Abstract
We have investigated the influence of transmembrane holding potential on the kinetics of interaction of a cationic ryanoid, 8β-amino-9α-hydroxyryanodine, with individual ryanodine receptor (RyR) channels and on the functional consequences of this interaction. In agreement with previous studies involving cationic, neutral, and anionic ryanoids, both rates of association and dissociation of the ligand are sensitive to transmembrane potential. A voltage-sensitive equilibrium between high- and low-affinity forms of the receptor underlies alterations in rates of association and dissociation of the ryanoid. The interaction of 8β-amino-9α-hydroxyryanodine with RyR influences the rate of cation translocation through the channel. With this ryanoid bound, the channel fluctuates between two clearly resolved subconductance states (α and β). We interpret this observation as indicating that with 8β-amino-9α-hydroxyryanodine bound, the pore of the RyR channel exists in two essentially isoenergetic conformations with differing ion-handling properties. The equilibrium between the α- and β-states of the RyR-8β-amino-9α-hydroxyryanodine complex is sensitive to transmembrane potential. However, the mechanisms determining this equilibrium differ from those responsible for the voltage-sensitive equilibrium between high- and low-affinity forms of the receptor.
INTRODUCTION
Ryanodine has been an indispensable tool in the investigation of the identity (Inui et al., 1987a,b; Smith et al., 1988; Lai et al., 1988a; Anderson et al., 1989), structure (Samsó and Wagenknecht, 1998; Orlova et al., 1997), and function (Meissner, 1986; Sutko et al., 1997) of one group of intracellular Ca2+-release channels referred to as ryanodine receptors (RyR) (Lai et al., 1988b; Bers, 2001). It has also been instrumental in the identification of the involvement of RyR-mediated Ca2+-release in a variety of cellular processes (Foskett and Wong, 1991; Bazotte et al., 1991; Swann, 1992; Walz et al., 1995; Ullmer et al., 1996; Bazotte et al., 1991) and in establishing the importance of RyR-mediated Ca2+ release from the sarcoplasmic reticulum in muscle excitation-contraction coupling (Marban and Wier, 1985; Beuckelmann and Wier, 1988; DuBell et al., 1993; Bers and Bridge, 1989).
The interaction of ryanodine with individual RyR channels results in altered function characterized by a dramatic increase in open probability and a modification in the rate of ion translocation through the channel (Rousseau et al., 1987; Nagasaki and Fleischer, 1988; Holmberg and Williams, 1989). By monitoring interactions of various analogs of ryanodine (ryanoids) with single RyR channels we have demonstrated that both the probability of interaction of the ryanoid with the channel and the rate of cation translocation after formation of the RyR-ryanoid complex are determined by the structure of the ligand (Tinker et al., 1996; Tanna et al., 1998, 2000, 2003). The structural features of the ryanoid that determine rates of cation translocation in the RyR-ryanoid complex differ from those controlling binding (Welch et al., 1997). Modified rates of ion translocation after the formation of the RyR-ryanoid complex result from changes in a range of features of ion handling and likely reflect alterations in the structure of the channel pore (Lindsay et al., 1994; Tanna et al., 2001; Tu et al., 1994).
Using ryanoids that dissociate readily from the receptor we have described the following basic features of ryanoid interaction with individual, voltage-clamped, RyR channels (Tanna et al., 1998):
The occurrence of a ryanoid-modified state results from the interaction of a single molecule of the ryanoid with the channel.
This binding site is only accessible from the cytosolic face of the channel and when the channel is in an open conformation.
The probability of interaction of a ryanoid is influenced strongly by transmembrane holding potential.
We have investigated the mechanism underlying the influence of holding potential on the probability of ryanoid interaction with RyR by monitoring the kinetics of interaction of neutral, cationic, and anionic ryanoids (Tanna et al., 1998, 2000, 2003). These investigations indicate that the major factor involved in this process is a voltage-driven alteration in the affinity of the RyR-ryanoid binding site.
For the majority of ryanoids so far examined, the interaction of the ligand, or a specific conformation of the ligand, results in the occurrence of a single modified conductance state (Tinker et al., 1996; Tanna et al., 2003). The ryanoid examined in this communication, 8β-amino-9α-hydroxyryanodine, is unusual. Previous investigations have demonstrated that interaction of this ryanoid with individual RyR channels results in an increase in open probability and a reduction in rates of ion translocation with transitions between two clearly resolved subconductance states (Tanna et al., 2002). Here we report that the probability of occurrence of these two states is influenced by transmembrane holding potential and investigate the mechanisms underlying this voltage dependence. These observations provide novel insights into the nature of the ryanoid-modified conductance state of RyR and into the mechanisms underlying ryanoid interaction with RyR.
MATERIALS AND METHODS
Materials
Phosphatidylethanolamine was purchased from Avanti Polar Lipids (Alabaster, AL), and phosphatidylcholine from Sigma-Aldrich (St. Louis, MO). The [3H]ryanodine was supplied by New England Nuclear (NEN Life Science, Boston, MA), and aqueous counting scintillant was obtained from Packard Instrument (Meriden, CT). Other reagents were purchased as the best available grade from VWR International, Dorset, UK, Sigma-Aldrich, or Packard. The 8β-amino-9α-hydroxyryanodine was synthesized as described earlier (Welch et al., 1997) and stored as a stock solution in 50% ethanol at −20°C.
Isolation of sheep cardiac heavy sarcoplasmic reticulum membrane vesicles and solubilization and purification of the ryanodine receptor
Heavy sarcoplasmic reticulum membrane vesicles were prepared using procedures described previously (Sitsapesan and Williams, 1990). Heavy sarcoplasmic reticulum membrane vesicles were solubilized with 3-[(3-cholamidopropyl)-dimethylammonio]-1-propane sulfonate and RyR2 isolated and reconstituted into unilamellar liposomes for incorporation into planar phospholipid bilayers as described previously (Lindsay and Williams, 1991).
Planar phospholipid bilayers
Phospholipid bilayers were formed from suspensions of phosphatidylethanolamine in n-decane and individual RyR2 channels incorporated into the bilayer following previously described methods (Tanna et al., 1998, 2000, 2003). Single channel current fluctuations were monitored with K+ as the charge-carrying species. Channel proteins incorporate into the bilayer in a fixed orientation so that the cytosolic face of the channel is exposed to the solution in the cis chamber and the luminal face of the channel is exposed to the solution in the trans chamber. Experiments were carried out at room temperature (21 ± 2°C). The interaction of 8β-amino-9α-hydroxyryanodine with the channel was studied by adding the indicated concentration of the ryanoid to the solution at the cytosolic face of the channel.
