Abstract
MscS is a bacterial mechanosensitive channel that shows voltage dependence. The crystal structure of MscS revealed that the channel is a homoheptamer with a large chamber on the intracellular site. Our previous experiments indicated that the cytoplasmic chamber of the channel is not a rigid structure and changes its conformation upon the channel activation. In this study, we have applied various sized cosolvents that are excluded from protein surfaces. It is well known that such cosolvents induce compaction of proteins and prevent thermal fluctuations. It is also known that they shift channel equilibrium to the state of lower volume. We have found that large cosolvents that cannot enter the channel interior accelerate channel inactivation when applied from the cytoplasmic side, but they slow down inactivation when applied from the extracellular side. We have also found that small cosolvents that can enter the channel cytoplasmic chamber prevent the channel from opening, unlike the large ones. These data support our idea that the channel cytoplasmic chamber shrinks upon inactivation but also give new clues about conformational changes of the channel upon transitions between its functional states.
INTRODUCTION
Mechanosensitive (MS) ion channels open due to membrane stretch. They have been found in many cell types of various organisms from bacteria to vertebrates (Hamill and Martinac, 2001; Martinac, 2004; Sukharev and Corey, 2004). Two bacterial channels, MscL and MscS (Sukharev et al., 1993), of known crystal structures (Bass et al., 2002; Chang et al., 1998) are the best characterized MS channels and serve as models to study mechanotransduction in general. They both reside in the bacterial inner membrane and serve as safety valves, releasing cytoplasmic osmolytes upon osmotic downshock (Levina et al., 1999). The quaternary structure of the MscS channel reveals that the functional channel is a heptamer, and each of seven subunits is composed of three transmembrane helices TM1, TM2, and TM3 (Fig. 1 A). The TM3 helices, rich in glycines and alanines, line the channel pore. The cytoplasmic domains of the channel are composed mostly of β-sheets and surround a large water-filled chamber with a diameter of ∼40 Å (Fig. 1, A and B). The chamber has seven pores, 14 Å in diameter each, located at the subunit interfaces and the additional opening of 8 Å formed by a β barrel at the bottom of the chamber (Fig. 1 B). It has been suggested that the structure reveals an open channel conformation (Bass et al., 2002), but a recent molecular dynamics study of water inside the MscS channel led the authors to conclude that the structure may represent an inactivated state of the channel (Anishkin and Sukharev, 2004). The MscS channels become inactive under sustained mechanical stimuli (Fig. 2 A). It has been shown that the fully inactive channels could be reactivated by a next suction step, providing the time between pulses was long enough and suction had been released between pulses (Koprowski and Kubalski, 1998). Thus MscS has at least three kinetic states—closed, open, and inactivated—and it is very likely they reflect different conformations of the channel molecule. Our and others' experiments indicate that the cytoplasmic chamber may undergo large conformational changes upon transition from the closed to open state of the channel (Koprowski and Kubalski, 2003; Miller et al., 2003). These changes should ultimately result in changes of the volume of the chamber (Edwards et al., 2004). The objective of the studies presented in this article was to set a series of experimental conditions under which changes in the volume of the MscS cytoplasmic chamber and/or in its entire ion-conducting pathway could be expected and resulting alterations of the channel kinetics would be observed.
FIGURE 1.
Quaternary structure of MscS. (A) Side view of the MscS heptamer shown in ribbons representation with one subunit marked in black. Transmembrane helices TM1, TM2, and TM3 are indicated. (B) The left panel presents the side view of the cross section of MscS shown in a surface representation. The box indicates the gate region formed by the TM3 helices. A-A marks the cross section through the cytoplasmic chamber of the channel, and the cross section is shown in the right panel. Sizes of the bottom (left panel) and of the side (right panel) openings are indicated. Sizes of PEGs used are shown as shaded spheres.
FIGURE 2.
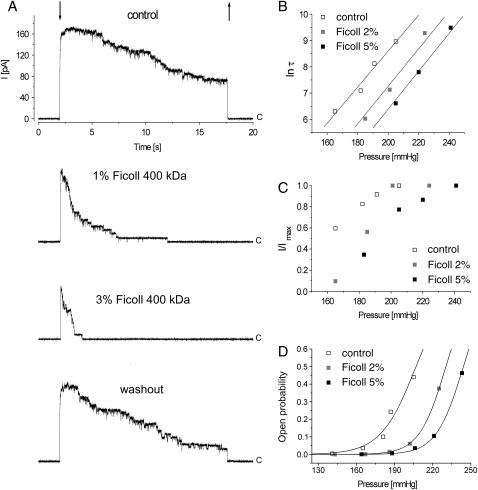
Effect of different concentrations of ficoll applied to the cytoplasmic side of MscS on the channel currents. (A) Ficoll changed reversibly the activities of the MscS channels, fewer channels opened, and the inactivation was faster. All traces were recorded from the same patch. Arrows indicate application and release of suction (downward and upward, respectively), and c marks the closed level of the channels. All traces were recorded at +15 mV and suction pulse was 215 mm Hg. The effect of 2% and 5% of ficoll on the inactivation time constant, τ, (B), and on total current, I, are shown as a ratio I/Imax, where Imax represents the current through all the channels in the patch (C). (D) The effect of 2% and 5% of ficoll on the channel open probabilities, Po, plotted against suction and fitted with Boltzmann curves. Data sets presented in B, C, and D represent one experiment.
