theory, decay could be initiated by a 5′-to-3′ exonuclease, like NMD in Saccharomyces cerevisiae (reviewed in ref. 1), or an endonuclease.
In the current study, mouse erythroleukemia (MEL) cells were stably transfected with either a normal or a nonsense-containing human β-globin allele. The use of MEL cells is far less involved than the use of transgenic mice, which have to be made anemic before RNA isolation and analysis. MEL cells displayed the same human β-globin mRNA decay products from the nonsense-containing allele as observed in transgenic mice. In fact, these products were also produced to a lesser extent by the nonsense-free allele, indicating that a premature termination codon activates a preexisting mRNA decay pathway. The 5′ termini of the decay products generally coincided with a UG dinucleotide, suggesting a role for this dinucleotide in endonucleolytic cleavage. Consistent with this conclusion, upstream counterparts of these decay products were also evident.
Materials and Methods
Cell Culture, DNA-Mediated Electroporation, and Dimethyl Sulfoxide (DMSO)-Induced Differentiation.
C88 MEL cells were cultured as described (16). After cells entered the logarithmic phase of growth (0.5–1.0 × 106 cells per ml), 2 × 107 were transfected with 75 μg of ScaI-linearized pLCR-hβ-globin plasmid by using a Gene Pulser (Bio-Rad). Plasmids harbored either a normal (Norm) or β0-thalassemic (Thal) human β-globin allele (12–14) under the control of the DMSO-responsive locus control region (16–18). Cell lines Norm 2 and Thal 10, which stably expressed high levels of nonsense-free and nonsense-containing human β-globin mRNA, respectively, were selected for analysis. For DMSO-induced differentiation, cells were collected and analyzed 96 h after the addition of DMSO to 2% (vol/vol). Alternatively (Figs. 1 B and C, 3, and 4), cells were propagated and induced at Lofstrand Laboratories (Gaithersburg, MD).
Figure 1.
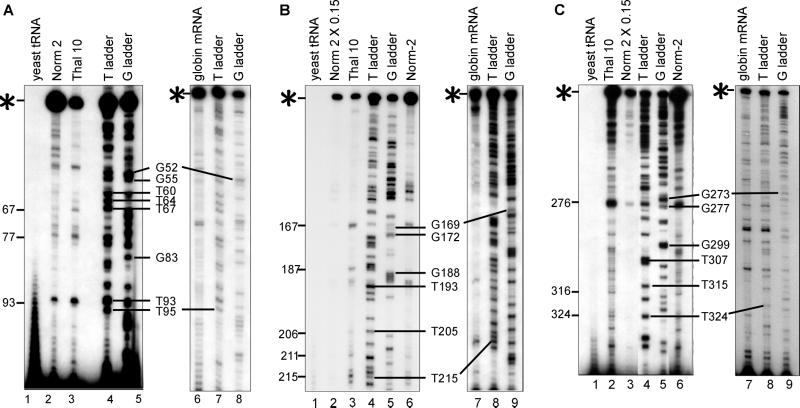
S1 nuclease mapping of 3′ decay products of normal and nonsense-containing human β-globin transcripts. (A) Mice transgenic for a nonsense-free (Norm) or nonsense-containing (Thal) human β-globin allele were made anemic, and RNA was isolated from peripheral blood (13). Additionally, MEL cells stably expressing the nonsense-free (Norm 2) or nonsense-containing (Thal 10) human β-globin allele were propagated in the absence (uninduced) or presence (induced) of 2% DMSO, and RNA was isolated from total (T), nuclear (N), and cytoplasmic (C) fractions (19). A 5′-32P-labeled 590-bp MseI–MseI fragment of human β-globin cDNA and a 5′-32P-labeled 183-bp BamHI–BamHI fragment of mouse α-globin cDNA were used as probes in S1 nuclease mapping (13). Full-length human β-globin mRNA protected 524 nt (hβ-Gl mRNA), mouse α-globin mRNA protected 183 nt (mα-Gl mRNA), and mRNA decay products are identified by arrows. The 159-nt protected fragment (□) derives from intron 2-containing human β-globin pre-mRNA (data not shown). Total (T), nuclear (N), and cytoplasmic (C) RNA from each transfectant was assayed by using the amounts specified above each lane (2, 15, or 30 μg). −RNA, no RNA; −S1, no S1 nuclease. The locations of the premature termination codon (PTC) and 5′ decay products are diagrammed. (B and C) A 5′-32P-labeled NaeI–BamHI fragment of a human β-globin cDNA was used as probe for S1 nuclease mapping. Full-length mRNA protected 351 nt (✶). In B, 10 μg of total RNA was used except for lane 2, where 1.5 μg was used. In C, 10 μg of total, 10 μg of poly(A)−, and 0.5 μg of poly(A)+ RNA were used. The sizes of the protected fragments are indicated according to the distance from the 5′ end of full-length β-globin mRNA to the left in B and right in C. M, molecular weight standards.