Single channel data acquisition
Single channel current fluctuations were displayed on an oscilloscope and stored on digital audio tape. For analysis, data were replayed, low-pass filtered at 1 kHz with an eight-pole Bessel filter, and digitized at 4 kHz using Satori V3.2 (Intracel, Cambridge, UK). Single channel current amplitudes and lifetimes were measured from digitized data. The representative traces shown in the figures were obtained from digitized data acquired with Satori V3.2 and transferred as an HPGL graphics file to a graphics software package (CorelDraw; Corel Systems, Ottawa, Canada) for annotation and printing.
The interaction of 8β-amino-9α-hydroxyryanodine with single channels
8β-amino-9α-hydroxyryanodine interacts with the high-affinity ryanodine-binding site on the SR Ca2+-release channel and modifies channel function; single channel conductance is reduced with K+ as the charge carrier and channel Po is increased (Tanna et al., 2002). The interaction of 8β-amino-9α-hydroxyryanodine with the channel is readily reversible on the timescale of a single channel experiment and, in the continued presence of the ryanoid, repeated transitions between periods of modified channel function and periods of normal gating and conductance are observed. Previously we have established that the interaction of reversible ryanoids (Tanna et al., 1998, 2000) with the channel and the resulting modification of channel function can be described by a simple bimolecular reaction scheme. As a consequence, apparent rate constants for the association (kon) and dissociation (koff) of 8β-amino-9α-hydroxyryanodine can be determined from the mean dwell times in the unmodified and modified conductance states (Eqs. 1 and 2):
 |
(1) |
and
 |
(2) |
Dwell times and the probability that the channel is in the ryanoid-modified state (Pmod) were determined by using Satori V3.2 as described previously (Tanna et al., 1998). Sections of the data were defined as the unmodified state (periods where the channel displayed transitions between the open and the closed levels) or the modified state (periods in which the channel displayed transitions between the modified and the closed levels). To obtain sufficient events, these parameters were obtained from steady-state recordings lasting at least 6 min.
The influence of transmembrane holding potential on the interaction of 8β-amino-9α-hydroxyryanodine with the RyR channel
If transition between the normal gating state of the RyR and the modified state resulting from the interaction of 8β-amino-9α-hydroxyryanodine is dependent on holding potential, Pmod will be described by the Boltzmann distribution (Eq. 3),
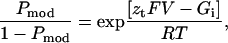 |
(3) |
where F is the Faraday constant, V is the transmembrane voltage, R is the gas constant, T is temperature (°K), zt is the voltage-dependence of the occurrence of the ryanoid-modified state, and Gi/RT is an expression of the equilibrium of the reaction at a holding potential of 0 mV.
For such relationships, the rate constants at a given voltage will be described as
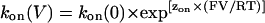 |
(4) |
and
 |
(5) |
where k(V) and k(0) are the rate constants at a particular voltage and at 0 mV, respectively, and z is the valence of the appropriate reaction. Plots of the natural logarithm of kon and koff against holding potential should be linear with slopes zonF/RT and −zoffF/RT and intercepts ln[kon(0)] and ln[koff(0)], respectively. The total voltage-dependence (ztotal) of the reaction is then zon + zoff.
Single channel open probability
Although the dissociation rates of ryanoids from the RyR are independent of channel Po, the rates of association of 8β-amino-9α-hydroxyryanodine (data not shown) and other ryanoids (Tanna et al., 1998, 2000, 2003) are directly proportional to channel Po. To minimize variability in Po, all experiments were carried out in the presence of up to 100 μM cytosolic EMD 41000 (McGarry and Williams, 1994). Since kinetic parameters determined for 8β-amino-9α-hydroxyryanodine are to be compared with equivalent parameters determined in earlier investigations with other ryanoids (Tanna et al., 1998, 2000, 2003), it is important to correct for variations in kon arising from unavoidable differences in Po between populations of channels used in all experiments. The value Po was monitored in sections of data during which no ryanoid was bound and kon values normalized to a Po of 1.0 (Tanna et al., 1998).
RESULTS
The influence of transmembrane holding potential on the interaction of 8β-amino-9α-hydroxyryanodine with the RyR channel
The interaction of the cationic ryanoid 8β-amino-9α-hydroxyryanodine with the high-affinity ryanodine-binding site on the RyR channel, in the presence of K+ as the permeant ion, results in the modification of rates of ion translocation. With the ryanoid bound we observe fluctuations between two distinct subconductance states, defined as α (fractional conductance 0.31) and β (fractional conductance 0.56) (Tanna et al., 2002). The interaction of 8β-amino-9α-hydroxyryanodine with the channel is reversible and consequently it is possible to observe the effect of transmembrane holding potential on the probability of interaction of this ryanoid with the channel. Current fluctuations of a single RyR channel in the presence of 10 μM 8β-amino-9α-hydroxyryanodine at transmembrane holding potentials ranging from ±30 mV are shown in Fig. 1. As transmembrane holding potential is taken to more positive voltages, the time the channel resides in the ryanoid-modified state increases. The relationship between Pmod and holding potential, in the range of −60 to 40 mV for several channels in the presence of 10 μM 8β-amino-9α-hydroxyryanodine, is shown in Fig. 2 A. The solid line is the best-fit Boltzmann distribution (Eq. 3) obtained by nonlinear regression with a value for zt of 2.73 (r = 0.96).
FIGURE 1.
The influence of holding potential on the probability of modification of RyR channel function by 8β-amino-9α-hydroxyryanodine. Traces were obtained from a single, representative, RyR2 channel in symmetrical 610 mM K+ with 10 μM 8β-amino-9α-hydroxyryanodine in the solution at the cytosolic face of the channel. O, open; C, closed; and →, modified states.
FIGURE 2.
