Abstract
Recent work by Veatch and Keller has described micron-scale liquid-liquid immiscibility in giant unilamellar vesicles composed of ternary mixtures of cholesterol, dipalmitoylphosphatidylcholine (DPPC), and dioleoylphosphatidylcholine (DOPC). Significantly, they do not observe micron-scale immiscibility in any of the three corresponding binary mixtures under the same conditions. It is shown here that this unexpected result can be accounted for by the formation of a complex between cholesterol and DPPC. The complex is miscible with DPPC and cholesterol, and immiscible with DOPC. A simple, idealized thermodynamic treatment of this model leads to theoretical ternary phase diagrams that are similar to the experimental diagram reported by Veatch and Keller. The model also accounts for significant qualitative features of the deuterium NMR spectra of these mixtures in bilayers.
A pair of recent studies (1, 2) provides new insight into intermolecular forces in bilayer membranes containing cholesterol and phospholipids. In these studies, it was found that although binary mixtures of cholesterol, dipalmitoylphosphatidylcholine (DPPC), or dioleoylphosphatidylcholine (DOPC) show no micron-scale liquid-liquid phase separation, ternary mixtures of these lipids do show this phase separation. This result suggests the formation of “condensed complexes” between cholesterol and DPPC, the complexes being immiscible with DOPC. Such complexes have been proposed previously in connection with the phase diagrams of cholesterol-phospholipid mixtures in monolayers (3, 4). Although a number of investigators have now observed liquid-liquid immiscibility in various ternary mixtures, the referenced work (1, 2) is of particular interest here since the investigators have not only determined the three-component phase diagram, but have also found two critical points at a single temperature and have established tie-lines, in part through the use of NMR.
The thermodynamic model used earlier to interpret cholesterol-phospholipid monolayer phase diagrams involves both intermolecular attractions (complex formation) and mean-field repulsions, leading to immiscibilities (3, 4). In a ternary mixture, there are three pairwise interactions of each kind, a potentially discouraging six parameters in all. However the monolayer results point unambiguously to the formation of complexes between cholesterol and saturated phospholipids with the longer fatty acid chains, such as DPPC. The monolayer data also indicate the absence of significant complex formation between cholesterol and unsaturated phospholipids such as DOPC (4). However, it is difficult to carry over the quantitative monolayer parameters to the bilayer case since both immiscibility parameters and equilibrium constants for complex formation are strongly dependent on monolayer pressure. In this report, we include a repulsion between DOPC and complex, and assume the other possible repulsions can be neglected.
The assumed form of the Gibbs free energy G is
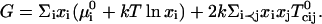 |
(1) |
Here the 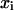 are the equilibrium concentrations (mole fractions) of the four components in the mixture: cholesterol, DPPC, DOPC, and complex. The standard chemical potentials of each component,
are the equilibrium concentrations (mole fractions) of the four components in the mixture: cholesterol, DPPC, DOPC, and complex. The standard chemical potentials of each component, 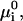 can be set equal to zero except for that of the complex, which is equal to
can be set equal to zero except for that of the complex, which is equal to 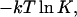 where K is the equilibrium constant for complex formation. The
where K is the equilibrium constant for complex formation. The 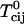 are critical temperatures for each ij binary pair. An important point in this work is that all these critical temperatures are set equal to zero, except for the pair complex-DOPC, now denoted simply
are critical temperatures for each ij binary pair. An important point in this work is that all these critical temperatures are set equal to zero, except for the pair complex-DOPC, now denoted simply 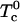 . The free energy then contains only two parameters: the equilibrium constant K and the critical temperature
. The free energy then contains only two parameters: the equilibrium constant K and the critical temperature 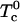 . For purposes of discussion, it is convenient to consider first the limiting case where the reaction of complex formation is complete,
. For purposes of discussion, it is convenient to consider first the limiting case where the reaction of complex formation is complete, 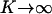 , and the immiscibility (repulsion) is large,
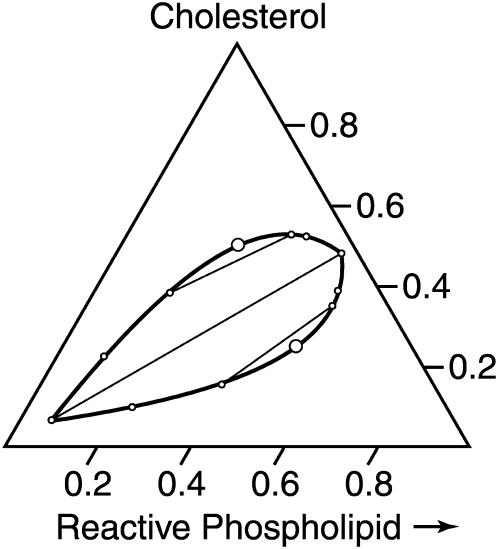
, and the immiscibility (repulsion) is large, 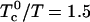 . In this case, the mixtures are simply ternary mixtures—DPPC, DOPC, and complex—or cholesterol, DOPC, and complex. The former corresponds to excess DPPC, and the latter to excess cholesterol. At the stoichiometric composition, the system is a binary mixture, complex and DOPC. The corresponding phase diagram is shown in Fig. 1.