Figure 3.
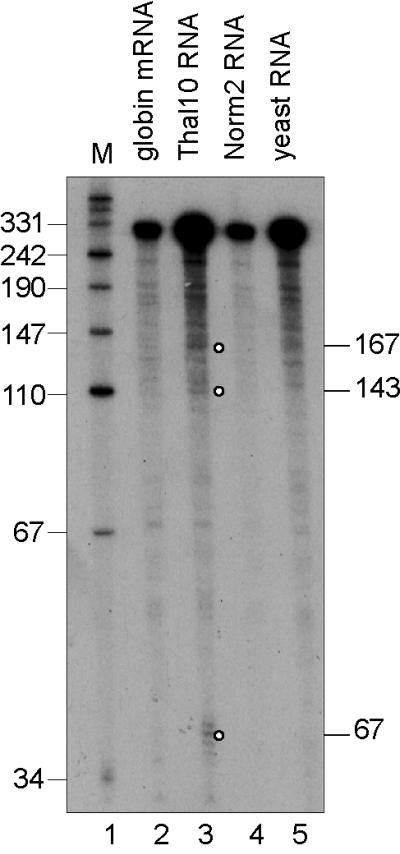
S1 nuclease mapping of 5′ decay products of normal and nonsense-containing human β-globin transcripts. S1 nuclease protection was performed as in Fig. 1 by using 0.74 ng of synthetic β-globin mRNA (lane 2) (21), 15 μg of poly(A)− MEL cell RNA, or 15 μg of yeast RNA and a 3′-labeled BfaI–BamHI probe that spans nucleotides 31–351 of human β-globin mRNA. Decay intermediates are identified with circles.
Figure 4.
Primer extension mapping the 5′ ends of the human β-globin mRNA decay products. Total RNA (10 μg), or synthetic human β-globin mRNA (7.2 ng) (21) was annealed to oligonucleotide DNAs (see Fig. 2 and Table 1) complementary to mRNA nucleotides 112–136 (A), 226–250 (B), or 341–365 (C) and assayed by primer extension. Sequencing ladders (T or G ladders) provided molecular size markers, and a control primer extension (synthetic globin RNA) identified sites of reverse transcriptase stalling. Numbers to the left map potential cleavage sites relative to the full-length 5′ end, which is defined as 1. Notably, because Thal 10 mRNA contains one less nucleotide at codon 44 relative to Norm 2 mRNA, the extension band resulting from cleavage at nucleotide 167 is shorter by one nucleotide. Results shown are representative of at least two independently performed experiments that used RNA from transfectants grown and harvested on different days. Full-length products are identified with an asterisk (✶).
RNA Isolation and Analysis.
Total, nuclear, and cytoplasmic MEL-cell RNAs (Fig. 1A) were isolated as described (19), or total MEL-cell RNA (Fig. 1 B and C; Fig. 3) was isolated by using RNAgents Total RNA Isolation System (Promega). Oligo(dT)-cellulose bound poly(A)+ and unbound poly(A)− fractions of total RNA (Fig. 1C) were obtained by using Poly(A) Pure (Ambion). For S1 nuclease mapping (20), RNA from either Norm 2 or Thal 10 transfectants or, as controls, the peripheral blood of anemic mice transgenic for either the Norm or Thal alleles (12–14) was dissolved in S1 hybridization buffer [80% deionized formamide/40 mM Pipes (pH 7.6)/0.4 M NaCl/1 mM EDTA] at a concentration of 5 μg/ml and incubated with 32P-end-labeled DNA fragments overnight at 52°C. Human β-globin transcripts were mapped by using a 5′-end-labeled 590-bp MseI–MseI fragment of human β-globin cDNA that extends from pSP64 vector sequences residing upstream of the cDNA into exon 3 and protects 524 nt of full-length mRNA (12), a 5′-end-labeled 635-bp NaeI–BamHI fragment of human β-globin cDNA (21) that extends from pSP64 vector sequences residing upstream of the cDNA into exon 2 and protects 351 nt of full-length mRNA, or a 3′-end-labeled BfaI–BamHI fragment of human β-globin cDNA that extends from cDNA nucleotides 31 to 351 and protects 320 nt of full-length mRNA. Mouse α-globin transcripts were mapped by using a 5′-end-labeled 183-bp BamHI–BamHI fragment of mouse α-globin cDNA that protects 183 nt of α-globin mRNA. After hybridization, samples were incubated with S1 nuclease (100 units; Life Technologies/GIBCO/BRL) at 25°C for 2 h, extracted with phenol/choloroform/isoamyl alcohol and then with choloroform/isoamyl alcohol, precipitated with ethanol, and electrophoresed in a denaturing 6% acrylamide gel. Protected fragments were quantified by PhosphorImager (Molecular Dynamics) and visualized by autoradiography (Fig. 1A). Alternatively, fragments were quantified (22) after the level of mouse β-globin mRNA in the RNA preparations was determined to be comparable (±20%). This was done by primer extension (22) using a primer complementary to nucleotides 112–126 of the mouse mRNA (data not shown).
