Abstract
In this study, we have examined how the headgroup size and properties affect the membrane properties of sphingomyelin and interactions with cholesterol. We prepared N-palmitoyl ceramide phosphoethanolamine (PCPE) and compared its membrane behavior with D-erythro-N-palmitoyl-sphingomyelin (PSM), both in monolayers and bilayers. The pure PCPE monolayer did not show a phase transition at 22°C (in contrast to PSM), but displayed a much higher inverse isothermal compressibility as compared to the PSM monolayer, indicating stronger intermolecular interactions between PCPEs than between PSMs. At 37°C the PCPE monolayer was more expanded (than at 22°C) and displayed a rather poorly defined phase transition. When cholesterol was comixed into the monolayer, a condensing effect of cholesterol on the lateral packing of the lipids in the monolayer could be observed. The phase transition from an ordered to a disordered state in bilayer membranes was determined by diphenylhexatriene steady-state anisotropy. Whereas the PSM bilayer became disordered at 41°C, the PCPE bilayer main transition occurred around 64°C. The diphenylhexatriene steady-state anisotropy values were similar in both PCPE and PSM bilayers before and after the phase transition, suggesting that the order in the hydrophobic core in both bilayer types was rather similar. The emission from Laurdan was blue shifted in PCPE bilayers in the gel phase when compared to the emission spectra from PSM bilayers, and the blue-shifted component in PCPE bilayers was retained also after the phase transition, suggesting that Laurdan molecules sensed a more hydrophobic environment at the PCPE interface compared to the PSM interface both below and above the bilayer melting temperature. Whereas PSM was able to form sterol-enriched domains in dominantly fluid bilayers (as determined from cholestatrienol dequenching experiments), PCPE failed to form such domains, suggesting that the size and/or properties of the headgroup was important for stabilizing sphingolipid/sterol interaction. In conclusion, our study has highlighted how the headgroup in sphingomyelin affect its membrane properties and interactions with cholesterol.
INDRODUCTION
Sphingomyelin is the major sphingolipid class present in the external leaflet of cellular plasma membranes. Interest in sphingomyelin (SM) has been raised in part from the findings that there appears to be a preferential interaction between sphingomyelin and cholesterol in both cell and model membranes (Slotte, 1999), and in part because this interaction is central for the formation of cholesterol and SM rich domains or rafts in membranes (Brown and London, 1997; Simons and Ikonen, 1997). The rafts are believed to play an important role in protein and lipid transport and sorting and in several signaling cascades (Brown and London, 2000; Dobrowsky, 2000; Fielding and Fielding, 2000; Simons and Toomre, 2000; Ikonen, 2001; Maier et al., 2001).
Naturally occurring SMs have the phosphocholine headgroup linked to the hydroxyl group on carbon 1 of a long-chain base. The most common long-chain base in cultured cells (e.g., human skin fibroblast and baby hamster kidney cells) is sphingosine (1,3-dihydroxy-2-amino-4-octadecene), followed by sphinganine (1,3-dihydroxy-2-amino-octadecane), both with the D-erythro-(2S,3R) configuration (Sarmiento et al., 1985; Ramstedt et al., 1999). Long and highly saturated acyl chains are linked to the amine group on carbon 2 of the long-chain base. The effect of various structural features of SMs on the interaction between sphingomyelin and cholesterol has been investigated during the years by our group. SM contains both hydrogen bond donating and accepting groups, which can contribute to inter- and intramolecular interactions. It has been shown that the 3-OH group of SM does not strongly affect the interaction between cholesterol and sphingomyelin in model membranes, since removal of the 3-OH group did not alter the cholesterol oxidase susceptibility of cholesterol in membranes (Grönberg et al., 1991; Kan et al.,1991). The presence of the NH group at C2 of SM, on the other hand, has been shown to be essential for the interaction of SM with cholesterol (Bittman et al., 1994). Recently it was suggested that in SM bilayers the 3-OH group forms mainly intramolecular hydrogen bonds with the phosphate ester oxygen, whereas the NH group also could act as a hydrogen donor in intermolecular hydrogen bonding between SM molecules (Talbott et al., 2000; Mombelli et al., 2003; Niemela et al., 2004). Clearly hydrogen bonding is important in the interfacial region of SM membranes, but its significance for cholesterol/SM association remains to be established.
The interaction between cholesterol and SM is enhanced by van der Waals interactions between cholesterol and the long saturated acyl chains commonly found in sphingomyelins (McIntosh et al., 1992). A cis unsaturation in the N-linked acyl chain of SM is known to weaken the interaction between cholesterol and SM (Ramstedt and Slotte, 1999). It was very recently demonstrated that N-oleoyl-sphingomyelin failed to form sterol-rich domains in bilayer membranes, apparently because of the high solubility of the unsaturated sphingomyelin in the bulk phosphatidylcholine phase (Epand and Epand, 2004). The sphingosine long-chain base has a trans double bond between carbons 4 and 5, and the removal of this trans double bond (yielding dihydrosphingomyelins (DHSM)) was shown to affect the lateral packing of the DHSM and to lead to more favorable interactions with cholesterol compared to SM which have a trans double bond (Kuikka et al., 2001; Nyholm et al., 2003).
The interaction of cholesterol with phospholipids is also known to be affected by the molecular structure of the polar headgroup. It was shown by scanning calorimetry that cholesterol interacts better with phosphatidylcholine than with phosphatidylethanolamine in fluid bilayers (van Dijck et al., 1976; van Dijck, 1979). The preferential interaction of cholesterol with phosphatidylcholine over phosphatidylethanolamine in bilayers was later confirmed by determination of the equilibrium distribution of cholesterol between vesicles of different lipid composition (Yeagle and Young, 1986; Niu and Litman, 2002). The maximum solubility of cholesterol in bilayers is higher for phosphatidylcholine membranes than for bilayers comprising phosphatidylethanolamine. The cholesterol solubility limit has been measured to 50 mol % (Collins and Phillips, 1982) or 66–67 mol % (Huang et al., 1999) in phosphatidylcholine bilayers, depending of the history of the sample, its preparation method, and the way cholesterol solubility was determined (for a review, see Bach and Wachtel, 2003). In phosphatidylethanolamine bilayers, the corresponding cholesterol solubility limit has been observed at 35–45 mol % (Cheetham et al., 1989) and 50 mol % (Huang et al., 1999; for a review, see Bach and Wachtel, 2003). This difference in solubility of cholesterol in phosphatidylcholine and phosphatidylethanolamine bilayers, respectively, was recently explained to result from the difference in headgroup size and hence capacity to shield the cholesterol molecule from exposure to water at the interface (Huang and Feigenson, 1999).
In this study, we were interested to examine how removal of the three methyl groups from the SM phosphocholine headgroup would affect the membrane properties of the molecule, and how interactions with cholesterol would be affected. Ceramide phosphoethanolamine is a naturally occurring lipid, although its concentration in membranes is very small and its biological role unknown (Broad and Dawson, 1973; Malgat et al., 1986; Maurice and Malgat, 1990; Olsen and Jantzen, 2001). We synthesized N-palmitoyl ceramide phosphoethanolamine (PCPE) from D-erythro-N-palmitoyl-sphingomyelin (PSM), and compared the biophysical properties of PCPE with the properties of PSM.