(A) The relationship between Pmod by 8β-amino-9α-hydroxyryanodine and holding potential. The value Pmod was determined by monitoring dwell times in the unmodified and modified conductance states in 6-min recordings with 10 μM 8β-amino-9α-hydroxyryanodine in the solution at the cytosolic face of the channel. Each point is the mean ± SE of 4–7 experiments. The solid line is the best-fit Boltzmann distribution obtained by nonlinear regression with the parameters quoted in the text. (B) Variation of association (kon) and dissociation (koff) rates of 8β-amino-9α-hydroxyryanodine with holding potential. Rates were determined from the experiments illustrated in A with 10 μM 8β-amino-9α-hydroxyryanodine in the solution at the cytosolic face of the channel. All values of kon are normalized to a Po of 1.0 (as described in Materials and Methods). Each point is the mean ± SE of 4–7 experiments. The solid lines were obtained by linear regression with the parameters quoted in the text. (C) The relationship of the dissociation constant, KD, calculated from the data in B as [KD = koff (s−1) / kon (μM−1 s−1)] of 8β-amino-9α-hydroxyryanodine with changing holding potential. The line drawn through the points was obtained by linear regression. In all cases, where not visible, error bars are included within the symbol.
Similarly, the rate of association (kon) and dissociation (koff) of 8β-amino-9α-hydroxyryanodine vary with applied holding potential: kon increases whereas koff decreases with a rise in voltage to positive holding potential (Fig. 2 B). The lines of best fit obtained by linear regression for plot of the lnkon and lnkoff against holding potential have slopes of 0.069 ± 0.003 (r = 0.96) and −0.037 ± 0.003 (r = 0.88), yielding zon and zoff values of 1.75 and 0.94, respectively. Together these produce a ztotal of 2.69. Values for kon and koff at 0 mV, obtained from the lines of best fit in Fig. 2 B, are 0.091 μM−1 s−1 and 0.036 s−1. Fig. 2 C shows the relationship of the dissociation constant of 8β-amino-9α-hydroxyryanodine [Kd = koff (s−1)/kon (μM−1 s−1)] to transmembrane holding potential. The extrapolated value of Kd at 0 mV is 0.38 μM.
The substructure within the 8β-amino-9α-hydroxyryanodine-modified state
The two distinct subconductance states observed while 8β-amino-9α-hydroxyryanodine is bound to the RyR channel reflect separate conformations of the conduction pathway with slightly different ion handling properties (Tanna et al., 2002). While assessing the influence of transmembrane voltage on the probability of 8β-amino-9α-hydroxyryanodine modification of RyR function, it became evident that altering transmembrane holding potential dramatically influences the probability of the channel residing in either the α-, or the β-subconductance state. Traces in Fig. 3 A show the occurrence of the α- and β-states after the interaction of 8β-amino-9α-hydroxyryanodine with a single RyR channel, as transmembrane holding potential is altered between ±80 mV. At 80 mV the channel resides predominantly in the β-state; as transmembrane holding potential is made more negative, the probability of occurrence of the α-state is increased. The probabilities of occurrence of α- and β-states (within the 8β-amino-9α-hydroxyryanodine-modified state) were determined at a range of holding potentials. With cursors placed at the α- and β-levels, dwell times in each state were defined by 50% threshold analysis as described previously for Po measurements (Sitsapesan and Williams, 1994), according to
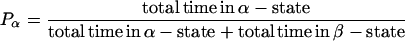 |
(6) |
and
 |
(7) |
The relationship between Pβ and transmembrane holding potential is shown in Fig. 3 B. The solid line is the best-fit Boltzmann distribution obtained by nonlinear regression,
 |
(8) |
where F is the Faraday constant, V is the transmembrane voltage, z is the voltage dependence of the occurrence of the β-state, and Gi/RT is an expression of the equilibrium of the reaction at the holding potential of 0 mV. The value of zβ derived from the slope of this line is 1.0.
FIGURE 3.
(A) Representative traces of 8β-amino-9α-hydroxyryanodine-modified subconductance states from a single channel with 10 μM cytosolic 8β-amino-9α-hydroxyryanodine at holding potentials ranging from ±80 mV. (B) The relationship between the probability of occurrence of the β-subconductance state and holding potential. Each point is the mean ± SE of 4–23 experiments. The solid line is the best-fit Boltzmann distribution obtained by nonlinear regression with the parameters quoted in the text. (C) Variation in the transition rates between α- and β-, and β- and α-subconductance states of the 8β-amino-9α-hydroxyryanodine-modified state with transmembrane holding potential. Each point is the mean ± SE of 4–23 experiments. The solid lines were obtained by linear regression with parameters quoted in the text. In both plots, where not visible, error bars are included within the symbol.
In previous investigations we have demonstrated that a ryanoid-modified state occurs as the result of an interaction of a single molecule of ryanoid (Tanna et al., 1998), or a single conformation of a ryanoid molecule (Tanna et al., 2001, 2003, 2003), with the high-affinity ryanoid binding site on the RyR channel. Our observations with 8β-amino-9α-hydroxyryanodine indicate that with this ryanoid bound, the RyR channel can fluctuate between two conformations with slightly different properties of ion handling (Tanna et al., 2002). Further, the probability of occurrence of these two states is dependent upon transmembrane holding potential.
The simplest mechanism to explain this behavior would involve a voltage-dependent equilibrium between α- and β-conformations of the RyR-ryanoid complex. In such a scheme the dwell times in both α- and β-states would be described by single exponential distributions. Dwell times in the α- and β-subconductance states of the RyR-8β-amino-9α-hydroxyryanodine complex were monitored for all modified states in 90 six-min runs at holding potentials within the range ±80 mV and the minimum number of exponential components required to fit the distributions determined by maximum likelihood fitting (data not shown). In 65% the distributions of dwell times in both α- and β-states of the RyR-8β-amino-9α-hydroxyryanodine complex are best described by single exponentials. This observation favors strongly a simple kinetic scheme in which variations within the 8β-amino-9α-hydroxyryanodine-modified state arise from a voltage-dependent equilibrium between a single α- and a single β-subconductance state.
The apparent rate constants for the transitions from the α- to the β-subconductance state (kαβ) and from the β- to the α-subconductance state (kβα) of the 8β-amino-9α-hydroxyryanodine-modified state can be determined from the mean dwell times obtained from lifetime analysis of the α- and β-subconductance states, as shown below:
 |
(9) |
and
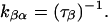 |
(10) |
The rate constants at a given voltage can be described as
 |
(11) |
and
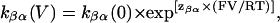 |
(12) |
where k(V) and k(0) are the rate constants at a particular voltage and at 0 mV, respectively, and z is the valence of the appropriate reaction. The value z may then be determined as the slope of the plot of the natural logarithm of the rate constant against transmembrane holding potential (zαβF/RT and −zβαF/RT) and k(0) may be determined from the intercept (ln[kαβ(0)] and ln[kβα(0)]). The total voltage dependence of the reaction is then given by zαβ + zβα. A plot of this form is shown in Fig. 3 C. The values kαβ and kβα both vary with applied transmembrane holding potential. kαβ increases, as transmembrane holding potential is made more positive, whereas the opposite is true for kβα. The solid lines drawn through the points in Fig. 3 C were obtained by linear regression and the values of zαβ and zβα obtained from the slopes of these lines are 0.19 and 0.28, respectively, giving a total valence of 0.47.