In several studies, an approach called “osmotic stress” (Parsegian et al., 1986; Zimmerberg and Parsegian, 1986) has been used to detect and measure volume changes of various ion channels during the course of their activation, inactivation, or closing. In this method, large molecules (cosolvents) that cannot enter the channel interior are added to the aqueous protein solution. It is assumed that the cosolvent exerts osmotic pressure on the channel volume and a more compact state is preferred as a result of a shift of the channel open-close equilibrium. In addition to the determination of volume changes upon gating, the method aims to estimate the number of water molecules released from the channel interior inaccessible to cosolvents upon transition from one conformational state to another. This experimental approach has been employed to estimate volume changes upon activation of voltage dependent anion channel (Zimmerberg and Parsegian, 1986), alamethicin channels (Vodyanoy et al., 1993), and potassium channels (Zimmerberg et al., 1990). It was also applied to Kv channels to investigate volume changes associated with C-type inactivation (Jiang et al., 2003). The “osmotic stress” analysis considers cosolvents as entirely inert or neutral molecules facilitating only a removal of water molecules from the channel interior. There is, however, a large body of evidence that suggests cosolvents are not neutral and they interact with proteins (Timasheff, 1998a, 1998b, 2002). The interaction can be positive (preferential binding) or negative (preferential exclusion resulting in preferential hydration). Cosolvents that preferentially bind to proteins stabilize them in an expanded, unfolded state (solubilizers). Urea, guanidine hydrochloride, or propyleneglycol are known to solubilize and denaturate proteins. Cosolvents that are preferentially excluded from proteins—polyethylenoglycans (PEGs), dextrans, ficoll, and sucrose—fix them in the native, compact state and restrain conformational fluctuations within the native state (stabilizers). The two groups of cosolvents were used to probe various molecular processes associated with proteins such as folding, allosteric effects, or physiological regulation. There is controversy over whether a quantitative analysis of the data obtained using the “osmotic stress” method is possible (Parsegian et al., 2000; Timasheff, 1998b, 2002). The number of water molecules released from the channel interior could not be estimated precisely, and it is usually overestimated, taking into account that cosolvents are excluded from the entire surface of the protein (Shimizu, 2004). Despite the debate on interpretation of the experimental results, there is a consensus that proteins change their conformation in the presence of cosolvents, and the state of smaller volume is favored upon supplementing a protein solution with stabilizers.
It has been shown that in the presence of different-sized PEGs and, to avoid any hydrostatic effect, at constant osmotic pressure of all solutions tested, alamethicin channels that are known to respond to mechanical stimuli (Bruner and Hall, 1983) had reduced open probability (Vodyanoy et al., 1993). The effect was stronger the higher the molecular weight of the applied PEGs. We have applied a very similar approach to the MS channel MscS—using cosolvents of different molecular weight and expecting that MscS kinetic states are linked to the conformational changes of the channel in their presence. We have found that at constant osmotic pressure of all solutions tested, the channels show faster adaptation upon supplementing the solutions with different types of stabilizers. We have applied PEGs of various molecular weights and found that fewer channels opened when PEGs of lower molecular weight were used; larger PEGs, however, affected mostly an inactivation rate. These results indicate that the “preferential exclusion” approach is a more favorable method in proper understanding and analysis of our data. We relate the observed changes in the channel behavior in the presence of cosolvents to the changes of its surface area. Based on the crystal structure of the channel, we suggest possible conformational changes of the channel molecule upon transitions between its functional states.
MATERIALS AND METHODS
Bacterial strains and protoplast preparation
All electrophysiological experiments were performed on Escherichia coli strain MJF 379—Frag1 kefA::kan. Frag1 is a wild-type strain, a derivative of E. coli K-12. The strain was kindly provided by I. R. Booth (University of Aberdeen, UK). Protoplasts were prepared as described earlier (Kubalski, 1995).
Chemicals and solutions
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Osmotic pressure of all experimental solutions containing ficoll, dextran, glucose, and PEGs was 760 mOsm/kg H2O and was adjusted with sorbitol using an osmometer (800, Trident, Warsaw, Poland). Additionally, we kept the osmotic pressure of each PEG on a constant level of 30 mOsm, and the resulting concentrations of PEG 200, 600, 1450, 3350, and 6000 were the following: 0.55%, 1.45%, 2.90%, 4.30%, and 4.90%, respectively.
Electrophysiology and data analysis
Single-channel recordings were obtained from inside out excised membrane patches, and the experimental procedure and equipment used were the same as described earlier (Koprowski and Kubalski, 1998). Bath and pipette control solutions were the same and contained 150 mM KCl, 400 mM sorbitol, 4 mM CaCl2, 5 mM MgCl2, and 5 mM HEPES, pH = 7.2.
Suction, at constant pipette voltage +15 mV, was applied pneumatically to the patch pipettes using a 10-ml syringe, together with two in-line, three-way valves, and was monitored by a pressure manometer PM015D (10.3 kPa or 1.5 psi; WPI, Sarasota, FL). This system allowed us to apply any suction step with an error of <±2% of the required level. Intervals of 60 s or longer were maintained between consecutive applications of suction. Data were acquired (with a sampling rate of 2.5 kHz), filtered at 1 kHz, and analyzed using pCLAMP6 software.