MATERIALS AND METHODS
Material
PSM was purified from egg yolk sphingomyelin (Avanti Polar Lipids, Alabaster, AL) by reverse-phase HPLC (Supelco Discovery C18-column, dimensions 250 × 21.2 mm, 5 μm particle size, with 100% methanol as the mobile phase, flow 9 ml/min). The identity of the product was verified by mass spectroscopy. Pure PSM was used for the synthesis of PCPE. 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), 1,2-dimyristoyl- sn-glycero-3-phosphocholine (DMPC), and 1-palmitoyl-2-stearoyl-(12-doxyl)-sn-glycero-3-phosphocholine (12SLPC) were obtained from Avanti Polar Lipids. Calcium chloride dihydrate was obtained from Merck (Darmstadt, Germany) and ethanolamine from J. T.Baker (Deventer, The Netherlands). 1,2-Dimyristoyl-sn-glycero-3-phosphoethanolamine (DMPE), phospholipase D from Streptomyces chromofuscus, cholesterol, and β-cyclodextrin (βCyD) were obtained from Sigma Chemicals (St.Louis, MO). βCyD solutions were prepared in pure water to a concentration of 40 mM. A stock solution of PCPE was prepared in chloroform/methanol (3:1, v/v), stock solutions of other lipids in hexane/2-propanol (3:2, v/v). All stock solutions were stored in the dark at −20°C, and warmed to ambient temperature before use.
1,6-Diphenyl-1,3,5-hexatriene (DPH) and 6-dodecanoyl-2-dimethylaminonaphthalene (Laurdan) were obtained from Molecular Probes (Leiden, The Netherlands). Cholesta-5,7,9(11)-trien-3-beta-ol (cholestatrienol, CTL) was synthesized from 7-dehydrocholesterol using the method published by Fischer et al. (1984). The identity of CTL was verified by GC-MS and the dry product was stored at −87°C until solubilized in ethanol. The purity of CTL was checked by reverse-phase HPLC on an RP-18 column (250 × 4 mm column dimensions, 5 μm particle size, methanol:acetonitrile 30:70 by volume as the mobile phase) before use. Stock solutions of fluorescent probes were stored at −20°C and used within a week.
Millipore UF Plus produced water (resistivity 18.2 MΩcm) was used. The solvents used for synthesis were stored over Molecular Sieves 4A (Merck).
Synthesis of palmitoyl ceramide phosphoethanolamine
PCPE was synthesized from PSM using phospholipase D from Streptomyces chromofuscus, which is a Ca2+-dependent enzyme known to be able catalyze both hydrolysis and transphosphatidylation of phospholipids (El Kirat et al., 2003). In brief, 14.2 μmol of PSM was solubilized in 6.25 ml toluene. After that 2.4 mmol ethanolamine, 60 μmol CaCl2, and 110 U phospholipase D, each solubilized in Tris buffer pH 8, were added to the reaction mixture in this order. The total volume ratio of solvents in the reaction mixture was toluene:Tris buffer (pH 8) 82:18 by volume. The reaction was carried out at 30°C for 26 h and the formation of the product was followed by TLC developed with chloroform:methanol:water (25:10:1.1 by volume) according to Skipski et al. (1964). PCPE was purified by high performance liquid chromatography on a LiChrospher Si60 column (5 μm particle size, 250 × 4 mm column dimensions) using chloroform:methanol:water (50:45:5 by volume) at a flow of 1 ml/min at room temperature. The identity and purity of the product was verified by mass spectroscopy in ESI negative ion mode.
Force-area isotherms
To characterize the interfacial properties of PCPE we used the surface barostat technique. Monolayers of pure PCPE or a binary mixture containing 25, 50, 75 mol % cholesterol in PCPE were compressed on water either at 22°C or 37°C using a KSV surface barostat (KSV Instruments, Helsinki, Finland). Data were collected using proprietary KSV software.
Inverse isothermal compressibility coefficient measurements
To characterize viscosity properties of the phospholipid monolayers the inverse isothermal compressibility coefficient (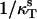 ) was determined as a function of the surface pressure using a computer-controlled KSV surface barostat. The phospholipids used were PSM, DMPC, DMPE, and PCPE. Pure monolayers were prepared at the air/water interface at 22°C and compressed at a barrier speed of 10 mm/min to a given target surface pressure. The range of the surface pressures studied varied between 3–30 mN/m and the pressure was increased in 3 mN/m increments. To obtain the inverse isothermal compressibility values the barrier was programmed to oscillate for 2 min with an area change set to 0.5% and the frequency at 40 mHz.
) was determined as a function of the surface pressure using a computer-controlled KSV surface barostat. The phospholipids used were PSM, DMPC, DMPE, and PCPE. Pure monolayers were prepared at the air/water interface at 22°C and compressed at a barrier speed of 10 mm/min to a given target surface pressure. The range of the surface pressures studied varied between 3–30 mN/m and the pressure was increased in 3 mN/m increments. To obtain the inverse isothermal compressibility values the barrier was programmed to oscillate for 2 min with an area change set to 0.5% and the frequency at 40 mHz.
The inverse isothermal compressibility coefficient was calculated from
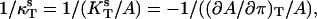 |
where 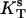 is the surface compressibility at a constant temperature T, A is area, and π is a surface pressure (Rosenholm et al., 2003).
is the surface compressibility at a constant temperature T, A is area, and π is a surface pressure (Rosenholm et al., 2003).
Cholesterol desorption kinetics
To study cholesterol affinity to PCPE in comparison with PSM and DMPE, we determined cholesterol desorption rates from mixed phospholipid/cholesterol monolayers to βCyD in the subphase. Mixed monolayers containing either one of the phospholipid and 50 mol % cholesterol were prepared at the air/water interface at 22°C. The trough used was of zero-order type, with a reaction chamber (23.9 ml volume, 28.3 cm2 area) separated by a glass bridge from a lipid reservoir. The monolayers were compressed to 20 mN/m with a KSV surface barostat. After a stable monolayer had been formed, βCyD (in a volume not exceeding 1 ml) was injected into the stirred reaction chamber without penetrating the monolayer. The final concentration of βCyD in the subphase was 1.7 mM. The removal of cholesterol from the monolayer to the subphase was determined from the area decrease of the monolayer at constant surface pressure, as described by Ohvo and Slotte (1996).