The forgoing analyses establish that the interaction of 8β-amino-9α-hydroxyryanodine with RyR2 induces a change in function that is characterized by fluctuations between two clearly defined conductance states while the ryanoid is bound. Further, the probability of occurrence of the α- and β-states is determined by transmembrane holding potential, with both rate constants (kαβ and kβα) clearly sensitive to changes in holding potential. However, a detailed inspection of the data reveals some minor quantitative anomalies.
In a two-state model Pβ would be expected to approach 1.0 at high positive potentials; in reality, Pβ saturates at a value of 0.69 (Fig. 3 B). Secondly, not all distributions of the dwell times in α- and β-subconductance states are best described by single exponentials. Finally, as is apparent from Fig. 3 C, the rate constant for the transition from the β- to the α-subconductance state, which should vary linearly with changing potential, clearly deviates from linearity at high positive transmembrane holding potentials. These inconsistencies suggest the likelihood of another voltage-dependent effect occurring when the RyR channel is held at positive voltages. Inspection of transitions between α- and β-subconductance states reveals the mechanism underlying the observed anomalies. The phenomenon arises because of closing events that occur while the ryanoid is bound to the channel, as shown in Fig. 4.
FIGURE 4.
Representative traces showing closings of a single channel from the 8β-amino-9α-hydroxyryanodine-modified subconductance state at holding potentials of +60 and −60 mV. C, closed; α, α-subconductance state; and β, β-subconductance state. The 50% threshold set between α- and β-subconductance states is indicated by the solid line.
Closings from the β-state cross the 50% threshold set between the α- and β-levels. These closings will alter measured dwell times in the β-state. They will also produce an overestimate of Pα and an underestimate of Pβ. Closings from the α-state do not introduce this anomaly (Fig. 4).
Therefore, the degree of the error is dependent on two factors—first, the probability of occurrence of the β-subconductance state; and second, the probability of the occurrence of closing events. As the probability of occurrence of the β-state rises as holding potential is taken to increasingly positive potentials, the erroneous shortening of β-state durations is most likely to occur at these holding potentials. The effect will be exacerbated by closings within the modified state and these will occur with increased frequency in channels with low Po (Tanna et al., 1998). Together these phenomena explain the deviation in the rate constant of transitions from the β- to the α-state observed at high positive potentials in Fig. 3 C. The data in this figure indicate no obvious reciprocal deviation in rates of transitions from the α- to β-states at high positive potentials. Closing events from the β-state will increase the number of α-state events; however, if dwell times in these false α-states have a similar distribution to those of the true α-state events, the effect on kαβ will be minimal. This phenomenon, together with the fragmentation of β-states in channels with low Po, is likely to explain the occurrence of some α- and β-lifetime histograms described by more than one exponential.
Although closings from the β-conductance state of the RyR-8β-amino-9α-hydroxyryanodine complex will introduce small quantitative anomalies at high positive holding potentials, the data presented in Fig. 3 establish clearly the mechanism underlying the variation in probability of occurrence of the α- and β-subconductance states with changing transmembrane holding potential.
Is there a preferred route of entry to and exit from the 8β-amino-9α-hydroxyryanodine-modified state?
In an attempt to gain more information on the mechanisms governing the formation of the 8β-amino-9α-hydroxyryanodine-modified states and the transition between these states, we have monitored entry into and exit from the modified state seen after the interaction of this ryanoid with RyR. Representative events are shown in Fig. 5. Fig. 5 A displays traces of both the association and dissociation of the ryanoid to and from the channel at 60 mV, whereas Fig. 5 B displays traces of both the association and dissociation of 8β-amino-9α-hydroxyryanodine to and from the channel at −60 mV. Of a total of 265 transitions inspected, at holding potentials within the range ± 80 mV, 93% of the association events involved transitions from the open state to the α-subconductance state, and 96% of the dissociation events involved transitions from the α-subconductance state to the open state.
FIGURE 5.
Traces showing the association and dissociation of 8β-amino-9α-hydroxyryanodine with and from the RyR channel at 60 mV (A) and −60 mV (B). In each case the lower panel shows association and dissociation events of the ryanoid (indicated by the shaded bars in the upper panels) on an expanded timescale. C, closed; O, open; α, α-subconductance state; and β, β-subconductance state.
DISCUSSION
The influence of transmembrane holding potential on the probability of interaction of 8β-amino-9α-hydroxyryanodine with a RyR channel
The measurement of rates of ryanoid association with and dissociation from individual RyR channels has revealed novel features of the mechanisms underlying ryanoid interaction. Of particular interest is the observation that rates of both ryanoid association and dissociation are influenced by transmembrane holding potential. This phenomenon was first observed with 21-amino-9α-hydroxyryanodine, a cationic ryanoid with a formal charge of +1 (Tanna et al., 1998) and has subsequently been observed with ryanoids with no formal charge (ryanodol) (Tanna et al., 2000) and a formal charge of −1 (10-O-succinoylryanodol) (Tanna et al., 2003). These investigations have demonstrated that the formal charge of the ryanoid has little influence on the modulation of ryanoid interaction with RyR by transmembrane holding potential. Irrespective of the charge of the ryanoid, rates of association increase and rates of dissociation decrease as holding potentials are taken from negative to positive values.
The influence of holding potential on rates of association and dissociation of the cationic ryanoid used in this investigation is entirely consistent with our earlier observations, and adds weight to the proposal that the variations in the probability of ryanoid interaction with RyR seen with changing transmembrane holding potential results predominantly from a voltage-driven alteration in the affinity of the receptor site on the channel protein.
The total voltage dependence of the interaction of a ryanoid with no formal charge (ryanodol) is 1.51 (Tanna et al., 2000). In other words, the formation of the high-affinity conformation of RyR involves the movement, within the receptor, of the equivalent of a formal charge of 1.51 across the full voltage drop across the channel. The equivalent parameter determined here for 8β-amino-9α-hydroxyryanodine is 2.69. Therefore, although it is clear that the major component in the action of transmembrane voltage on the interaction of ryanoids with RyR is a change in receptor affinity, differences in voltage dependence of neutral and charged ryanoids such as 8β-amino-9α-hydroxyryanodine could indicate the contribution of an additional voltage-dependent component in the binding of these ligands. Possible sources for this additional voltage dependence will be discussed in a subsequent section of this discussion.