Preparation of Polysome Extract and Mono S Fractions.
The polysome crude extracts used in Fig. 5 were prepared as described (23, 24). For Mono S column chromatography, DMSO-induced Norm 2 MEL cells (14 × 108) were suspended in 12 ml of 10% glycerol/20 mM Tris⋅HCl buffer (pH 7.8)/10 mM MgCl2/0.1 mM EDTA/0.2 mM DTT/0.2 μg/ml each of antipain and leupeptin (Sigma). After 20 min on ice the cells were homogenized with 20 strokes of a Dounce homogenizer, B pestle, and the homogenate was centrifuged for 5 min at 2,500 × g. The resulting supernatant was layered over 1.5 ml of 30% sucrose in the same buffer and centrifuged for 2 h at 40,000 rpm in a Ti rotor 50 The resulting polysome pellet was suspended in 2 ml of 2.5% glycerol, 20 mM Tri⋅HCl buffer (pH 7.6), 1.5 mM MgCl2/0.25 mM EDTA/1 mM DTT/0.2 μg/ml each of antipain and leupeptin. Triton X-100 was then added to 0.2%, and the solution was shaken intermittently for 1.5 h before freezing overnight. Two milliliters was centrifuged at 40,000 rpm in a Beckman 50 Ti rotor for 2 h. The supernatant was dialyzed against 1 liter of 50 mM sodium phosphate buffer (pH 7.2)/1 mM DTT containing antipain and leupeptin as described above. Protein (7 mg/ml) was loaded onto a FPLC Mono S column (1 ml) and eluted with a gradient of 0.02 to 0.6 M NaCl in 50 mM sodium phosphate buffer (pH 7.2)/1 mM DTT, and antipain and leupeptin as described above. Fifty fractions (1 ml) were collected. Mono S fractions contained approximately 15–20 μg/ml protein.
Figure 5.
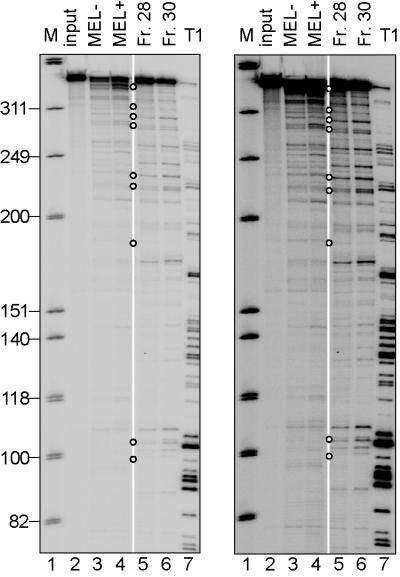
β-globin mRNA endonuclease activity associated with MEL-cell polysomes. (Left) An in vitro-synthesized, 5′-32P-labeled transcript consisting of the 5′-most 347 nt of human β-globin mRNA (plus 20 nt of vector) was incubated for 30 min at 37°C with no added protein (lane 2, input), polysome extract from uninduced (MEL−, lane 3) or 48-hr DMSO-induced (MEL+, lane 4) MEL cells, or two fractions containing β-globin mRNA endonuclease activity recovered from Mono S fractionation of the polysome extract (lanes 5 and 6). Lane 7 shows a partial T1 digest of the β-globin transcript; an open circle (○) shows products with counterparts in vivo. Right is a longer exposure of Left.
Endonuclease Activity Assay.