Steady-state fluorescence anisotropy
To compare packing properties of PCPE and PSM bilayer membrane in both pure and cholesterol containing vesicles, the steady-state anisotropy of DPH as a function of temperature was determined. The bilayer vesicles were prepared from pure PSM or PCPE, or from a mixture of 33 mol % cholesterol and 66 mol % PSM or PCPE, and 1 mol % DPH as a reporter molecule. The lipids mixtures were dried under nitrogen, dispersed in Dulbecco's phosphate-buffered saline, and heated above the transition temperature of the particular lipid. The warm samples were vortexed briefly and sonicated for 2 min (20% duty cycle, power output 15 W) using a Branson probe sonifier W-250 (Branson Ultrasonics, Danbury, CT). The samples containing the fluorescent probe were protected from light during all steps. Excitation was carried out at 355 nm and the emission was recorded at 425 nm as the temperature was scanned from 5°C to 80°C at a speed of 5°C/min using a PTI QuantaMaster 1 spectrofluorimeter (Photon Technology International, Lawrenceville, NJ). The steady-state anisotropy, r, was determined as described in Lakowicz (1999).
Laurdan emission spectra
To obtain further information about interfacial properties of PCPE bilayer we measured the emission spectra of Laurdan in both pure and cholesterol-containing vesicles and compared those with the spectra obtained in PSM bilayers. The bilayer vesicles containing pure PSM or PCPE, or a mixture of 33 mol % cholesterol and 66 mol % PSM or PCPE, and 1 mol % Laurdan as a reporter molecule were prepared by probe sonication as described above. Laurdan emission spectra were recorded at 5°C below and above the gel-liquid phase transition of PSM (36°C and 46°C, respectively) and PCPE (59°C and 69°C, respectively) using a PTI QuantaMaster 1 spectrofluorimeter. The emission spectra were collected between 390 and 550 nm, whereas the samples were excited at 365 nm. All spectra were recorded as the average of at least three scans.
To quantified Laurdan emission spectra changes in PCPE bilayer we calculated excitation generalized polarization (GPex) and plotted it against temperature. Excitation generalized polarization is defined as (Parasassi et al., 1990):
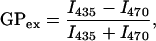 |
where I435 and I470 are the emission intensities at the characteristic wavelength of the gel phase (435 nm) and the liquid crystalline phase (470 nm), respectively. The bilayer vesicles containing pure PCPE or PSM and 1 mol % Laurdan were prepared by probe sonication as described above. Laurdan emission spectra were recorded stepwise by 5°C between 35°C and 85°C in PCPE vesicles and between 10°C and 65°C in PSM vesicles using a PTI QuantaMaster 1 spectrofluorimeter. The emission spectra were collected between 390 and 550 nm, whereas the sample was excited at 365 nm.
Quenching of steady-state fluorescence
The quenching of steady-state cholestatrienol fluorescence by 12SLPC was measured on a PTI QuantaMaster 1 spectrofluorimeter essentially following the procedure described by Ahmed et al. (1997). Briefly, vesicles with a total lipid concentration of 50 μM with 1 mol % CTL were used. Fluorescence emission intensity was measured in sample F (quenched) consisting of POPC: 12SLPC: phospholipid: chol: CTL (35:30:30:4:1, molar ratio), and compared to Fo samples (nonquenched), in which 12SLPC was replaced by POPC. The bilayer vesicles were prepared as described above. The solvent water was saturated with argon before being used to minimize the risk of oxidation and the samples containing fluorescent probe were protected from light during all steps. The phospholipids used were PSM, PCPE, or POPC and the fluorescence emission intensity of CTL was measured with excitation and emission wavelengths of 324 nm and 374 nm, respectively as a function of temperature.
RESULTS
Force/area isotherms
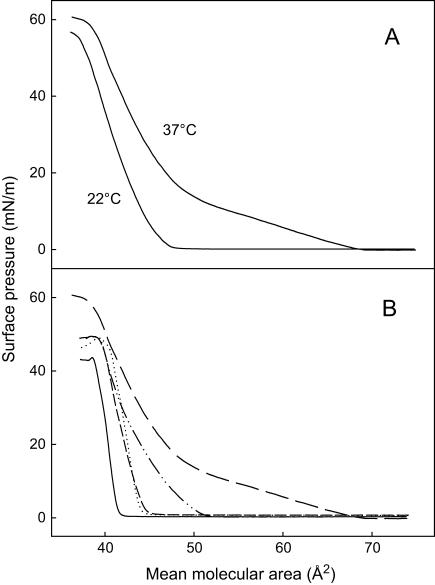
To characterize the interfacial properties of PCPE, pure or mixed monolayers of PCPE and cholesterol were prepared at the water/air interface and studied by the surface barostat technique. The surface pressure versus mean molecular area isotherms of PCPE at 22°C and 37°C are shown in Fig. 1 A. At 22°C the PCPE monolayer was mainly in a condensed state, since the isotherm did not reveal a clear phase transition. The collapse pressure of a pure PCPE monolayer was ∼55mN/m and the mean molecular area at collapse was slightly below 40 Å2/molecule. The isotherm of PCPE at 37°C revealed an expanded-to-condensed phase transition (starting at 15 mN/m; Fig. 1 A). Interestingly, the collapse pressure of PCPE was slightly higher at 37°C than 22°C, as was the collapse area. The influence of increasing amount of cholesterol in the binary mixture on PCPE monolayer properties at 37°C is shown in Fig. 1 B. Cholesterol had a considerable condensing effect on the PCPE monolayer film. Based on these results, it was clear that cholesterol was to some extent miscible with PCPE and affected its lateral packing properties.
FIGURE 1.
Force-area isoterms of binary mixtures of PCPE and cholesterol. (A) The surface pressure versus mean molecular area isotherms for PCPE at 22°C and 37°C. (B) The surface pressure versus mean molecular area isotherms for mixtures containing 0 (long-dashed line), 25 (dash-dot-dash line), 50 (short-dashed line), 75 (dotted line), or 100 (solid line) mol % cholesterol in PCPE at 37°C. The monolayers were compressed on water at a speed not exceeding 10 Å2/molecule/min.
Inverse isothermal compressibility coefficient
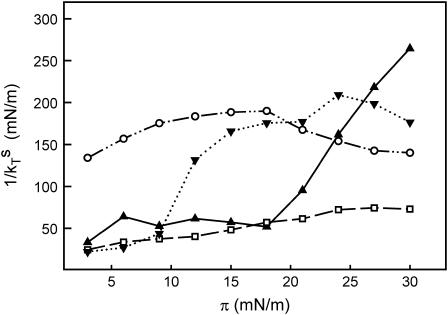
Since the pure PCPE monolayer appeared to be very viscous, we decided to determine the inverse isothermal compressibility coefficient as a function of surface pressure. This quantity is known to depend on the state of the film and to increase as a function of the degree of condensation and thus be related to monolayer viscosity (Davies and Rideal, 1961). Typical values of the inverse isothermal compressibility coefficient for surfactant film in the liquid-expanded state are 10–50 mN/m, in the liquid condensed state 100–250 mN/m, and in the solid-condensed state 1000–2000 mN/m (Davies and Rideal, 1961). In Fig. 2, we plotted the inverse isothermal compressibility coefficient of PSM, PCPE, DMPC, and DMPE monolayers against the surface pressure. The transition from a liquid-expanded to a liquid-condensed phase was clearly detectable for DMPE monolayers at ∼9 mN/m and for PSM monolayers at ∼18 mN/m, which is in good agreement with force/area isoterms of these lipids (Weidemann and Vollhardt, 1995; Ramstedt and Slotte, 1999). The inverse isothermal compressibility of DMPC monolayers increased slightly with increasing surface pressure, reaching an inverse isothermal compressibility of 74 mN/m at a monolayer surface pressure of 30 mN/m. The inverse isothermal compressibility for PCPE monolayers was markedly higher than the values observed with the other lipids, especially at low monolayer surface pressures. This observation confirmed the high viscosity observed in pure PCPE monolayers.