. In this case, the mixtures are simply ternary mixtures—DPPC, DOPC, and complex—or cholesterol, DOPC, and complex. The former corresponds to excess DPPC, and the latter to excess cholesterol. At the stoichiometric composition, the system is a binary mixture, complex and DOPC. The corresponding phase diagram is shown in Fig. 1.
FIGURE 1.
Calculated phase diagram for a ternary mixture of cholesterol, a reactive phospholipid (DPPC), and an unreactive phospholipid (DOPC). The reaction is assumed to go to completion. The large dots give locations of the two critical points, and the straight lines illustrate tie-lines.
This calculated phase diagram has a striking similarity to those published in references 1 and 2 in that the tie-lines are inclined at 30° to the reactive phospholipid axis, and a line between the critical points is inclined 60° in the opposite direction. This slope of the tie-line is directly related to the assumed 1:1 stoichiometry of the complex. (The region below the stoichiometric tie-line corresponding to excess reactive phospholipid may change to a three-phase region if the reactive phospholipid is a solid.)
With increasing temperature, the two critical points (large open circles in Fig. 1) merge into a single upper critical point. The two-phase region vanishes at this point. In the elementary mean-field theory of critical points in binary mixtures, a critical point is reached when the entropy of mixing (given by the log terms in Eq. 1) just balances the energy of repulsion (term proportional to 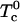 ). For very large K, this upper critical point is reached when the temperature is increased until
). For very large K, this upper critical point is reached when the temperature is increased until 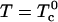 . The critical composition is then 1/3, 1/3, 1/3. However, the upper critical point can also be reached by thermal dissociation of the complexes. As an example, when the ratio
. The critical composition is then 1/3, 1/3, 1/3. However, the upper critical point can also be reached by thermal dissociation of the complexes. As an example, when the ratio 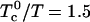 as in Fig. 1, the critical end-point is reached and the two-phase region disappears if the equilibrium constant decreases until
as in Fig. 1, the critical end-point is reached and the two-phase region disappears if the equilibrium constant decreases until 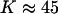 . The critical composition at this end-point is 0.371 mol fraction cholesterol, close to the classical critical composition 1/3 cited above. Of course, both
. The critical composition at this end-point is 0.371 mol fraction cholesterol, close to the classical critical composition 1/3 cited above. Of course, both 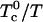 and K change with temperature. For example, in contrast to the extreme parameters used for Fig. 1, the two-phase region disappears and the critical end-point is reached with
and K change with temperature. For example, in contrast to the extreme parameters used for Fig. 1, the two-phase region disappears and the critical end-point is reached with 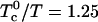 and
and 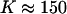 .
.
Significant additional insight into the molecular properties of these mixtures is provided by the deuterium NMR studies of Veatch et al. (2). These investigators measured quadrupole splittings in the deuterium NMR of labeled phospholipids and cholesterol as a function of temperature and bilayer composition. From such splittings, one can derive order parameters. In general, order parameters range between zero (no order) and 1 (complete order). In liquid crystals, the degree of order is intermediate between these extremes. To discuss the NMR results in terms of the model of condensed complexes, it is convenient to introduce the idea of relative order parameters S. In the case of the reactive phospholipid, we let 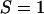 represent the order of a reactive phospholipid molecule that is in a complex, and
represent the order of a reactive phospholipid molecule that is in a complex, and 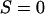 the order of the phospholipid molecule that is not complexed. To illustrate this approach, we describe below an illustrative calculation of order parameters for the reactive phospholipid molecules in a mixture such as cholesterol, DPPC, and DOPC.
the order of the phospholipid molecule that is not complexed. To illustrate this approach, we describe below an illustrative calculation of order parameters for the reactive phospholipid molecules in a mixture such as cholesterol, DPPC, and DOPC.