The mean single-channel open probability, Po, during the pressure pulse was calculated by integrating the current passing through all active channels, I, during the recording time and dividing this integral by the current through a single open channel, i, multiplied by the number of active channels, N, according to the formula Po = I/Ni. Data points were plotted and fitted to Boltzmann functions using Origin 4.0 (Microcal Software, Northampton, MA). The positions of the midpoints of Boltzmann curves and their average shifts are presented as the mean ±SD.
RESULTS
A transition from the closed to an open state of the MscS channels can be expressed by the number of activated channels upon application of a defined suction step to the patch and represented by the maximal total current Imax at this suction. At sustained suction, MscS inactivates and undergoes a transition from the open to an inactivated state. This process is described by a single exponential function fitted to experimental traces, and the time constant, τ, is defined as the inverse of the inactivation rate. In the experiments presented below, we describe changes of Imax and/or τ in the presence of various cosolvents added to the channel bath solutions.
Effect of ficoll on MscS single-channel currents
Ficoll (molecular weight 400,000) at concentrations 1% (n = 3), 2% (n = 12), 3% (n = 9), 5% (n = 6), and 10% (n = 2) was applied to the protoplast membrane patches to the cytoplasmic side of the membrane. Ficoll increased τ (Fig. 2, A and B) and reduced Imax (Fig. 2, A and C). Both effects were reversible. The channel inactivation was faster, and we conclude that inactivation is a preferred state in the presence of ficoll. Since ficoll is preferentially excluded from protein surfaces—and proteins are expected to lower their surfaces in its presence—we conclude that the inactivated state of the channel is associated with a configuration in which the channel surface interacting with ficoll is lower. Since ficoll molecules are too large to enter the channel interior, the channel surface is represented in this experiment mostly by the external surface of the large chamber on the cytoplasmic side of the membrane.
The ficoll effect on Imax suggests that the activation of the channel is impaired. This effect can be observed in a limited range of pressures since Imax saturates at higher pressures. MscS cannot be opened when it is in the inactivated state (Koprowski and Kubalski, 1998). Thus lower Imax in the presence of ficoll may indicate that activation is impaired due to the inability of the channel protein to undergo a transition from the inactivated to the closed state and/or due to the possibility that upon activation the channel increases its external surface (being in contact with ficoll) and this increment is partly blocked by the presence of ficoll.
The dependence of the single-channel open probabilities, Po, on suction in control and in the presence of 2% and 5% of ficoll is shown in Fig. 2 D. The experimental points were fitted to Boltzmann curves, and the midpoints of the Boltzmann curves in control and in solutions with 2% and 5% ficoll were 207, 230, and 244 mm Hg, respectively. The average shift of the midpoint after channel exposure to 2% ficoll was 20 ± 7.2 mm Hg (n = 4).
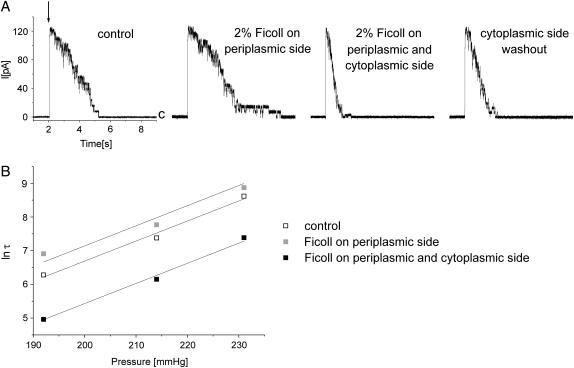
In another set of experiments, we applied 2% ficoll from the periplasmic side of the membrane. In three experiments, in which the experimental pipette was perfused with the ficoll containing solution, Imax was not affected (Fig. 3). The inactivation, however, unlike in the experiments when cytoplasmic parts of the channel were exposed to ficoll, was slower. Ficoll does not enter the cytoplasmic chamber of the channel since it cannot pass the channel gate, therefore its effect is associated with the parts of the channel exposed to the periplasm. This result could be interpreted in the following way: assuming that the periplasm-exposed parts of the channel enlarge their surface upon inactivation, the presence of ficoll would counteract this phenomenon. The channel could inactivate but the process was slower. In the course of the experiment, we added ficoll to the cytoplasmic side of the membrane (ficoll concentration was symmetric on both sides of the membrane), and this resulted in faster inactivation than in control. We assume that large cytoplasmic parts of the channel change their surface upon exposure to ficoll, and by the additivity principle both effects compensate each other's action. Since the channel surface areas in contact with ficoll are larger on the cytoplasmic side of the membrane, the effect of reduction of the inactivation rate prevails.
FIGURE 3.
Effect of ficoll applied to the periplasmic side of MscS on the channel currents. (A) Ficoll changed reversibly the time constant of inactivation, τ, inactivation was slower, and the number of activated channels was unaffected. Addition of ficoll to the cytoplasmic side of the channel accelerated inactivation. All traces were recorded from the same patch at +15 mV. Suction pulse (indicated by the downward arrow) was 232 mm Hg and its duration was 12 s. c marks the closed level of the channels. (B) Change of the inactivation time constant, τ, as a function of pressure in the experiment shown in A.