The modified state resulting from the interaction of 8β-amino-9α-hydroxyryanodine with RyR involves transitions between two conductance states
The interaction of ryanodine with an individual RyR channel results in the occurrence of a characteristic reduced conductance state with very high open probability. Modifications in the rate of ion translocation after the interaction of ryanodine with RyR result from a complex series of alterations in the ion-handling features of the channel, most likely arising from conformational changes in the pore (Lindsay et al., 1994; Tu et al., 1994).
Altering the structure of ryanodine produces ryanoids that interact with the high-affinity ryanodine binding site on RyR and produce qualitatively similar alterations in channel function. However, the rate of ion translocation through the RyR-ryanoid complex is dependent upon the structure (Tinker et al., 1996; Welch et al., 1997, 1997) or conformation (Tanna et al., 2001, 2003) of the ligand and is determined by both electrostatic and steric features at defined loci on the molecule (Welch et al., 1997). The demonstration that altered rates of ion translocation result from conformational changes in the pore after ryanodine binding (Lindsay et al., 1994) makes it logical to propose that the different modified conductance states seen on the formation of different RyR-ryanoid complexes reflect different pore conformations determined by the structure or conformation of the specific ryanoid. In support of this proposal, recent experiments indicate that the location of the site of interaction of TEA+ within the voltage drop across the pore is altered by ryanoid interaction, and that the degree of site relocation is broadly inversely proportional to the rate of ion translocation in the RyR-ryanoid complex (Tanna et al., 2001).
Altered pore structure, and hence altered rates of ion translocation, after the interaction of a ryanoid with RyR, could arise from one of two likely mechanisms—induced fit or prior isomerization. For induced fit the binding energy made available by the interaction of a ryanoid with RyR would produce an alteration in pore structure, with the final conformation of the pore being determined by the structure or conformation of the ryanoid. For prior isomerization, the ligand would bind and stabilize a conformation of the receptor that has an extremely low probability of occurrence in the absence of ryanoid. In such a scheme, different ryanoids would bind to and stabilize only one of a manifold of conformers. In either mechanism the interaction of a specific ryanoid conformer would induce or stabilize a particular pore structure.
The data reported here for 8β-amino-9α-hydroxyryanodine indicate that it is possible for the structure of the pore to change while the ryanoid is bound to the receptor. At first sight it might appear that the observation of two modified conductance states after the formation of the RyR-8β-amino-9α-hydroxyryanodine complex is inconsistent with the induction or stabilization of distinct conformations of the channel pore. However, these mechanisms could account for the occurrence of two conductance states after the formation of the RyR-8β-amino-9α-hydroxyryanodine complex if the underlying pore conformations are of a similar energy and, as a consequence, had similar probabilities of occurrence with 8β-amino-9α-hydroxyryanodine bound. The variation in probability of occurrence of the α- and β-states with changing voltage would then reflect alterations in the relative energy of the two conformations of the pore, fuelled by the effects of the electric field on the RyR-8β-amino-9α-hydroxyryanodine complex.
The relationships between these different states under various experimental conditions are summarized in Fig. 6 A. In this scheme, C represents the closed state of the channel, O and O* are unmodified open states with low and high affinity for ryanoid, and α and β are isomers of the ryanoid-modified state. The equilibriums between O and O* and α and β are sensitive to transmembrane potential. In the absence of ryanoid, α- and β-states are not occupied.
FIGURE 6.
(A) A cartoon representing relationships between states of the RyR2 channel with changing holding potential and in the absence and the presence of 8β-amino-9α-hydroxyryanodine. The energy levels are not to scale. See text for details. (B) A minimal ordered mechanism accounting for the interaction of 8β-amino-9α-hydroxyryanodine with an individual RyR2 channel under voltage-clamp conditions. O and O* are unmodified open states with low and high (indicted by the asterisk) affinity for ryanoid, and α and β are isomers of the ryanoid-modified state. The equilibriums between O and O* and α and β are sensitive to transmembrane potential.
State I represents RyR at −60 mV in the absence of ryanoid and at a low open probability. State II represents RyR at −60 mV after the addition of a ligand such as EMD41000 that elevates open probability (Po > 0.9) but in the absence of ryanoid (Pmod = 0). State III represents RyR at a holding potential of −20 mV at a high Po and in the presence of 8β-amino-9α-hydroxyryanodine (Pmod = 0.5, Pβ = 0.3). State IV represents RyR at high Po in the presence of 8β-amino-9α-hydroxyryanodine at a holding potential of +60 mV (Pmod = 1.0, Pβ = 0.75).
It is of interest to note that behavior similar to that reported here for the RyR-8β-amino-9α-hydroxyryanodine complex has been monitored in another species of intracellular Ca2+-release channel in the absence of modifying ligands. Rapid, voltage-dependent transitions between subconductance states have been observed in individual type-1 and type-3 inositol trisphosphate receptor (InsP3R) channels in Xenopus oocyte nuclei (Mak and Foskett, 1997; Mak et al., 2000). In agreement with the mechanisms proposed here to account for the transitions between α- and β-conductance states in the RyR-8β-amino-9α-hydroxyryanodine complex, Mak and Foskett suggested that these states represent partial open conformations of the InsP3R and that transitions between the states involved a relatively low activation energy barrier. These observations support the proposal that conformations of channel pores, giving rise to modified conductance properties, can exist in the absence of modifying ligands such as ryanoids and that, by extension, such conformations might be stabilized by the interaction of a ryanoid.
The mechanism of interaction of 8β-amino-9α-hydroxyryanodine with RyR
In the preceding section of this discussion we considered the energy relationships between some of the forms of RyR in the absence and presence of 8β-amino-9α-hydroxyryanodine; however, these considerations do not address the mechanisms involved in the interaction of this ryanoid with its receptor on the channel. Any mechanism must account for several experimental observations:
The open probability of the channel in the absence of ryanoid is insensitive to changes in applied potential.
The ryanoid binding site is only available when the channel is open.
The affinity of the receptor for ryanoid is modulated by applied potential.
The ryanoid-modified channel undergoes a potential-sensitive isomerization.
Ryanoid modification generally leads to the α-modified conductance state irrespective of holding potential.
Dissociation of the ryanoid is generally from the α-modified conductance state.