The human β-globin cDNA plasmid pSPkβC (a gift from Rich Spritz, University of Colorado, Boulder) was linearized with BamHI, and 5′-end-labeled RNA was prepared by using [γ-32P]GTP (23), treated with 2 units of DNase I for 15 min at 37°C, and gel purified. Activity assays were performed in 20 μl containing 50 mM Tris (pH 7.2), 50 mM MgC12, and 10 mM DTT by using 5 μg of polysome extract or 2.5 μg of Mono S fractions of polysome extract plus 105 cpm of 5′-end-labeled transcript. Reactions were performed at 37°C for the indicated times and terminated by the addition of SDS to a final concentration of 1% and 20 μg of proteinase K followed by incubation for 15 min at 65°C. Samples were then extracted with phenol/chloroform (50:50) and precipitated with ethanol. RNA was recovered, electrophoresed in denaturing 6% polyacrylamide/urea gels, and visualized by autoradiography.
Results
Undifferentiated and DMSO-Differentiated MEL Cells Generate Human β-Globin mRNA Decay Products.
C88 MEL cells were stably transfected with one of two human β-globin alleles that had been used to generate transgenic mice (12–14): a nonsense-free (Norm) allele or a nonsense-containing β0-thalassemic (Thal) allele that harbored a single nucleotide deletion within codon 44. This Thal allele encodes mRNA that terminates translation within codons 60 and 61 and is subject to NMD in the bone marrow of affected patients and erythroid tissues of transgenic mice (12–14, 25, 26). Before transfection, each allele was inserted downstream of the β-globin locus control region of the GSC1417 β-globin gene expression plasmid (17, 18).
MEL cells were harvested either immediately after (uninduced), or 96 h after (DMSO-induced) the addition of DMSO, which induces erythroid differentiation. Total, nuclear, and cytoplasmic RNAs were isolated and analyzed by using S1 nuclease mapping. As controls, RNA from the peripheral blood of anemic mice transgenic (TG) for either the Norm or Thal allele (12–14) was also analyzed. Human β-globin transcripts were mapped by using a 5′-end-labeled 590-bp MseI–MseI fragment and, as a control, endogenous mouse β-globin transcripts were mapped by using a 5′-end-labeled 183-bp BamHI–BamHI fragment.
As expected, full-length β-globin mRNA was detected as a 524-nt S1 nuclease-resistant band in all transfectants and both TG samples (Fig. 1A, hβ-Gl mRNA). α-Globin mRNA was detected as a 183-bp S1 nuclease-resistant band in all but the nuclear fractions of uninduced transfectants, indicating that induction was required for detection in nuclear RNA (Fig. 1A, mα-Gl mRNA). Also evident were additional S1 nuclease-resistant bands in total and cytoplasmic but not nuclear fractions of both uninduced and induced Thal 10 transfectants that corresponded to mRNA lacking the 5′-terminal 50–210 nt (Fig. 1A). Therefore, these bands correspond to the mRNA decay products evident in mice transgenic for the different Thal alleles (12–14). Consistent with this interpretation, three of the bands comigrated with bands evident in TG-Thal but not TG-Norm peripheral blood (Fig. 1A). After DMSO induction, there was less full-length human β-globin mRNA in the Thal 10 transfectant compared with the Norm 2 transfectant and, relative to before DMSO induction, there was an approximately 5-fold increase in the amount of the mRNA decay products relative to full-length mRNA in the Thal 10 transfectant (e.g., compare uninduced and induced bands in Thal 10 cytoplasmic fractions). Studies concomitantly performed using cells expressing a reverted version of the Thal allele (13), which contained a single base pair insertion that restores translation termination to normal, revealed results identical to those obtained with the Norm 2 transfectant (data not shown).
More accurate mapping of the decay products was obtained by using RNA from DMSO-induced transfectants and a 5′-end-labeled NaeI–BamHI fragment as a probe (21). As expected, full-length β-globin mRNA protected 351 nt of the probe from S1 nuclease digestion (Fig. 1B). After induction, the Thal 10 transfectant expressed only 15% as much full-length human β-globin mRNA as the Norm 2 transfectant (Fig. 1B, lanes 1–3). Comparing equal amounts of expressed human β-globin mRNA (Fig. 1B, lanes 2 and 3) rather than equal amounts of input RNA (Fig. 1B, lanes 1 and 3) revealed that the decay products are, in fact, evident for the Norm 2 transfectant. The ratio of decay products to full-length mRNA was about 5-fold to10-fold higher for the Thal 10 transfectant than the Norm 2 transfectant, indicating that the nonsense codon enhances the activity of a decay pathway that also targets nonsense-free mRNA. The increased sensitivity of this probe resulted in the identification of additional (a total of 12) decay products. The 5′ ends of these decay products begin 67 to 316 nt from the 5′ end of full-length mRNA (Fig. 1B, numbers to left) and correspond to all decay products detected with the MseI–MseI probe. Notably, 9 of the 12 ends reside within a sequence containing a UG dinucleotide (Fig. 2, Table 1). Consistent with the finding that the mRNA decay products of TG-Thal mice are polyadenylated (12–14), the majority of the decay products were recovered by using oligo(dT)-cellulose (Fig. 1C).