The effect of ficoll when it was applied from the periplasmic side of the membrane was not strong, and any strong statements are not justified. This result, however, is significant, since the effect of ficoll is asymmetric to that observed when it was applied from inside. This indicates that the observed effects may not be associated with the cosolvent-driven changes of the membrane properties, and they reflect a change of a preferred conformation of the channel protein in the presence and in the absence of ficoll.
Effects of dextran and glucose
In the next series of experiments, we wanted to test if other cosolvents/stabilizers of different molecular weight, and whose interactions with proteins are of different chemical nature, affect the channel kinetics the way ficoll did. We applied dextran (molecular weight 35,000; n = 11) and glucose (molecular weight 160; n = 8) to the cytoplasmic side of the membrane, and osmotic pressure of each solution was the same as the osmotic pressure of the ficoll solution (760 mOsm/kgH2O). We found that dextran and glucose—as ficoll—reduced the inactivation rate, but the effects were inversely proportional to their molecular weight, i.e., inactivation of the channels in dextran was faster than that in glucose and slower than that observed in the presence of ficoll. Fig. 4 shows the effect on inactivation of each cosolvent as a function of its fractional osmotic pressure. Imax in these experiments was more strongly affected in the presence of glucose than in the presence of cosolvents of higher molecular weight (not shown; effect of glucose on Imax is presented in Fig. 7).
FIGURE 4.
Ficoll, dextran, and glucose applied from the cytoplasmic side of MscS changed the inactivation time constant, τ, of the channels in a manner inversely proportional to their molecular weight. Change of τ is presented as a function of a fractional osmolarity of each cosolvent. Black, shaded, and open symbols represent effects of ficoll, dextran, and glucose, respectively, and each symbol type (squares, triangles, or circles) represents one experiment.
FIGURE 7.
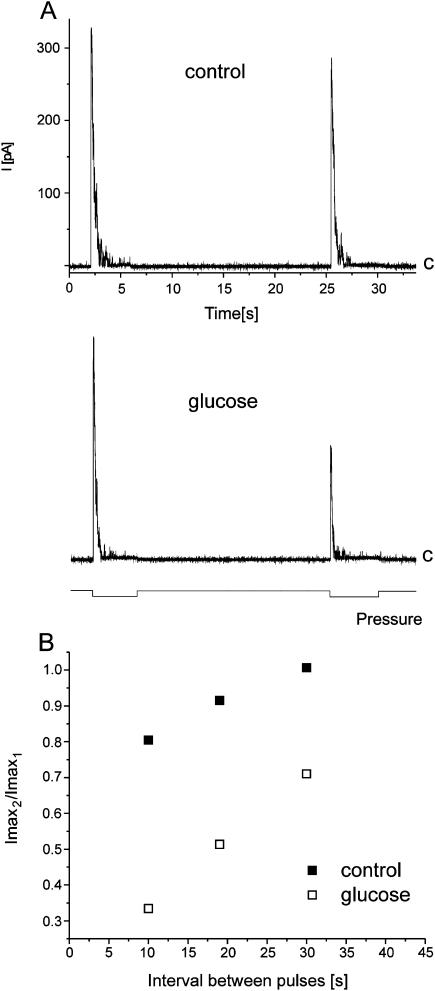
Transition of the channel from the inactivated to the closed state was affected in the presence of glucose as revealed by the two-pulse protocol. Two identical suction pulses (120 mm Hg) were applied 10, 20, and 30 s apart. The traces shown in A were recorded from the same patch at +15 mV, and the time interval between the suction pulses was 20 s (as indicated at the bottom of the panel). The ratio Imax2/Imax1 (Imax2 and Imax1 are the total currents observed during the second and the first pulse, respectively) changed in the presence of glucose, and it is plotted against the time interval between pulses (B). The traces in A and the data points in B come from the same experiment.
The channel open probabilities, Po, at different pressures were fitted to Boltzmann curves. After the channels were exposed to dextran or glucose, the curves were shifted toward higher pressures. The average change of their position was 12.4 ± 2.8 (n = 4) and 14.3 ± 7 mm Hg (n = 3) in 4.57% dextran and in 10 mM glucose, respectively.
Effects of PEGs of different molecular weight
We wanted to verify our previous observation that cosolvents of higher molecular weights had a more pronounced effect on the time course of the channel inactivation, τ, than those of lower size. We also wanted to show that the observed difference could result from a size of the cosolvent and not from its chemical properties. For this reason we used a set of PEGs of different molecular weights. PEGs 200 (n = 21), 600 (n = 10), 1450 (n = 9), 3350 (n = 7), and 6000 (n = 40) were applied to the cytoplasmic side of the membrane patch containing MscS channels. Initially the patch was exposed to the control bath solution, and then the solutions containing various PEGs were subsequently introduced to the chamber (Fig. 5 A). Our experiments showed that PEGs of lower molecular weight (200, 600) affected mostly Imax (Fig. 5 B). The effect of PEGs of higher molecular weights (1450–6000) on Imax was slight or there was a lack of it; the inactivation rate, however, was much more reduced than in the presence of PEGs of lower molecular weights (Fig. 5). These results are very similar to those obtained with glucose, dextran, and ficoll and show that cosolvents of smaller molecular weight affect primarily Imax, whereas cosolvents of higher molecular weight have a more pronounced effect on the rate of inactivation. These results may also indicate that the two processes—activation, a transition from the closed to open state (represented by Imax) and inactivation, a transition from the open to inactivated state (represented by τ)—are associated with different surface changes of the channel molecule.