FIGURE 2.
Inverse isothermal compressibility coefficient of phospholipid monolayers plotted against the surface pressure. Monolayers of PSM (—▴—), DMPC (–□–), DMPE (·⋯▾⋯·), and PCPE (—··○··—)were compressed on water at 22°C at a speed of 10 mm/min to a given target pressure. The barrier was programmed to oscillate for 2 min with an area change set to 0.5% and the frequency at 40 mHz. The curves are representative curves of two separate experiments.
Cholesterol desorption from mixed monolayers
One way to measure the interaction between cholesterol and a colipid in a membrane is to determinate the rate of cholesterol desorption from mixed monolayers to βCyD in the subphase (Ohvo and Slotte, 1996). Therefore we prepared mixed monolayers containing 50 mol % cholesterol together with either PSM, DMPE, or PCPE and determined the rate of cholesterol desorption to βCyD in the subphase (1.7 mM) at constant surface pressure (20 mN/m and 22°C). As the results presented in Table 1 show, the observed desorption rates were higher from PCPE mixed monolayers as compared to DMPE mixed monolayers, indicating that cholesterol had a looser interaction with PCPE than DMPE. The strongest interaction was seen with PSM, as already known from many similar studies (Ramstedt and Slotte, 1999; Kuikka et al., 2001). Consequently, the removal of three methyl groups from PSM weakened interactions with cholesterol, or led to a partial lateral segregation of the two lipids. Cholesterol desorption is very fast from the pure cholesterol monolayers, as shown in Table 1.
TABLE 1.
Cholesterol desorption from mixed cholesterol/phospholipid monolayers to βCyD in the subphase
| Monolayer phospholipid | Desorption rate (pmol cm−2 min−1) |
|---|---|
| Pure cholesterol | 39.2 ± 0.62 |
| PSM | 0.88 ± 0.16 |
| DMPE | 5.4 ± 0.32 |
| PCPE | 7.6 ± 0.49 |
Mixed monolayers containing 50 mol % of cholesterol were prepared at air/water interface at 22°C. The monolayers were compressed to 20 mN/m and maintained at a constant surface pressure. Cholesterol desorption to βCyD (1.7 mM) in the subphase was determined as a time function. The values are given as the average ± SD from at least three different monolayer experiments.
Steady-state anisotropy of DPH in vesicles
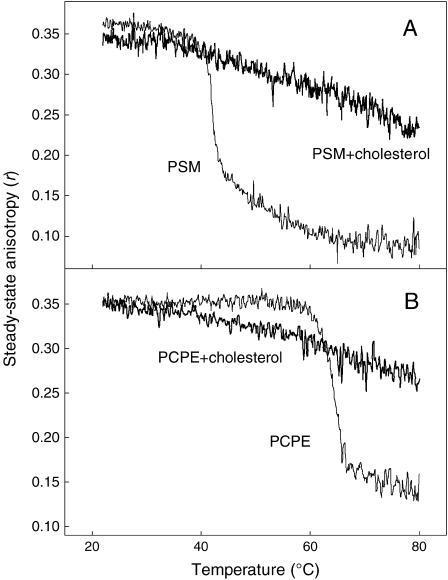
The anisotropy of DPH was measured as a function of temperature to obtain information about packing properties in the hydrophobic core of PCPE bilayer membrane in both the presence and absence of cholesterol. DPH is a fluorescent hydrophobic molecule that orients itself along the acyl chains of the phospholipids in the bilayer (Shinitzky and Barenholz, 1974), and consequently is sensitive to packing properties in the hydrophobic core of the bilayer membrane (Shinitzky and Barenholz, 1978). In Fig. 3 A, the steady-state anisotropy is plotted against temperature for pure PSM and 33 mol % cholesterol in PSM bilayer membrane. The gel to liquid disordered phase transition of pure PSM was clearly reported as a steep decrease in the anisotropy of DPH. The midtemperature of this transition was at 41°C for the PSM bilayer, which is in good agreement with previously published steady-state anisotropy and differential scanning calorimetry data (Kuikka et al., 2001). For a PCPE bilayer (Fig. 3 B), a corresponding transition was observed around 64°C. The extent of DPH anisotropy change going from the ordered to the disordered phase was rather similar for both lipids as well as the order in bilayer hydrophobic core in gel and liquid disordered phase. Inclusion of 33 mol % cholesterol removed the phase transition in both PCPE and PSM membrane.
FIGURE 3.
Steady-state anisotropy (rss) of DPH in bilayer membranes. The bilayers were prepared from pure PSM and from a mixture 33 mol % cholesterol in PSM (panel A), or from pure PCPE and from a mixture 33 mol % cholesterol in PCPE bilayers (panel B). The anisotropy of DPH is drawn as a function of temperature.
Emission spectra of Laurdan in vesicles
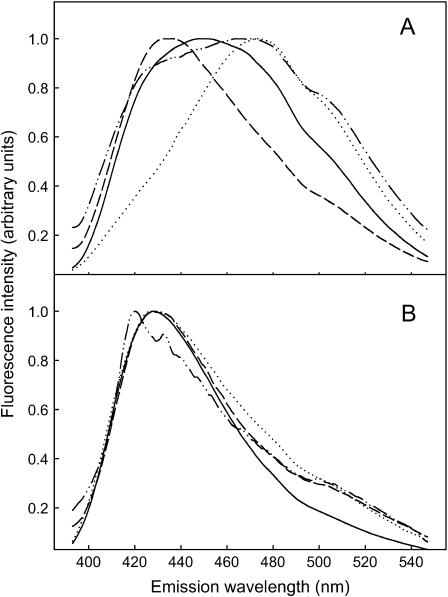
The interfacial properties of the bilayer membrane were studied using Laurdan as an interfacial environment-sensitive probe. The emission spectra of Laurdan were measured in both pure and cholesterol-containing vesicles at 5°C below and above the phase transition temperature of the lipid as determined by steady-state anisotropy. In vesicles containing pure phospholipids (Fig. 4 A), Laurdan at Tm −5°C had a more blue-shifted emission in PCPE vesicles as compared to PSM vesicles. Above the transition (at Tm + 5°C) the emission spectra was more red-shifted in both membrane types, although PCPE retained much more of the blue-shifted component compared to PSM. These results suggest that Laurdan experienced a more hydrophobic environment in the PCPE bilayer at both temperatures compared to the situation with Laurdan in PSM bilayers. The addition of cholesterol to both PCPE and PSM bilayers (at 33 mol %) more or less abolished the difference which Laurdan experienced in the pure phospholipid bilayers (Fig. 4 B). Both below and above the Tm of the mixed phospholipid bilayer, the Laurdan emission spectra were blue shifted with a very small red-shifted component.