Figure 2.
Sequence of human β-globin mRNA, where nucleotides in bold are complementary to DNA primers used in primer extensions.
Table 1.
Human β-globin mRNA decay products
| Distance, nt
|
Cleavage site | ||
|---|---|---|---|
| Primer extension | S1 nuclease mapping | Electrophoretic mobility | |
| 67 | 67 | 67 | cuga |
| 77 | 77 | gucu | |
| 93 | 92 | cugu | |
| 143 | 143 | gcug | |
| 167 | 167 | ccag | |
| 187 | 186 | 186/187 | uugg |
| 205 | cuga | ||
| 214 | 214 | guua | |
| 259 | 259 | gugc | |
| 269 | 269 | guga | |
| 272 | augg | ||
| 276 | 276 | cugg | |
| 282 | ucac | ||
| 316 | 316 | ugag | |
| 324 | 324 | 324 | cugc |
Numbers refer to distances of the cleavage sites from the 5′ end of full-length mRNA as determined by using primer extension or S1 nuclease mapping of Thal 10 MEL-cell RNA, or electrophoretic mobility of in vitro-labeled human β-globin RNA incubated with polysome extract from uninduced or DMSO-induced MEL cells. Endonucleolytic cleavage sites are more accurately derived (±2 nt) by using primer extension and electrophoretic mobility assays.
The mRNA Decay Products Are Generated by Endonucleolytic Activity.
The β-globin mRNA decay products identified above could result either from the pausing of a 5′-3′ exonuclease, or endonucleolytic cleavage. It is difficult to identify decay products upstream of an endonuclease cleavage site because of their extreme lability to highly processive 3′-5′ exonucleases (3, 27). To search for upstream counterparts of the decay products, S1 nuclease mapping was performed by using poly(A)− RNA from DMSO-induced Thal 10 transfectants and a 3′-end-labeled probe spanning nucleotides 31–351 of human β-globin mRNA. This approach identified 5′ counterparts of the decay products resulting from cleavage at nucleotides 67, 143, and 167 of β-globin mRNA in Thal 10, but not Norm 2 cells (Fig. 3). The 320-nt band found in yeast RNA resulted from duplex DNA rehybridization. The other 5′ decay intermediates were below the level of detection of this assay. These data indicate that the observed decay products resulted from endonucleolytic cleavage of β-globin mRNA.
The 5′ Termini of the mRNA Decay Products Coincide Primarily with UG Dinucleotides.
Primer extension was performed to more accurately map endonucleolytic cleavage sites. Primers consisted of 25-nt DNAs complementary to β-globin mRNA nucleotides 112–136, 226–250, or 341–365 (Fig. 2, bold). The first two primers were specific for human β-globin transcripts, whereas the third primer annealed to both human and mouse β-globin transcripts. A sequencing ladder that facilitated 5′ end determination was generated by extending each primer in the presence of Norm 2 RNA, and either ddATP (T ladder) or ddCTP (G ladder) together with the remaining three dNTPs (Fig. 4 A–C, ladders are labeled to the right of each panel as the number of nucleotides from the 5′ end of full-length mRNA). Control reactions were performed with yeast RNA (Fig. 4 A–C, lanes 1) and synthetic β-globin mRNA (Fig. 4A, lane 6; Fig. 4B, lane 7, and Fig. 4C, lane 7) to identify reverse transcriptase pause sites.
When primer 112–136 was used, the level of full-length mRNA from the Thal 10 transfectant constituted 15% of the level of full-length β-globin mRNA from the Norm 2 transfectant (Fig. 4A, compare lanes 2 and 3), as expected from results obtained by S1 nuclease mapping (Fig. 1B). Three extension products that derived from mRNA decay products were identified based on two criteria: (i) absence from the extension of synthetic β-globin mRNA (Fig. 4A, lane 6), and (ii) reduced abundance in Norm 2 RNA relative to Thal 10 RNA (Fig. 4A, compare lanes 2 and 3). The 45-nt extension product was also present in synthetic globin mRNA so it was not included. The 5′ ends of these extension products mapped to cleavage sites at nucleotides 67, 77, and 93 of β-globin mRNA.