FIGURE 5.
Effects of PEGs of different molecular weights applied from the cytoplasmic side of the MscS channels. (A) PEGs 200 and 600 reduced the number of active channels and PEGs 1450, 3350, and 6000 affected mostly the inactivation time constant, τ. All traces were recorded from the same patch at +15 mV. Suction pulse (indicated by the downward arrow) was 170 mm Hg and its duration was 12 s. c marks the closed level of the channels. Changes of Imax (B) and τ (C) are plotted as a function of molecular weights of PEGs applied. Data in B and C represent the experiment shown in A, and the number at each data point indicates a diameter of a PEG molecule. PEGs inside the boxes (B and C) were able to penetrate the cytoplasmic chamber of MscS by the bottom or side openings. Effects of PEG 200 (D) and PEG 6000 (E) on the channel open probabilities were plotted against suction and fitted to Boltzmann curves.
Open probabilities, Po, from experiments with PEG 200 and PEG 6000 are shown in Fig. 5 (D and E, respectively). The data points were fitted with Boltzmann curves, and the midpoints of the curves in the control and in the presence of PEG were 180 and 190 mm Hg for PEG 200 (Fig. 5 D) and 169 and 220 mm Hg for PEG 6000 (Fig. 5 E). The average shift of the midpoint of the Boltzmann curve was 7.9 ± 4.5 and 61.7 ± 10.4 mm Hg in the presence of PEG 200 and PEG 6000, respectively (n = 3, in both cases).
Our routine experimental procedure was such that each suction pulse was applied 1–2 min after suction of the previous pulse was released. This time was necessary for a recovery of the channels from the inactivated state. In experiments when the experimental chamber was perfused, we waited 5 min after the end of perfusion and before application of the next suction pulse. In a few cases we observed that after removal of the control solution from the chamber and introduction of the PEG 200 solution, the initial response to suction of the channels was a double-step response and contained a fast initial peak, which disappeared when the next suction pulse was applied (Fig. 6). At first, we considered this fast step as an artifact, but since we have observed it several times we thought it could be associated with the time course of our experiments and eventually with the entrance of PEG 200 molecules into the MscS cytoplasmic chamber. We changed the procedure and shortened the time between removal of the control bath devoid of PEG and application of the first suction pulse in the presence of PEG 200. Under these conditions, the initial peaklike response was seen repeatedly. Our conclusion is the following: after transfer of the channel protein from the water bath solution to the cosolvent (PEG 200) solution, there was a gradient of cosolvent concentration outside and inside of the channel cytoplasmic chamber until eventually PEG 200 entered the chamber through its openings. When concentration of PEG 200 outside the chamber was larger than inside, the cosolvent interacted with the outer surface of the chamber (as larger PEGs do), which resulted in the initial peak response. After PEG 200 entered the chamber and the inner and outer side of the chamber were in contact with it, the peak disappeared. This hypothesis is supported by the fact that the channels responded to pressure in a peaklike fashion in the presence of PEGs 1450–6000, which could not penetrate the chamber, and their contact with the channel was restricted to its outer surface. In conclusion, under asymmetric distribution of the cosolvent and when the outer side of the cytoplasmic chamber interacted with it, a decrease of the inactivation rate was a predominant effect observed. When there was a symmetric distribution of cosolvent and the channel interior including the pore region interacting with it, we observed a decrease in Imax.
FIGURE 6.
Effect of PEG 200 on the MscS currents was time-dependent. After introducing PEG 200 into the experimental chamber, we observed the initial peaklike response (B), which disappeared in the course of the experiment (C). For explanation, see the text. All traces were recorded from the same patch at +15 mV. Suction pulse (indicated by the downward arrow) was 155 mm Hg and its duration was 12 s. c marks the closed level of the channels.
The initial peak response was observed when the time between the introduction of PEG 200 into the chamber and the first application of pressure was short. Long exposure of the closed channels to PEG 200 eliminated this response. This result indicates that PEG 200 could enter the inner volume of the chamber in the closed state of the channel and thus the openings of the cytoplasmic chamber exist in this state. This finding is supported by the recent molecular dynamics study of MscS revealing that the side openings did not assume a completely closed state in all simulations explored (Sotomayor and Schulten, 2004).