The sensitivities to potential of the transitions between the low- and high-affinity open states of the channel and the isomerization of the ryanoid-modified channel are different.
High positive transmembrane holding potentials favor the occurrence of both the high-affinity state of the open RyR channel and the β-conductance state of the RyR-8β-amino-9α-hydroxyryanodine complex. However, these processes involve different mechanisms; the increased affinity of the receptor for the ryanoid involves the movement of charge within the receptor molecule. The sensitivity of the equilibrium between the two conductance states in the RyR-8β-amino-9α-hydroxyryanodine complex to holding potential requires the ryanoid to be bound. If positive holding potentials favored the probability of occurrence of the β-state before ryanoid interaction, increasingly positive holding potentials would decrease the probability of occurrence of the α-state and, contrary to experimental observation, the probability of ryanoid interaction would be decreased as holding potential was varied from negative to positive values.
A stochastic simulator was used to evaluate mechanisms in which the binding of ryanoid is either a random or ordered process. In line with our experimental observations we have limited these mechanisms to include only interactions of ryanoid with open conformations of RyR. Several random mechanisms were examined; however, they were not consistent with our finding that interaction of ryanoid with RyR leads predominantly to the α-modified conductance state. As a consequence we propose the ordered mechanism shown in Fig. 6 B as a minimal scheme to account for the complex interactions of 8β-amino-9α-hydroxyryanodine with RyR at varying holding potentials. The values used for the kinetic simulations were taken from the data in this article. The rate constants for the opening and closing of the channel are 400 s−1 and 40 s−1, respectively, to give a Po = 0.95. The various rate constants were taken from Eqs. 4, 5, 11, and 12. The values for z are those reported here. These equations will break down at some point as other processes become kinetically important (or rate-limiting, e.g., diffusion). Estimation of the values for ligand binding and release are complicated, since one is estimating both the intrinsic rate of ligand binding and the amount of O* available to bind ryanoid. The intrinsic rate of dissociation was estimated from the data at the highest positive potential (+40 mV) giving values of kon = 1 × 106 and koff = 7 × 10−3. (Note this gives a kinetic dissociation constant equal to 15 nM, close to that of ryanodine. This suggests that the difference in binding of ryanoids does not arise from differences in intrinsic affinity, but because the ryanoids bind preferentially to different forms of the RyR.) These values of rate constants are considered voltage-independent. The voltage sensitivity of Pmod is due entirely to the voltage-induced changes in the population of O and O*. The values of ko (Fig. 3 A) for the interconversion of O and O* were arbitrarily chosen at 1000 s−1 and (Fig. 3 B) for the interconversion of α and β are estimated from Fig. 3 C to be 400 s−1.
Although this mechanism can be criticized, since it predicts that Po will be sensitive to potential, kinetic simulations demonstrate that a 100-fold change in the equilibrium constant of the O, O* transition produces a change of only 0.1 in Po. This effect decreases as basal Po is increased and so, under the conditions used in the experiments reported here, effects on Po are unlikely to be detected. The kinetic simulation predicts rapid fluctuations between open and closed states and long periods in the ryanoid-modified state with rapid fluctuations between α- and β-states during the modified periods. The effect of potential on both Pmod and Pβ values of the model follow the relationship seen in our single channel recordings. We have also simulated this ordered scheme using a thermodynamic model and found it to be consistent with our experimental observations.
The demonstration that the equilibrium between α- and β-states is a property of the RyR-ryanoid complex suggests a possible mechanism whereby the addition of the cationic ryanoid, such as 8β-amino-9α-hydroxyryanodine, to the binding site within the channel creates a voltage-sensing complex that fluctuates between slightly different pore conformations in response to changes in holding potential. Such a mechanism would require that the site of interaction of ryanoids with RyR be within the region of the channel molecule over which transmembrane voltage falls and this is most likely to be within the pore of the channel. A location for the high-affinity ryanoid binding site within the pore of RyR is supported by the recent observations that mutations within structural elements of RyR2 that are likely to contribute to the formation of the pore disrupt the binding of [3H]-ryanodine (Chen et al., 2002) and increase the rate of dissociation of ryanodine from individual channels under voltage-clamp conditions (Wang et al., 2003). Responses of a cationic voltage-sensing complex to changes in the electric field within the voltage drop across RyR could contribute to the higher overall voltage-dependence of 8β-amino-9α-hydroxyryanodine-induced channel modification when compared with a neutral ryanoid such as ryanodol.
What factors determine the rate of dissociation of a ryanoid from the RyR-ryanoid complex?
One of the most significant observations to emerge from our investigations of the interaction of derivatives and congeners of ryanodine with individual RyR channels is that rates of ryanoid dissociation from the RyR-ryanoid complex can vary enormously. This observation led to the crude classification of ryanoids as either irreversible (e.g., ryanodine) or reversible (e.g., 8β-amino-9α-hydroxyryanodine) modifiers of channel function (Tinker et al., 1996).
In addition to the voltage-dependent alteration in the affinity of the receptor, rates of ryanoid dissociation will be determined by the nature of the RyR-ryanoid complex. Observations with 8β-amino-9α-hydroxyryanodine presented here indicate that the conformation of the receptor plays a major role in determining koff of the ligand from the complex. With this ryanoid bound, the receptor can exist in two conformations, manifest as different conductance states, and rates of dissociation of the ligand from the two conformations are profoundly different. The probability of dissociation of this ryanoid from the β-conformation is at best 0.04. If, hypothetically, the interaction of 8β-amino-9α-hydroxyryanodine stabilized only the β-conformation of RyR, it is probable that this ryanoid would be classified as irreversible. Dissociation of the ryanoid from RyR requires a shift in conformation to the α-state.
An inspection of the interaction of several reversible and irreversible ryanoids reveals a possible common mechanism underlying reversibility. In our experience, reversible ryanoids stabilize more than one conformation of RyR. In some cases different modified conductance states can be clearly resolved, as in the case of 8β-amino-9α-hydroxyryanodine (Figs. 1, 3, 5, and 6); in others, different states are poorly resolved and the modified state appears noisy when compared with the fully open and closed states of the channel. Examples of this are shown in Fig. 7. The vast majority of modified conductance states observed after the interaction of 21-amino-9α-hydroxyryanodine with RyR are noisy; variation in current amplitude around the mean of the modified state is significantly greater than that of either the closed or open states of the channel (Tanna et al., 2002). The source of this excess noise becomes apparent when, infrequently, modified conductance events are observed in which the channel resides predominantly in one conductance state, equivalent to one extreme of the noise, with occasional transitions to another state equivalent to the other extreme of the noise. An example of this behavior is shown in Fig. 7 A. It is noticeable that dwell times of 21-amino-9α-hydroxyryanodine in states of this form are considerably shorter than those in the more frequently observed noisy state. This could indicate that, as is the case with 8β-amino-9α-hydroxyryanodine, the conformation giving rise to the predominant state in events such as those shown in Fig. 7 A would have a high ryanoid dissociation rate.