FIGURE 4.
Emission spectra of Laurdan in bilayer membranes. The emission spectra of Laurdan in pure PSM and PCPE bilayers are shown in panel A both 5°C below and above the Tm. Panel B shows Laurdan emission in PSM and PCPE membranes containing 33 mol % cholesterol both 5°C below and above the Tm. All emission spectra in the graph were background subtracted, corrected, and normalized. The solid line is PSM below the transition, and the dotted line is PSM above the transition. The dashed line is PCPE below the transition, and the dash-dot-dash line is PCPE above the transition.
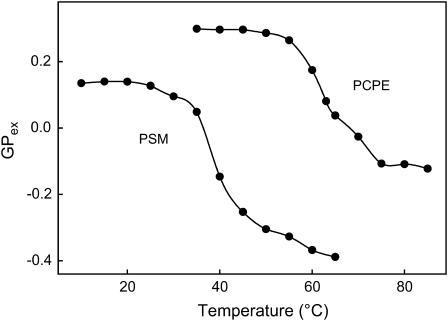
To obtain further information about how the phase transition of PSM and PCPE bilayers affected the emission spectra of Laurdan, we plotted the excitation GP value as a temperature function (Fig. 5). The transition temperature as a decrease of GPex value was clearly detected with the midtemperature at ∼62°C for PCPE bilayer and ∼39°C for PSM bilayer, the latter in agreement with previously reported data (Bagatolli et al., 1998, 1999). The difference between transition temperature of PCPE bilayer as determined by DPH (64°C) and by Laurdan (62°C) originates from different position of probes in membrane bilayer. Whereas DPH senses different packing properties in the hydrophobic core of membrane bilayer, Laurdan is sensitive to polarity in interfacial region of membrane bilayer. The GPex value was higher for PCPE compared with PSM both below and above transition temperature indicating a more condensed and less hydrated interface of PCPE bilayer membrane.
FIGURE 5.
GPex values of Laurdan in PCPE and PSM bilayers as a function of temperature. Laurdan emission spectra in PCPE vesicles were recorded stepwise by 5°C between 35°C and 85°C, in PSM vesicles between 10°C and 65°C. Vesicles contained 1 mol % Laurdan, and the GPex value was calculated as described above.
No shift of the maximum emission wavelength of Laurdan was observed in vesicles containing 33 mol % cholesterol in PCPE for all temperatures used (stepwise by 5°C between 35°C and 85°C, data not shown). This confirmed our result from steady-state anisotropy measurements, that inclusion of 33 mol % cholesterol in PCPE removes the gel to liquid crystalline phase transition of PCPE.
Formation of the sterol-rich domains
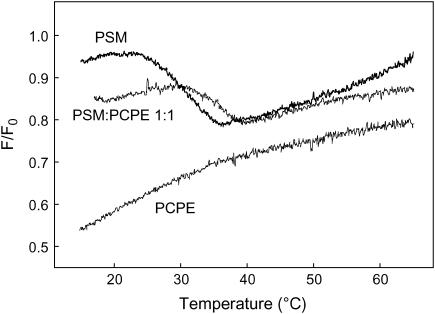
CTL is a fluorescent probe known to have very similar orientation in membrane as natural cholesterol and more importantly to be able to induce a comparable condensation of phospholipids membranes (Scheidt et al., 2003). The formation of sterol-rich domains in mixed lipid bilayers was studied by determination of the fraction of CTL emission that was quenchable by 12SLPC (a quencher located outside Lo). Fluorescence emission intensity was measured in sample F (quenched) consisting of POPC: 12SLPC: phospholipid: chol: CTL (35:30:30:4:1, molar ratio), and compared to Fo samples (nonquenched), in which 12SLPC was replaced by POPC. To follow the melting of possible sterol-rich domains in membrane bilayers, the ratio F/Fo was plotted as a function of temperature (Fig. 6). The melting of the sterol-rich domains formed by PSM (30 mol %) was clearly detected as a decrease of F/Fo ratio. In contrast no melting of sterol-enriched domains was detected in membranes containing PCPE (30 mol %). In bilayers containing both PSM and PCPE (15 mol % each) the magnitude of the decrease in F/Fo was about half of that seen in PSM containing bilayers, suggesting that PCPE did not contribute to solubilizing sterol in the sphingolipid-enriched domains. However, the presence of PCPE in the sphingolipid-enriched domains led to a slight stabilization of these against a temperature-induced melting, as shown by the shift of the melting curve to the right.
FIGURE 6.
Quenching of CTL emission by 12SLPC in phospholipids bilayers as a function of temperature. Sample F (quenched) consisted of POPC/12SLPC/phospholipids/cholesterol/CTL (35:30:30:4:1 molar ratio); 12SLPC was replaced by POPC in sample Fo (unquenched). The phospholipids used were PSM, PCPE, or PSM/PCPE 1:1 molar ratio. The temperature was increased by 5°C/min and the F/Fo ratio was calculated.
DISCUSSION
In this work, we have examined how the removal of the three methyl groups from the SM phosphocholine headgroup influenced membrane properties of the resulting lipid. It was of particular interest to examine whether the extended hydrogen-bonding properties of this ceramide-based lipid could compensate for any of the changes in molecular properties induced by the loss of the three choline methyl groups.
When PCPE was spread to a pure monolayer at the air/water interface at ambient temperature, its isotherm (Fig. 1 A) was more condensed than the corresponding PSM isotherm (Ramstedt and Slotte, 1999). Although the PSM isotherm shows a liquid expanded to condensed phase transition at 22°C (Ramstedt and Slotte, 1999), with the PCPE monolayer it was necessary to increase the temperature to 37°C to induce a phase transition. The smaller cross-sectional area of the phosphoethanolamine headgroup in PCPE allowed closer contact between the molecules in the hydrophobic region of the membrane and therefore lead to more tight packing of the acyl chains (Yu and Hui, 1992). The effect of the extended hydrogen-bonding properties of PCPE was seen in the monolayers as an increased viscosity. The measured isothermal inverse compressibility coefficient was significantly higher in PCPE monolayers than in corresponding PSM monolayer, especially in the low surface pressure range (between 2.5 and 15 mN/m; Fig. 2). A similarly high isothermal inverse compressibility coefficient was not measured in a DMPE monolayer, suggesting that hydrogen-bonding involving the free NH2-group of the ethanolamine did not explain the high isothermal inverse compressibility coefficient in PCPE monolayer. Rather, it is likely that intermolecular cohesion in PCPE monolayers was extensively stabilized by hydrogen bonding in the interfacial region, possibly involving the free OH on carbon 3, and the NH-linked acyl chain at carbon 2 of the sphingosine backbone of PCPE (Ramstedt and Slotte, 2002).