When primer 226–250 was used, the level of full-length mRNA from the Thal 10 transfectant also constituted 15% of the level of full-length β-globin mRNA from the Norm 2 transfectant (Fig. 4B, compare lanes 2, 3, and 6). The 5′ ends of primer extension products that met the two criteria mapped to cleavage sites at nucleotides 167, 187, and 205 of β-globin mRNA. When primer 341–365 was used, the level of full-length mRNA from the Thal 10 transfectant also constituted 15% of the level of full-length β-globin mRNA from the Norm 2 transfectant considering that the primer is complementary to both human and mouse β-globin RNAs (Fig. 4C, compare lanes 2 and 3). The 5′ ends of primer extension products that met the two criteria mapped to cleavage sites at nucleotides 276 and, to a lesser extent, 324 (Fig. 4C).
Tabulation of the cleavage sites identified by S1 nuclease mapping and primer extension revealed sites in common at nucleotides 67, 92/93, 167, 186/187, 316, and 324, all but one of which reside close to a UG dinucleotide (Fig. 2, Table 1). Notably, primer extension products may be underrepresented because those that also fell at polymerase pause sites were discounted. We conclude that human β-globin mRNA may be cleaved by an endonuclease similar, if not identical, to the one detected in sarcoma 180 cell extracts (11) and related to Xenopus liver PMR1 (9).
Polysome-Containing Extracts of MEL Cells Are Characterized by UG-Selective RNase Activity.
For more detailed analysis of the cleavage reaction, an in vitro endonuclease cleavage assay was established in which a 5′-end-labeled β-globin transcript was incubated with polysomes prepared from the Thal 10 transfectant. Polysome extracts from either uninduced or DMSO-induced cells generated numerous β-globin mRNA cleavage products (Fig. 5, lanes 3 and 4). When sized by comparison with a partial T1 digest of the transcript (Fig. 5, lane 7) and a DNA sequencing ladder (data not shown), nine of the products (Fig. 5, open circles) matched those identified in vivo (Figs. 1 and 4; see Fig. 2 and Table 1 for comparison). Seven products resulted from UG-site cleavages. The generation of more decay products in vitro than in vivo also typifies albumin mRNA (27) and may reflect nonspecific degradation because of the absence of RNP proteins on in vitro-synthesized transcripts relative to cellular transcripts, or a failure to cap the decay products in vitro.
Polysome extracts of Norm 2 cells were fractionated by using Mono S chromatography (24). Remarkably, fractions 28–30 generated a pattern of β-globin mRNA decay products that was similar to that obtained by using crude polysome extract (Fig. 5, lanes 3–6), suggesting that enzyme identification might soon be possible.
Discussion
We show here that nonsense-containing human β-globin mRNA produced in cultured MEL cells is abnormally low in abundance (Figs. 1 and 4). An abnormally low abundance generally typifies nonsense-containing mammalian mRNAs, provided the nonsense codon resides at least 50–55 nt upstream of the last exon–exon junction (28), as characterizes 60–61 Ter studied here. A recent study also quantitated the amounts of full-length mRNA present in MEL cells transfected with normal and nonsense-containing human β-globin alleles (29). For reasons unknown, nonsense codons within the 5′ half of exon 1 and, therefore, more than 50–55 nt upstream of the last exon–exon junction failed to reduce mRNA abundance.
The abnormally low abundance of nonsense-containing human β-globin mRNAs in erythroid tissues of transgenic mice is concomitant with the generation of mRNA decay products lacking sequences from the mRNA 5′ end (13, 14). In agreement with this finding, mRNA decay products were also detected in both uninduced and DMSO-induced MEL cells (Figs. 1 and 4). These products were also detected, albeit to a lesser extent, in MEL cells producing human β-globin mRNA lacking a nonsense codon (Figs. 1 and 4). Therefore, nonsense codons augment a decay process that targets normal β-globin mRNA. Detection of the 5′ counterparts of decay products demonstrates decay of a nonsense-containing mRNA through an endonucleolytic pathway (Fig. 3). Both S1 nuclease mapping and primer extension revealed that the 5′ ends of most decay products reside at or near a UG dinucleotide (Fig. 2, Table 1), suggesting involvement of a UG-site-selective endoribonuclease.