FIGURE 7.
Different levels of noise of modified conductance states after the interaction of ryanoids with individual RyR2 channels (see text for details).
Fig. 7 B shows an example of a noisy modified conductance state induced by ryanodol. Although the noise of this modified state is clearly significantly greater than that of the open or closed states of the channel we have not observed well-resolved transitions to another identifiable conductance state. With both 21-amino-9α-hydroxyryanodine and ryanodol bound, rates of dissociation would be determined by both the voltage-dependent affinity of the receptor and the probability of occurrence of the less stable RyR-ryanoid conformation which, based on our findings with 8β-amino-9α-hydroxyryanodine, is itself likely to be determined by transmembrane potential.
In contrast, ryanoids classified as irreversible in single channel experiments appear to stabilize a single conformation of RyR. Examples of this class of ryanoids include ryanodine (Fig. 7 C), 21-azido-9α-hydroxyryanodine (Fig. 7 D), and 21-p-nitrobenzoylamino-9α-hydroxyryanodine (Fig. 7 E). However, it is important to appreciate that we cannot exclude the possibility that the interactions of all ryanoids with RyR stabilize more than one conformation of the channel with different rates of ryanoid dissociation. If this were the case the scheme presented in Fig. 6 B for 8β-amino-9α-hydroxyryanodine would be applicable to all ryanoids, and differing rates of dissociation of the ligands could reflect differences in the voltage-dependence of the probability of occurrence of different conformations of the RyR-ryanoid complex.
Acknowledgments
We are very grateful to Dr. Kevin Foskett for discussions on multiple level subconductance states in InsP3R.
This work was supported by funds from the British Heart Foundation; National Science Foundation (MCB 9817605); the Molecular Modeling/Graphics Core Facility, University of Nevada, Reno; and the National Institutes of Health (HL077976).
References
- Anderson, K., F. A. Lai, Q.-Y. Liu, E. Rousseau, H. P. Erickson, and G. Meissner. 1989. Structural and functional characterization of the purified cardiac ryanodine receptor-Ca2+ release channel complex. J. Biol. Chem. 264:1329–1335. [PubMed] [Google Scholar]
- Bazotte, R. B., B. Pereira, S. Higham, V. Shoshan-Barmatz, and N. Kraus Friedmann. 1991. Effects of ryanodine on calcium sequestration in the rat liver. Biochem. Pharmacol. 42:1799–1803. [DOI] [PubMed] [Google Scholar]
- Bers, D. M. 2001. Excitation-Contraction Coupling and Cardiac Contractile Force. Kluwer, Dordrecht, The Netherlands.
- Bers, D. M., and J. H. B. Bridge. 1989. Relaxation of rabbit ventricular muscle by Na-Ca exchange and sarcoplasmic reticulum Ca-pump: ryanodine and voltage sensitivity. Circ. Res. 65:334–342. [DOI] [PubMed] [Google Scholar]
- Beuckelmann, D. J., and W. G. Wier. 1988. Mechanism of release of calcium from sarcoplasmic reticulum of guinea-pig cardiac cells. J. Physiol. 405:233–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S. R. W., P. Li, M. C. Zhao, X. L. Li, and L. Zhang. 2002. Role of the proposed pore-forming segment of the Ca2+ release channel (ryanodine receptor) in ryanodine interaction. Biophys. J. 82:2436–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBell, W. H., B. Lewartowski, H. A. Spurgeon, H. S. Silverman, and E. G. Lakatta. 1993. Repletion of sarcoplasmic reticulum Ca after ryanodine in rat ventricular myocytes. Am. J. Physiol. 265:H604–H615. [DOI] [PubMed] [Google Scholar]
- Foskett, J. K., and D. Wong. 1991. Free cytoplasmic Ca2+ concentration oscillations in thapsigargin-treated parotid acinar cells are caffeine- and ryanodine-sensitive. J. Biol. Chem. 266:14535–14538. [PubMed] [Google Scholar]
- Holmberg, S. R. M., and A. J. Williams. 1989. Single channel recordings from human cardiac sarcoplasmic reticulum. Circ. Res. 65:1445–1449. [DOI] [PubMed] [Google Scholar]
- Inui, M., A. Saito, and S. Fleischer. 1987a. Isolation of the ryanodine receptor from cardiac sarcoplasmic reticulum and identity with the feet structures. J. Biol. Chem. 262:15637–15642. [PubMed] [Google Scholar]
- Inui, M., A. Saito, and S. Fleischer. 1987b. Purification of the ryanodine receptor and identity with feet structures of junctional terminal cisternae of sarcoplasmic reticulum from fast skeletal muscle. J. Biol. Chem. 262:1740–1747. [PubMed] [Google Scholar]
- Lai, F. A., H. P. Erickson, E. Rousseau, Q.-Y. Liu, and G. Meissner. 1988a. Purification and reconstitution of the Ca release channel from skeletal muscle. Nature. 331:315–319. [DOI] [PubMed] [Google Scholar]
- Lai, F. A., H. P. Erickson, E. Rousseau, Q.-Y. Liu, and G. Meissner. 1988b. Evidence for a Ca2+ channel within the ryanodine receptor complex from cardiac sarcoplasmic reticulum. Biochem. Biophys. Res. Commun. 151:441–449. [DOI] [PubMed] [Google Scholar]
- Lindsay, A. R. G., A. Tinker, and A. J. Williams. 1994. How does ryanodine modify ion-handling in the sheep cardiac sarcoplasmic reticulum Ca2+-release channel? J. Gen. Physiol. 104:425–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay, A. R. G., and A. J. Williams. 1991. Functional characterisation of the ryanodine receptor purified from sheep cardiac muscle sarcoplasmic reticulum. Biochim. Biophys. Acta. 1064:89–102. [DOI] [PubMed] [Google Scholar]
- Mak, D.-O. D., and J. K. Foskett. 1997. Single-channel kinetics, inactivation, and spatial distribution of inositol trisphosphate (IP3) receptors in Xenopus oocyte nucleus. J. Gen. Physiol. 109:571–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak, D. O. D., S. McBride, V. Raghuram, Y. Yue, S. K. Joseph, and J. K. Foskett. 2000. Single-channel properties in endoplasmic reticulum membrane of recombinant type 3 inositol trisphosphate receptor. J. Gen. Physiol. 115:241–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marban, E., and W. G. Wier. 1985. Ryanodine as a tool to determine the contributions of calcium entry and calcium release to the calcium transient and contraction of cardiac Purkinje fibers. Circ. Res. 56:133–138. [DOI] [PubMed] [Google Scholar]