Double-pulse protocol
Each of the experiments presented above exploited a simplest experimental design: we applied suction pulses to the MscS channels in the presence of various cosolvents present on the cytoplasmic or/and periplasmic side of the membrane. In these experiments, we were able to observe changes in activation and inactivation of the channels resulting from preferential interactions of the cosolvents being in contact with them. Analysis of the data allowed for detection of the channel surface area changes upon transitions from the closed to open state and from the open to inactivated one. To observe changes of the channel surface upon transition from the inactivated to the closed state, which is a time-dependent process, we employed a double-pulse protocol. In this protocol, two identical suction pulses separated by 10–90 s were applied. We expected that Imax of the channel response to the second pulse might be affected in the presence of cosolvents. We tested glucose and ficoll, and we found that ficoll (n = 6) did not change the properties of the response. Glucose affected Imax (three experiments), and a set of traces from one of them is shown in Fig. 7 A. Both traces were recorded from the same patch perfused during an experiment with a solution containing 30 mM glucose. In this experiment, time intervals between pulses were 10, 20 (shown in Fig. 7 A), and 30 s. Imax2/Imax1 (Imax1 and Imax2 represent maximal currents upon responses to the first and second pulse, respectively) from this experiment are plotted against the time interval between the first and the second pulse (Fig. 7 B). The effect, as expected, was stronger when the time interval between the pulses was shorter. From the traces shown in Fig. 7 A, one can notice that glucose also affected inactivation (in the control, τ1 and τ2 were 0.27 s and 0.26 s, and in the presence of glucose 0.20 s and 0.10 s, respectively; τ1 and τ2 represent the inactivation time constants during the first and the second suction pulse, respectively), however, in other experiments a similar effect was not observed. The lack of consistency among all double-pulse experiments may be due to the protocol employed in which low suction pulses (sufficient just to activate all channels in the patch) were applied to ensure a fast recovery from an inactivated state. From our previous studies, we know that upon application of higher pressures, spontaneous process of inactivation was not observed (Koprowski and Kubalski, 1998).
In an additional double-pulse experiment that was performed in the presence of another small cosolvent, PEG 200, a similar effect on Imax was observed (not shown).
DISCUSSION
Addition of cosolvents to the biological reactive system modulates the reaction by a displacement of the system equilibrium. These additives change preferential interactions of the solvent components with the reacting protein, in our case with the channel molecule. All cosolvents used in this study belong to the group of cosolvents that are preferentially excluded from the channel surface and thereby cause the channel preferential hydration. Large cosolvents that did not enter the channel interior, except by causing a change in the channel free energy by being preferentially excluded from its external surface, also exerted their effect on the channel interior by reducing the activity of water inside the chamber. In these cases the observed effects consisted of two components: the stabilizing component, reflecting an increase of the channel surface free energy, and the volume-related component, a result of the difference in osmolarity between the channel interior and the external solution. The effects of large molecules of different chemical natures but applied at the same osmotic pressure (dextran and ficoll) should be similar if they depended on their osmotic gradient across the chamber wall. We have observed, however, that in their presence the rate of the channel inactivation was changed in an inversely proportional manner to their molecular weight (Figs. 4 and 5 C). This suggests that the preferential exclusion of cosolvents from the channel external surfaces was a predominant process in effect in these experiments.
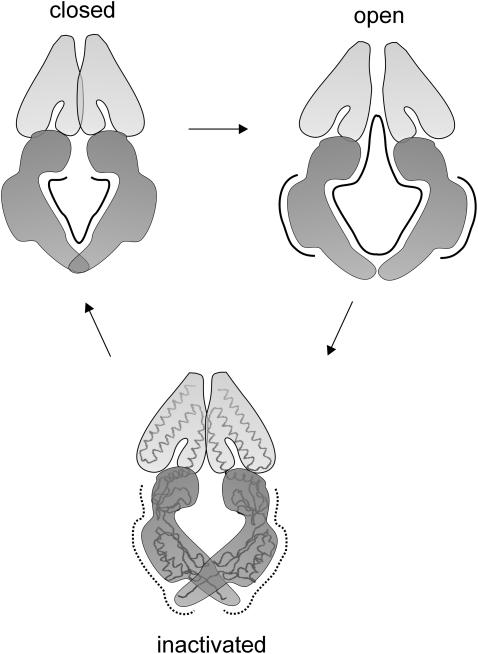
Surface changes of proteins are associated with changes of their conformations, which in the case of ion channels may reflect their functional states. Our results indicate that the surface changes of the MscS channel in the presence of cosolvents reflect conformational changes of the channel upon its activation, inactivation, and closure. When the cytoplasmic side of the membrane was exposed to nonpenetrating cosolvents, the process of inactivation was faster. Activation was impaired in their presence but to a lesser degree than in the presence of cosolvents that could enter the cytoplasmic chamber of the channel. Application of ficoll (a large-molecule cosolvent) from the extracellular side of the membrane slowed down the inactivation. Recovery of the channel from an inactivated state was slower when cosolvents of smaller molecular size were present at the cytoplasmic side of the membrane. Based on these results, we propose a model indicating which surface areas of the channel are affected when the channel goes from one state to another (Fig. 8). It has been recently suggested that the MscS crystal structure represents a nonconductive state of the channel (Anishkin and Sukharev, 2004). Another recent study revealed widening of the channel upon molecular dynamics simulation, when restraints imposed on the channel to keep it in the crystal structure conformation were abolished and the surface tension was applied (Sotomayor and Schulten, 2004). These data suggest that the channel may become larger than revealed by the crystal structure, and since the pore radius increases in the expanded conformation, this conformation may represent the open channel state. For these reasons, in our model (Fig. 8) the crystal form of the channel is presented as the conformation of its inactivated state. The solid lines in Fig. 8 indicate the surface areas of the channel which become larger upon the channel transition to the conformation indicated. The dotted lines indicate surfaces which become smaller. In summary:
Activation (represented by Imax) is associated with an increase of the area of the channel inner surface (the chamber and the transmembrane gate) accessible to small cosolvents and possibly with an increase of the volume of this entire part of the channel. Large cosolvents that interact with the outer side of the channel cytoplasmic domains also affected Imax but to a lesser degree.