We assume an equilibrium constant of 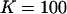 to form a 1:1 complex, and (as in Fig. 1)
to form a 1:1 complex, and (as in Fig. 1) 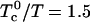 when
when 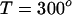 . Further, let the heat of reaction
. Further, let the heat of reaction 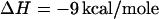 (4). The initial composition of the mixture corresponds to the stoichiometric critical composition, ∼0.372 mol fraction cholesterol, and 0.372 mol fraction reactive phospholipid. The critical temperature of this mixture is found to be Tc ≅ 311.8 K, substantially below
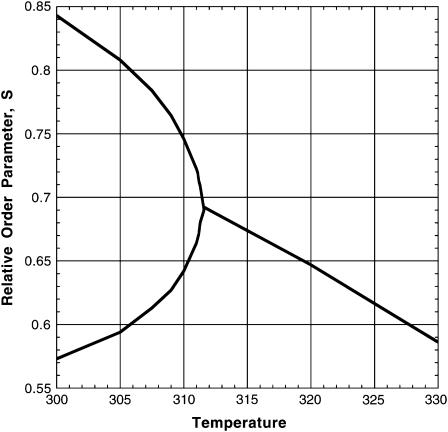
(4). The initial composition of the mixture corresponds to the stoichiometric critical composition, ∼0.372 mol fraction cholesterol, and 0.372 mol fraction reactive phospholipid. The critical temperature of this mixture is found to be Tc ≅ 311.8 K, substantially below 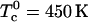 . This difference is due to thermal dissociation of the complex. The calculated relative order parameter plot is shown in Fig. 2. The plot in Fig. 2 has a striking qualitative similarity to the observed NMR quadrupole splittings (2). Above the critical temperature, there is a single average order parameter that decreases with increasing temperature due to thermal dissociation of the complex. Below the critical temperature, there are two order parameters, one for each phase. This splitting of the order parameter plot can be thought of in terms of two steps. In the first step, there is a phase separation to minimize repulsion between complex and unsaturated phospholipid. In the second step, the complex partially dissociates in the complex dilute phase. It is this step that leads to the reduction in order of one phase. (This reduction in order in one of the phases does not take place if the equilibrium constant is very large, e.g.,
. This difference is due to thermal dissociation of the complex. The calculated relative order parameter plot is shown in Fig. 2. The plot in Fig. 2 has a striking qualitative similarity to the observed NMR quadrupole splittings (2). Above the critical temperature, there is a single average order parameter that decreases with increasing temperature due to thermal dissociation of the complex. Below the critical temperature, there are two order parameters, one for each phase. This splitting of the order parameter plot can be thought of in terms of two steps. In the first step, there is a phase separation to minimize repulsion between complex and unsaturated phospholipid. In the second step, the complex partially dissociates in the complex dilute phase. It is this step that leads to the reduction in order of one phase. (This reduction in order in one of the phases does not take place if the equilibrium constant is very large, e.g., 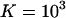 .) Unfortunately, these calculated order parameters are not easily related to all the observed quadrupole splittings due to the effects of rapid molecule exchange between phase separated domains when the domains are small (2, 5).
.) Unfortunately, these calculated order parameters are not easily related to all the observed quadrupole splittings due to the effects of rapid molecule exchange between phase separated domains when the domains are small (2, 5).
FIGURE 2.
Calculated relative order parameter S for the reactive phospholipid molecules as a function of temperature (K). The critical temperature and composition are ∼311.8° and 0.372 mol fraction for both cholesterol and reactive phospholipid. Below the critical point, two phases are present with the different order parameters.
We conclude that selective complex formation between cholesterol and DPPC coupled with a repulsion between the complex and DOPC is a useful first approximation for understanding the properties of these mixtures.
Acknowledgments
I thank Sarah Veatch and Sarah Keller for discussions.
References
- (1).Veatch, S. L., and S. L. Keller. 2003. Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol. Biophys. J. 85:3074–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Veatch, S. L., I. V. Polozov, K. Gawrisch, and S. L. Keller. 2004. Lipid domains in vesicles investigated by NMR and fluorescence microscopy. Biophys. J. 86:2910–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Radhakrishnan, A., and H. M. McConnell. 1999. Cholesterol-phospholipid complexes in membranes. Biophys. J. 77:1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).McConnell, H., and A. Radhakrishnan. 2003. Condensed complexes of cholesterol and phospholipids. Biochim. Biophys. Acta. 1610:159–173. [DOI] [PubMed] [Google Scholar]
- (5).Vist, M. R., and J. H. Davis. 1990. Phase equilibria of cholesterol/dipalmitoylphosphatidylcholine mixtures: 2H nuclear magnetic resonance and differential scanning calorimetry. Biochemistry. 29:451–464. [DOI] [PubMed] [Google Scholar]