Addition of cholesterol to the PCPE monolayer at 37°C caused a condensation of the lateral packing of PCPE, a phenomenon well documented for other expanded phospholipid monolayers (Smaby et al., 1994). The cholesterol-induced condensation of PCPE suggests that cholesterol was able to interact with the PCPE in the monolayers and hence was at least partially miscible with PCPE (Dorfler, 1990). To quantify the relative strength of interaction between cholesterol and PCPE we measured rates of cholesterol desorption from mixed monolayers to βCyD in the subphase (Ohvo and Slotte, 1996). Whereas cholesterol desorption from a pure cholesterol monolayer is fast (39.2 pmol cm−2 min−1; Table 1), interaction between cholesterol and colipids in the monolayer reduce this desorption in proportion to the interaction between cholesterol and the colipid (Ohvo-Rekilä et al., 2002). Desorption from sphingomyelin-containing membranes is documented to be slow, and in this study we measured the rate to be ∼0.9 pmol cm−2 min−1 for equimolar PSM/cholesterol monolayers (Table 1). This rate was similar to the rate determined before for PSM (Ramstedt and Slotte, 1999). Desorption from equimolar PCPE mixed monolayers was much faster (7.6 pmol cm−2 min−1) than the rate determined for the PSM monolayer, suggesting that the removal of the three methyl groups from the choline moiety destabilized the sphingolipid/cholesterol interaction. Such a destabilizing effect of the headgroup on cholesterol interaction was also reported for monounsaturated phosphatidylcholines and phosphatidylethanolamines (the desorption rate being 16.9 and 23.2 pmol cm−2 min−1 for POPC and POPE, receptively; Ohvo-Rekilä et al., 2002). It is further known that removal of the three methyl groups from phosphatidylcholines reduce cholesterol solubility in bilayer membranes (Collins and Phillips, 1982; Cheetham et al., 1989; Huang et al., 1999; Bach and Wachtel, 2003). It is thus clear that the headgroup size will affect both cholesterol solubility and cholesterol/colipid interaction in membranes. It also appears that hydrogen-bonding possibilities from the ceramide back-bone to cholesterol did not provide additional stabilization to cholesterol/PCPE interaction, since the rate of cholesterol desorption increased so dramatically by removal of the three methyl groups from the choline moiety (Table 1).
To further characterize the properties of PCPE in bilayer membranes, we examined the bilayer melting temperature, the interfacial hydration, and domain-forming properties of PCPE (with cholesterol) and compared these with PSM. Due to both stronger headgroup interactions and more close packing, the gel to liquid crystalline transition temperatures of saturated phosphatidylethanolamine bilayers are ∼20–30°C higher than the transition temperatures of phosphatidylcholine bilayers with the same acyl chain composition (van Dijck et al., 1976). The melting of PCPE bilayers, as determined from changes in the steady-state anisotropy of DPH, occurred at a 23°C higher temperature compared to the melting of PSM bilayers, which correlated rather closely with the trend reported for phosphatidylcholine/phosphatidylethanolamine pairs. The observed transition temperature was ∼64°C for PCPE bilayers and 41°C for PSM bilayers, the latter temperature corresponding well with the Tm obtained by differential scanning calorimetry (Kuikka et al., 2001). The removal of three methyl groups from the PSM headgroup did not markedly influence the order in the acyl chain region, since the DPH steady-state anisotropy was similar in PSM and PCPE vesicles both before and after the gel-liquid transition. Cholesterol at 33 mol % abolished the melting transition of PCPE bilayer in a similar way as for PSM bilayers (Kuikka et al., 2001).
To compare interfacial properties in bilayer membranes prepared from either PCPE or PSM, we determined the emission spectrum of Laurdan, since its emission is sensitive to the polarity of the environment of the fluorescent moiety (Parasassi et al., 1991). The transition from the gel to the liquid crystalline phase in vesicle bilayers leads to a red shift in Laurdan emission spectra as a consequence of facilitated penetration of water molecules into the lipid interface after melting of the bilayer (Parasassi et al., 1994a,b). Below the melting temperature, Laurdan gave a more blue-shifted emission spectra in PCPE bilayers as compared to PSM membranes, indicating that the PCPE membrane interface was more hydrophobic (less hydrated) compared to the PSM system. The Laurdan emission was similarly blue-shifted in the gel phase of dipalmitoyl phosphatidylethanolamine as compared with DPPC (Epand and Leon, 1992), suggesting that the phosphatidylethanolamine membrane interface was less polar than the phosphatidylcholine interface. The more hydrophobic interface in phosphatidylethanolamine membranes apparently relates to the fact that the headgroup of phosphatidylethanolamine is less hydrated as compared to the headgroup of phosphatidylcholine (Sen and Hui, 1988). In the melted bilayers, Laurdan emission was more red-shifted, but much more so for PSM than for PCPE, suggesting that even in the melted state the PCPE bilayer interface was much more hydrophobic and less hydrated than the corresponding PSM bilayer interface. This difference in interfacial polarity was clearly seen from the calculated GPex values for pure PCPE and PSM bilayers below and above the main transition temperature. The presence of 33 mol % cholesterol in the bilayer membranes led to blue-shifted Laurdan emissions, mainly because cholesterol is known to reduce the hydration of the membrane interface and hence the dipolar relaxation, to which Laurdan is sensitive in its excited state (Parasassi et al., 1994a).
Finally, to study how the headgroup size affected the properties of the sphingomyelin to form liquid-ordered domains with cholesterol, we utilized a quenching assay involving a fluorescent sterol (CTL) and a quencher lipid (12SLPC). We have previously shown that CTL and cholesterol can form sterol-rich domains with PSM, in which CTL is partially protected from quenching by 12SLPC (Y. J. E. Björkqvist, T. K. M. Nyholm, J. P. Slotte, and B. Ramstedt, unpublished; see also Fig. 6). Using this assay, PCPE failed to form sterol-rich domains in which CTL would be protected from quenching by 12SLPC (Fig. 6), suggesting that the headgroup size is crucially important for PSM to form and maintain sterol-rich domains in fluid bilayer membranes. The observation that PCPE failed to form sterol-rich domains with cholesterol agrees well with the desorption data (Table 1), which also suggested that PCPE interacted less favorably with cholesterol as compared to PSM.
Taken together, the results presented in this article clearly show that removal of the three methyl groups from the choline moiety of PSM leads to the formation of a molecule that can no longer interact favorably with cholesterol, despite the hydrogen-bonding properties of the sphingolipid.
Acknowledgments
We thank Dr. Bodil Ramstedt for valuable comments on the manuscript, and Sari Tiljander-Karlsberg for help in some preliminary experiments.
This study was supported by generous grants from the Academy of Finland, the Sigrid Juselius Foundation, the Oskar Öflund Foundation, and Medicinska Understödsföreningen Liv och Hälsa.