Inactivation is associated with a decrease of the external surface of the cytoplasmic chamber of the channel and probably with a decrease of its volume. It is likely that the periplasm-exposed parts of the channel enlarge their surface upon inactivation.
Upon closure (a transition from inactivated to closed state), the channel increases its inner surface area.
FIGURE 8.
MscS surface areas change upon transitions between different conformational states as revealed by the channel behavior in the presence of cosolvents. Solid lines indicate the surfaces that expand upon transitions, and dotted lines those that shrink. Activation is associated with an increase of the outer and inner surfaces of the channel including the gate. The area of external surfaces of the channel decreases when it inactivates. Upon closure of the channel, the area of its inner surface decreases. The crystal form of MscS represents here the inactivated state.
We have applied glucose, ficoll, and dextran at the same osmotic pressure (760 mOsm/kgH2O) to the MscS channels from their cytoplasmic side. Glucose, the smallest molecule from all three cosolvents tested, was expected to enter the channel chamber and, according to the “osmotic stress” analysis, not affect the channel activity. Both dextran and ficoll, due to their high molecular weight, did not enter the channel chamber. Glucose affected mostly Imax, whereas dextran and ficoll decreased the rate of the channel inactivation. We hypothesized that size and not the chemical nature of the cosolvent molecule was the primary cause of the observed effects. Experiments with various-sized PEGs confirmed our hypothesis and implied that “steric exclusion” (Timasheff, 1998a) was a mechanism in effect in these experiments since molecular sizes of all cosolvents used were larger than water. The difference came from the fact that smaller cosolvents entered the chamber and were able to interact with the inner surface of the channel as well as with the pore region. The larger ones interacted with the external surface of the cytoplasmic chamber but to a different extent due to their size. It is worthwhile to note at this point that the single-channel amplitudes were not changed in the presence of small cosolvents. We did not observe a reduction of the channel conductance, and thus we may conclude that glucose and PEG 200 did not penetrate the MscS pore region. The Boltzmann curves fitted to the experimental points obtained before and after application of cosolvents were shifted toward higher levels of pressure. The shift of the midpoints of the Boltzmann curves was higher when larger-sized cosolvents were applied. This implies that in their presence the change in the rate of the channel inactivation had a more pronounced effect on the channel open probability than a decrease of Imax observed upon exposure of the channels to the smaller cosolvents. Glucose affected the transition from an inactivated to the closed state, and we argue that it counteracts a possible increase of the surface of the inner side of the channel (accessible to glucose) upon this transition. Cosolvents of larger size when applied from the cytoplasmic side of the membrane had no effect. This suggests that the primary changes in the surface area upon exiting the inactivated state occur within the channel ion-conducting pathway and they may be associated with rearrangements of the TM3 helices and their close proximities.
Small cosolvents penetrating the channel are effective in reduction of Imax when the channel undergoes a transition from the closed to open state. We assume that an increase of the channel inner surface, including the gate, is most significant upon activation. An increase of the external surface also occurs during activation, and it could be observed in the reduction of Imax in the presence of ficoll and dextran at lower applied pressures (Fig. 2 A). At higher pressures, Imax saturated and the effect of its reduction was not seen, but then we were able to observe the effect of the cosolvents on the rate of inactivation (Fig. 2, B and C).
From experiments on alamethicin channels, it is known that small PEGs that enter the channel pore did not influence channel activity, and all three large PEGs used (molecular weights 2000, 3400, and 17,000) changed the channel open probability in a similar fashion (Vodyanoy et al., 1993). At first glance, these results may seem to be incompatible to ours, since in our hands the effects of large cosolvents not entering the channel interior depended on their size. It is very likely that the difference reflects diversity of both channels in size and organization. Unlike MscS, alamethicin channels are composed of short membrane-spanning subunits lacking extramembranous domains (Fox and Richards, 1982) that preferably interact with large-molecule cosolvents.
Our experiments were performed in the presence of sorbitol, and we are aware that its presence also affected the channel behavior. In all our experimental solutions, summarized osmolarity of each cosolvent and sorbitol was maintained at a constant level of ∼460 mOsm/kgH2O (the total osmolarity of all solutions was kept constant and it was 760 mOsm/kgH2O). Sorbitol is smaller than glucose; their diameters are 5.8 Å and 7.5 Å, respectively, and they both can penetrate the channel interior. Addition of glucose to the sorbitol solution exerted a well-pronounced effect on the channel behavior, and we assume that in this case too “steric exclusion” was a principal source of the observed changes.
Acknowledgments
We thank Dr I. R. Booth from the University of Aberdeen for providing the E. coli strain used in this study.
This work was supported by grant KBN 6P04C 002 20 from the State Committee for Scientific Research and funding from the Nencki Institute of Experimental Biology. Piotr Koprowski was a fellow of the Foundation for Polish Science.