Previous work identified a polysome-associated UG-selective endonuclease (PMR1) that remains associated with mRNA after EDTA dissociation of polysomes (9, 24, 30). The properties of PMR1 are strikingly similar to those of a previously identified UG-selective endonuclease that cleaves rabbit β-globin mRNA (11). Like PMR1 (27), the polysome-associated endonuclease activity from MEL cells cleaves in vitro at many, but not all, of the same sites as seen in vivo. While the identity of the β-globin mRNA endonuclease remains to be determined, we conclude that the activity identified here catalyzes the decay of nonsense-free β-globin mRNA in erythroid cells and is activated by the presence of a premature termination codon.
In S. cerevisiae, the primary pathway of mRNA decay involves deadenylation, followed by decapping by Dcp1p and 5′-to-3′ degradation of the mRNA body by Xrn1p (1). NMD triggers the rapid decay of mRNAs that prematurely terminate translation by activating deadenylation-independent decapping by Dcp1p followed by Xrn1p-catalyzed 5′-3′ degradation. Commonalities between NMD in yeast and mammalian cells are reflected in (i) requirements for Upf1, Upf2/Nmd2, and Upf3 proteins and (ii) a destabilizing element located downstream of the premature termination codon that, in yeast, binds Hrp1p or a functionally equivalent protein, and in mammals binds the exon junction complex that is deposited as a consequence of pre-mRNA splicing (refs. 31 and 32, and references therein). While the polarity of NMD in mammalian cells is currently unknown, Upf1 immunopurifies with decapping protein (F. Lejeune, M. Kiledjian, and L.E.M., unpublished data; J. Lykke-Andersen, personal communication) suggesting the involvement of 5′-to-3′ decay. It remains to be determined whether the deadenylation-independent endonucleolytic cleavage reported here for nonsense-containing and, to a lesser extent, nonsense-free human β-globin mRNA in mouse erythroid tissues typifies other mRNAs or involves the Upf proteins.
mRNA endonucleases appear to function primarily in cells that are differentiated to produce high levels of a specific gene product(s) and, as such, display selectivity toward these targets rather than targeting all mRNAs within a cell (reviewed in ref. 2). Because nonsense-containing mRNAs in nonerythroid cells are not detectably degraded by an endonucleolytic activity, we suggest that the human β-globin mRNA decay products reported here are generated by a specialized decay pathway that may be superimposed on or supercede the general NMD pathway.
Acknowledgments
We thank Jim Malter and Rich Spritz for human β-globin mRNA expression vectors. This work was supported by Public Health Service Research Grants GM 59614 (to L.E.M.) and GM 38277 and GM 55407 (to D.R.S.), and by the Oak Ridge National Laboratory Director's Research and Development Fund 3211-002Q (to A.S.)
Abbreviations
- MEL
mouse erythroleukemia
- PMR1
polysome ribonuclease 1
- NMD
nonsense-mediated mRNA decay
- thal
thalassemic
- TG
transgenic
References
- 1.Tharun S, Parker R. In: mRNA Metabolism and Post-Transcriptional Gene Regulation. Harford J, Morris D R, editors. New York: Wiley; 1997. pp. 181–200. [Google Scholar]
- 2.Schoenberg D R, Chernokalskaya E. In: mRNA Metabolism and Post-Transcriptional Gene Regulation. Harford J, Morris D R, editors. New York: Wiley; 1997. pp. 217–240. [Google Scholar]
- 3.Meinsma D, Scheper W, Holthuizen P E, Van den Brande J L, Sussenbach J S. Nucleic Acids Res. 1992;20:5003–5009. doi: 10.1093/nar/20.19.5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binder R, Horowitz J A, Basilion J P, Koeller D M, Klausner R D, Harford J B. EMBO J. 1994;13:1969–1980. doi: 10.1002/j.1460-2075.1994.tb06466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binder R, Hwang S P, Ratnasabapathy R, Williams D L. J Biol Chem. 1989;264:16910–16918. [PubMed] [Google Scholar]