References
- Ahmed, S. N., D. A. Brown, and E. London. 1997. On the origin of sphingolipid/cholesterol-rich detergent soluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent insoluble, liquid ordered lipid phase in model membranes. Biochemistry. 36:10944–10953. [DOI] [PubMed] [Google Scholar]
- Bach, D., and E. Wachtel. 2003. Phospholipid/cholesterol model membranes: formation of cholesterol crystallites. Biochim. Biophys. Acta. 1610:187–197. [DOI] [PubMed] [Google Scholar]
- Bagatolli, L. A., E. Gratton, and G. D. Fidelio. 1998. Water dynamics in glycosphingolipid aggregates studied by Laurdan fluorescence. Biophys. J. 75:331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagatolli, L. A., T. Parasassi, G. D. Fidelio, and E. Gratton. 1999. A model for the interaction of 6-lauroyl-2-(N,N-dimethylamino)naphthalene with lipid environments: implication for spectral properties. Photochem. Photobiol. 70:557–564. [PubMed] [Google Scholar]
- Bittman, R., C. R. Kasireddy, P. Mattjus, and J. P. Slotte. 1994. Interaction of cholesterol with sphingomyelin in monolayers and vesicles. Biochemistry. 33:11776–11781. [DOI] [PubMed] [Google Scholar]
- Broad, T. E., and R. M. C. Dawson. 1973. Formation of ceramide phosphorylethanolamine from phosphatidylethanolamine in the rumen protozoon Entodinium caudatum. Biochem. J. 134:659–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, D. A., and E. London. 1997. Structure of detergent-resistant membrane domains: does phase separation occur in biological membranes? Biochem. Biophys. Res. Commun. 240:1–7. [DOI] [PubMed] [Google Scholar]
- Brown, D. A., and E. London. 2000. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 275:17221–17224. [DOI] [PubMed] [Google Scholar]
- Cheetham, J. J., E. Wachtel, D. Bach, and R. M. Epand. 1989. Role of stereochemistry of the hydroxyl group of cholesterol and the formation of nonbilayer structures in phosphatidylethanolamines. Biochemistry. 28:8928–8934. [DOI] [PubMed] [Google Scholar]
- Collins, J. J., and M. C. Phillips. 1982. The stability and structure of cholesterol-rich codispersions of cholesterol and phosphatidylcholine. J. Lipid Res. 23:291–298. [PubMed] [Google Scholar]
- Davies, J. T., and E. K. Rideal. (Editors). 1961. Interfacial Phenomena. Academic Press, New York.
- Dobrowsky, R. T. 2000. Sphingolipid signaling domains floating on rafts or buried in caves? Cell. Signal. 12:81–90. [DOI] [PubMed] [Google Scholar]
- Dorfler, H. D. 1990. Mixing behavior of binary insoluble phospholipid monolayers. Analysis of the mixing properties of binary lecithin and cephalin systems by application of several surface and spreading techniques. Adv. Colloid Interface Sci. 31:1–110. [DOI] [PubMed] [Google Scholar]
- El Kirat, K., A. F. Prigent, J. P. Chauvet, B. Roux, and F. Besson. 2003. Transphosphatidylation activity of Streptomyces chromofuscus phospholipase D in biomimetic membranes. Eur. J. Biochem. 270:4523–4530. [DOI] [PubMed] [Google Scholar]
- Epand, R. M., and R. F. Epand. 2004. Non-raft forming sphingomyelin-cholesterol mixtures. Chem. Phys. Lipids. 132:37–46. [DOI] [PubMed] [Google Scholar]
- Epand, R. M., and B. T.-C. Leon. 1992. Hexagonal phase forming propensity detected in phospholipid bilayers with fluorescent probes. Biochemistry. 31:1550–1554. [DOI] [PubMed] [Google Scholar]
- Fielding, C. J., and P. E. Fielding. 2000. Cholesterol and caveolae: structural and functional relationships. Biochim. Biophys. Acta. 1529:210–222. [DOI] [PubMed] [Google Scholar]
- Fischer, R. T., F. A. Stephenson, A. Shafiee, and F. Schroeder. 1984. Delta 5,7,9(11)-cholestatrien-3-beta-ol: a fluorescent cholesterol analogue. Chem. Phys. Lipids. 36:1–14. [DOI] [PubMed] [Google Scholar]
- Grönberg, L., Z.-s. Ruan, R. Bittman, and J. P. Slotte. 1991. Interaction of cholesterol with synthetic sphingomyelin derivates in mixed monolayers. Biochemistry. 30:10746–10754. [DOI] [PubMed] [Google Scholar]
- Huang, J., J. T. Buboltz, and G. W. Feigenson. 1999. Maximum solubility of cholesterol in phosphatidylcholine and phosphatidylethanolamine bilayers. Biochim. Biophys. Acta. 1417:89–100. [DOI] [PubMed] [Google Scholar]
- Huang, J., and G. W. Feigenson. 1999. A microscopic interaction model of maximum solubility of cholesterol in lipid bilayers. Biophys. J. 76:2142–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen, E. 2001. Roles of lipid rafts in membrane transport. Curr. Opin. Cell Biol. 13:470–477. [DOI] [PubMed] [Google Scholar]
- Kan, C.-C., Z.-s. Ruan, and R. Bittman. 1991. Interaction of cholesterol with sphingomyelin in bilayer membranes: evidence that the hydroxyl group of sphingomyelin does not modulate the rate of cholesterol exchange between vesicles. Biochemistry. 30:7759–7766. [DOI] [PubMed] [Google Scholar]
- Kuikka, M., B. Ramstedt, H. Ohvo-Rekilä, J. Tuuf, and J. P. Slotte. 2001. Membrane properties of D-erythro-N-acyl sphingomyelins and their corresponding dihydro species. Biophys. J. 80:2327–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowicz, J. R 1999. Principles of Fluorescence Spectroscopy. Kluvert Academic/Plenum Publishers, New York.