References
- Anishkin, A., and S. Sukharev. 2004. Water dynamics and dewetting transitions in the small mechanosensitive channel MscS. Biophys. J. 86:2883–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass, R. B., P. Strop, M. Barclay, and D. C. Rees. 2002. Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science. 298:1582–1587. [DOI] [PubMed] [Google Scholar]
- Bruner, L. J., and J. E. Hall. 1983. Pressure effects on alamethicin conductance in bilayer membranes. Biophys. J. 44:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, G., R. H. Spencer, A. T. Lee, M. T. Barclay, and D. C. Rees. 1998. Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science. 282:2220–2226. [DOI] [PubMed] [Google Scholar]
- Edwards, M. D., I. R. Booth, and S. Miller. 2004. Gating the bacterial mechanosensitive channels: MscS a new paradigm? Curr. Opin. Microbiol. 7:163–167. [DOI] [PubMed] [Google Scholar]
- Fox, R. O. Jr., and F. M. Richards. 1982. A voltage-gated ion channel model inferred from the crystal structure of alamethicin at 1.5-A resolution. Nature. 300:325–330. [DOI] [PubMed] [Google Scholar]
- Hamill, O. P., and B. Martinac. 2001. Molecular basis of mechanotransduction in living cells. Physiol. Rev. 81:685–740. [DOI] [PubMed] [Google Scholar]
- Jiang, X., G. C. Bett, X. Li, V. E. Bondarenko, and R. L. Rasmusson. 2003. C-type inactivation involves a significant decrease in the intracellular aqueous pore volume of Kv1.4 K+ channels expressed in Xenopus oocytes. J. Physiol. 549:683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski, P., and A. Kubalski. 1998. Voltage-independent adaptation of mechanosensitive channels in Escherichia coli protoplasts. J. Membr. Biol. 164:253–262. [DOI] [PubMed] [Google Scholar]
- Koprowski, P., and A. Kubalski. 2003. C termini of the Escherichia coli mechanosensitive ion channel (MscS) move apart upon the channel opening. J. Biol. Chem. 278:11237–11245. [DOI] [PubMed] [Google Scholar]
- Kubalski, A. 1995. Generation of giant protoplasts of Escherichia coli and an inner-membrane anion selective conductance. Biochim. Biophys. Acta. 1238:177–182. [DOI] [PubMed] [Google Scholar]
- Levina, N., S. Tötemeyer, N. R. Stokes, P. Louis, M. A. Jones, and I. R. Booth. 1999. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 18:1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinac, B. 2004. Mechanosensitive ion channels: molecules of mechanotransduction. J. Cell Sci. 117:2449–2460. [DOI] [PubMed] [Google Scholar]
- Miller, S., M. D. Edwards, C. Ozdemir, and I. R. Booth. 2003. The closed structure of the MscS mechanosensitive channel. Cross-linking of single cysteine mutants. J. Biol. Chem. 278:32246–32250. [DOI] [PubMed] [Google Scholar]
- Parsegian, V. A., R. P. Rand, N. L. Fuller, and D. C. Rau. 1986. Osmotic stress for the direct measurement of intermolecular forces. Methods Enzymol. 127:400–416. [DOI] [PubMed] [Google Scholar]
- Parsegian, V. A., R. P. Rand, and D. C. Rau. 2000. Osmotic stress, crowding, preferential hydration, and binding: a comparison of perspectives. Proc. Natl. Acad. Sci. USA. 97:3987–3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu, S. 2004. Estimating hydration changes upon biomolecular reactions from osmotic stress, high pressure, and preferential hydration experiments. Proc. Natl. Acad. Sci. USA. 101:1195–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotomayor, M., and K. Schulten. 2004. Molecular dynamics study of gating in the mechanosensitive channel of small conductance MscS. Biophys J. 87:3050–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukharev, S. I., and D. P. Corey. 2004. Mechanosensitive channels: multiplicity of families and gating paradigms. Sci. STKE. 2004:re4. [DOI] [PubMed]
- Sukharev, S. I., B. Martinac, V. Y. Arshavsky, and C. Kung. 1993. Two types of mechanosensitive channels in the Escherichia coli cell envelope: solubilization and functional reconstitution. Biophys. J. 65:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timasheff, S. N. 1998a. Control of protein stability and reactions by weakly interacting cosolvents: the simplicity of the complicated. Adv. Protein Chem. 51:355–432. [DOI] [PubMed] [Google Scholar]
- Timasheff, S. N. 1998b. In disperse solution, “osmotic stress” is a restricted case of preferential interactions. Proc. Natl. Acad. Sci. USA. 95:7363–7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timasheff, S. N. 2002. Protein-solvent preferential interactions, protein hydration, and the modulation of biochemical reactions by solvent components. Proc. Natl. Acad. Sci. USA. 99:9721–9726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodyanoy, I., S. M. Bezrukov, and V. A. Parsegian. 1993. Probing alamethicin channels with water-soluble polymers. Size-modulated osmotic action. Biophys. J. 65:2097–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg, J., F. Bezanilla, and V. A. Parsegian. 1990. Solute inaccessible aqueous volume changes during opening of the potassium channel of the squid giant axon. Biophys. J. 57:1049–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg, J., and V. A. Parsegian. 1986. Polymer inaccessible volume changes during opening and closing of a voltage-dependent ionic channel. Nature. 323:36–39. [DOI] [PubMed] [Google Scholar]