- McGarry, S. J., and A. J. Williams. 1994. Activation of the sheep cardiac sarcoplasmic reticulum Ca2+-release channel by analogues of sulmazole. Br. J. Pharmacol. 111:1212–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner, G. 1986. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J. Biol. Chem. 261:6300–6306. [PubMed] [Google Scholar]
- Nagasaki, K., and S. Fleischer. 1988. Ryanodine sensitivity of the calcium release channel of sarcoplasmic reticulum. Cell Calcium. 9:1–7. [DOI] [PubMed] [Google Scholar]
- Orlova, E. V., I. I. Serysheva, M. Van Heel, S. L. Hamilton, and W. Chiu. 1997. Two structural configurations of the skeletal muscle calcium release channel. Nature Struct. Biol. 3:547–551. [DOI] [PubMed] [Google Scholar]
- Rousseau, E., J. S. Smith, and G. Meissner. 1987. Ryanodine modifies conductance and gating behavior of single Ca2+ release channel. Am. J. Physiol. 253:C364–C368. [DOI] [PubMed] [Google Scholar]
- Samsó, M., and T. Wagenknecht. 1998. Contributions of electron microscopy and single-particle techniques to the determination of the ryanodine receptor three-dimensional structure. J. Struct. Biol. 121:172–180. [DOI] [PubMed] [Google Scholar]
- Sitsapesan, R., and A. J. Williams. 1994. Gating of the native and purified cardiac SR Ca2+-release channel with monovalent cations as permeant species. Biophys. J. 67:1484–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitsapesan, R., and A. J. Williams. 1990. Mechanisms of caffeine activation of single calcium-release channels of sheep cardiac sarcoplasmic reticulum. J. Physiol. 423:425–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, J. S., T. Imagawa, J. J. Ma, M. Fill, K. P. Campbell, and R. Coronado. 1988. Purified ryanodine receptor from rabbit skeletal muscle is the calcium-release channel of sarcoplasmic reticulum. J. Gen. Physiol. 92:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutko, J. L., J. A. Airey, W. Welch, and L. Ruest. 1997. The pharmacology of ryanodine and related compounds. Pharmacol. Rev. 49:53–98. [PubMed] [Google Scholar]
- Swann, K. 1992. Different triggers for calcium oscillations in mouse eggs involve a ryanodine-sensitive calcium store. Biochem. J. 287:79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanna, B., W. Welch, L. Ruest, J. L. Sutko, and A. J. Williams. 1998. Interactions of a reversible ryanoid (21-amino-9α-hydroxy-ryanodine) with single cardiac ryanodine receptor channels. J. Gen. Physiol 112:55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanna, B., W. Welch, L. Ruest, J. L. Sutko, and A. J. Williams. 2000. The interaction of a neutral ryanoid with the ryanodine receptor channel provides insights into the mechanisms by which ryanoid binding is modulated by voltage. J. Gen. Physiol. 116:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanna, B., W. Welch, L. Ruest, J. L. Sutko, and A. J. Williams. 2001. Ryanoid modification of the cardiac muscle ryanodine receptor channel results in relocation of the tetraethylammonium binding site. J. Gen. Physiol. 117:385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanna, B., W. Welch, L. Ruest, J. L. Sutko, and A. J. Williams. 2002. Excess noise in modified conductance states following the interaction of ryanoids with cardiac ryanodine receptor channels. FEBS Lett. 516:35–39. [DOI] [PubMed] [Google Scholar]
- Tanna, B., W. Welch, L. Ruest, J. L. Sutko, and A. J. Williams. 2003. An anionic ryanoid, 10-O-succinoylryanodol, provides insights into the mechanisms governing the interaction of ryanoids and the subsequent altered function of ryanodine-receptor channels. J. Gen. Physiol. 121:551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinker, A., J. L. Sutko, L. Ruest, P. Deslongchamps, W. Welch, J. A. Airey, K. Gerzon, K. R. Bidasee, H. R. Besch, Jr., and A. J. Williams. 1996. Electrophysiological effects of ryanodine derivatives on the sheep cardiac sarcoplasmic reticulum calcium-release channel. Biophys. J. 70:2110–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu, Q., P. Vélez, M. S. Brodwick, and M. Fill. 1994. Streaming potentials reveal a short ryanodine-sensitive selectivity filter in cardiac Ca2+ release channel. Biophys. J. 67:2280–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullmer, C., H. G. W. M. Boddeke, K. Schmuck, and H. Lübbert. 1996. 5-HT2B receptor-mediated calcium release from ryanodine-sensitive intracellular stores in human pulmonary artery endothelial cells. Br. J. Pharmacol. 117:1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz, B., O. Baumann, B. Zimmermann, and E. V. Ciriacy-Wantrup. 1995. Caffeine- and ryanodine-sensitive Ca2+-induced Ca2+ release from the endoplasmic reticulum in honeybee photoreceptors. J. Gen. Physiol. 105:537–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R., L. Zhang, J. Bolstad, N. Diao, C. Brown, L. Ruest, W. Welch, A. J. Williams, and S. R. W. Chen. 2003. Residue Gln4863 within a predicted transmembrane sequence of the Ca2+ release channel (ryanodine receptor) is critical for ryanodine interaction. J. Biol. Chem. 278:51557–51565. [DOI] [PubMed] [Google Scholar]
- Welch, W., A. J. Williams, A. Tinker, K. E. Mitchell, P. Deslongchamps, J. Lamothe, K. Gerzon, K. R. Bidasee, H. R. Besch, Jr., J. A. Airey, J. L. Sutko, and L. Ruest. 1997. Structural components of ryanodine responsible for modulation of sarcoplasmic reticulum calcium channel function. Biochemistry. 36:2939–2950. [DOI] [PubMed] [Google Scholar]