- Maier, O., T. A. Slimane, and D. Hoekstra. 2001. Membrane domains and polarized trafficking of sphingolipids. Semin. Cell Dev. Biol. 12:149–161. [DOI] [PubMed] [Google Scholar]
- Malgat, M., A. Maurice, and J. Baraud. 1986. Sphingomyelin and ceramide-phosphoethanolamine synthesis by microsomes and plasma membranes from rat liver and brain. J. Lipid Res. 27:251–260. [PubMed] [Google Scholar]
- Maurice, A., and M. Malgat. 1990. Evidence for the biosynthesis of ceramide-phosphoethanolamine in brain synaptic plasma membrane vesicles and sciatic nerve microsomes from normal and Trembler mice. Neurosci. Lett. 118:177–180. [DOI] [PubMed] [Google Scholar]
- McIntosh, T. J., S. A. Simon, D. Needham, and C.-H. Huang. 1992. Structure and cohesive properties of sphingomyelin/cholesterol bilayers. Biochemistry. 31:2012–2020. [DOI] [PubMed] [Google Scholar]
- Mombelli, E., R. Morris, W. Taylor, and F. Fraternali. 2003. Hydrogen-bonding propensities of sphingomyelin in solution and in a bilayer assembly: a molecular dynamics study. Biophys. J. 84:1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemela, P., M. T. Hyvonen, and I. Vattulainen. 2004. Structure and dynamics of sphingomyelin bilayer: insight gained through systematic comparison to phosphatidylcholine. Biophys. J. 87:2976–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu, S.-L., and B. L. Litman. 2002. Determination of membrane cholesterol partition coefficient using a lipid vesicle-cyclodextrin binary system: effect of phospholipids acyl chain unsaturation and headgroup composition. Biophys. J. 83:3408–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholm, T. K. H., M. Nylund, and J. P. Slotte. 2003. A calorimetric study of binary mixtures of dihydrosphingomyelin and sterols, sphingomyelin, or phosphatidylcholine. Biophys. J. 84:3138–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohvo, H., and J. P. Slotte. 1996. Cyclodextrin-mediated removal of sterols from monolayers: effect of sterol structure and phospholipids on desorption rate. Biochemistry. 35:8018–8024. [DOI] [PubMed] [Google Scholar]
- Ohvo-Rekilä, H., B. Ramstedt, P. Leppimaki, and J. P. Slotte. 2002. Cholesterol interactions with phospholipids in membranes. Prog. Lipid Res. 41:66–97. [DOI] [PubMed] [Google Scholar]
- Olsen, I., and E. Jantzen. 2001. Sphingolipids in bacteria and fungi. Anaerobe. 7:103–112. [Google Scholar]
- Parasassi, T., G. De Stasio, A. d'Ulbaldo, and E. Gratton. 1990. Phase fluctuation in phospholipid membranes revealed by Laurdan fluorescence. Biophys. J. 57:1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasassi, T., G. De Stasio, G. Ravagnan, R. M. Rush, and E. Gratton. 1991. Quantitation of lipid phase in phospholipid vesicles by the generalized polarization of Laurdan fluorescence. Biophys. J. 60:179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasassi, T., M. Di Stefano, M. Loiero, G. Ravagnan, and E. Gratton. 1994a. Cholesterol modifies water concentration and dynamics in phospholipids bilayers: a fluorescence study using Laurdan probe. Biophys. J. 66:763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasassi, T., M. Di Stefano, M. Loiero, G. Ravagnan, and E. Gratton. 1994b. Influence of cholesterol on phospholipid bilayers phase domains as detected by Laurdan fluorescence. Biophys. J. 66:120–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramstedt, B., P. Leppimaki, M. Axberg, and J. P. Slotte. 1999. Analysis of natural and synthetic sphingomyelins using high-performance thin-layer chromatography. Eur. J. Biochem. 266:997–1002. [DOI] [PubMed] [Google Scholar]
- Ramstedt, B., and J. P. Slotte. 1999. Interaction of cholesterol with sphingomyelins and acyl-chain-matched phosphatidylcholines: a comparative study of the effect of the chain length. Biophys. J. 76:908–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramstedt, B., and J. P. Slotte. 2002. Membrane properties of sphingomyelins. FEBS Lett. 531:33–37. [DOI] [PubMed] [Google Scholar]
- Rosenholm, J. B., P. Ihalainen, and J. Peltonen. 2003. Thermodynamic characterization of Langmuir monolayers of thiolipids: a conceptual analysis. Colloid. Surface. A. 228:119–130. [Google Scholar]
- Sarmiento, F., G. Schwarzmann, and K. Sandhoff. 1985. Direct evidence by carbon-13 NMR spectroscopy for the erythro configuration of the sphingoid moiety in Gaucher cerebroside and other natural sphingolipids. Eur. J. Biochem. 146:59–64. [DOI] [PubMed] [Google Scholar]
- Scheidt, H. A., P. Müller, A. Herrmann, and D. Huster. 2003. The potential of fluorescent and spin-labeled steroid analogs to mimic natural cholesterol. J. Biol. Chem. 278:45563–45569. [DOI] [PubMed] [Google Scholar]
- Sen, A., and S. W. Hui. 1988. Direct measurement of headgroup hydration of polar lipids in inverted micelles. Chem. Phys. Lipids. 49:179–184. [DOI] [PubMed] [Google Scholar]
- Shinitzky, M., and Y. Barenholz. 1974. Dynamics of the hydrocarbon layer in liposomes of lecithin and sphingomyelin containing diacetylphosphate. J. Biol. Chem. 249:2652–2657. [PubMed] [Google Scholar]
- Shinitzky, M., and Y. Barenholz. 1978. Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim. Biophys. Acta. 515:367–394. [DOI] [PubMed] [Google Scholar]
- Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature. 387:569–572. [DOI] [PubMed] [Google Scholar]
- Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1:31–39. [DOI] [PubMed] [Google Scholar]
- Skipski, V. P., R. F. Peterson, and M. Barclay. 1964. Quantitative analysis of phospholipids by thin-layer chromatography. Biochem. J. 90:374–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotte, J. P. 1999. Sphingomyelin-cholesterol interactions in biological and model membranes. Chem. Phys. Lipids. 102:13–27. [DOI] [PubMed] [Google Scholar]
- Smaby, J. M., H. L. Brockman, and R. E. Brown. 1994. Cholesterol's interfacial interactions with sphingomyelins and phosphatidylcholines: hydrocarbon chain structure determines the magnitude of condensation. Biochemistry. 33:9135–9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott, C. M., I. Vorobyov, D. Borchman, K. G. Taylor, D. B. DuPre, and M. C. Yappert. 2000. Conformational studies of sphingolipids by NMR spectroscopy. II. Sphingomyelin. Biochim. Biophys. Acta. 1467:326–337. [DOI] [PubMed] [Google Scholar]
- van Dijck, P. W. M., B. de Kruijff, L. L. M. van Deenen, J. de Gier, and R. Demel. 1976. The preference of cholesterol for phosphatidylcholine in mixed phosphatidylcholine-phosphatidylethanolamine bilayers. Biochim. Biophys. Acta. 455:576–588. [DOI] [PubMed] [Google Scholar]
- van Dijck, P. W. M. 1979. Negatively charged phospholipids and their position in the cholesterol affinity sequence. Biochim. Biophys. Acta. 555:89–101. [DOI] [PubMed] [Google Scholar]
- Weidemann, G., and D. Vollhardt. 1995. Long-range tilt orientation order in phospholipid monolayers: the inner structure of dimyristoyl-phosphatidylethanolamine domains. Thin Solid Films. 264:94–103. [Google Scholar]
- Yeagle, P. L., and J. E. Young. 1986. Factors contributing to the distribution of cholesterol among phospholipids vesicles. J. Biol. Chem. 261:8175–8181. [PubMed] [Google Scholar]
- Yu, H., and S. W. Hui. 1992. Methylation effects on the microdomain structures of phosphatidylethanolamine monolayers. Chem. Phys. Lipids. 62:69–78. [DOI] [PubMed] [Google Scholar]