- 6.Wang Z, Kiledjian M. EMBO J. 2000;19:295–305. doi: 10.1093/emboj/19.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee C H, Leeds P, Ross J. J Biol Chem. 1998;273:25261–25271. doi: 10.1074/jbc.273.39.25261. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham K S, Dodson R E, Nagel M A, Shapiro D J, Schoenberg D R. Proc Natl Acad Sci USA. 2000;97:12498–12502. doi: 10.1073/pnas.220425497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chernokalskaya E, Dompenciel R E, Schoenberg D R. Nucleic Acids Res. 1997;25:735–742. doi: 10.1093/nar/25.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chernokalskaya E, Dubell A N, Cunningham K S, Hanson M N, Dompenciel R E, Schoenberg D R. RNA. 1998;4:1537–1548. doi: 10.1017/s1355838298980451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bandyopadhyay R, Coutts M, Krowczynska A, Brawerman G. Mol Cell Biol. 1990;10:2060–2069. doi: 10.1128/mcb.10.5.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim S, Mullins J J, Chen C M, Gross K W, Maquat L E. EMBO J. 1989;8:2613–2619. doi: 10.1002/j.1460-2075.1989.tb08401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim S K, Maquat L E. EMBO J. 1992;11:3271–3278. doi: 10.1002/j.1460-2075.1992.tb05405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim S K, Sigmund C D, Gross K W, Maquat L E. Mol Cell Biol. 1992;12:1149–1161. doi: 10.1128/mcb.12.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maquat L E. In: Translational Control of Gene Expression. Sonenberg N, Hershey J W B, Mathews M B, Ab G, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 2000. pp. 849–868. [Google Scholar]
- 16.Antoniou M. In: Methods in Molecular Biological Gene Transfer and Expression Protocols. Murray E J, editor. Clifton, NJ: Humana; 1991. pp. 421–434. [Google Scholar]
- 17.Antoniou M, Grosveld F. Genes Dev. 1990;4:1007–1013. doi: 10.1101/gad.4.6.1007. [DOI] [PubMed] [Google Scholar]
- 18.Collis P, Antoniou M, Grosveld F. EMBO J. 1990;9:233–240. doi: 10.1002/j.1460-2075.1990.tb08100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belgrader P, Cheng J, Zhou X, Stephenson L S, Maquat L E. Mol Cell Biol. 1994;14:8219–8228. doi: 10.1128/mcb.14.12.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kollias G, Hurst J, deBoer E, Grosveld F. Nucleic Acids Res. 1987;15:5739–5747. doi: 10.1093/nar/15.14.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajagopalan L E, Malter J S. J Biol Chem. 1996;271:19871–19876. doi: 10.1074/jbc.271.33.19871. [DOI] [PubMed] [Google Scholar]
- 22.Doktycz M J, Larimer F W, Pastrnak M, Stevens A. Proc Natl Acad Sci USA. 1998;95:14614–14621. doi: 10.1073/pnas.95.25.14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoenberg D R, Cunningham K S. Methods. 1999;17:60–73. doi: 10.1006/meth.1998.0708. [DOI] [PubMed] [Google Scholar]
- 24.Dompenciel R E, Garnepudi V R, Schoenberg D R. J Biol Chem. 1995;270:6108–6118. doi: 10.1074/jbc.270.11.6108. [DOI] [PubMed] [Google Scholar]
- 25.Maquat L E, Kinniburgh A J, Rachmilewitz E A, Ross J. Cell. 1981;27:543–553. doi: 10.1016/0092-8674(81)90396-2. [DOI] [PubMed] [Google Scholar]
- 26.Kinniburgh A J, Maquat L E, Schedl T, Rachmilewitz E, Ross J. Nucleic Acids Res. 1982;10:5421–5427. doi: 10.1093/nar/10.18.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanson M N, Schoenberg D R. J Biol Chem. 2001;276:12331–12337. doi: 10.1074/jbc.M010483200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagy E, Maquat L E. Trends Biochem Sci. 1998;23:198–199. doi: 10.1016/s0968-0004(98)01208-0. [DOI] [PubMed] [Google Scholar]
- 29.Romao L, Inacio A, Santos S, Avila M, Faustino P, Pacheco P, Lavinha J. Blood. 2000;96:2895–2901. [PubMed] [Google Scholar]
- 30.Cunningham K S, Hanson M N, Schoenberg D R. Nucleic Acids Res. 2001;29:1156–1162. doi: 10.1093/nar/29.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.González C I, Ruiz-Echevarria M J, Vasudevan S, Henry M F, Peltz S W. Mol Cell. 2000;5:489–499. doi: 10.1016/s1097-2765(00)80443-8. [DOI] [PubMed] [Google Scholar]
- 32.Lejeune F Y, Ishigaki Y, Maquat L E. EMBO J. 2002;21:3536–3545. doi: 10.1093/emboj/cdf345. [DOI] [PMC free article] [PubMed] [Google Scholar]